Behavioural variant frontotemporal dementia is characterized by an increase in primary reward-seeking behaviours, including pursuit of food, drug, and sexual rewards. Perry et al. reveal that increased reward-seeking correlates with lower volume in the right ventral putamen and pallidum, which are known reward circuit structures.
Keywords: frontotemporal dementia, reward processing, hypersexuality, overeating, alcohol
Abstract
Behavioural variant frontotemporal dementia is characterized by abnormal responses to primary reward stimuli such as food, sex and intoxicants, suggesting abnormal functioning of brain circuitry mediating reward processing. The goal of this analysis was to determine whether abnormalities in reward-seeking behaviour in behavioural variant frontotemporal dementia are correlated with atrophy in regions known to mediate reward processing. Review of case histories in 103 patients with behavioural variant frontotemporal dementia identified overeating or increased sweet food preference in 80 (78%), new or increased alcohol or drug use in 27 (26%), and hypersexuality in 17 (17%). For each patient, a primary reward-seeking score of 0–3 was created with 1 point given for each target behaviour (increased seeking of food, drugs, or sex). Voxel-based morphometry performed in 91 patients with available imaging revealed that right ventral putamen and pallidum atrophy correlated with higher reward-seeking scores. Each of the reward-related behaviours involved partially overlapping right hemisphere reward circuit regions including putamen, globus pallidus, insula and thalamus. These findings indicate that in some patients with behavioural variant frontotemporal dementia, low volume of subcortical reward-related structures is associated with increased pursuit of primary rewards, which may be a product of increased thalamocortical feedback.
Introduction
Some symptoms in behavioural variant frontotemporal dementia (FTD) suggest abnormal reward processing. Patients may overeat and crave sweet food (Miller et al., 1995). Though patients are frequently hyposexual (Miller et al., 1995), some may become hypersexual (Mendez and Shapira, 2013). The use of alcohol or drugs of abuse has been reported (Cruz et al., 2008), but not well characterized. Although such behaviours could relate to altered reward processing, they might also emerge from less specific deficits including problems with inhibitory control or lack of awareness of bodily states. If reward processing changes are the cause this would be consistent with the anatomical vulnerability in early behavioural variant FTD, which includes known reward-processing areas, such as orbitofrontal cortex, ventral striatum, frontoinsula, anterior cingulate and dorsomedial thalamus (Seeley et al., 2008; Haber and Knutson, 2010).
The objectives of this study were to identify behaviours in patients with behavioural variant FTD that suggest abnormal reward processing and determine whether these behaviours are linked to anatomical changes in reward-processing regions. Linkage of these behaviours to portions of neural circuits with well-described roles in reward processing may ultimately help to explain their aetiology.
Differences may exist between processing a primary reward (stimuli which inherently produce pursuit behaviours) and a secondary reward (stimuli, such as money, that are paired with or can be used to obtain another reward). We chose to focus on changes in eating, sexual behaviour and drug use because these are primary rewards with evidence of pursuit to consumption. By considering three behaviours together we proposed to identify the common effect of reward-seeking independent of the sensory or cognitive components specific to each behaviour. We hypothesized that reward circuit structural changes would underlie all three behaviours.
Materials and methods
Subjects
All patients with behavioural variant FTD who had been evaluated at the University of California San Francisco Frontotemporal Dementia Program Project Grant were studied (evaluation details in Supplementary material). All patients who met behavioural variant FTD research criteria were included (Neary et al., 1998). Patients with motor neuron disease were included if they met behavioural variant FTD criteria. One hundred and twenty-five patients were initially screened. Nineteen were excluded based on having a current non-behavioural variant FTD diagnosis, including other neurodegenerative syndromes (e.g. primary progressive aphasia variants, corticobasal syndrome and progressive supranuclear palsy). Three were excluded because their research notes were not available for review. After these exclusions 103 patients were included. Written informed consent was obtained from patients or surrogates according to procedures approved by the UCSF Committee on Human Research.
Data abstraction
All available notes and research visit summaries were reviewed by one author (D.C.P.). Behaviours connected with reward processing were recorded, including changes in eating, sexual behaviour and alcohol or drug use. A determination of increased reward-seeking was based on the presence of behaviours that require active pursuit of reward to the point of consumption, rather than opportunistic responses to environment or changes in habits. Environmental dependency or utilization behaviour may localize more broadly to prefrontal cortex and habitual actions to dorsal frontal regions and striatum rather than being reward circuit mediated. Behaviours were counted only if they occurred at or after the neurodegenerative syndrome onset.
Eating behaviour
Increased preference for sweets and overeating were characterized as associated with increased pursuit of reward. Restrictive dieting and rigid eating habits were not included among reward-seeking behaviours.
Drug and alcohol use
Increased or new alcohol or drug use was also considered as reflective of increased reward seeking. Patients with a longstanding substance abuse history were not counted as having disease-related reward-seeking behaviour.
Sexual behaviour
Behaviours were considered hypersexual if they reflected heightened motivation to pursue sexual reward. This included an increased interest in sexually explicit material such as pornography, increased seeking of sexual activity inside or outside of committed relationships, and public masturbation. Sexual comments and impulsive, inappropriate touching of strangers were not counted as hypersexuality. Although these common behavioural variant FTD behaviours are sexual, they are also tied to social disinhibition, impulsivity and environmental dependency (Baird et al., 2007). Their exclusion allows for a more clear evaluation of those behaviours where sexual gratification is the goal. Hyposexual behaviours were not counted.
Combinations of primary reward-seeking behaviour
Primary reward-seeking was quantified by giving 1 point for each behaviour (overeating/increased sweet preference, hypersexuality, and increased drug and alcohol use). This created a primary reward-seeking score ranging from 0 (no reward-seeking behaviours) to 3 (seeking multiple primary rewards).
Correlation of reward-seeking with neuropsychological and neuropsychiatric measures
Additional analyses assessed the relationship between reward scores and other measures of cognition and behaviour (Supplementary material).
Genetics and pathology
Information was gathered when available on autopsy-confirmed diagnoses or the presence of genetic mutations known to cause autosomal-dominant behavioural variant FTD (Supplementary material).
Image acquisition
Of 103 patients reviewed, 91 had useble neuroimaging (demographic features in Table 1). Images were acquired on one of three scanners, 48 patients at 1.5 T, 15 at 4 T, and 28 on a 3 T scanner (Supplementary material).
Table 1.
Demographic features of subjects in the imaging analysis
| Subjects with behavioural variant FTD | |
|---|---|
| Age at time of scan | 59.7 (8.4) |
| Male (%) | 58 (63.7) |
| Education (years) | 15.7 (3.1) |
| Race, n = 87 (% white) | 77 (88.5) |
| MMSE (/30) | 22.9 (7.3) |
Results for behavioural variant FTD subjects displayed as mean (standard deviation) except for male sex and race. n = 91 unless otherwise specified. MMSE = Mini-Mental State Examination.
Images selected were either the first UCSF research MRI after patients displayed reward-seeking symptoms or their first research MRI for those who never displayed reward-related symptoms.
Imaging analysis
Voxel-based morphometry (Ashburner and Friston, 2000) was performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) (Supplementary material). Multiple regression was performed to assess for changes in combined grey and white matter volume associated with higher primary reward-seeking scores. Multiple regression was also performed for each separate behaviour to assess for decreased volume associated with the presence of overeating/sweet preference, drug or alcohol use, and hypersexuality. The threshold for statistical significance was set at P < 0.05 after family wise error (FWE) correction for multiple comparisons, and statistical maps were examined at a level of P < 0.001 uncorrected for multiple comparisons. Age, sex, total intracranial volume and Mini-Mental State Examination (Folstein et al., 1975) score were covariates in each regression, and scanner type was included as a binary factor (1.5 T, for the most common scanner, or non-1.5 T for the other two scanners).
Results
Behavioural findings
Eating behaviour
Among all 103 patients, 80 (77.7%) had overeating or increased sweet preference (70 among those in the imaging analysis). Eighteen only preferred sweets, 23 only overate, and 39 showed both symptoms. In six cases the eating changes developed during follow-up; they were present at initial evaluation in the other 74. Rigid dietary routines were observed in 23 subjects, but were not counted as reward-seeking.
Drug and alcohol use
New or increased alcohol or drug use occurred in 27 (26.2%) patients (25 in the imaging analysis). This was present at initial evaluation in all but one case. Alcohol use was the most common (21 patients), but increased cigarette smoking was found in nine, and opiate (three patients) and benzodiazepine (one patient) use were also described. Four patients drank until becoming stuporous or passing out, three drove while intoxicated, and five had been enrolled in substance abuse rehabilitation programmes. One drank in spite of taking Antabuse™. Seven had substance use that predated the onset of neurodegenerative symptoms, but these were not counted as new reward-seeking behaviours.
Sexual behaviour
Seventeen patients (16.5%) showed hypersexuality (14 in the imaging analysis), including seven with increased interest in sexually explicit material. In one case the behaviour developed after initial presentation. One patient visited strip clubs up to twice daily. Two patients exhibited public masturbation. Five had extramarital affairs. Seven of 103 were described as hyposexual, though among patients who exhibited hypersexual behaviour there were several who also did not pursue sexual relations with their partners.
Combinations of primary reward-seeking behaviour
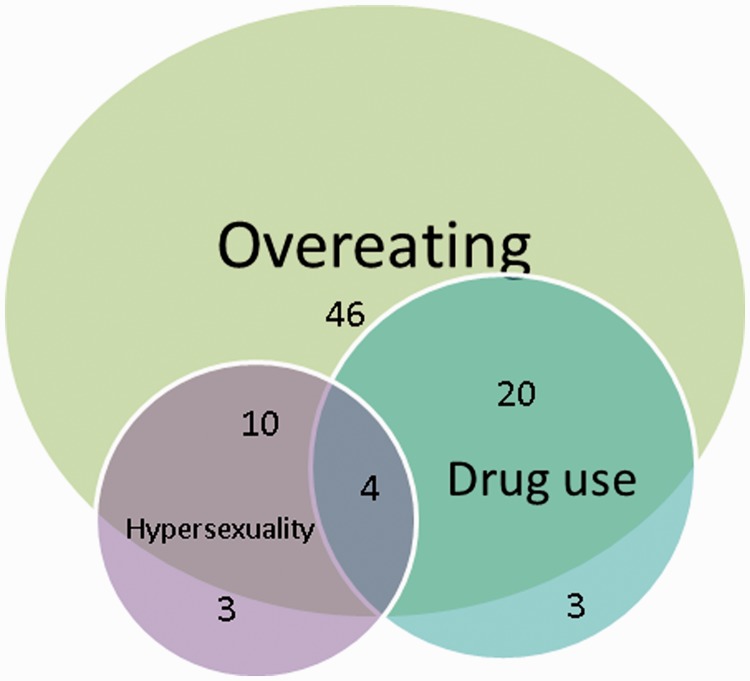
Eighty-four (82%) of 103 exhibited at least one primary reward-seeking behaviour. Thirty exhibited two behaviours and four showed all three (Fig. 1). Seventeen exhibited no primary reward-seeking behaviour.
Figure 1.
Co-occurrence of reward seeking behaviours. Diagram shows the overlap in pursuit of the primary reward seeking behaviours overeating, drug or alcohol use, and hypersexuality. Numbers reflect number of patients exhibiting behaviours out of total n = 103.
Imaging findings
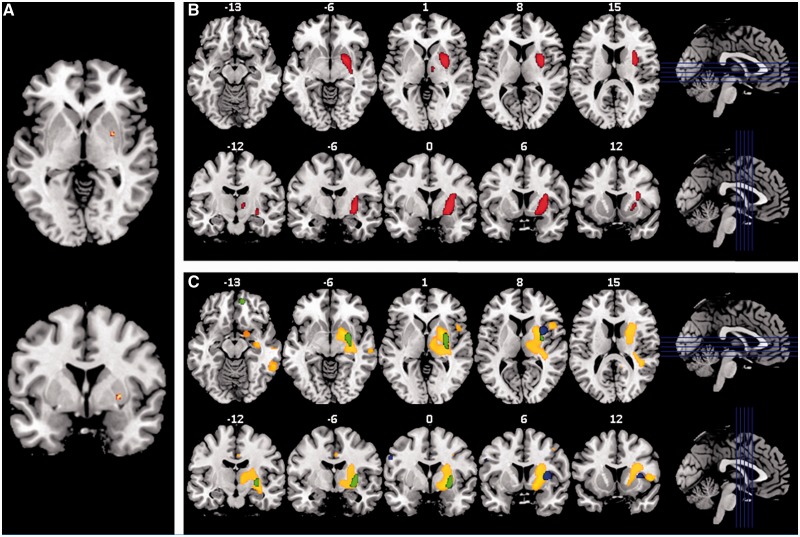
Brain regions in which volume loss correlated with higher scores included the right ventral putamen extending into the ventral pallidum with a statistically significant peak in mid-anterior putamen [T = 4.59, Montreal Neurological Institute coordinates (28, 0, 0), Fig. 2A]. Additional regions that exceeded a P < 0.001 threshold uncorrected for multiple comparisons are displayed in Fig. 2B.
Figure 2.
Voxel-based morphometry of primary reward seeking score and of individual component behaviours. (A) T map thresholded at PFWE < 0.05 and overlaid on MNI template brain showing single area of decreased volume in the right ventral pallidum associated with higher primary reward-seeking score, which is a composite of the presence of overeating, drug use, and hypersexuality. (B) Axial and coronal slices of T map thresholded at P < 0.001 uncorrected for multiple comparisons overlaid on MNI template brain showing—in red—areas of volume loss associated with higher primary reward seeking score. (C) Axial and coronal slices of T map thresholded at P < 0.01 uncorrected for multiple comparisons overlaid on MNI template brain showing areas of volume loss associated with the presence of individual primary reward-seeking behaviours. Blue shows hypersexuality. Green shows alcohol or drug seeking. Yellow/orange shows overeating and sweet food preference. The right side of axial and coronal images corresponds to the right side of the brain.
When each behaviour was evaluated individually no clusters survived FWE correction. To further explore whether the anatomy for each behaviour might differ substantially from the others the threshold was decreased to P < 0.01 uncorrected for multiple comparisons and results for all three behaviours were overlaid on the same template. This showed that each behaviour was associated with right hemisphere atrophy and that all three affected subcortical reward circuit structures, particularly the right putamen (Fig. 2C).
Discussion
In reviewing 103 patients with behavioural variant FTD we found that 82% exhibited behaviours suggestive of increased primary reward-seeking. Overeating and sweet craving occurred most frequently, but drug use (particularly alcohol) was also common, and some patients exhibited hypersexual behaviour. Imaging analysis revealed a significant correlation between primary reward-seeking and atrophy of the right ventral putamen extending into the right pallidum. Considering these structures’ known reward circuit roles, our findings support the hypothesis that these behaviours are caused, at least in part, by abnormal reward processing.
The portions of the putamen identified receive innervation from cortical regions involved in reward (orbitofrontal cortex and anterior cingulate) (Haber and Knutson, 2010). The ventral pallidum is central to reward processing (Smith et al., 2009) as it is the major output target of the ventral striatum, with which it is reciprocally connected. Ventral pallidum also bears reciprocal connections to the ventral tegmental area and projects to the dorsomedial nucleus of the thalamus, hypothalamus and lateral habenular nucleus, a key structure in decreasing dopaminergic activity in response to punishment or the absence of reward (Haber and Knutson, 2010).
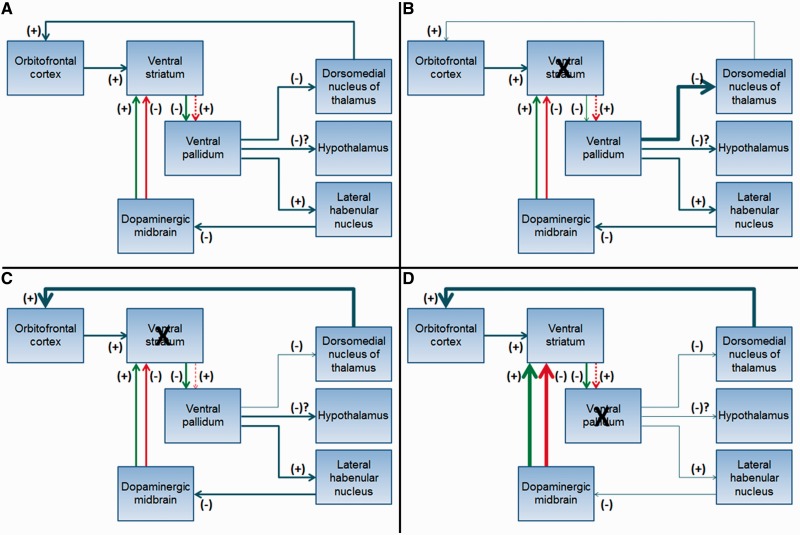
Primary reward-seeking in behavioural variant FTD could relate to increased reward circuit activity, although an alternative hypothesis is that seeking more potent food, drug or sexual stimuli is compensatory behaviour for low reward circuit activity. Decreased ventral putamen or pallidum volume could either result in increased or decreased reward circuit activity (Fig. 3) depending on lesion location and the vulnerable neuronal population. Striatal degeneration would decrease globus pallidus inhibition through a direct pathway (Fig. 3B), resulting in stronger thalamic inhibition and ultimately less cortical activation, which would only lead to increased reward seeking as a compensatory behaviour. Alternatively, weaker pallidal stimulation through an indirect pathway would ultimately increase cortical activation (Fig. 3C). If degeneration of the putamen is leading to increased reward-seeking, and if it is mediated by increased thalamocortical excitation, then the reward circuit indirect pathway would need to be more affected than the direct pathway. Although direct and indirect pathways involving ventral striatum exist (Hikida et al., 2010), whether one neuronal population is more vulnerable in behavioural variant FTD is unknown, and not addressed by this study.
Figure 3.
Schematic diagram of potential reward circuit abnormalities in behavioural variant FTD. Green arrows depict the direct pathway. Red arrows depict the indirect pathway. The dashed line indicates that additional synaptic connections exist between these structures but are not illustrated for simplicity. (A) Normal reward circuit connections at baseline. (B) The effect of a ventral striatal lesion on the direct pathway. (C) The effect of a ventral striatal lesion on the indirect pathway. (D) The effect of a ventral pallidum lesion.
Degeneration of the pallidum could result in increased thalamocortical activity in a more conceptually straightforward manner (Fig. 3D). Ventral pallidum degeneration could also affect primary reward processing through altered hypothalamic feedback, leading to lack of satiety, or decreased output to the lateral habenular nucleus, so the dopaminergic system is not appropriately turned down when reward does not match prediction. The ventral pallidum is involved in regulating eating (Johnson et al., 1996), drug and alcohol seeking (Kemppainen et al., 2012), and sexual behaviour (Mendez et al., 2004). One patient with Parkinson’s disease became hypersexual after a right pallidotomy (Mendez et al., 2004).
The laterality of the findings may also be significant. Emotional lateralization models attribute negative emotion or withdrawal behaviours to the right hemisphere. Right hemisphere degeneration could lead to an increase in approach behaviours mediated by relatively preserved left hemisphere structures.
The areas we found to be associated with reward-seeking behaviours are a subset of the vulnerable regions in behavioural variant FTD (Seeley et al., 2008; Halabi et al., 2013), indicating that reward is one of many components underlying behavioural variant FTD behaviours. Though the findings for each individual behaviour did not reach significance, they suggest that common right hemisphere, largely subcortical anatomy was responsible for overeating, drug use and hypersexuality, and that the combination of the three resulted in the significant pooled reward score finding. Although each behaviour may result from a combination of factors, by examining multiple reward types together we targeted the reward-seeking component and found a well-localized result. For example, the anatomy involved in eating behaviour was more widely distributed compared with the anatomy associated with reward-seeking. Multiple processes contribute to eating regulation, such as interoceptive function from the insula, in addition to changes in reward processing. Previous neuroimaging analyses in behavioural variant FTD linked overeating with right ventral insula and right striatal atrophy (Woolley et al., 2007), and sweet craving with right frontoinsular atrophy (Whitwell et al., 2007). We examined overeating and sweet preference together and also found an association with right insula and striatum degeneration. A third study of eating in behavioural variant FTD focused on the hypothalamus (Piguet et al., 2011). Although our study did not examine the diencephalon with high spatial resolution, the regions associated with eating behaviour in our study also extended into the hypothalamus at a more permissive threshold of P < 0.01. The anatomical correlates of alcohol and drug use and hypersexuality have not previously been evaluated in behavioural variant FTD. Hypersexuality has been described in some patients with right temporal variant behavioural variant FTD (Mendez and Shapira, 2013), and as ventral basal ganglia degeneration accompanies this variant, our findings suggest right hemisphere reward circuit changes as a possible explanation for those cases.
Our main findings involved deep nuclei; however, cortical regions also process reward. Orbitofrontal cortex is important for reward representation and primary reward-seeking was associated with atrophy of a small, right orbitofrontal cluster that did not reach significance. The uncorrected map shows some insular cortex involvement, suggesting decreased sensitivity to negative consequences since this region has been implicated in processing punishment (Seymour et al., 2007).
Although substance abuse in behavioural variant FTD has been observed at a similar frequency to our findings (Ikeda et al., 2002), it is often conceptualized as hyperoral behaviour, rather than reward-seeking. Though the frequency of hypersexuality is comparable to previous studies (Mendez and Shapira, 2013), the higher rate of hypersexuality than hyposexuality in this study differs from previous reports (Miller et al., 1995) and could reflect a higher frequency of the behaviours than suspected, an ascertainment bias in reporting, or differing definitions of hypersexual, as many of the sexual behaviours documented were self-centred rather than requiring meaningful socio-emotional, interpersonal interaction. The high frequency of eating behaviours in this study is consistent with previous reports (Miller, et al., 1995; Ikeda et al., 2002).
The retrospective nature of the study is a limitation. Determinations regarding whether behaviours were present depended on documentation. In this research study patients undergo structured evaluation in which these behaviours are likely to be elicited and recorded. Prospective assessment of another behavioural variant FTD sample with validated reward-processing tasks could confirm these findings. These tasks should include rewards and punishments to address whether behaviours such as overeating, hypersexuality and drug use are due to increased reward sensitivity or decreased sensitivity to negative consequences (such as obesity, alienation of spouse or partner, and prosecution for driving while intoxicated). The present study supports a reward-processing interpretation of reward-seeking behaviour in behavioural variant FTD and suggests this could be a relevant model for understanding other behaviours in this and other neuropsychiatric disorders.
Funding
This study was supported by grants P01AG019724, R01AG032306 and K24AG045333 from the NIH National Institute on Ageing.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviation
- FTD
frontotemporal dementia
References
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Baird AD, Wilson SJ, Bladin PF, Saling MM, Reutens DC. Neurological control of human sexual behaviour: insights from lesion studies. J Neurol Neurosurg Psychiatry. 2007;78:1042–9. doi: 10.1136/jnnp.2006.107193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz M, Marinho V, Fontenelle LF, Engelhardt E, Laks J. Topiramate may modulate alcohol abuse but not other compulsive behaviors in frontotemporal dementia: case report. Cogn Behav Neurol. 2008;21:104–6. doi: 10.1097/WNN.0b013e31816bdf73. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabi C, Halabi A, Dean DL, Wang PN, Boxer AL, Trojanowski JQ, et al. Patterns of striatal degeneration in frontotemporal dementia. Alzheimer Dis Assoc Disord. 2013;27:74–83. doi: 10.1097/WAD.0b013e31824a7df4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Brown J, Holland AJ, Fukuhara R, Hodges JR. Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;73:371–6. doi: 10.1136/jnnp.73.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Parente MA, Stellar JR. NMDA-induced lesions of the nucleus accumbens or the ventral pallidum increase the rewarding efficacy of food to deprived rats. Brain Res. 1996;722:109–17. doi: 10.1016/0006-8993(96)00202-8. [DOI] [PubMed] [Google Scholar]
- Kemppainen H, Raivio N, Kiianmaa K. Role for ventral pallidal GABAergic mechanisms in the regulation of ethanol self-administration. Psychopharmacology (Berl) 2012;223:211–21. doi: 10.1007/s00213-012-2709-x. [DOI] [PubMed] [Google Scholar]
- Mendez MF, O'Connor SM, Lim GT. Hypersexuality after right pallidotomy for parkinson's disease. J Neuropsychiatry Clin Neurosci. 2004;16:37–40. doi: 10.1176/jnp.16.1.37. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Shapira JS. Hypersexual behavior in frontotemporal dementia: a comparison with early-onset Alzheimer's disease. Arch Sex Behav. 2013;42:501–9. doi: 10.1007/s10508-012-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL, Darby AL, Swartz JR, Yener GG, Mena I. Dietary changes, compulsions and sexual behavior in frontotemporal degeneration. Dementia. 1995;6:195–9. doi: 10.1159/000106946. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Piguet O, Petersen A, Yin Ka Lam B, Gabery S, Murphy K, Hodges JR, et al. Eating and hypothalamus changes in behavioral-variant frontotemporal dementia. Ann Neurol. 2011;69:312–19. doi: 10.1002/ana.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65:249. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, Singer T, Dolan R. The neurobiology of punishment. Nat Rev Neurosci. 2007;8:300–11. doi: 10.1038/nrn2119. [DOI] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196:155–67. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Sampson EL, Loy CT, Warren JE, Rossor MN, Fox NC, et al. VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. Neuroimage. 2007;35:207–13. doi: 10.1016/j.neuroimage.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Gorno-Tempini ML, Seeley WW, Rankin K, Lee SS, Matthews BR, et al. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69:1424–33. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.