Abstract
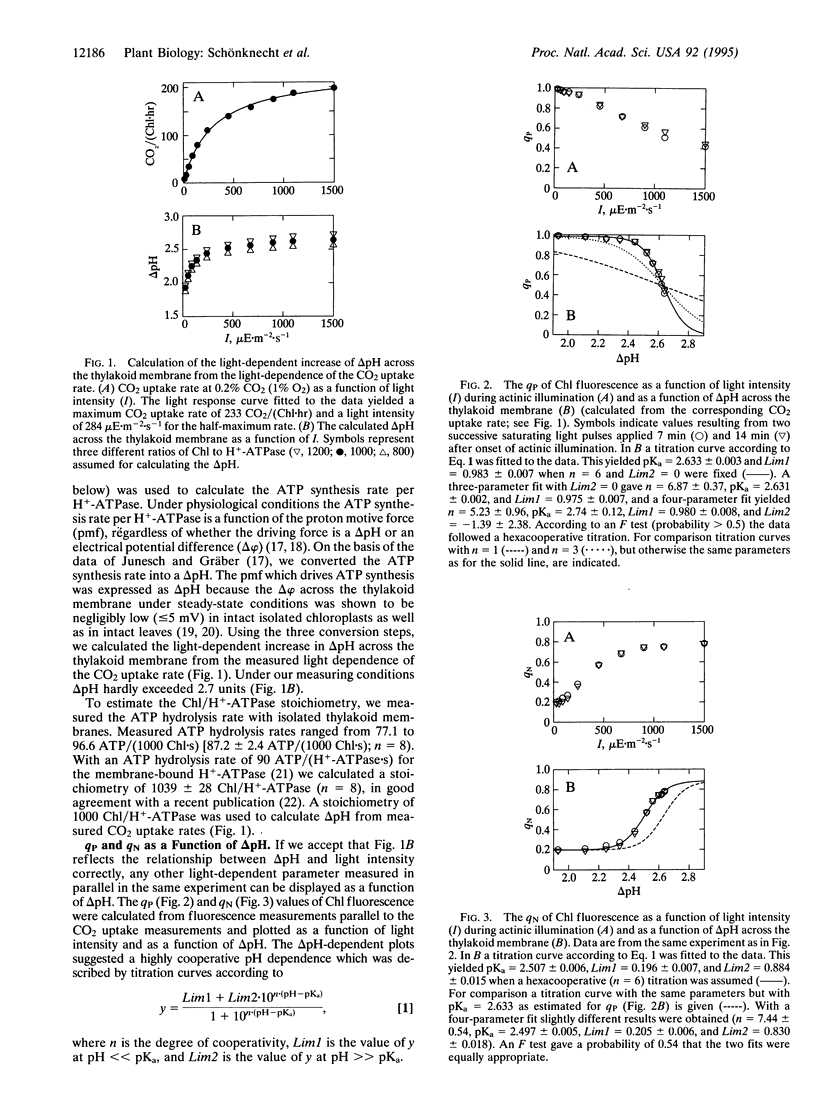
Under conditions (0.2% CO2; 1% O2) that allow high rates of photosynthesis, chlorophyll fluorescence was measured simultaneously with carbon assimilation at various light intensities in spinach (Spinacia oleracea) leaves. Using a stoichiometry of 3 ATP/CO2 and the known relationship between ATP synthesis rate and driving force (Delta pH), we calculated the light-dependent pH gradient (Delta pH) across the thylakoid membrane in intact leaves. These Delta pH values were correlated with the photochemical (qP) and nonphotochemical (qN) quenching of chlorophyll fluorescence and with the quantum yield of photosystem II (phiPSII). At Delta pH > 2.1 all three parameters (qP, qN, and phiPSII) changed very steeply with increasing DeltapH (decreasing pH in the thylakoid). The observed pH dependences followed hexacooperative titration curves with slightly different pKa values. The significance of the steep pH dependences with slightly different pKa values is discussed in relation to the regulation of photosynthetic electron transport in intact leaves.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthon G. E., Jagendorf A. T. Methanol-induced release of tightly bound adenine nucleotides from thylakoid-associated CF1. Biochim Biophys Acta. 1984 Aug 31;766(2):354–362. doi: 10.1016/0005-2728(84)90251-2. [DOI] [PubMed] [Google Scholar]
- Briantais J. M., Vernotte C., Picaud M., Krause G. H. A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochim Biophys Acta. 1979 Oct 10;548(1):128–138. doi: 10.1016/0005-2728(79)90193-2. [DOI] [PubMed] [Google Scholar]
- Junesch U., Gräber P. The rate of ATP-synthesis as a function of delta pH and delta psi catalyzed by the active, reduced H(+)-ATPase from chloroplasts. FEBS Lett. 1991 Dec 9;294(3):275–278. doi: 10.1016/0014-5793(91)81447-g. [DOI] [PubMed] [Google Scholar]
- Junge W., Hong Y. Q., Qian L. P., Viale A. Cooperative transient trapping of photosystem II protons by the integral membrane portion (CF0) of chloroplast ATP-synthase after mild extraction of the four-subunit catalytic part (CF1). Proc Natl Acad Sci U S A. 1984 May;81(10):3078–3082. doi: 10.1073/pnas.81.10.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfundel E. E., Dilley R. A. The pH Dependence of Violaxanthin Deepoxidation in Isolated Pea Chloroplasts. Plant Physiol. 1993 Jan;101(1):65–71. doi: 10.1104/pp.101.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possmayer F. E., Gräber P. The pHin and pHout dependence of the rate of ATP synthesis catalyzed by the chloroplast H(+)-ATPase, CF0F1, in proteoliposomes. J Biol Chem. 1994 Jan 21;269(3):1896–1904. [PubMed] [Google Scholar]
- Rottenberg H., Grunwald T., Avron M. Determination of pH in chloroplasts. I. Distribution of ( 14 C) methylamine. Eur J Biochem. 1972 Jan 31;25(1):54–63. doi: 10.1111/j.1432-1033.1972.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Rumberg B., Siggel U. pH changes in the inner phase of the thylakoids during photosynthesis. Naturwissenschaften. 1969 Mar;56(3):130–132. doi: 10.1007/BF00601025. [DOI] [PubMed] [Google Scholar]
- Sigalat C., Haraux F., de Kouchkovsky Y. Flow-force relationships in lettuce thylakoids. 1. Strict control of electron flow by internal pH. Biochemistry. 1993 Sep 28;32(38):10193–10200. doi: 10.1021/bi00089a040. [DOI] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Vredenberg W. J., Tonk W. J. On the steady-state electrical potential difference across the thylakoid membranes of chloroplasts in illuminated plant cells. Biochim Biophys Acta. 1975 Jun 17;387(3):580–587. doi: 10.1016/0005-2728(75)90095-x. [DOI] [PubMed] [Google Scholar]



