Optic nerve injuries cause death of retinal ganglion cells and degeneration of their axons. Vigneswara et al. show that combined delivery of caspase-2 and caspase-6 inhibitors into the eye promotes retinal ganglion cell survival and axon regeneration in a rodent model, an effect that is mediated through the JAK/STAT pathway.
Keywords: CASP2, CASP6, axon regeneration, apoptosis, retinal ganglion cells, optic nerve
Abstract
We have previously shown that crushing the optic nerve induces death of retinal ganglion cells by apoptosis, but suppression of CASP2, which is predominantly activated in retinal ganglion cells, using a stably modified short interfering RNA CASP2, inhibits retinal ganglion cell apoptosis. Here, we report that combined delivery of short interfering CASP2 and inhibition of CASP6 using a dominant negative CASP6 mutant activates astrocytes and Müller cells, increases CNTF levels in the retina and leads to enhanced retinal ganglion cell axon regeneration. In dissociated adult rat mixed retinal cultures, dominant negative CASP6 mutant + short interfering CASP2 treatment also significantly increases GFAP+ glial activation, increases the expression of CNTF in culture, and subsequently increases the number of retinal ganglion cells with neurites and the mean retinal ganglion cell neurite length. These effects are abrogated by the addition of MAB228 (a monoclonal antibody targeted to the gp130 component of the CNTF receptor) and AG490 (an inhibitor of the JAK/STAT pathway downstream of CNTF signalling). Similarly, in the optic nerve crush injury model, MAB228 and AG490 neutralizes dominant negative CASP6 mutant + short interfering CASP2-mediated retinal ganglion cell axon regeneration, Müller cell activation and CNTF production in the retina without affecting retinal ganglion cell survival. We therefore conclude that axon regeneration promoted by suppression of CASP2 and CASP6 is CNTF-dependent and mediated through the JAK/STAT signalling pathway. This study offers insights for the development of effective therapeutics for promoting retinal ganglion cell survival and axon regeneration.
Introduction
Unlike PNS axons, CNS axons fail to regenerate after injury. Many growth-limiting factors have been implicated, including apoptosis of axotomized neurons, limiting supplies of neurotrophic factors and the presence of an axon growth inhibitory environment in the CNS neuropil. Optic nerve injury induces progressive retinal ganglion cell (RGC) death (Villegas-Perez et al., 1993; Berkelaar et al., 1994; Kermer et al., 1998), so that 70–75% of RGCs are lost within 7 days (Berry et al., 1996, 1999; Agudo et al., 2008; Ahmed et al., 2011; Vigneswara et al., 2012) and 80–90% by 28 days, primarily as a result of apoptosis (Garcia-Valenzuela et al., 1994; Rabacchi et al., 1994; Isenmann et al., 1997). Caspases are a family of cysteine-dependent proteases well known for their orchestration of apoptosis, although they also have non-apoptotic roles. They are expressed as pro-caspases and activated by either proximity-induced dimerization (initiator caspases) or proteolytic cleavage (effector caspases) (Pop and Salvesen, 2009). This enables active caspase enzymes to cleave regulatory proteins that have roles in DNA replication (Casciola-Rosen et al., 1994; Song et al., 1996), DNA repair (Lazebnik et al., 1994), cell survival signalling (Leist et al., 1997), cytoskeletal reorganization and cellular disassembly (Porter et al., 1997; Bokoch, 1998).
CASP3 and CASP9 are regarded as crucial in apoptosis of RGC after axotomy (Kermer et al., 1998, 1999, 2000; Chaudhary et al., 1999; Weishaupt et al., 2003); however, after optic nerve crush, cleaved CASP3 is expressed in cells of the inner nuclear layer of the retina but is not present in RGCs. Instead, RGCs exclusively express cleaved CASP2 (Ahmed et al., 2011; Vigneswara et al., 2012) and suppression of CASP2, by either a chemically stabilized CASP2 short interfering RNA (siCASP2) or a pharmacological inhibitor, significantly protects RGCs from death for at least 14 days after injury (Ahmed et al., 2011; Vigneswara et al., 2012). Despite significant RGC neuroprotection by both of these pharmacological strategies, RGC axon regeneration is unaffected, suggesting that different signalling pathways regulate neuron survival and axon regeneration.
It was recently claimed that CASP6 and CASP8 are upregulated in RGCs after optic nerve transection and that inhibition of both caspases with pharmacological inhibitors promotes RGC survival and axon regeneration (Monnier et al., 2011). This suggests a possible role for CASP6 and CASP8 in the cellular pathways that regulate both RGC survival and axon regeneration. However, pharmacological caspase inhibitors lack specificity and may lead to spurious conclusions (Berger et al., 2006; Timmer and Salvesen, 2007; McStay et al., 2008). In addition, our previous attempts to demonstrate immunolocalization of cleaved CASP6 or cleaved CASP8 in RGC after optic nerve crush, using the same antibody sources as previously described (Monnier et al., 2011), have failed to yield positive results (Ahmed et al., 2011; Vigneswara et al., 2012). Despite the absence of cleaved CASP6 and cleaved CASP8 in RGCs and their low abundance in the retina, we have observed slight increases in retinal levels after optic nerve crush (Vigneswara et al., 2012). This suggests that CASP6 and CASP8 are modulated in response to optic nerve crush in other cells of the retina, such as Müller cells and astrocytes, thereby producing indirect effects on RGCs.
Müller cells and astrocytes of the retina undergo reactive gliosis after optic nerve crush, which is characterized by proliferation, changes in cell shape caused by altered intermediate filament production and changes in gene expression (MacLaren, 1996; Dyer and Cepko, 2000; Kirsch et al., 2010). In addition, Müller cells and astrocytes produce CNTF and LIF, key anti-apoptotic and axogenic factors for RGC, in areas affected by injury, which in turn activate downstream JAK/STAT3 signalling locally by autocrine or paracrine mechanisms (Winter et al., 1995; Escartin et al., 2006, 2007). Both of these cytokines share the signal transduction subunits gp130 and LIF receptor β (LIFRβ), which constitute a functional LIF receptor complex along with the CNTF receptor to activate the JAK/STAT3 pathway. Increased CNTF levels reinforce Müller cell and astrocyte hypertrophy and GFAP expression, in turn enhancing the endogenous levels of CNTF (DeChiara et al., 1995; Kahn et al., 1995; Winter et al., 1995; Escartin et al., 2006, 2007; Kirsch et al., 2010).
As CASP6 activity is required for axon degeneration of other neuron types induced by trophic factor deprivation and cerebral ischaemia (Nikolaev et al., 2009; Akpan et al., 2011; Simon et al., 2012), it is possible that it may also take part in indirectly blocking RGC axon regeneration by attenuating glial activation and CNTF production. Hence, we hypothesize that suppression of CASP6, leading to increased levels of CNTF in the retina as a consequence of enhanced glial activation after optic nerve crush, will enhance RGC axon regeneration. Here, we delivered a specific inhibitor of CASP6 (CASP6 dominant negative, C6DN), coupled to a cell penetrating peptide, Penetratin 1 (Pen1) either alone or in combination with a chemically stabilized siRNA to CASP2 (siCASP2) and measured the effects on RGC neuroprotection and axon regeneration. We showed that CASP2 inhibition provided significantly more RGC neuroprotection after optic nerve crush than CASP6 inhibition. Moreover, the combination of C6DN and siCASP2 did not potentiate RGC survival over that observed with siCASP2 alone. However, when both caspases were simultaneously inhibited, RGC axon regeneration was indirectly and significantly increased through activation of retinal glial-dependent CNTF secretion. We have, therefore, elucidated a novel mechanism by which combined inhibition of CASP2 and CASP6 promotes both RGC survival and axon regeneration after optic nerve injury.
Materials and methods
Optic nerve crush
All animal procedures were licensed and approved by the UK Home Office and the University of Birmingham Ethical Review Committee. Adult, female 6–8-week-old Sprague-Dawley rats (180–220 g) were anaesthetized with isofluorane inhalation anaesthesia (Janssen Pharmaceuticals), the optic nerve was exposed through a supra-orbital approach and crushed bilaterally 2 mm from the globe using watchmaker’s forceps as described previously (Berry et al., 1996, 1999; Ahmed et al., 2005, 2006, 2010; Logan et al., 2006; Douglas et al., 2009; Vigneswara et al., 2012). None of the animals developed cataracts, confirming that the lens had not been injured during surgery.
Penetratin 1–CASP6 dominant negative
The mutant CASP6 (Cys163Ala) dominant negative (C6DN) expression construct was a kind gift of G. S. Salvesen, Sanford-Burnham Institute, La Jolla, CA. C6DN was purified in our laboratories as previously described (Denault and Salvesen, 2003). Pen1 was custom synthesized by PolyPeptide Laboratories. Pen1 and C6DN were linked by incubating equimolar amounts at 37°C for 24 h to generate disulphide bonds. Linkage was confirmed by non-reducing 20% PAGE with western blotting using anti-His antibodies.
In vivo experiments
In the preliminary Pen1-C6DN dose-finding experiment, groups comprised six rats/treatment (i.e. 12 eyes/treatment): (i) intact; (ii) optic nerve crush (ONC) + Pen1 (vehicle control) (Pen1, 0 µM C6DN); (iii) 2 µM Pen1-C6DN; (iv) 4 µM Pen1-C6DN; (v) 5 µM Pen1-C6DN; and (vi) 7 µM Pen1-C6DN. To monitor CASP2 and CASP6 activation over the first 7 days, groups of six rats/treatment (12 eyes/treatment) were sacrificed at 4 and 7 days after optic nerve crush, whereas a further three rats (six eyes) were used as intact controls. In further experiments, groups of six rats/treatment (12 eyes/treatment) were used to determine the effects of siCASP2 (gift from Quark Pharmaceuticals) and pre-optimized Pen1-C6DN on the levels of CASP2 and cleaved Lamin A/C, a substrate for active CASP6 and comprised: (i) ONC + Pen1; (ii) ONC + Pen1-C6DN; (iii) ONC + Pen1 + siCASP2; and (iv) ONC + Pen1-C6DN + siCASP2. To determine the effects of siCASP2 and pre-optimized Pen1-C6DN singly and in combination on RGC survival, groups of six rats/treatment (12 eyes/treatment) were used and groups comprised: (i) ONC + PBS; (ii) ONC + Pen1; (iii) ONC + Pen1-C6DN; (iv) ONC + Pen1 + siCASP2; (v) ONC + Pen1-C6DN + siCASP2; and (vi) intact controls. To determine the effects of siCASP2 and pre-optimized Pen1-C6DN on RGC axon regeneration and Müller cell activation in the retina six rats/treatment (12 eyes/treatment) were used and groups comprised: (i) ONC + Pen1; (ii) ONC + Pen1-C6DN; (iii) ONC + Pen1 + siCASP2; and (iv) ONC + Pen1-C6DN + siCASP2. To determine the levels of CNTF in the eye, groups of six rats/treatment were used and groups comprised: (i) ONC + Pen1; (ii) ONC + Pen1-C6DN; (iii) ONC + Pen1 + siCASP2; and (iv) ONC + Pen1-C6DN + siCASP2. To determine the effects of MAB228 and AG490 on RGC survival, axon regeneration, retinal Müller cell activation and CNTF localization, groups of 12 rats/treatment (six rats for Fluoro-Gold™ labelling and six rats for GAP43 and GFAP/CNTF immunohistochemistry) were used and groups comprised: (i) ONC + Pen1-C6DN + siCASP2 + IgG; (ii) ONC + Pen1-C6DN + siCASP2 + MAB228; and (iii) ONC + Pen1-C6DN + siCASP2 + AG490. Finally, to determine the levels of CNTF in the eye after treatment with MAB228 and AG490, groups of six rats/treatment were used and groups comprised: (i) ONC + Pen1-C6DN + siCASP2 + IgG; (ii) ONC + Pen1-C6DN + siCASP2 + MAB228; and (iii) ONC + Pen1-C6DN + siCASP2 + AG490.
Intravitreal injections
In a preliminary experiment, Pen1-C6DN was titrated to determine the optimal dose required to promote maximal RGC survival. Immediately after optic nerve crush, animals (n = 12 eyes/group) received Pen1 vehicle or increasing concentrations of C6DN from 2, 4, 5 and 7 μM Pen1-C6DN using glass micropipettes. In further experiments, 5 μM of Pen1 or Pen1-C6DN was injected. Intravitreal injections were repeated every 7 days based on previous experiments with other caspase inhibitors (Ahmed et al., 2011). Twenty micrograms per eye of siCASP2 was injected along with either 5 μM Pen1 or Pen1-C6DN. The optimal dose of MAB228 was predetermined by its ability to reduce CNTF levels in treated eyes, with optimal concentrations determined as 5 μg/eye (not shown), the inhibitor of Janus kinase 2 (JAK) AG490 was injected at 17 mM/eye (Muller et al., 2007) and CNTF was injected at a dose of 1.5 μg/eye (Muller et al., 2007). All intravitreal injections were made up in a final volume of 5 μl/eye and repeated every 7 days.
Retinal whole mounts
At 19 days after optic nerve crush, 2 μl of 4% Fluoro-Gold™ (Cambridge Bioscience) in PBS was injected into the optic nerve, between the lamina cribrosa and the site of optic nerve crush in all experimental groups (Berry et al., 1996, 1999; Ahmed et al., 2010, 2011; Vigneswara et al., 2012). Animals were sacrificed 48 h later and intracardially perfused with 4% formaldehyde (TAAB Laboratories) in PBS. Retinae were harvested and flat mounted onto Superfrost™ Plus microscope slides (VWR International). Retinal whole mounts were dried onto glass slides and mounted in VectaMount™ (Vector Laboratories). Samples were then randomized by a second investigator and photographs were captured using a Zeiss Axioplan 2 fluorescent microscope equipped with a digital Axiocam HRc camera, controlled through Axiovision 4 software (all from Zeiss). The number of Fluoro-Gold™-labelled RGCs was counted using the automated particle counting software in ImagePro (Version 6.0) (Media Cybernetics) from photographs of 12 rectangular (0.36 × 0.24 mm) areas/retina, three from each retinal quadrant, placed radially at inner (1/6 eccentricity), mid-periphery (1/2 eccentricity) and outer retina (5/6 eccentricity) from the centre of the optic disc. The number of Fluoro-Gold™-labelled RGCs in the 12 images/retina were divided by the counting area and resultant numbers were pooled to calculate mean densities of Fluoro-Gold™-labelled RGCs/mm2 for each retina (Peinado-Ramon et al., 1996; Vigneswara et al., 2012).
Anterograde labelling of regenerating retinal ganglion cell axons with rhodamine B isothiocyanate
Anterograde labelling of regenerating axons using rhodamine B isothiocyanate (RITC; Sigma) was performed as described previously (Thanos et al., 1987). Briefly, 5 μl of a 2.5% solution of RITC was intravitreally injected using glass micropipettes 2 days before sacrifice. Animals were killed by overdose of CO2, perfused with 4% formaldehyde in PBS and the optic nerves were prepared for cryosectioning as described below.
Tissue preparation and sectioning
After intracardiac perfusion with 4% formaldehyde in PBS, eyes and optic nerve were removed and prepared as described previously (Douglas et al., 2009; Ahmed et al., 2010; Vigneswara et al., 2012). Briefly, eyes and optic nerves were post-fixed in 4% formaldehyde (TAAB) in PBS, incubated in a graded series of sucrose solutions in PBS and then embedded in O.C.T. mounting medium (Raymond A Lamb Ltd) before freezing at −80°C. Later, 15-µm thick parasagittal and longitudinal sections of eye and optic nerve, respectively, were cut on a cryostat (Bright Instruments), adhered onto glass slides and stored at −20°C until required.
Immunohistochemistry
Immunohistochemistry was performed on sections of retina and optic nerve as described previously (Douglas et al., 2009; Ahmed et al., 2010, 2011; Vigneswara et al., 2012). Briefly, sections were washed in PBS and non-specific binding was blocked for 20 min before incubation with the relevant primary antibody. Monoclonal anti-GAP43 (1:500 dilution in PBS containing 3% bovine serum albumin and 0.05% Tween-20; Invitrogen) was used to localize regenerating axons; monoclonal anti-GFAP and a polyclonal anti rat-CNTF (1:500; Promega) was used to stain for astrocytes and CNTF in retinal sections and in retinal cultures; monoclonal anti-βIII-tubulin (1:200; Sigma) was used to stain for RGC and their neurites in retinal cultures. Sections were washed in PBS and incubated with appropriate Alexa Fluor® 488 and Texas Red®-labelled secondary antibody (Invitrogen) for 1 h at room temperature, washed, mounted in Vectashield® mounting medium with DAPI (Vector Laboratories) and examined under an Axioplan2 epifluorescent microscope (Zeiss).
Quantification of Müller cell processes
Retinal Müller cell activation was quantified as described previously (Ahmed et al., 2010). Briefly, after GFAP immunohistochemistry, GFAP+ Müller cell processes were counted along a 250 µm horizontal line in retinal sections, placed orthogonal to the radial plane through the middle of the internal plexiform layer. The mean GFAP+ cell counts for each condition (n = 12 retinal sections/condition) were calculated and expressed as mean ± SEM.
Adult rat retinal cultures
Five days after optic nerve crush and intravitreal treatments of pre-optimized reagents (concentrations detailed above), animals (n = 6 eyes/group) were sacrificed by CO2 overdose, retinae harvested and dissociated using a Papain dissociation system following the manufacturer’s instructions (Worthington Biochemical), as described previously (Ahmed et al., 2006, 2010; Logan et al., 2006; Douglas et al., 2009; Vigneswara et al., 2013). Retinal cells from untreated rats were also prepared in a similar way. Retinal cells were plated at a density of 125 × 103/well into 8-well chamber slides precoated with laminin and poly-D-lysine and cultured in 300 μl Neurobasal®-A medium supplemented with B27 supplement and gentimicin (all from Invitrogen). Cells were cultured for 4 days at 37°C and 5% CO2 before either removal of culture medium and cells for ELISA (see below for description) or fixation in 4% formaldehyde in PBS for immunocytochemistry. Experiments comprised of n = 3 wells/treatment and each experiment was repeated on three separate occasions and hence results for each data point are the mean ± SEM from nine wells/treatment.
Immunocytochemistry
Fixed cells were immunostained for GFAP and βIII-tubulin to determine the number of GFAP+ astrocytes and Müller cells and to measure the number of RGC with neurites and the neurite lengths, as described previously (Ahmed et al., 2010). Briefly, cells were washed in PBS, permeabilized and blocked using PBS containing 3% bovine serum albumin and 0.1% Triton™ X-100 for 30 min at room temperature before incubation with the mouse anti-GFAP (1:500 dilution, Sigma) or mouse anti-βIII-tubulin antibodies (1:200 dilution, Sigma) for 1 h at room temperature in a humidified chamber. Cells were then washed in PBS and incubated for 1 h at room temperature with Alexa Fluor® 488 anti-mouse IgG (1:400 dilution; Invitrogen). After further washes in PBS, sections were mounted under coverslips using Vectamount® containing DAPI (Vector Laboratories) and viewed under a Zeiss Axioplan 2 fluorescent microscope equipped with an Axiocam HRc and Axiovision software, as described earlier. Immunocytochemistry controls with primary antibody omitted were included in each run and the negative control slides were used to set the background threshold levels for non-specific staining (not shown) during image capture.
Retinal ganglion cell survival and neurite outgrowth and quantification of astrocyte activation
The mean number of surviving βIII-tubulin+ RGCs, mean neurite length, the number of RGCs with neurites and the number of GFAP+ astrocytes/Müller cells were quantified as described previously (Ahmed et al., 2006, 2010; Logan et al., 2006; Douglas et al., 2009; Vigneswara et al., 2013). Briefly, each anonymized chamber slide was divided into nine quadrants and images of RGCs, their neurites and GFAP+ astrocytes/Müller cells were captured randomly from each quadrant. Axiovision (Zeiss) was then used to measure the neurite lengths, whereas ImagePro (Version 6.3; Media Cybernetics) was used to quantify the number of βIII-tubulin+ RGC with neurites longer than the RGC diameter and the number of GFAP+ astrocytes/Müller cells. Neurite outgrowth from at least 180 RGCs/treatment was measured, except in untreated and Pen1-vehicle treated cultures in which 100 RGCs were assessed (i.e. all RGCs that grew neurites longer than the RGC diameter).
Enzyme-linked immunosorbent assay for CNTF
For detection of CNTF in vitro, cultured cells and culture medium were homogenized in cell lysis buffer and clarified by centrifugation. Lysates were then assayed for CNTF and compared to culture medium-only conditions to account for any background CNTF. Retinae harvested from in vivo experiments were homogenized in cell lysis buffer, clarified by centrifugation and the supernatant was frozen at −20°C until required for assay. A commercially available rat CNTF ELISA kit (R&D Systems) was used to detect CNTF in cultured retinal cell lysates, following the manufacturer’s instructions.
Protein extraction and western blotting
Six rats (12 eyes/treatment) were sacrificed by an overdose of CO2 and total protein from retinae and where appropriate, optic nerve, were extracted in cell lysis buffer and processed for western blotting as previously described (Ahmed et al., 2005, 2006, 2010). Western blots were probed overnight at 4°C with antibodies against: rabbit anti-human CASP2 (Abcam); rabbit anti-Lamin A/C and rabbit anti-human GAPDH, all from Cell Signalling Technology. Relevant protein bands were detected with an appropriate HRP-labelled secondary antibody (GE Healthcare) and detected using an enhanced chemiluminescence system (ECL) (GE Healthcare). Blots were stripped and re-probed as required.
Densitometry
Western blots were quantified by densitometry as described previously (Ahmed et al., 2006, 2010; Douglas et al., 2009). Briefly, blots were scanned into Adobe Photoshop and TIFF files were analysed in ScionImage (version 4.0.2, Scion Corp.) using the built-in gel plotting macros. The integrated density of each band of interest in each lane was calculated for three separate blots from three independent experiments.
Statistical analysis
The significance of differences between sample means were calculated using GraphPad Prism (GraphPad Software Inc., Version 4.0) by one-way ANOVA followed by post hoc testing with Dunnett’s method.
Results
Characterization of a specific inhibitor of CASP6
To determine the functional relevance of the upregulation of cleaved CASP6 expression after optic nerve crush we inhibited CASP6 activity in the retina. Currently available small molecule caspase inhibitors are not specific enough to dissect the contribution of individual caspases (McStay et al., 2008). We used a previously characterized C6DN construct (Edgington et al., 2012) to block activation/activity of CASP6. For access into the retina, C6DN was disulphide-linked to Pen1, a cell-penetrating peptide (Davidson et al., 2004), the disulphide bond is broken by the reducing environment of the cell cytoplasm, thus releasing the peptide cargo and allowing it to act at its cellular target.
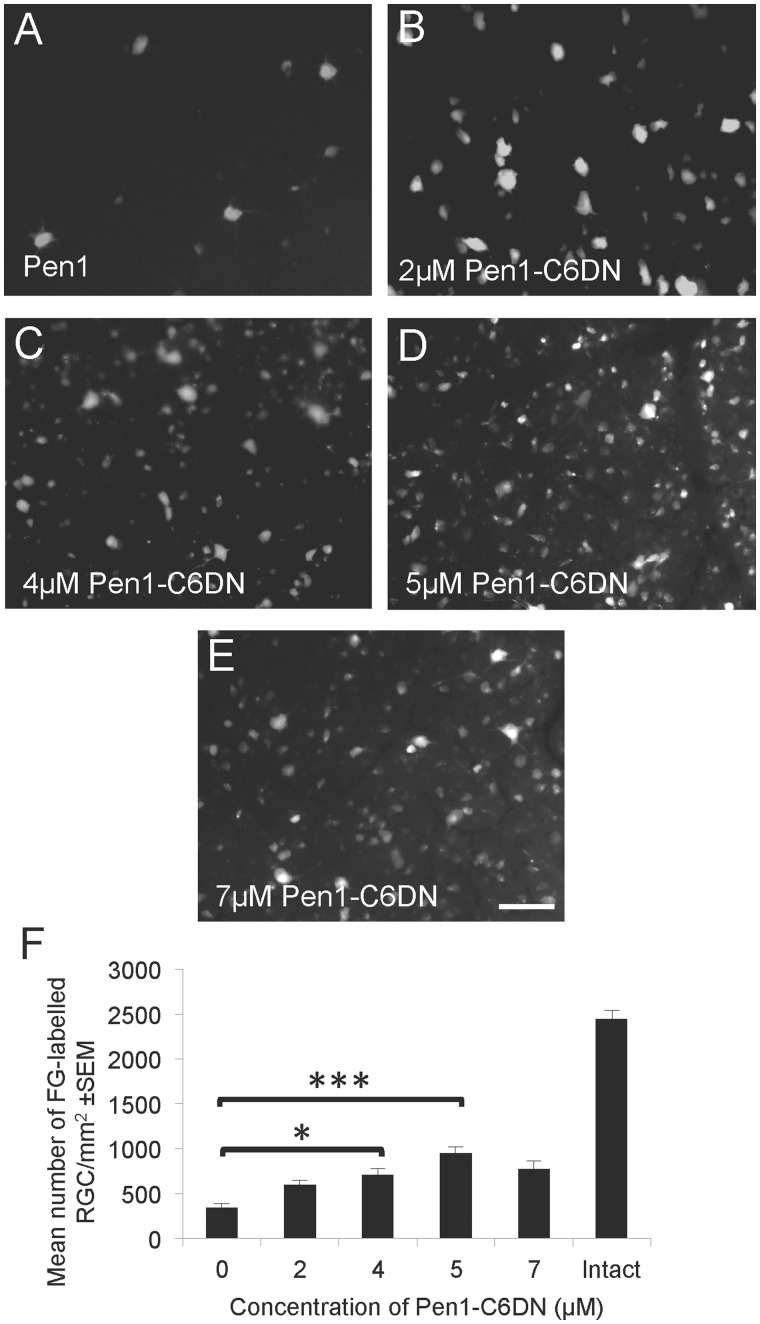
The neuroprotective efficacy of this construct was examined by assessing RGC survival after optic nerve crush and intravitreal delivery of a range of doses of Pen1-C6DN, by counting the number of Fluoro-Gold™ back-labelled RGCs in retinal whole mounts. Compared to the vehicle treatment (Pen1), which left 400 ± 45 Fluoro-Gold™-labelled RGCs/mm2, Pen1-C6DN (2, 4 and 5 μM) caused a dose-dependent statistically significant increase in the numbers of surviving Fluoro-Gold™-labelled RGC, to a maximal level of 987 ± 65 RGCs/mm2 (Fig. 1A–F). Concentrations of Pen1-C6DN >5 μM did not significantly increase the number of Fluoro-Gold™-labelled RGC; thus, maximal RGC protection (60%) was observed with 5 μM Pen1-C6DN. These results demonstrate that specific blockade of CASP6 activity, using Pen1-C6DN, significantly enhances RGC survival.
Figure 1.
Dose response for Pen1-C6DN. Optic nerves were crushed and the retinae treated with a range of doses of Pen1-C6DN from 0–7 μM C6DN at Days 0, 7 and 14 after optic nerve crush. At 19 days after optic nerve crush Fluoro-Gold™ (FG) was injected into the proximal optic nerve stump and allowed to retrogradely fill surviving RGCs. Two days later, animals were killed, retinae were harvested, whole mounts were made and the number of Fluoro-Gold™ labelled RGCs was quantified by image analysis. (A–E) Representative photomicrographs of Fluoro-Gold™-labelled RGC showing increased RGC survival with increasing concentrations of Pen1-C6DN up to 5 μM. (F) Quantification to demonstrate that 5 μM Pen1-C6DN promoted optimal RGC survival. *P < 0.05, ***P < 0.0001. Scale bar = 50 μm.
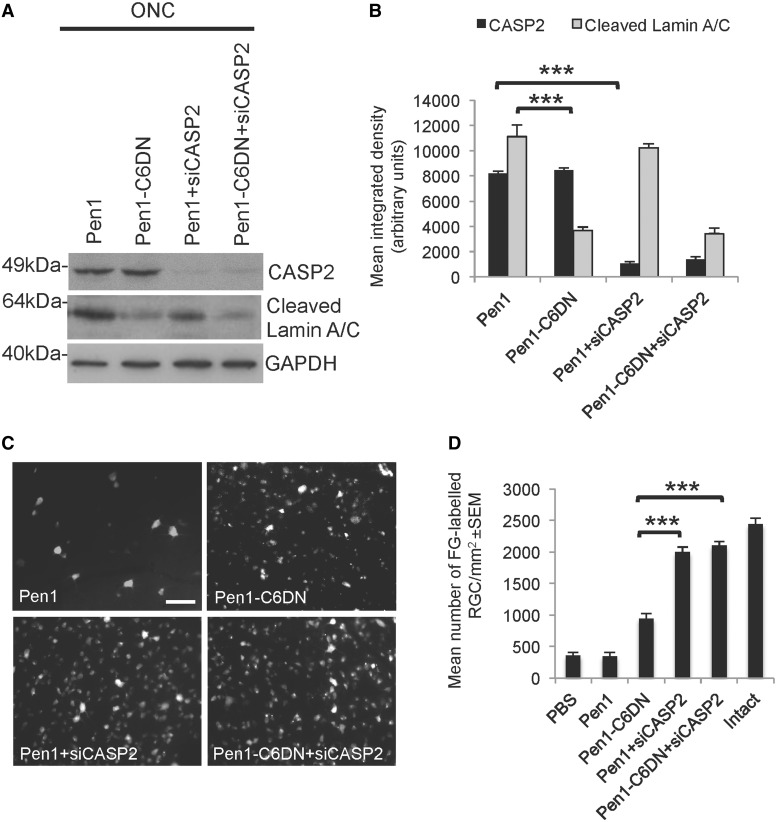
To ensure that each intervention was affecting only the targeted caspase, we examined the expression of CASP2 in retinae treated with Pen1 and Pen1-C6DN by western blot and detected no decrease in CASP2 levels (Fig. 2A and B). Pen1-C6DN decreased basal cleaved Lamin A/C (a substrate of CASP6; Orth et al., 1996; Takahashi et al., 1996; Ruchaud et al., 2002; Mintzer et al., 2012) levels whereas, in Pen1 and Pen1 + siCASP2-treated retinae, basal cleaved Lamin A/C levels were unaffected (Fig. 2A and B), indicating constitutive CASP6 activity in non-RGCs as we were previously unable to demonstrate immunoreactive CASP6 in RGCs after optic nerve crush (Ahmed et al., 2011; Vigneswara et al., 2012). These results demonstrate that our CASP2 and CASP6-specific inhibitors do not have cross reactivity but that each specifically regulates their targeted caspase.
Figure 2.
C6DN and siCASP2 promote significant RGC survival. Five micromolar Pen1-C6DN was intravitreally delivered either alone or in combination with siCASP2 immediately after ONC and at 7 and 14 days after optic nerve crush. At 21 days, animals were killed and retinae were harvested either for western blot analysis or to count the number of Fluoro-Gold™ (FG)-labelled RGC in retinal whole mounts. (A and B) Western blot and subsequent densitometry to show that suppression of CASP6 using 5 μM Pen1-C6DN prevents cleavage of Lamin A/C whereas siCASP2 suppresses the levels of CASP2 in treated eyes. (C and D) Fluoro-Gold™ labelling of RGC to demonstrate that Pen1-C6DN protects ∼50% of RGC from apoptosis whereas intravitreal delivery of either Pen1 + siCASP2 or Pen1-C6DN + siCASP2 promoted >95% of RGC survival. GAPDH was used as a protein loading control. ***P < 0.0001. Scale bar: C = 50 μm.
To assess the neuroprotective properties of the combined Pen1-C6DN and siCASP2, we intravitreally injected optimal doses of Pen1-C6DN together with our previously optimized dose of siCASP2 (Ahmed et al., 2011) after optic nerve crush, and 21 days later assessed the number of Fluoro-Gold™ backfilled surviving RGCs. In eyes treated with the vehicle control (Pen1), 405 ± 34 RGCs/mm2 remained at 21 days after optic nerve crush (Fig. 2C and D), whereas in Pen1-C6DN treated eyes 979 ± 34 RGCs/mm2 remained (Fig. 2C and D). However, Pen1 + siCASP2 protected 2145 ± 54 RGCs/mm2, whereas Pen1-C6DN + siCASP2 did not significantly improve RGC neuroprotection over that observed for siCASP2 alone (Fig. 2C and D). Compared to intact controls, Pen1-C6DN promoted 60% RGC neuroprotection whereas siCASP2 alone or Pen1-C6DN + siCASP2 protected >95% of RGC from apoptosis at 21 days after optic nerve crush. These results suggest that both Pen1 + siCASP2 and Pen1-C6DN + siCASP2 promote optimal RGC survival.
Pen1-C6DN + siCASP2 promote retinal ganglion cell axon regeneration
Most work supports the contention that active CASP2 and CASP6 modulate different signalling pathways, thus we tested whether simultaneous blocking of these caspases using a combined treatment of Pen1-C6DN and siCASP2 would promote RGC axonal regeneration. We have previously shown that siCASP2 decreased CASP2 expression by almost 90% after optic nerve crush but there was little associated RGC axonal regeneration (Ahmed et al., 2011). In other models of axonal degeneration (trophic factor deprivation and stroke) CASP6 activation initiates axonal degeneration (Nikolaev et al., 2009; Akpan et al., 2011). To determine whether CASP6 might have a similar function in RGC degeneration, we inhibited CASP6 function using C6DN and combined this with delivery of siCASP2 to promote optimal RGC survival and thus enhance the possibility of reduced RGC loss and axon degeneration. At 7 and 14 days after optic nerve crush, animals received intravitreal injections of siCASP2 and/or Pen1-C6DN, and we analysed RGC axon regeneration through the crush site at 21 days after optic nerve crush using antibodies against GAP43, a marker for regenerating axons (Berry et al., 1996; Leon et al., 2000).
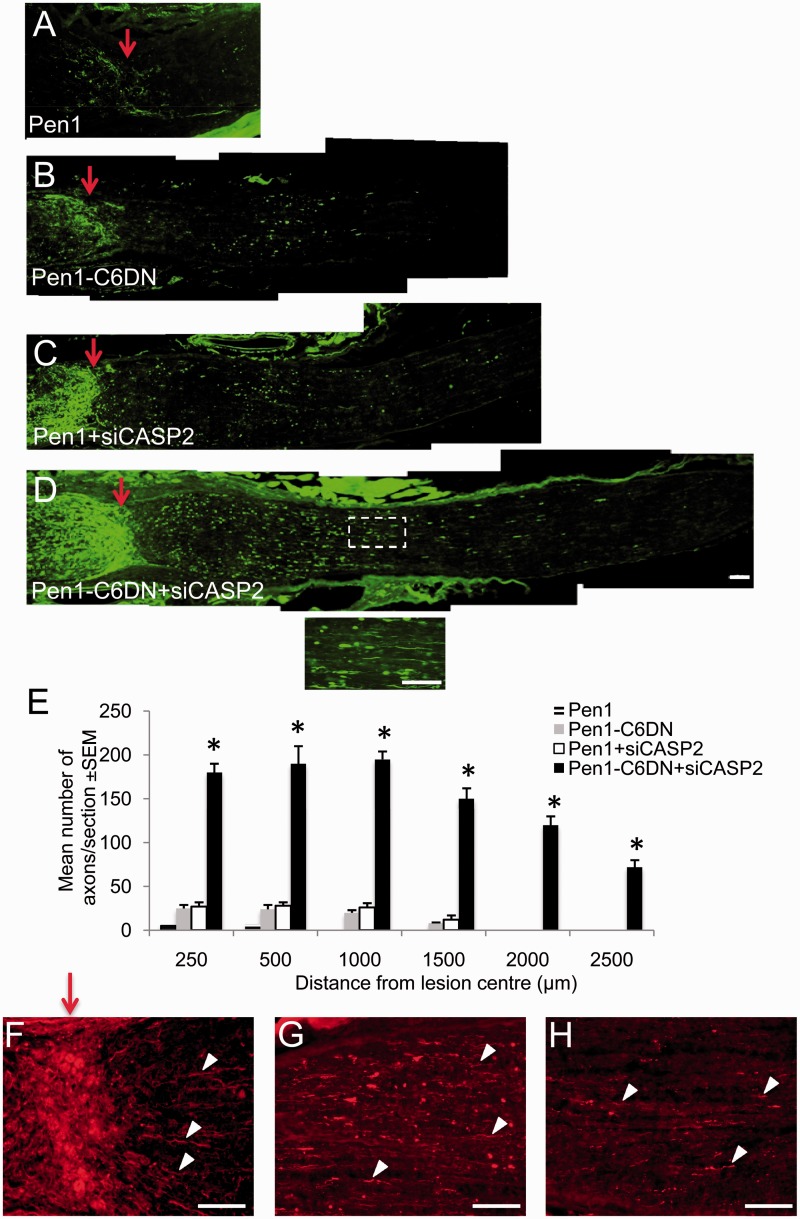
When the optic nerve of Pen1-treated animals were sectioned longitudinally and immunostained for GAP43 (Fig. 3A and E) after ONC, few GAP43+ axons were seen in the proximal optic nerve segment, and none traversed the lesion site to enter the distal optic nerve segment. In Pen1-C6DN treated optic nerve (Fig. 3B and E), more GAP43+ axons occupied the proximal optic nerve, but few traversed the lesion site, although some of these penetrated into the distal optic nerve up to 1500 µm from the lesion centre. The mean number of axons/optic nerve were low after the monotherapies, so that the numbers of GAP43+ axons were similar in the Pen1 + siCASP2-treated and Pen1-C6DN-treated groups, with only occasional axons penetrating >1000 µm from the lesion centre and into the distal optic nerve (Fig. 3C and E). The greatest numbers of GAP43+ axons were present in the Pen1-C6DN + siCASP2-treated groups, with 195 ± 9 and 72 ± 8 axons/section growing at 1000 µm and 2500 µm, respectively, from the lesion (Fig. 3D and E). These results suggest that, although Pen1-C6DN or siCASP2 alone promote little RGC axon regeneration, combined delivery of Pen1-C6DN + siCASP2 promotes significant RGC axon regeneration.
Figure 3.
Intravitreal delivery of optimized Pen1-C6DN + siCASP2 promotes RGC axon regeneration. After ONC, optimized Pen1-C6DN either alone or in combination with siCASP2 was intravitreally injected at 0, 7 and 14 days. Animals were killed at Day 21, optic nerves dissected out and processed for immunohistochemistry. GAP43 stained images to show regenerating RGC axons in (A) Pen1, (B), Pen1-C6DN, (C) Pen1 + siCASP and (D) Pen1-C6DN + siCASP2. Inset shows high power magnification of boxed region in D. (E) Quantification to show significant numbers of RGC axons at different distances beyond the lesion epicentre in Pen1-C6DN + siCASP2-treated optic nerve. *P < 0.0001 compared with Pen1 or Pen1-C6DN/Pen1 + siCASP2, Scale bars = 100 μm. Anterograde rhodamine B labelling to confirm regenerating RGC axons in the distal optic nerve stump (examples shown by arrowheads) after treatment with Pen1-C6DN + siCASP immediately past the lesion site (F), at 1000 μm (G) and at 2000 μm from the lesion site (H). Red arrows show lesion site.
Anterograde labelling with rhodamine B isothiocyanate confirmed similar numbers of regenerating RGC axons emerging from the lesion site and growing through the distal optic nerve stump (arrowheads) to 1000 and 2000 μm from the lesion site (Fig. 3F–H). For example, the number of RITC-labelled axons at 250, 1000 and 2000 μm from the lesion site were, 200 ± 14, 210 ± 12 and 30 ± 10 axons/section, respectively (not shown).
Pen1-C6DN + siCASP2 delivery enhanced retinal glia activation and upregulated CNTF production in glia and occasional retinal ganglion cells
Studies of retinal glial activation in models of RGC axon regeneration have shown that glial activation is correlated with axon regeneration (Berry et al., 1996; Leon et al., 2000; Lorber et al., 2002, 2005, 2008, 2009, 2012; Yin et al., 2003; Pernet and Di Polo, 2006; Muller et al., 2007; Ahmed et al., 2010). We have also shown that after optic nerve crush, inflammation-mediated glial activation in either the vitreous or in the optic nerve injury site promoted significant RGC survival, but that only vitreal inflammation with associated retinal glial activation was correlated with axon regeneration. This observation suggests that activated retinal glia secrete factors that are conducive for RGC axon regeneration (Ahmed et al., 2010). Moreover, activated retinal astrocytes express CNTF in response to lens injury or intravitreal zymosan injections; the downstream JAK/STAT3 pathway is strongly activated in regenerating RGCs and the lens injury-induced switch of RGCs to a regenerative state is dependent on CNTF and JAK/STAT3 signalling (Muller et al., 2007). Thus, we investigated whether intravitreal delivery of Pen1-C6DN + siCASP2 after optic nerve crush also promoted retinal glial activation and CNTF expression.
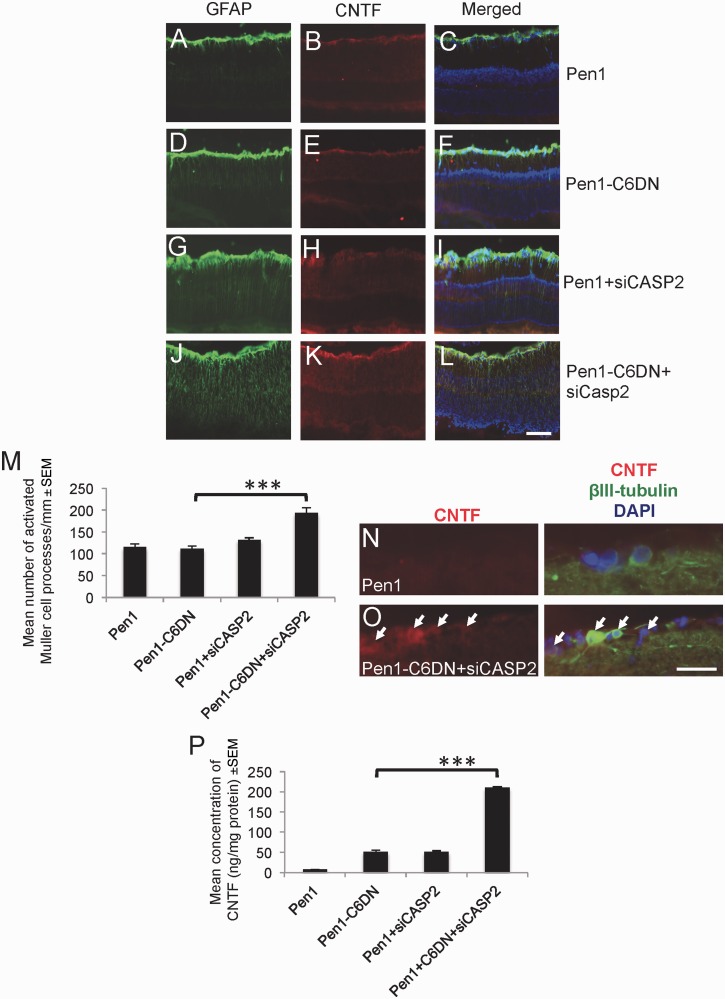
GFAP+ astrocytes/Müller cell end-feet were similarly activated and low levels of CNTF appeared in the nerve fibre layer after optic nerve crush in vehicle control Pen1-treated (Fig. 4A–C) and in Pen1-C6DN-treated eyes (Fig. 4D and E). In Pen1 + siCASP2-treated eyes (Fig. 4G–I), more GFAP+ astrocytes/Müller cells and CNTF were observed in the nerve fibre layer compared with Pen1 and Pen1-C6DN treated eyes. The greatest levels of GFAP+ and CNTF+ staining were observed in Pen1-C6DN + siCASP2-treated eyes, with more numerous GFAP+ Müller cell processes spanning the entire radial width of the retina (Fig. 4J–L), reflected by two times more activated Müller cell processes counted in these eyes compared to the numbers in other treatments (Fig. 4M). In addition, occasional RGC were immunopositive for CNTF only after combined suppression of CASP2 and -6, suggesting that some RGC may also respond to the effects of the combined treatments (Fig. 4N and O).
Figure 4.
Intravitreal delivery of optimized Pen1-C6DN + siCASP2 activates retinal astrocytes and Müller cells and increases CNTF in treated eyes. At 21 days after ONC and treatment with C6DN alone or in combination with siCASP2, eyes were processed for immunohistochemistry. (A–L) Representative images of the retinae of Pen1, Pen1-C6DN and Pen1 + siCASP2 treated eyes, demonstrating GFAP+ activated astrocytes and Müller cells with associated CNTF. (M) Combined Pen1-C6DN + siCASP2 treated eyes showed the highest numbers of activated astrocytes/Müller cells and CNTF localization in the retina. Immunolocalization of CNTF in βIII-tubulin+ RGC in (N) Pen1 and (O) Pen1-C6DN + siCASP2-treated retinae. (P) ELISA detected increased levels of retinal CNTF in eyes treated with combined Pen1-C6DN + siCASP2. ***P < 0.0001. Scale bars: A–L = 100 μm; N and O = 50 μm.
ELISA confirmed higher titres of CNTF in the retina of eyes treated with Pen1-C6DN + siCASP2 than that measured in the eyes from any other treatments (Fig. 4P). Together, these results suggest that the bi-therapy of Pen1-C6DN + siCASP2 activates retinal astrocytes/Müller cells to produce high titres of CNTF and that this may explain the enhanced RGC axon regeneration observed after this combined treatment.
We next investigated another possible mechanism of the enhanced CNTF production by retinal glia and RGCs, as CASP6 suppression can upregulate proinflammatory cytokine release by microglia that may then facilitate enhanced CNTF release from activated retinal cells. Immunohistochemistry for OX42, a marker of microglia, demonstrated higher levels of microglial activation after CASP2 and CASP6 monotherapy suppression, and that combined suppression of CASP2 and CASP6 synergistically increased this microglial activation (Supplementary Fig. 1A–E). These results suggest that microglial activation and subsequent proinflammatory cytokine production may contribute to the activation of retinal glia and subsequent CNTF release.
Retinal ganglion cell cultures prepared from ONC + Pen1-C6DN + siCASP2-treated eyes show enhanced glial activation and significant titres of CNTF in culture medium
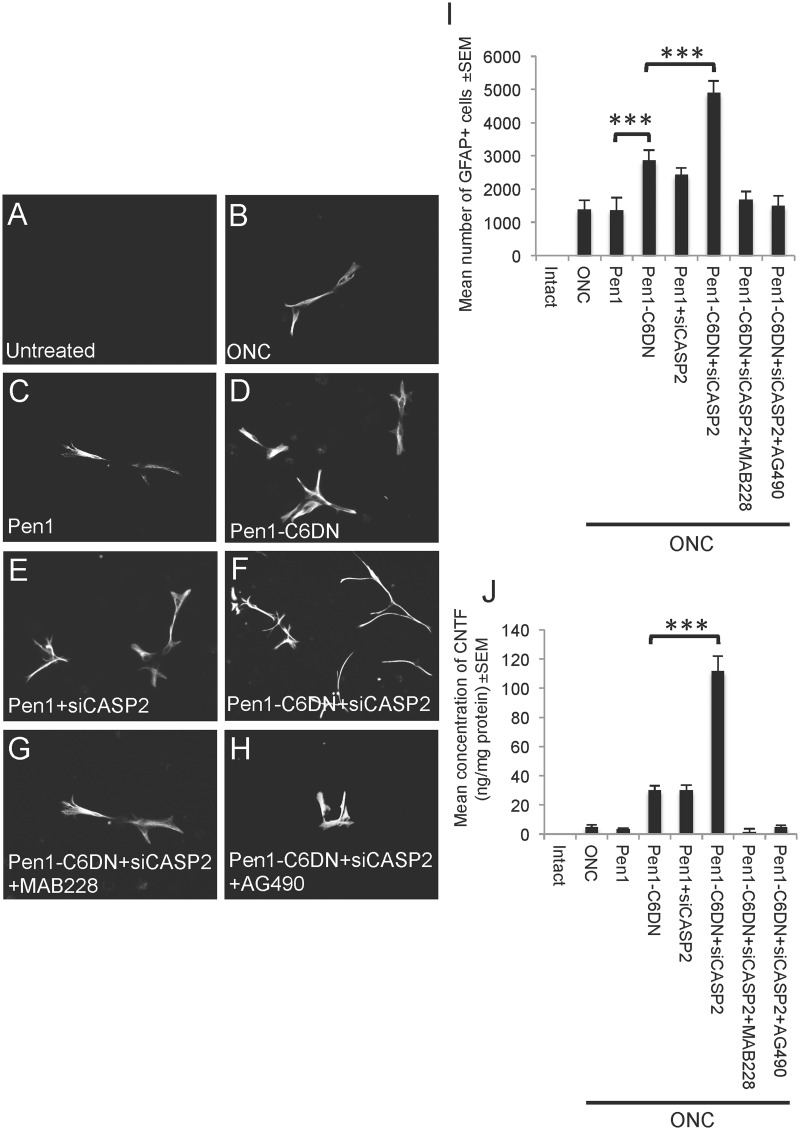
Many of the responses observed in vivo after optic nerve crush and treatment may be studied in vitro using adult retinal cultures. To further assess whether Pen1-C6DN + siCASP2 promoted glial activation and indirectly enhanced neurite outgrowth of surviving RGCs, retinal cultures from intact untreated eyes and from eyes 5 days after optic nerve crush and treatment with Pen1 vehicle, Pen1-C6DN, Pen1 + siCASP2 and Pen1-C6DN + siCASP2 treatment were prepared. After 3 days in culture, there were no activated GFAP+ glia detected in retinal cell cultures derived from untreated eyes (Fig. 5A and I). In retinal cell cultures prepared after optic nerve crush (Fig. 5B and I) and optic nerve crush before Pen1 treatment (Fig. 5C and I), 1396 ± 259 and 1367 ± 379 activated GFAP+ glia were present, respectively. Cultures prepared from eyes after optic nerve crush and mono-therapy with Pen1-C6DN and Pen1 + siCASP2, contained enhanced numbers of activated GFAP+ glia (2867 ± 379 and 2433 ± 208, Fig. 5D, E and I), respectively, whereas cultures prepared from Pen1-C6DN + siCASP2-treated eyes contained the greatest numbers of GFAP+ glia (4900 ± 355 glia, Fig. 5F and I). These results demonstrate that Pen1-C6DN + siCASP2 treatment led to the survival of enhanced numbers of activated GFAP+ glia in retinal cell cultures and this confirmed the in vivo findings.
Figure 5.
Adult retinal cultures prepared 5 days after ONC and intravitreal treatment with optimized Pen1-C6DN or Pen1-C6DN + siCASP2 show increased numbers of GFAP+ glia that co-localize CNTF. (A and I) Untreated retinal cultures do not contain GFAP+ glia, while increasing numbers of GFAP+ glia were observed in retina dissociated after 5 days with intravitreal treatment of (B and I) Pen1, (C and I) Pen1-C6DN, (D and I) Pen1-siCASP2 and (E and I) Pen1-C6DN + siCASP2. (F and I) Retinal cell cultures prepared from Pen1-C6DN + siCASP2-treated eyes that have also had suppression of CNTF signalling with either the MAB228 (5 μg/eye) (G and I) or the JAK/STAT pathway inhibitor AG490 (17 mM/eye) (H and I), contain significantly reduced numbers of GFAP+ glia to the baseline levels observed after optic nerve crush or ONC + Pen1 treatment. (H) The numbers of GFAP+ glia positively correlated with the levels of CNTF production in culture, whereas treatment with MAB228 or AG490 suppressed CNTF production to baseline levels. ***P < 0.0001, Scale bar = 50μm.
As reactive astrocyte gliosis can be induced by elevated levels of CNTF (Winter et al., 1995; Escartin et al., 2007; Kirsch et al., 2010) we next investigated if blockade of the gp130 signalling component of the CNTF receptor complex using MAB228 or of the JAK/STAT3 pathway using AG490 suppressed the Pen1-C6DN + siCASP2-mediated survival of activated glia in retinal cell cultures. In cultures prepared from eyes after optic nerve crush and treatment for 5 days with MAB228 and AG490, we observed that the Pen1-C6DN + siCASP2-mediated enhancement of GFAP+ glia numbers in retinal cultures was depressed to control optic nerve crush and ONC + Pen1 levels (Fig. 5G, I and H and I, respectively) by the inhibitors of CNTF signalling.
As we observed high levels of CNTF after ONC and treatment with Pen1-C6DN + siCASP2 in vivo, we used ELISA to monitor CNTF titres in defined cell culture medium after treatment. We demonstrated that in the media of cultures prepared from untreated, ONC- and Pen1-treated retinae, low levels of CNTF were detected (Fig. 5J) but, in cultures from both Pen1-C6DN and Pen1 + siCASP2-treated retinae, ∼38 ± 8 ng/mg of CNTF protein were present in the media. The levels of released CNTF were 3-fold higher in cultures prepared from retinae after Pen1-C6DN + siCASP2 bi-therapy (115 ± 12 ng/mg of protein). However, addition of the inhibitors of CNTF signalling, MAB228 or AG490, depressed the numbers of Pen1-C6DN + siCASP2 activated glia and the released CNTF levels to those observed in the cultures from control optic nerve crush and Pen1-treated eyes. These results suggest that Pen1-C6DN + siCASP2 activates retinal glia and CNTF production, whereas blockade of CNTF signalling not only suppresses the numbers of activated glia, but also CNTF production in culture.
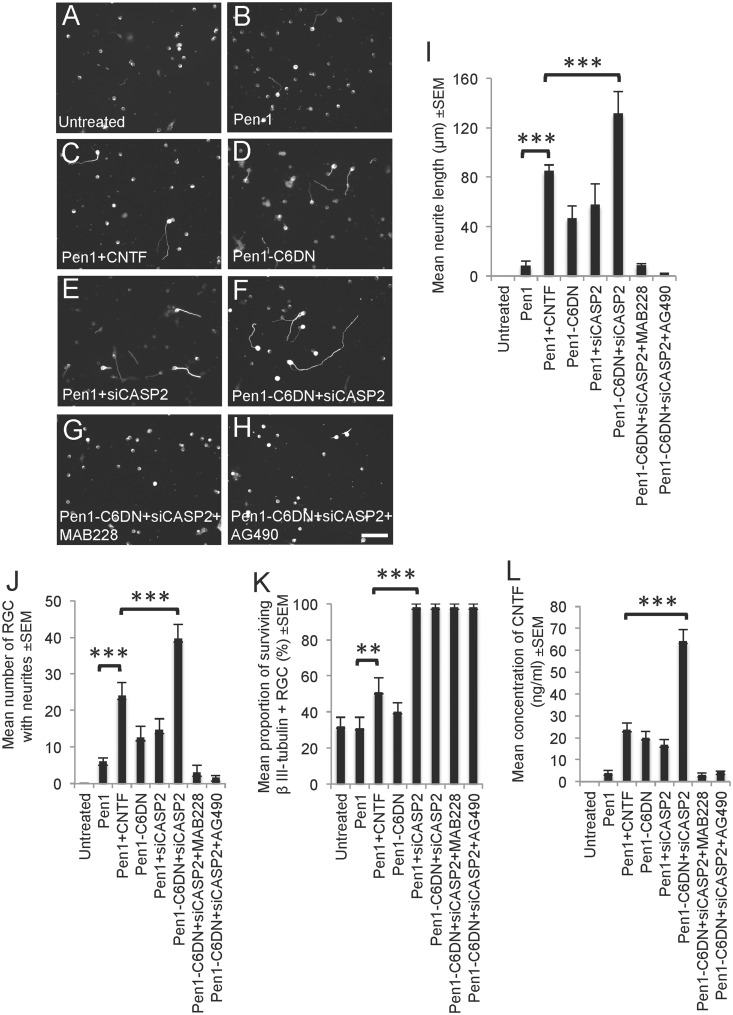
Treatment of retinal cell cultures with Pen1-C6DN + siCASP2 promotes RGC neurite outgrowth
As Pen1-C6DN + siCASP2 treatment activates retinal glia and secretion of CNTF in vivo and in vitro, we predicted that RGC survival and neurite outgrowth would also be enhanced in these cultures. We therefore investigated whether Pen1-C6DN + siCASP2 bi-therapy led to stimulated RGC survival and neurite outgrowth in retinal cell cultures, and whether MAB228 and AG490 treatment impacted on the RGC survival and neurite outgrowth. In retinal cultures from untreated and Pen1-treated eyes (Fig. 6A and I–K), few RGCs survived and grew neurites. After Pen1 + CNTF treatment, RGC survival was increased by 1.7-fold, whereas the number of RGC with neurites increased to 25 ± 5 RGCs with mean neurite lengths of 100 ± 10 μm recorded, compared with Pen1 alone (Fig. 6B and I–K). The addition of Pen1-C6DN (Fig. 6C, I and J) and Pen1 + siCASP2 on their own (Fig. 6D, I and J) promoted 50% fewer RGCs with neurites (10–12) than that seen with CNTF, with RGC neurite lengths of between 45 and 50 μm. RGC survival was similar in Pen1-C6DN and Pen1 + CNTF treatment groups, whereas Pen1 + siCASP2 provided >2-fold more RGC survival than CNTF or Pen1-C6DN (Fig. 6K). Combined Pen1-C6DN + siCASP2 treatment significantly increased the number of RGC with neurites and the mean neurite length to 130 ± 8 μm (Fig. 6E and I) compared with CNTF (Fig. 6I and J). RGC survival in the Pen1-C6DN + siCASP2 and Pen1 + siCASP2 groups was similar, but was significantly greater than that seen with the CNTF, Pen1-C6DN or control groups (Fig. 6K). Despite the observation that RGC neurite outgrowth was completely suppressed to basal levels in Pen1-C6DN + siCASP2 cultures treated with MAB228 and AG490 (Fig. 6G–J), RGC survival remained at the same high levels as other groups containing siCASP2 (Fig. 6K). ELISA to detect the levels of CNTF present in culture media from the different treatment groups showed that cultures prepared from eyes treated with Pen1-CNTF contained 23 ± 3 ng/ml, whereas those treated with Pen1-C6DN and Pen1 + siCASP2 contained 20 ± 3 and 16 ± 2.5 ng/ml, respectively (Fig. 6L). However, the highest levels of CNTF (64 ± 5.5 ng/ml) were detected in cultures prepared from Pen1-C6DN + siCASP2-treated eyes, equating to 3-fold more CNTF than after treatment with Pen1-CNTF. These results suggest that RGC survival is maximal in the presence of siCASP2, whereas C6DN + siCASP2-mediated glial activation and subsequent high titres of CNTF secretion enhances RGC neurite outgrowth.
Figure 6.
Adult retinal cell cultures prepared 5 days after ONC and intravitreal treatment with Pen1-C6DN or Pen1-C6DN + siCASP2 show increased RGC neurite outgrowth. In (A) untreated cultures and (B) cultures prepared 5 days after Pen1 vehicle treatment, few if any RGC grew neurites, whereas retinal cultures prepared from animals treated with (C) Pen1-CNTF (1.5 μg/eye) increased the (I) mean neurite length, (J) mean number of RGC with neurites and (K) RGC survival. In retinal cultures prepared from eyes treated with Pen1-C6DN and (E and I–K) Pen1-siCASP2, similarly increased mean neurite length and number of RGC with neurites were observed, whereas RGC survival was 55% and 98%, respectively. In retinal cultures prepared from animals treated with (F and I–K) Pen1-C6DN + siCASP2, significantly more neurite outgrowth was observed in terms of RGC neurite length and the number of RGC with neurites, whereas RGC neuroprotection was also 98%. However, simultaneous treatment with either (G and I–K) MAB228 or (H and I–K) AG490 in Pen1-C6DN + siCASP2-treated cultures abrogated RGC neurite outgrowth without affecting RGC viability. (L) ELISA from medium treated with Pen1-C6DN + siCASP2 showed significantly high levels of CNTF compared to those treated with CNTF or other monotherapies. **P < 0.001, ***P < 0.0001, Scale bar = 40 μm.
Blocking gp130 or the JAK/STAT pathway prevents Pen1-C6DN + siCASP2-mediated retinal ganglion cell axon growth and suppresses glial activation and CNTF production
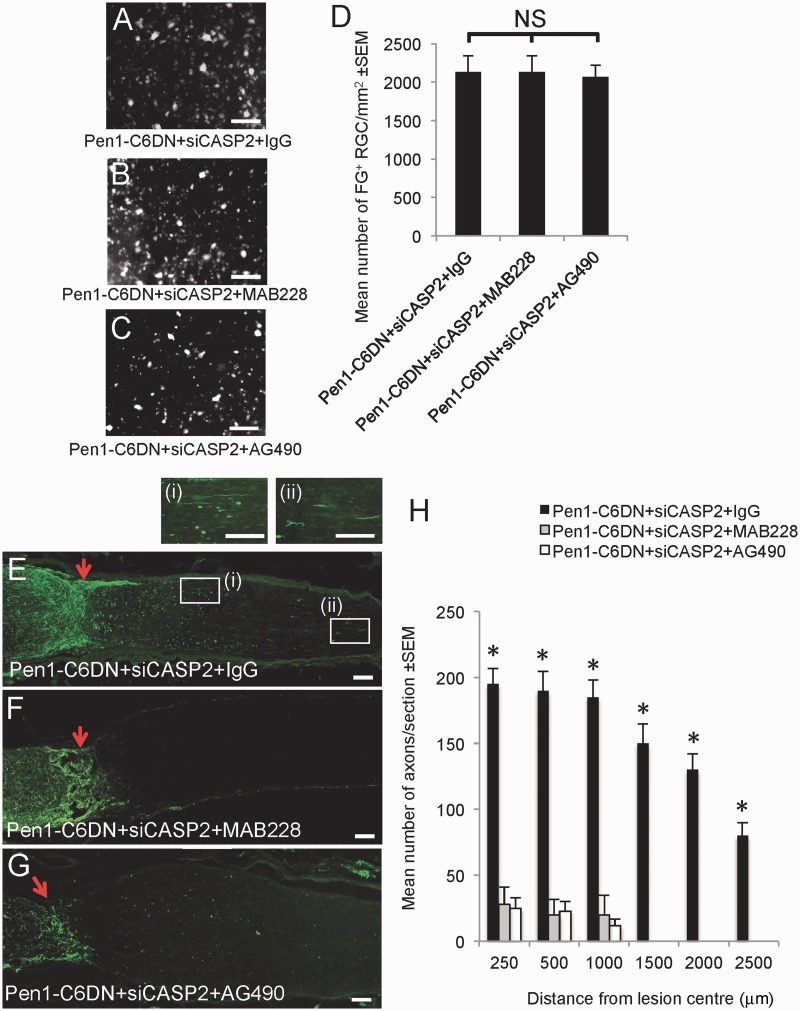
As suppression of gp130 and the JAK/STAT pathway blocked Pen1-C6DN + siCASP2-mediated RGC neurite outgrowth in vitro without affecting RGC survival, we asked whether MAB228 and AG490 could also attenuate Pen1-C6DN + siCASP2-induced RGC axon growth in vivo and assessed the impact of these treatments on RGC survival after optic nerve crush. We show that after intravitreal injection of Pen1-C6DN + siCASP2, RGC survival was unaffected by MAB228 or AG490 treatment and remained at the same high levels as treatment with a control IgG (Fig. 7A–D). RGC axon regeneration in Pen1-C6DN + siCASP2 + IgG control treatment group was the same as that observed earlier (cf. Fig. 7E and H with Fig. 3). However, the Pen1-C6DN + siCASP2-stimulated RGC axon regeneration was almost completely blocked by intravitreal injection of either MAB228 (Fig. 7F and H) or AG490 (Fig. 7G and H), suggesting that the CNTF pathway was primarily involved in Pen1-C6DN + siCASP2-mediated RGC axon regeneration.
Figure 7.
In vivo demonstration that Pen1-C6DN + siCASP2-stimulated RGC axon regeneration is abrogated by MAB228 and AG490, without affecting RGC survival. After ONC animals were intravitreally injected with optimized Pen1-C6DN + siCASP2 and rat IgG (5 µg/eye), MAB228 (5 µg/eye) and AG490 (17 mM/eye). (A–D) RGC survival was quantified by Fluoro-Gold™ (FG) counting in retinal whole mounts and showed that blocking Pen1-C6DN + siCASP2-mediated RGC axon growth by MAB228 or AG490 did not impact on (A–D) RGC survival but did block (E–H) RGC axon regeneration. *P < 0.0001 compared with Pen1-C6DN + siCASP2 + MAB228 or Pen1-C6DN + siCASP2 + AG490. Scale bars: A–C = 50 μm; E–G and insets (i) and (ii) = 100μm.
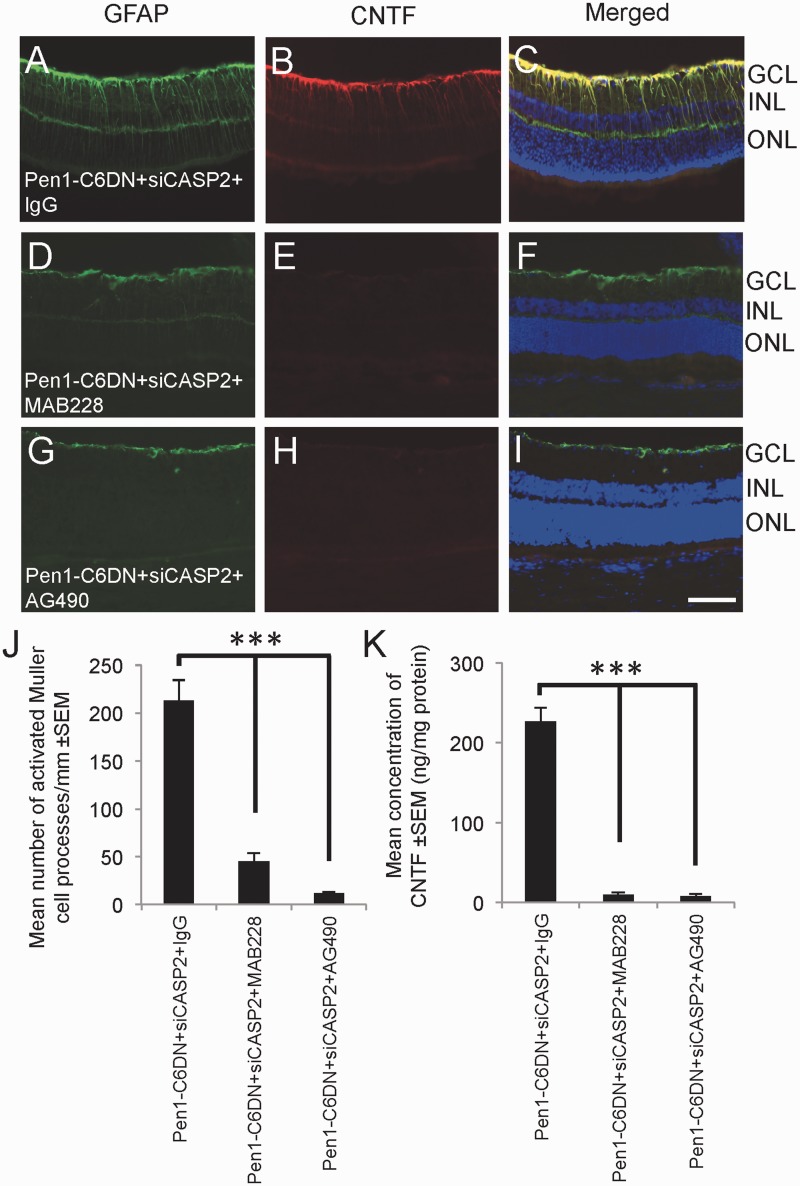
We then investigated whether MAB228 and AG490 also blocked Pen1-C6DN + siCASP2-mediated glial activation and CNTF production in the retina. After intravitreal Pen1-C6DN + siCASP2 plus control IgG injections, abundant GFAP and CNTF+ staining was observed in astrocytes of the nerve fibre layer and Müller cell radial processes (Fig. 8A–C). However, in Pen1-C6DN + siCASP2 + MAB228 (Fig. 8D–F) and Pen1-C6DN + siCASP2 + AG490-treated eyes (Fig. 8G–I), GFAP and CNTF+ staining was markedly attenuated. The mean number of activated Müller glia processes in Pen1-C6DN + siCASP + IgG treated retinae was 210 ± 5 processes/mm compared to only 45 ± 5 and 8 ± 3 processes/mm in Pen1-C6DN + siCASP2 + MAB228 and Pen1-C6DN + siCASP2 + AG490, respectively (Fig. 8J). ELISA to quantify the levels of CNTF in Pen1-C6DN + siCASP2 + IgG-treated retinae measured 223 ± 5 ng/mg of CNTF but the growth factor was barely detectable in both Pen1-C6DN + siCASP2 + MAB228 and Pen1-C6DN + siCASP2 + AG490-treated retinae (Fig. 8K).
Figure 8.
Blocking Pen1-C6DN + siCASP2-stimulated RGC axon regeneration by MAB228 and AG490 also blocks (A–J) glial activation and reduces CNTF production in the eye (K). ***P < 0.0001. Scale bar = 50μm. GCL = ganglion cell layer; INL = inner nuclear layer; ONL = outer nuclear layer.
Taken together, these results suggest that Pen1-C6DN + siCASP2-mediated RGC axon regeneration is suppressed when gp130 and JAK/STAT are blocked, and this correlates with reduced retinal glial activation and CNTF levels. Thus, suppression of CASP6 using the Pen1-C6DN, while capable of promoting optimum RGC survival when combined with CASP2 suppression), might be a promising way of indirectly enhancing RGC axon regeneration after optic nerve trauma.
Discussion
The data presented here demonstrate that, despite low levels of detectable CASP6 activation after optic nerve crush, inhibition of CASP6 promoted 60% RGC survival whereas inhibition of CASP2 promoted >95% RGC survival. Combined inhibition of CASP6 and -2 protected the majority of RGCs from death but also promoted extensive retinal gliosis and significant RGC neurite outgrowth/axon regeneration through the optic nerve lesion site and along the distal optic nerve. RGC axon regeneration promoted by combined suppression of CASP6 and -2 was mediated by glial-derived CNTF as blockade of the CNTF receptor or inhibition of JAK/STAT signalling, suppressed the associated glial activation, blocked CNTF production, inhibited RGC axon regeneration and preserved RGC viability. Taken together, our results show that combined suppression of CASP2 and CASP6 is RGC neuroprotective and activates a novel indirect RGC axogenic effect mediated by caspase-dependent gliosis and subsequent release of CNTF and JAK/STAT signalling.
Retinal ganglion cell survival and caspases
Despite reports that retinal CASP3, -8, and -9 activity are important in mediating RGC survival after optic nerve transection (Kermer et al., 1998, 1999; Chaudhary et al., 1999; Weishaupt et al., 2003; Cheung et al., 2004), high levels of cleaved CASP2 were exclusively localized to RGC after optic nerve crush whereas cleaved CASP3 was absent in RGC but present in other cells of the inner nuclear layer (Ahmed et al., 2011). This led us to suggest that CASP3 is not required for RGC death, but may be required for death of other cells in the retina after optic nerve crush (Ahmed et al., 2011). More recently, we reported increased levels of cleaved CASP3 in retinal lysates after optic nerve crush but little or no changes in cleaved forms of CASP7 or -8, while there was a slight increase in cleaved CASP6 (Vigneswara et al., 2012). Although retinal levels were increased after optic nerve crush, cleaved CASP3 and CASP6 immunoreactivity was absent from RGC (Ahmed et al., 2011; Vigneswara et al., 2012), suggesting that the activation of CASP3 and -6 observed in retinal lysates was unrelated to the death of RGC. Furthermore, we used an unbiased caspase trapping assay to show that CASP2 was activated in the retina after optic nerve crush (Vigneswara et al., 2012) and suppression of CASP2 with a short interfering RNA protected 98% of RGCs from apoptosis at 7 days (Ahmed et al., 2011).
In our current study, the same siCASP2 maintained >95% RGC protection from apoptosis, either alone or in combination with suppression of CASP6 using the CASP6 dominant negative (C6DN), for at least 21 days after optic nerve crush. Meanwhile, suppression of CASP6 alone using C6DN protected 60% of RGC from death at 21 days after optic nerve crush, similar to the levels reported using the non-specific Z-VEID-FMK CASP6 inhibitor (Monnier et al., 2011). Hence, we show here that inhibition of CASP6 resulted in lower levels of RGC neuroprotection than CASP2 inhibition, probably reflecting the muted responsiveness of constitutive CASP6 levels after optic nerve crush (Vigneswara et al., 2012). These results suggest that CASP6 is not the main regulator of RGC apoptosis. Of relevance, Monnier et al. (2011) have speculated CASP6 activation in a small RGC subpopulation, and hence the effects of CASP6 suppression after optic nerve crush are limited. By contrast, significantly increased levels of CASP2 are found in most RGC of the retina after optic nerve crush, which die by CASP2-dependent mechanisms (Ahmed et al., 2011; Vigneswara et al., 2012). Accordingly, suppression of CASP2 results in near 100% protection from post-optic nerve crush RGC death, suggesting that CASP2 is the principle regulator of RGC death (Ahmed et al., 2011). Combined CASP6 and CASP2 suppression did not induce stronger neuroprotection than that observed with CASP2 suppression alone, as we argue that RGC death is predominantly mediated by CASP2 and not CASP6. This assertion is given further credence by observations that suppression of other caspases after optic nerve crush, including CASP3, -6 or -8 cause only up to 35–60% RGC survival (Kermer et al., 1998, 1999; Monnier et al., 2011).
The suboptimal levels of RGC neuroprotection seen after C6DN treatment suggest that the development of an effective short interfering RNA to CASP6 may increase RGC survival by achieving greater levels of CASP6 knockdown. For example, C6DN suppressed Lamin A/C cleavage, a primary target of CASP6 activity, by only 60% compared with treatment groups that did not contain C6DN (Fig. 2A). Greater levels of CASP6 knockdown may more effectively suppress Lamin A/C cleavage indicating a more effective inhibition of CASP6 activity; more effective inhibition of CASP6 could lead to a greater stimulation of CNTF production, and better RGC survival and axon regeneration.
CASP2 activation has a multifaceted role in stress-induced apoptosis, including the induction of DNA damage, death receptor stimulation, heat shock, cytoskeletal disruption and oxidative stress (Troy et al., 2000; Ho et al., 2008), perhaps explained by the predicted cleavage specificity of CASP2 which resembles that of the effector CASP3, despite structural similarities to initiator caspases (Chauvier et al., 2007). Other reports indicate that CASP2-deficient neurons are resistant to apoptosis induced by amyloid-β (Troy et al., 2000; Troy and Ribe, 2008) and that transient global ischaemia leads to activation of CASP2 in hippocampal neurons (Niizuma et al., 2008). CASP2 mediates death of dorsal root ganglion neurons after sciatic nerve transection in vivo and also in a serum-withdrawal dorsal root ganglion neuron culture model, where suppression of CASP2 and not CASP3 using short interfering RNA significantly enhanced dorsal root ganglion neuron survival (Vigneswara et al., 2013). This is similar to earlier studies which showed that, although both CASP2 and CASP3 are activated by trophic factor withdrawal in sympathetic neurons, only CASP2 is required for execution of neuronal death (Troy et al., 1997, 2001). Retinal ischaemia induces activation of CASP2 in RGC and BDNF-mediated neuroprotection is associated with reduced CASP2 immunoreactivity (Kurokawa et al., 1999; Singh et al., 2001).
The involvement of caspases in axon regeneration
The possible involvement of caspases in mediating CNS axon integrity have been put forward based on the observations that CASP3 and CASP6 contribute to axonal degeneration (Nikolaev et al., 2009; Simon et al., 2012). For example, CASP6 was implicated in orchestrating axonal degeneration and it has been suggested that an extracellular fragment of amyloid-β protein (APP) acts through death receptor (DR)-6 and CASP6 to cause the axonal degeneration observed in Alzheimer’s disease (Nikolaev et al., 2009; Vohra et al., 2010). CASP3 is reported to orchestrate axon degeneration and as CASP6 is only effectively activated by CASP3, CASP3 deletion in vitro is fully protective against sensory axon degeneration and CASP3 and -6 knockout mice show delayed pruning of retinocollicular axons (Simon et al., 2012). However, Nikolaev et al. (2009) and Simon et al. (2012) both use either embryonic Day 12.5 or Day 13 dorsal root ganglion tissues for their assays, which are much more sensitive to NGF-dependent effects compared with adult dorsal root ganglion neurons that did not localize CASP3 or cleaved CASP3 in their neurites in culture and neither did knockdown of CASP3 have any effect on adult dorsal root ganglion neuron survival or neurite integrity (Vigneswara et al., 2013). In fact, suppression of CASP2 in cultured adult dorsal root ganglion neurons promoted significant survival of these neurons (Vigneswara et al., 2013), therefore suggesting a differential requirement for caspases in embryos compared to adults.
We determined whether CASP6 suppression in combination with enhanced survival of adult rat RGCs, promoted by inhibiting CASP2, could lead to enhanced RGC axon regeneration after optic nerve crush. We showed that inhibition of CASP6 along with downregulation of CASP2 promoted significant RGC axon regeneration and that this regeneration was ∼10-fold greater than that observed using a pseudopeptide caspase inhibitor in previous studies (Monnier et al., 2011). Although this represents a step in the right direction in terms of axon regeneration, Pen1-C6DN + siCASP2 only supported the regeneration of 1.4% of the total number of RGC axons, assuming that the number of axons in an intact optic nerve is 100 000 (Joos et al., 2010). Nevertheless, these levels of RGC axon regeneration are greater than that observed with Zymosan + cAMP (Kurimoto et al., 2010) and similar to that observed in Pten knockout mice (Park et al., 2008). However, RGC axon regeneration in Pten/Socs3 double mutant knockout mice was greater and represented regeneration of 6% of the total RGC axon population (Sun et al., 2011).
Notably, combined inhibition of CASP2 and CASP6 significantly activated retinal glia, including Müller cells and astrocytes, and stimulated the production of high levels of CNTF both in vivo and in vitro. Moreover, in combined CASP2 and CASP6 suppressed retinal cultures and in eyes, blocking CNTF receptor function and JAK/STAT signalling both prevented glial activation, suppressed CNTF production and blocked RGC neurite/axon regeneration. These observations all suggest a link between CASP6 and retinal glia. Although in this study we failed to localize significant levels of immunoreactive CASP6 to retinal glia, CASP6 was detectable by western blot of whole retinae (Vigneswara et al., 2012). However, Monnier et al. (2011) localized CASP6 in a subpopulation of RGCs and also to non-neuronal cells in the ganglion cell layer and to cells in the inner nuclear layer, presumed to be astrocytes and Müller cells, respectively.
GFAP+ glia were not present in retinal cell cultures prepared from intact eyes, however, after optic nerve crush a small population of GFAP+ astrocytes was present. Furthermore, inhibition of CASP6 and CASP2 promoted GFAP and CNTF expression in the glia present in the mixed retinal cultures, indicating an indirect mechanism of enhancing RGC survival and axon regeneration. Hence, our data imply that CASP6 is present in glia, where it constitutively signals responses such as cleavage of GFAP intermediate filaments (Mouser et al., 2006), probably maintaining glial quiescence and thus suppressed CNTF production. This proposition is supported by our in vitro observations of low numbers of GFAP+ glia in untreated retinal cultures where CASP6 is presumed active, and the stimulation of glial activation and CNTF production after CASP6 suppression (Fig. 5). Therefore, CASP6 suppression is likely to be beneficial for astrocyte activation and survival.
Thus, the combination of CASP2 and CASP6 stimulates retinal gliosis, which in turn upregulates the expression CNTF, and probably other neurotrophic factors, that then promote RGC survival and axon regeneration. Although the ability of exogenous CNTF to promote RGC axon regeneration is inconsistent, CNTF promotes RGC axon regeneration, an effect that is enhanced when combined with raised cAMP levels, whereas long-distance axon regeneration is supported by CNTF gene transfer to RGCs (Muller et al., 2009; Pernet et al., 2013). Furthermore, combinations of neurotrophic factors such as CNTF, BDNF, NGF, NTF3 and FGF2, promote synergistic RGC survival and axon regeneration (Mey and Thanos, 1993; Mansour-Robaey et al., 1994; Peinado-Ramon et al., 1996; Klocker et al., 2000; Logan et al., 2006). Therefore it is likely that combinations of these neurotrophic factors, together with suppression of CASP2 and CASP6 activity, might promote more robust RGC axon regeneration than observed with C6DN + siCASP2 alone.
We have shown previously that the cues for post-injury RGC survival and axon regeneration, which are mediated by inflammation, are different, as both retinal and optic nerve inflammation promoted RGC survival but only retinal inflammation was RGC axogenic (Ahmed et al., 2010). Our current study also implies differential mechanisms for RGC survival and axon regeneration and suggests that retinal glia are important to both processes. For example, we suggest that the activation of retinal glia that occurs after optic nerve crush is enhanced by Pen1-C6DN + siCASP2, increasing the release of CNTF. CNTF then acts not only on RGC in a paracrine manner to increase their regenerative capacity, but also induces by an autocrine mechanism further reactive gliosis in astrocytes and Müller cells (DeChiara et al., 1995; Kahn et al., 1995; Winter et al., 1995; Escartin et al., 2006, 2007), which in turn enhances CNTF production, contributing to an additional regenerative ability of RGC (Fig. 9). CNTF is probably also released by RGCs through autocrine mechanisms (Fig. 9) contributing to the higher titres of CNTF in the combined Pen1-C6DN + siCASP2-treated retinae. This proposed mechanism might explain why enhanced RGC axon regeneration occurs after Pen1-C6DN + siCASP2 treatment. In contrast, blocking gp130 or the JAK/STAT pathway does not prevent the low levels of CNTF production by injury-induced gliosis but seems to suppress the C6DN-induced gliosis and subsequent high titre release of CNTF, which in turn reduces the regenerative response of RGC.
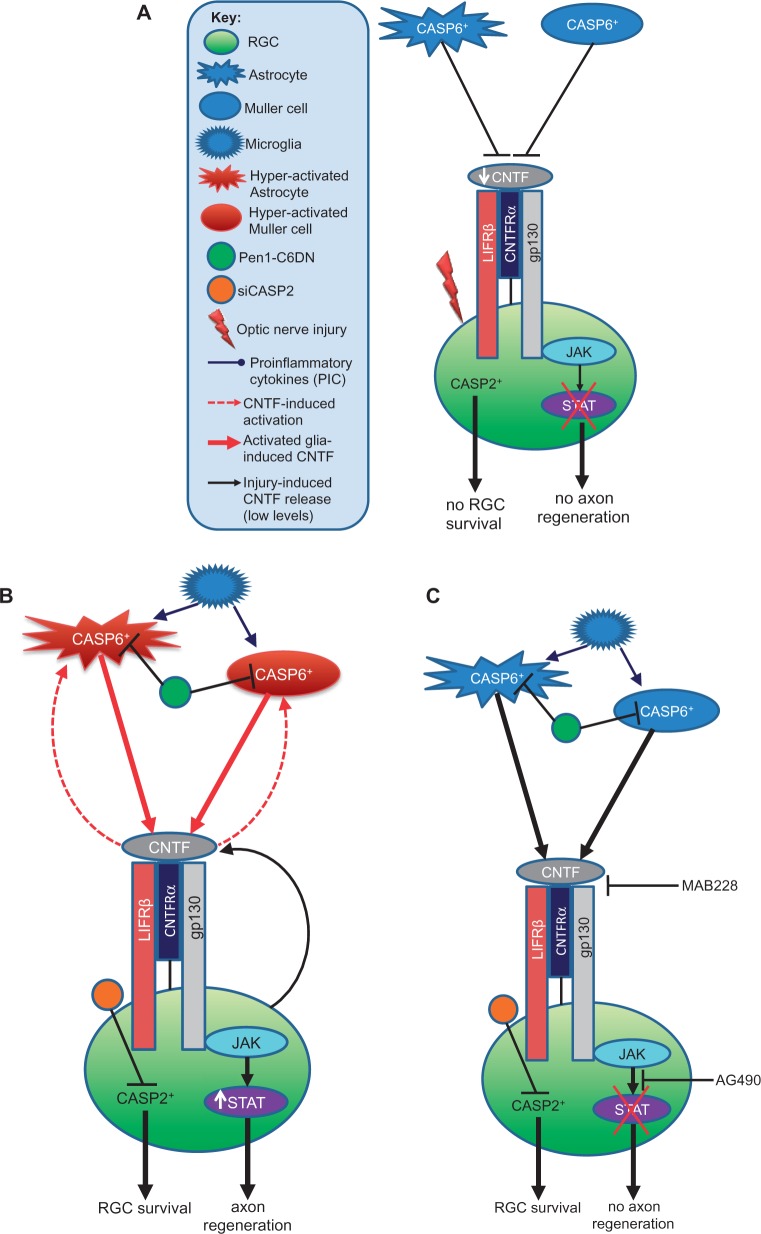
Figure 9.
Proposed mechanism of CNTF-induced gliosis in the retina after ONC and Pen1-C6DN + siCASP2 treatment. (A) After optic nerve crush alone, the low levels of injury-induced CNTF bind to the CNTF receptor and suboptimally activate the JAK/STAT pathway and, and hence little or no axon regeneration occurs. (B) After optic nerve crush and intravitreal delivery of Pen1-C6DN + siCASP2, however, glial activation occurs in response to both injury and the presence of Pen1-C6DN + siCASP2. This gliosis leads to CNTF release, which further stimulates reactive gliosis in an autocrine manner, enhancing CNTF release. In addition, treatment with Pen1-C6DN + siCASP2 also promotes release of proinflammatory cytokines from microglia that stimulate further release of CNTF from astrocytes and Müller cells. These high titres of CNTF activate the CNTF receptor and promote significant RGC axon regeneration through the JAK/STAT pathway. (C) In contrast, blocking the gp130 component of the CNTF receptor with MAB228 or blocking the JAK/STAT signalling pathway with AG490 blocks the autocrine response, suppresses proinflammatory cytokine-induced CNTF release leading to reduced CNTF titres, depressed reactive gliosis and hence RGC axon regeneration is prevented.
Mechanisms of CASP6-modulated retinal gliosis and CNTF expression
We hypothesize that constitutive CASP6 expression ‘clamps’ retinal glia in a quiescent state, preventing their activation and production of CNTF. Optic nerve crush does not fully release the glia from CASP6 control, and thus RGC axons do not regenerate as CNTF titres remain low. Only after CASP6 inhibition are glia able to become fully activated and produce axogenic titres of CNTF (Fig. 9). Suppression of CASP6 increases the release of pro-inflammatory cytokines from both astrocytes and microglia, including interleukins, interferons and tumour necrosis factors as well as chemokines (Hitchkiss and Nicholson, 2006). We have observed that suppression of CASP6 activates retinal microglia (Supplementary Fig. 1) and thus may contribute to the release of pro-inflammatory cytokines. Receptor binding of proinflammatory cytokines released from activated astrocytes and microglia activates a variety of intracellular signalling pathways, including the c-Jun N-terminal kinase (JNK), p38 mitogen activated protein kinase (p38/MAPK), PI3 kinase, extracellular signalling-related kinase (ERK) and activation of CASP1 and -3 (Van Eldik et al., 2007; Anisman, 2009). Activation of JNK and p38/MAPK is known to contribute to the accumulation of GFAP in astrocytes (Tang et al., 2006) and thus release from retinal glia of gp130 receptor ligands (LIF and IL6) that interact with CNTF to signal RGC survival through the JAK/STAT pathway. Indeed, CNTF is normally released by astrocytes and Müller cells of the retina after ONC, probably mediated by the release of inflammation-induced cytokines such as IL1β and TNFα, all of which enhance CNTF release (Kamiguchi et al., 1995; Muller et al., 2007; Lorber et al., 2008, 2012). CNTF induces changes in astrocyte responses including upregulation of GFAP, cellular hypertrophy and metabolic changes that lead to the expression of further CNTF (Winter et al., 1995; Escartin et al., 2007).
In conclusion, our findings demonstrate that bi-therapies that suppress CASP2 and CASP6 inhibit RGC apoptosis and promote retinal gliosis, leading to the release of high titres of CNTF, which promotes RGC axon regeneration. These studies suggest that the collective targeting of CASP2 and CASP6 has therapeutic potential in treating human adult CNS trauma and disease.
Supplementary Material
Acknowledgements
The authors would like to gratefully acknowledge that the siCASP2 was generously provided by Quark Pharmaceuticals Inc, CA, USA.
Glossary
Abbreviations
- C6DN
CASP6 dominant negative
- Pen1
Penetratin 1
- RGC
retinal ganglion cells
- siCASP2
short interfering CASP2
Funding
This work was supported by a project grant no 092539/Z/10/Z from The Wellcome Trust to Z.A. and the National Institutes of Health, grants no NS43089, NS081333 to C.M.T.
Supplementary material
Supplementary material is available at Brain online.
References
- Agudo M, Perez-Marin MC, Lonngren U, Sobrado P, Conesa A, Canovas I, et al. Time course profiling of the retinal transcriptome after optic nerve transection and optic nerve crush. Mol Vis. 2008;14:1050–63. [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z, Dent RG, Leadbeater WE, Smith C, Berry M, Logan A. Matrix metalloproteases: degradation of the inhibitory environment of the transected optic nerve and the scar by regenerating axons. Mol Cell Neurosci. 2005;28:64–78. doi: 10.1016/j.mcn.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Suggate EL, Brown ER, Dent RG, Armstrong SJ, Barrett LB, et al. Schwann cell-derived factor-induced modulation of the NgR/p75NTR/EGFR axis disinhibits axon growth through CNS myelin in vivo and in vitro. Brain. 2006;129(Pt 6):1517–33. doi: 10.1093/brain/awl080. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Aslam M, Lorber B, Suggate EL, Berry M, Logan A. Optic nerve and vitreal inflammation are both RGC neuroprotective but only the latter is RGC axogenic. Neurobiol Dis. 2010;37:441–54. doi: 10.1016/j.nbd.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Kalinski H, Berry M, Almasieh M, Ashush H, Slager N, et al. Ocular neuroprotection by siRNA targeting caspase-2. Cell Death Dis. 2011;2:e173. doi: 10.1038/cddis.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akpan N, Serrano-Saiz E, Zacharia BE, Otten ML, Ducruet AF, Snipas SJ, et al. Intranasal delivery of caspase-9 inhibitor reduces caspase-6-dependent axon/neuron loss and improves neurological function after stroke. J Neurosci. 2011;31:8894–904. doi: 10.1523/JNEUROSCI.0698-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- Berger AB, Sexton KB, Bogyo M. Commonly used caspase inhibitors designed based on substrate specificity profiles lack selectivity. Cell Res. 2006;16:961–3. doi: 10.1038/sj.cr.7310112. [DOI] [PubMed] [Google Scholar]
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–74. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M, Carlile J, Hunter A. Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve. J Neurocytol. 1996;25:147–70. doi: 10.1007/BF02284793. [DOI] [PubMed] [Google Scholar]
- Berry M, Carlile J, Hunter A, Tsang W, Rosenstiel P, Sievers J. Optic nerve regeneration after intravitreal peripheral nerve implants: trajectories of axons regrowing through the optic chiasm into the optic tracts. J Neurocytol. 1999;28:721–41. doi: 10.1023/a:1007086004022. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Caspase-mediated activation of PAK2 during apoptosis: proteolytic kinase activation as a general mechanism of apoptotic signal transduction? Cell Death Differ. 1998;5:637–45. doi: 10.1038/sj.cdd.4400405. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen LA, Miller DK, Anhalt GJ, Rosen A. Specific cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein is a characteristic biochemical feature of apoptotic cell death. J Biol Chem. 1994;269:30757–60. [PubMed] [Google Scholar]
- Chaudhary P, Ahmed F, Quebada P, Sharma SC. Caspase inhibitors block the retinal ganglion cell death following optic nerve transection. Brain Res Mol Brain Res. 1999;67:36–45. doi: 10.1016/s0169-328x(99)00032-7. [DOI] [PubMed] [Google Scholar]
- Chauvier D, Ankri S, Charriaut-Marlangue C, Casimir R, Jacotot E. Broad-spectrum caspase inhibitors: from myth to reality? Cell Death Differ. 2007;14:387–91. doi: 10.1038/sj.cdd.4402044. [DOI] [PubMed] [Google Scholar]
- Cheung ZH, Chan YM, Siu FK, Yip HK, Wu W, Leung MC, et al. Regulation of caspase activation in axotomized retinal ganglion cells. Mol Cell Neurosci. 2004;25:383–93. doi: 10.1016/j.mcn.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Davidson TJ, Harel S, Arboleda VA, Prunell GF, Shelanski ML, Greene LA, et al. Highly efficient small interfering RNA delivery to primary mammalian neurons induces MicroRNA-like effects before mRNA degradation. J Neurosci. 2004;24:10040–6. doi: 10.1523/JNEUROSCI.3643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara TM, Vejsada R, Poueymirou WT, Acheson A, Suri C, Conover JC, et al. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell. 1995;83:313–22. doi: 10.1016/0092-8674(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Denault JB, Salvesen GS. Expression, purification, and characterization of caspases. Curr Protoc Protein Sci. 2003 doi: 10.1002/0471140864.ps2113s30. Chapter 21: Unit 21 13. [DOI] [PubMed] [Google Scholar]
- Douglas MR, Morrison KC, Jacques SJ, Leadbeater WE, Gonzalez AM, Berry M, et al. Off-target effects of epidermal growth factor receptor antagonists mediate retinal ganglion cell disinhibited axon growth. Brain. 2009;132(Pt 11):3102–21. doi: 10.1093/brain/awp240. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Cepko CL. Control of Muller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000;3:873–80. doi: 10.1038/78774. [DOI] [PubMed] [Google Scholar]
- Edgington LE, van Raam BJ, Verdoes M, Wierschem C, Salvesen GS, Bogyo M. An optimized activity-based probe for the study of caspase-6 activation. Chem Biol. 2012;19:340–52. doi: 10.1016/j.chembiol.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Brouillet E, Gubellini P, Trioulier Y, Jacquard C, Smadja C, et al. Ciliary neurotrophic factor activates astrocytes, redistributes their glutamate transporters GLAST and GLT-1 to raft microdomains, and improves glutamate handling in vivo. J Neurosci. 2006;26:5978–89. doi: 10.1523/JNEUROSCI.0302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Pierre K, Colin A, Brouillet E, Delzescaux T, Guillermier M, et al. Activation of astrocytes by CNTF induces metabolic plasticity and increases resistance to metabolic insults. J Neurosci. 2007;27:7094–104. doi: 10.1523/JNEUROSCI.0174-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Valenzuela E, Gorczyca W, Darzynkiewicz Z, Sharma SC. Apoptosis in adult retinal ganglion cells after axotomy. J Neurobiol. 1994;25:431–8. doi: 10.1002/neu.480250408. [DOI] [PubMed] [Google Scholar]
- Ho LH, Read SH, Dorstyn L, Lambrusco L, Kumar S. Caspase-2 is required for cell death induced by cytoskeletal disruption. Oncogene. 2008;27:3393–404. doi: 10.1038/sj.onc.1211005. [DOI] [PubMed] [Google Scholar]
- Isenmann S, Wahl C, Krajewski S, Reed JC, Bahr M. Up-regulation of Bax protein in degenerating retinal ganglion cells precedes apoptotic cell death after optic nerve lesion in the rat. Eur J Neurosci. 1997;9:1763–72. doi: 10.1111/j.1460-9568.1997.tb01534.x. [DOI] [PubMed] [Google Scholar]
- Joos KM, Li C, Sappington RM. Morphometric changes in the rat optic nerve following short-term intermittent elevations in intraocular pressure. Invest Ophthalmol Vis Sci. 2010;51:6431–40. doi: 10.1167/iovs.10-5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn MA, Ellison JA, Speight GJ, de Vellis J. CNTF regulation of astrogliosis and the activation of microglia in the developing rat central nervous system. Brain Res. 1995;685:55–67. doi: 10.1016/0006-8993(95)00411-i. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H, Yoshida K, Sagoh M, Sasaki H, Inaba M, Wakamoto H, et al. Release of ciliary neurotrophic factor from cultured astrocytes and its modulation by cytokines. Neurochem Res. 1995;20:1187–93. doi: 10.1007/BF00995382. [DOI] [PubMed] [Google Scholar]
- Kermer P, Klocker N, Labes M, Bahr M. Inhibition of CPP32-like proteases rescues axotomized retinal ganglion cells from secondary cell death in vivo. J Neurosci. 1998;18:4656–62. doi: 10.1523/JNEUROSCI.18-12-04656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermer P, Klocker N, Labes M, Thomsen S, Srinivasan A, Bahr M. Activation of caspase-3 in axotomized rat retinal ganglion cells in vivo. FEBS Lett. 1999;453:361–4. doi: 10.1016/s0014-5793(99)00747-4. [DOI] [PubMed] [Google Scholar]
- Kermer P, Ankerhold R, Klocker N, Krajewski S, Reed JC, Bahr M. Caspase-9: involvement in secondary death of axotomized rat retinal ganglion cells in vivo. Brain Res Mol Brain Res. 2000;85:144–50. doi: 10.1016/s0169-328x(00)00256-4. [DOI] [PubMed] [Google Scholar]
- Kirsch M, Trautmann N, Ernst M, Hofmann HD. Involvement of gp130-associated cytokine signaling in Muller cell activation following optic nerve lesion. Glia. 2010;58:768–79. doi: 10.1002/glia.20961. [DOI] [PubMed] [Google Scholar]
- Klocker N, Kermer P, Weishaupt JH, Labes M, Ankerhold R, Bahr M. Brain-derived neurotrophic factor-mediated neuroprotection of adult rat retinal ganglion cells in vivo does not exclusively depend on phosphatidyl-inositol-3'-kinase/protein kinase B signaling. J Neurosci. 2000;20:6962–7. doi: 10.1523/JNEUROSCI.20-18-06962.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto T, Yin Y, Omura K, Gilbert HY, Kim D, Cen LP, et al. Long-distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and pten gene deletion. J Neurosci. 2010;30:15654–63. doi: 10.1523/JNEUROSCI.4340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa T, Katai N, Shibuki H, Kuroiwa S, Kurimoto Y, Nakayama C, et al. BDNF diminishes caspase-2 but not c-Jun immunoreactivity of neurons in retinal ganglion cell layer after transient ischemia. Invest Ophthalmol Vis Sci. 1999;40:3006–11. [PubMed] [Google Scholar]
- Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–7. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–6. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–26. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan A, Ahmed Z, Baird A, Gonzalez AM, Berry M. Neurotrophic factor synergy is required for neuronal survival and disinhibited axon regeneration after CNS injury. Brain. 2006;129(Pt 2):490–502. doi: 10.1093/brain/awh706. [DOI] [PubMed] [Google Scholar]
- Lorber B, Berry M, Logan A, Tonge D. Effect of lens lesion on neurite outgrowth of retinal ganglion cells in vitro. Mol Cell Neurosci. 2002;21:301–11. doi: 10.1006/mcne.2002.1175. [DOI] [PubMed] [Google Scholar]
- Lorber B, Berry M, Logan A. Lens injury stimulates adult mouse retinal ganglion cell axon regeneration via both macrophage- and lens-derived factors. Eur J Neurosci. 2005;21:2029–34. doi: 10.1111/j.1460-9568.2005.04034.x. [DOI] [PubMed] [Google Scholar]
- Lorber B, Berry M, Logan A. Different factors promote axonal regeneration of adult rat retinal ganglion cells after lens injury and intravitreal peripheral nerve grafting. J Neurosci Res. 2008;86:894–903. doi: 10.1002/jnr.21545. [DOI] [PubMed] [Google Scholar]
- Lorber B, Berry M, Douglas MR, Nakazawa T, Logan A. Activated retinal glia promote neurite outgrowth of retinal ganglion cells via apolipoprotein E. J Neurosci Res. 2009;87:2645–52. doi: 10.1002/jnr.22095. [DOI] [PubMed] [Google Scholar]
- Lorber B, Guidi A, Fawcett JW, Martin KR. Activated retinal glia mediated axon regeneration in experimental glaucoma. Neurobiol Dis. 2012;45:243–52. doi: 10.1016/j.nbd.2011.08.008. [DOI] [PubMed] [Google Scholar]
- MacLaren RE. Development and role of retinal glia in regeneration of ganglion cells following retinal injury. Br J Ophthalmol. 1996;80:458–64. doi: 10.1136/bjo.80.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci USA. 1994;91:1632–6. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McStay GP, Salvesen GS, Green DR. Overlapping cleavage motif selectivity of caspases: implications for analysis of apoptotic pathways. Cell Death Differ. 2008;15:322–31. doi: 10.1038/sj.cdd.4402260. [DOI] [PubMed] [Google Scholar]
- Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602:304–17. doi: 10.1016/0006-8993(93)90695-j. [DOI] [PubMed] [Google Scholar]
- Mintzer R, Ramaswamy S, Shah K, Hannoush RN, Pozniak CD, Cohen F, et al. A whole cell assay to measure caspase-6 activity by detecting cleavage of lamin A/C. PLoS One. 2012;7:e30376. doi: 10.1371/journal.pone.0030376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier PP, D'Onofrio PM, Magharious M, Hollander AC, Tassew N, Szydlowska K, et al. Involvement of caspase-6 and caspase-8 in neuronal apoptosis and the regenerative failure of injured retinal ganglion cells. J Neurosci. 2011;31:10494–505. doi: 10.1523/JNEUROSCI.0148-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouser PE, Head E, Ho Ha K, Rohn TT. Caspase-mediated cleavage of glial fibrillary acidic protein within degenerating astrocytes of the Alzheimer's disease brain. Am J Pathol. 2006;168:936–46. doi: 10.2353/ajpath.2006.050798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Hauk TG, Fischer D. Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain. 2007;130(Pt 12):3308–20. doi: 10.1093/brain/awm257. [DOI] [PubMed] [Google Scholar]
- Muller A, Hauk TG, Leibinger M, Marienfeld R, Fischer D. Exogenous CNTF stimulates axon regeneration of retinal ganglion cells partially via endogenous CNTF. Mol Cell Neurosci. 2009;41:233–46. doi: 10.1016/j.mcn.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Niizuma K, Endo H, Nito C, Myer DJ, Kim GS, Chan PH. The PIDDosome mediates delayed death of hippocampal CA1 neurons after transient global cerebral ischemia in rats. Proc Natl Acad Sci USA. 2008;105:16368–73. doi: 10.1073/pnas.0806222105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–9. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Orth K, Chinnaiyan AM, Garg M, Froelich CJ, Dixit VM. The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate lamin A. J Biol Chem. 1996;271:16443–6. [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–6. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado-Ramon P, Salvador M, Villegas-Perez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:489–500. [PubMed] [Google Scholar]
- Pernet V, Di Polo A. Synergistic action of brain-derived neurotrophic factor and lens injury promotes retinal ganglion cell survival, but leads to optic nerve dystrophy in vivo. Brain. 2006;129(Pt 4):1014–26. doi: 10.1093/brain/awl015. [DOI] [PubMed] [Google Scholar]
- Pernet V, Joly S, Dalkara D, Jordi N, Schwarz O, Christ F, et al. Long-distance axonal regeneration induced by CNTF gene transfer is impaired by axonal misguidance in the injured adult optic nerve. Neurobiol Dis. 2013;51:202–13. doi: 10.1016/j.nbd.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284:21777–81. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AG, Ng P, Janicke RU. Death substrates come alive. Bioessays. 1997;19:501–7. doi: 10.1002/bies.950190609. [DOI] [PubMed] [Google Scholar]
- Rabacchi SA, Bonfanti L, Liu XH, Maffei L. Apoptotic cell death induced by optic nerve lesion in the neonatal rat. J Neurosci. 1994;14:5292–301. doi: 10.1523/JNEUROSCI.14-09-05292.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchaud S, Korfali N, Villa P, Kottke TJ, Dingwall C, Kaufmann SH, et al. Caspase-6 gene disruption reveals a requirement for lamin A cleavage in apoptotic chromatin condensation. EMBO J. 2002;21:1967–77. doi: 10.1093/emboj/21.8.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DJ, Weimer RM, McLaughlin T, Kallop D, Stanger K, Yang J, et al. A caspase cascade regulating developmental axon degeneration. J Neurosci. 2012;32:17540–53. doi: 10.1523/JNEUROSCI.3012-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Savitz SI, Hoque R, Gupta G, Roth S, Rosenbaum PS, et al. Cell-specific caspase expression by different neuronal phenotypes in transient retinal ischemia. J Neurochem. 2001;77:466–75. doi: 10.1046/j.1471-4159.2001.00258.x. [DOI] [PubMed] [Google Scholar]
- Song Q, Lees-Miller SP, Kumar S, Zhang Z, Chan DW, Smith GC, et al. DNA-dependent protein kinase catalytic subunit: a target for an ICE-like protease in apoptosis. EMBO J. 1996;15:3238–46. [PMC free article] [PubMed] [Google Scholar]
- Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, et al. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480:372–5. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Alnemri ES, Lazebnik YA, Fernandes-Alnemri T, Litwack G, Moir RD, et al. Cleavage of lamin A by Mch2 alpha but not CPP32: multiple interleukin 1 beta-converting enzyme-related proteases with distinct substrate recognition properties are active in apoptosis. Proc Natl Acad Sci USA. 1996;93:8395–400. doi: 10.1073/pnas.93.16.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Xu Z, Goldman JE. Synergistic effects of the SAPK/JNK and the proteasome pathway on glial fibrillary acidic protein (GFAP) accumulation in Alexander disease. J Biol Chem. 2006;281:38634–43. doi: 10.1074/jbc.M604942200. [DOI] [PubMed] [Google Scholar]
- Thanos S, Vidal-Sanz M, Aguayo AJ. The use of rhodamine-B-isothiocyanate (RITC) as an anterograde and retrograde tracer in the adult rat visual system. Brain Res. 1987;406:317–21. doi: 10.1016/0006-8993(87)90799-2. [DOI] [PubMed] [Google Scholar]
- Timmer JC, Salvesen GS. Caspase substrates. Cell Death Differ. 2007;14:66–72. doi: 10.1038/sj.cdd.4402059. [DOI] [PubMed] [Google Scholar]
- Troy CM, Stefanis L, Greene LA, Shelanski ML. Nedd2 is required for apoptosis after trophic factor withdrawal, but not superoxide dismutase (SOD1) downregulation, in sympathetic neurons and PC12 cells. J Neurosci. 1997;17:1911–8. doi: 10.1523/JNEUROSCI.17-06-01911.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy CM, Rabacchi SA, Friedman WJ, Frappier TF, Brown K, Shelanski ML. Caspase-2 mediates neuronal cell death induced by beta-amyloid. J Neurosci. 2000;20:1386–92. doi: 10.1523/JNEUROSCI.20-04-01386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy CM, Rabacchi SA, Hohl JB, Angelastro JM, Greene LA, Shelanski ML. Death in the balance: alternative participation of the caspase-2 and -9 pathways in neuronal death induced by nerve growth factor deprivation. J Neurosci. 2001;21:5007–16. doi: 10.1523/JNEUROSCI.21-14-05007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy CM, Ribe EM. Caspase-2: vestigial remnant or master regulator? Sci Signal. 2008;1:pe42. doi: 10.1126/scisignal.138pe42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldik LJ, Thompson WL, Ralay Ranaivo H, Behanna HA, Martin Watterson D. Glia proinflammatory cytokine upregulation as a therapeutic target for neurodegenerative diseases: function-based and target-based discovery approaches. Int Rev Neurobiol. 2007;82:277–96. doi: 10.1016/S0074-7742(07)82015-0. [DOI] [PubMed] [Google Scholar]
- Vigneswara V, Berry M, Logan A, Ahmed Z. Pharmacological inhibition of caspase-2 protects axotomised retinal ganglion cells from apoptosis in adult rats. PLoS One. 2012;7:e53473. doi: 10.1371/journal.pone.0053473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneswara V, Berry M, Logan A, Ahmed Z. Pigment epithelium-derived factor is retinal ganglion cell neuroprotective and axogenic after optic nerve crush injury. Invest Ophthalmol Vis Sci. 2013;54:2624–33. doi: 10.1167/iovs.13-11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Perez MP, Vidal-Sanz M, Rasminsky M, Bray GM, Aguayo AJ. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol. 1993;24:23–36. doi: 10.1002/neu.480240103. [DOI] [PubMed] [Google Scholar]
- Vohra BP, Sasaki Y, Miller BR, Chang J, DiAntonio A, Milbrandt J. Amyloid precursor protein cleavage-dependent and -independent axonal degeneration programs share a common nicotinamide mononucleotide adenylyltransferase 1-sensitive pathway. J Neurosci. 2010;30:13729–38. doi: 10.1523/JNEUROSCI.2939-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaupt JH, Diem R, Kermer P, Krajewski S, Reed JC, Bahr M. Contribution of caspase-8 to apoptosis of axotomized rat retinal ganglion cells in vivo. Neurobiol Dis. 2003;13:124–35. doi: 10.1016/s0969-9961(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Winter CG, Saotome Y, Levison SW, Hirsh D. A role for ciliary neurotrophic factor as an inducer of reactive gliosis, the glial response to central nervous system injury. Proc Natl Acad Sci USA. 1995;92:5865–9. doi: 10.1073/pnas.92.13.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, et al. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–93. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.