The functional significance of brain plasticity seen in carpal tunnel syndrome is unclear. Using functional MRI and bio-behavioural testing, Maeda et al. link blurred primary somatosensory cortical representations of median nerve innervated fingers with symptomatology and impaired psychomotor performance and discrimination accuracy. Neuroplasticity in these patients is thus indeed maladaptive.
Keywords: functional magnetic resonance imaging (fMRI), median nerve neuropathy, tactile stimulation, psychomotor performance, finger agnosia
Abstract
Carpal tunnel syndrome, a median nerve entrapment neuropathy, is characterized by sensorimotor deficits. Recent reports have shown that this syndrome is also characterized by functional and structural neuroplasticity in the primary somatosensory cortex of the brain. However, the linkage between this neuroplasticity and the functional deficits in carpal tunnel syndrome is unknown. Sixty-three subjects with carpal tunnel syndrome aged 20–60 years and 28 age- and sex-matched healthy control subjects were evaluated with event-related functional magnetic resonance imaging at 3 T while vibrotactile stimulation was delivered to median nerve innervated (second and third) and ulnar nerve innervated (fifth) digits. For each subject, the interdigit cortical separation distance for each digit’s contralateral primary somatosensory cortex representation was assessed. We also evaluated fine motor skill performance using a previously validated psychomotor performance test (maximum voluntary contraction and visuomotor pinch/release testing) and tactile discrimination capacity using a four-finger forced choice response test. These biobehavioural and clinical metrics were evaluated and correlated with the second/third interdigit cortical separation distance. Compared with healthy control subjects, subjects with carpal tunnel syndrome demonstrated reduced second/third interdigit cortical separation distance (P < 0.05) in contralateral primary somatosensory cortex, corroborating our previous preliminary multi-modal neuroimaging findings. For psychomotor performance testing, subjects with carpal tunnel syndrome demonstrated reduced maximum voluntary contraction pinch strength (P < 0.01) and a reduced number of pinch/release cycles per second (P < 0.05). Additionally, for four-finger forced-choice testing, subjects with carpal tunnel syndrome demonstrated greater response time (P < 0.05), and reduced sensory discrimination accuracy (P < 0.001) for median nerve, but not ulnar nerve, innervated digits. Moreover, the second/third interdigit cortical separation distance was negatively correlated with paraesthesia severity (r = −0.31, P < 0.05), and number of pinch/release cycles (r = −0.31, P < 0.05), and positively correlated with the second and third digit sensory discrimination accuracy (r = 0.50, P < 0.05). Therefore, reduced second/third interdigit cortical separation distance in contralateral primary somatosensory cortex was associated with worse symptomatology (particularly paraesthesia), reduced fine motor skill performance, and worse sensory discrimination accuracy for median nerve innervated digits. In conclusion, primary somatosensory cortex neuroplasticity for median nerve innervated digits in carpal tunnel syndrome is indeed maladaptive and underlies the functional deficits seen in these patients.
Introduction
Carpal tunnel syndrome (CTS), a median nerve entrapment neuropathy, is characterized by pain and paraesthesia in median nerve innervated areas. Recent studies have shown that CTS is also characterized by functional (Druschky et al., 2000; Tecchio et al., 2002; Napadow et al., 2006; Dhond et al., 2012) and structural (Maeda et al., 2013) neuroplasticity in the primary somatosensory cortex (S1) of the brain. Several studies have noted enlarged and/or blurred cortical representations in contralateral S1 for the fingers, or digits of the hand, affected by CTS. For instance, functional MRI studies have found reduced distance between cortical representations of the second and third digits (D2/D3) in subjects with CTS and in addition, prolonged sensory conduction velocities for median nerve innervated digits (Napadow et al., 2006). This contracted D2/D3 separation distance for subjects with CTS was recently confirmed by our magneto-encephalography study in a separate patient cohort (Dhond et al., 2012). Furthermore, alterations in neuroplasticity with CTS may also extend to structural properties of S1 grey matter. Our recent whole-brain analysis found that grey matter volume was reduced in subjects with CTS and that (i) this reduction was confined specifically to the hand area of S1, contralateral to the more affected hand; and (ii) was associated with the median nerve sensory velocity (Maeda et al., 2013). Such changes in neuroplasticity likely reflect the synaptic reorganization noted in animal deafferentation models (Merzenich et al., 1983; Wall et al., 1986, 1992; Florence and Kaas, 1995; Tommerdahl et al., 1996) as well as in human immobilization (Lissek et al., 2009; Weibull et al., 2011) and deafferentation (Werhahn et al., 2002) models. However, the linkage between such neuroplasticity and functional deficits in CTS is currently unknown.
Although CTS has been characterized by elevated tactile detection thresholds (Thonnard et al., 1999; Tucker et al., 2007), it is doubtful that this change in detection threshold could be due to factors associated with skin physiology (such as observed with age; Zhang et al., 2011a), but is rather more likely associated with centrally mediated deficits that lead to changes in acute and/or chronic S1 cortical organization. A number of tactile sensory discriminative metrics, other than tactile detection thresholds, have been demonstrated to be sensitive to alterations in centrally mediated information processing. For example, observations obtained in non-CTS pain populations from tactile tasks that challenge sensory discriminative performance between adjacent digits is compromised, and this is most likely the result of impaired inhibition, which in turn leads to some form of maladaptive neuroplasticity (Zhang et al., 2011b; Nguyen et al., 2013). Observations from these tactile discrimination performance tasks, which were designed to engage interactions between adjacent and near-adjacent cortical ensembles of adjacent digit cortical representations, have paralleled findings obtained from stimulus-evoked activity in the somatosensory cortex of non-human primates (Francisco et al., 2008). Moreover, these sensory discriminative tasks are impacted in human performance when the balance between excitation and inhibition is altered locally in S1 cortex (Rai et al., 2012; Lee et al., 2013). Previous reports have demonstrated that decreasing the spatial distance between two vibrotactile stimuli delivered to the skin—and consequently, decreasing the cortical distance between the evoked response—has a significant impact on the sensory percept of those two stimuli (Tannan et al., 2006, 2007; Zhang et al., 2008). Those findings imply that a shorter cortical distance between digit representations would hypothetically result in decreased sensory discriminative performance in tasks requiring discrimination between the represented skin sites. In our study, we predicted that a decrease in cortical separation distance between D2 and D3 would lead to a decrease in tactile discrimination capacity between those digits. As we found a significant overlap between those digit representations in our pilot study of subjects with CTS, we anticipated that these subjects would have a decrease in sensory discriminative performance that would be related to D2/D3 separation.
Furthermore, sensory discrimination phenomena such as finger agnosia manifests as difficulty in distinguishing different fingers, and has been localized to disruption of the angular gyrus and other regions of the parietal lobe (Rusconi et al., 2005). Altered S1 reorganization of somatotopic representations in CTS may also support the deficits in fine motor control seen in these patients (Fernandez-de-las-Penas et al., 2009a; de la Llave-Rincon et al., 2011), likely through disrupted sensorimotor integration (Shinoura et al., 2005). Examples of deficient sensorimotor integration have been noted by visuomotor tasks involving pinch grip control (Radwin et al., 2004). For instance, reduced speed and accuracy in pinch-release performance has been reported for CTS-affected fingers (Jeng et al., 1994). Recently, more sophisticated multi-digit manipulation protocols have also been applied to subjects with CTS, demonstrating that these subjects are also deficient in dexterous manipulation (Zhang et al., 2011) from an inability to adequately control finger force distribution (Zhang et al., 2013). While S1 physiology and sensorimotor feedback underlies fine motor control, it is currently unknown whether the blurring of cortical representations for median nerve innervated digits in CTS represents maladaptive neuroplasticity and is related to behavioural deficits in sensory discrimination performance and fine motor control.
In this cross-sectional study, we aimed to link neuroimaging metrics such as D2/D3 S1 cortical separation distance with CTS symptoms, somatosensory discrimination capacity, and psychomotor performance. We also aimed to confirm our previously reported alterations in S1 organization in CTS using a much larger sample. We used high-resolution functional MRI to evaluate brain response to vibrotactile stimulation on median and ulnar nerve innervated digits. We also evaluated tactile discrimination and fine motor skill capacity for tasks involving these same digits. We hypothesized that reduced D2/D3 separation distance in S1 would be associated with worsened symptomatology (particularly paraesthesia), poor somatosensory discrimination accuracy, and reduced fine motor skill performance for median nerve innervated digits in subjects with CTS.
Materials and methods
Subjects
Male and female CTS and healthy control subjects, aged 20–60 years, were enrolled. Subjects in both groups responded to study advertisement or, for subjects with CTS, recruitment from patient data registries, and eligible healthy control subjects were chosen in preference to match the evolving age and gender distribution of the patient cohort in the study. Subjects with CTS needed to have a history of pain and/or paraesthesia in median nerve innervated territories, >3 months duration. All subjects were examined for eligibility by a physiatrist at Spaulding Rehabilitation Hospital, which included a physical exam for Phalen’s (Phalen, 1966) and Durkan’s sign (Durkan, 1991), and testing of median and ulnar sensory nerve conduction velocities (Cadwell Sierra EMG/NCS Device). For subjects with CTS, inclusion criteria for nerve conduction velocities consisted of >3.7 ms sensory latency for median nerve or >0.5 ms sensory latency compared to ulnar sensory conduction. Subjects diagnosed with bilateral CTS were tested on the more affected hand.
Exclusion criteria for both groups consisted of contraindications to MRI, history of diabetes mellitus, cardiovascular, respiratory, or neurological illnesses, rheumatoid arthritis, wrist fracture with direct trauma to median nerve, current usage of prescriptive opioid medication, severe thenar atrophy, previous acupuncture treatment for CTS, nerve entrapment other than median nerve, cervical radiculopathy or myelopathy, generalized peripheral neuropathy, blood dyscrasia or coagulopathy or current use of anticoagulation therapy. Symptom severity and functional status were evaluated with the Boston Carpal Tunnel Syndrome Questionnaire (BCTSQ; Levine et al., 1993), where pain was evaluated as the average of ratings on the first through fifth questions, whereas paraesthesia was evaluated as the average of ratings on the sixth through 10th questions.
All study protocols were approved by Massachusetts General Hospital and Partners Human Research Committee and all subjects provided written informed consent.
Psychomotor performance testing
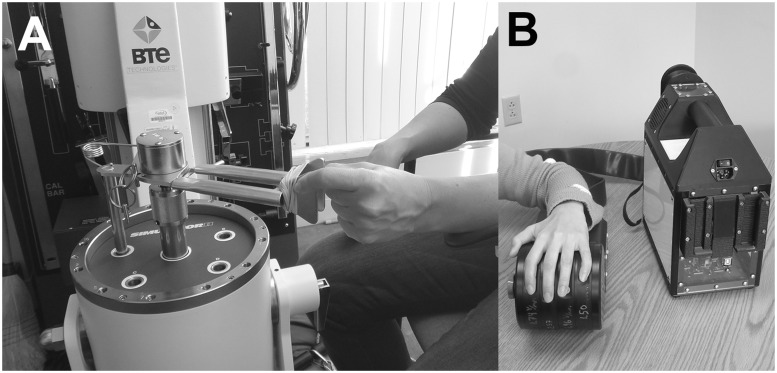
Psychomotor performance testing was completed using the BTE work simulator with the pinch strength attachment (Fig. 1A; BTE Technologies Simulator II, BTE Technologies) adapted with laptop monitoring and custom software using the Labview platform (ver.7, National Instruments). The task was made to replicate previously published psychomotor performance tasks, which demonstrated significant deficits in subjects with CTS (Jeng et al., 1994). Subjects first performed two repetitions of a maximum voluntary contraction using pinching of their thumb and index finger on the more affected hand. Subjects were in a seated position with the arm bent at 90° and elbow supported on an armrest. The psychomotor performance task used the higher maximum voluntary contraction value from the two repetitions and then set a ‘high’ (25% maximum voluntary contraction) and ‘low’ (2% maximum voluntary contraction) threshold for each subject. The psychomotor performance task was a pinch and release performance task which tested subjects’ speed and accuracy at this pinch and release manoeuvre. Subjects were given visual feedback and were instructed to pinch stronger than the high threshold and release below the low threshold as quickly as possible. Subjects were able to practice the task twice before testing. The entire task was 8 s in duration, was performed twice, and the number of successful pinch and release manoeuvres per second was calculated from the final 5 s of each test. Accuracy was assessed by also calculating the overshoot and undershoot forces beyond the high and low thresholds, respectively. We also calculated the percentage of pinch/release cycles completed successfully, to evaluate if speed calculations may have been adversely affected by differences in accuracy between groups.
Figure 1.
Functional deficits in subjects with CTS were assessed with (A) psychomotor performance testing using the BTE Technologies Simulator II adapted in-house for visuomotor pinch/release tasks, and (B) sensory discrimination testing using the CM4 (Cortical Metrics, LLC), a portable four-finger vibrotactile stimulator using voice coil technology.
Sensory discrimination testing
During an experimental session, before MRI scanning, the subject was seated comfortably in a chair with one arm resting on an armrest attached to the head unit of a portable four-site vibrotactile stimulator (Fig. 1B; CM4, Cortical Metrics, LLC; for full description see Holden et al., 2012). The subject placed the hand over the tactile stimulator with the volar surface of each finger’s (D2 to D5) distal phalanx on 5 mm diameter probe tips. In the case of subjects with CTS, the more affected hand was used. The probe tips were independently controlled and the stimulator was capable of delivering a wide range of frequencies and amplitudes.
Subjects were evaluated with a four-finger forced choice protocol. This protocol was composed of four trials per digit (16 trials total). During each trial, a short duration stimulus (amplitude: 100 µm, frequency: 25 Hz, duration: 500 ms) was delivered randomly to one of the four digits. Subjects were instructed to press a key corresponding to the digit stimulated immediately after the stimulus was detected. Each trial, other than the first trial, began 2 s after subject response of the previous trial. Response time (from onset of stimulus to subject response) and response accuracy (percentage of correct trials) for D2 and D3 (median nerve innervated), as well as D5 (ulnar nerve innervated) were recorded.
Somatosensory cortical mapping with functional magnetic resonance imaging
Following sensory discrimination testing, subjects underwent MRI evaluation on a 3.0 T Siemens Trio equipped with 32-channel head coil. A structural MRI scan used for localization was followed by functional MRI evaluation. Structural MRI data were acquired with a multi-echo MPRAGE T1-weighted pulse sequence (repetition time = 2530 ms, echo time 1/echo time 2 = 1.64/30.0 ms, inversion time = 1200 ms, flip angle = 7°, field of view = 256 × 256, slices = 176, sagittal acquisition, spatial resolution = 1 × 1 × 1 mm3). Event-related functional MRI was used in conjunction with vibrotactile stimulation at three different digits (D2, D3 and D5). A separate scan run was performed for each digit and the order of stimulation was pseudo-randomized. Functional MRI data were acquired using a gradient echo blood oxygen level-dependent T2*-weighted pulse sequence adapted for improved spatial resolution (repetition time/echo time = 2000/30 ms, field of view = 200 × 200 mm, 32 coronal slices, voxel size = 2.1 × 2.1 × 2.5 mm, flip angle = 90°). Slices were oriented roughly parallel to the central sulcus contralateral to the stimulated digit, maximizing spatial resolution along the dimension of any expected shifts along the post-central gyrus. Vibrotactile stimulation was provided by a magnetic resonance-compatible device constructed in-house in conjunction with a shell provided by Cortical Metrics. The device contained four piezoelectric transducers (one for each digit, T220-A4NM-303 Y, Piezo Systems Inc.) fitted with roughened edge cylindrical plastic probes (5mm diameter). Stimulation was controlled by software coded in-house (Labview 7.1, National Instruments) in conjunction with relay integrated circuitry and an analogue signal generator (HM8030_5, HAMEG Instruments). The piezoelectric transducers were driven by a sine-wave signal at 30 Hz, which was amplified to achieve a piezoelectric element deflection of 0.51 mm. Each one of the three digits (D2, D3 and D5) on the more affected hand was stimulated in separate runs with an event-related design comprised of 27 2 s duration stimulation events separated by a randomized interstimulus interval (6-12 s, total scan time = 306 s). Subjects lay supine in the scanner with earplugs to attenuate gradient noise. Subjects were informed they would receive intermittent finger stimulation and instructed to close their eyes and focus their attention on the stimulated finger. Following scanning, subjects were asked to report which finger was stimulated and the intensity of stimulation on a scale of 0 (no sensation) to 10 (very strong but not painful).
Data analysis
Demographic, clinical assessment, sensory discrimination testing data, and psychomotor performance testing data were evaluated for normality (Shapiro-Wilk test) and for equal variance between groups (Levene’s test). These data were then contrasted between CTS and healthy control subjects using either a Student’s t-test or Mann-Whitney U-test (if either distribution normality or intergroup variance equality was not found) at a significance level of P < 0.05 (SPSS version 20). For assessment of sensory discrimination response time, subjects whose response accuracy was worse than 2 standard deviations (SD) from the mean were excluded to improve data quality. Additionally, response times were computed from trials in which subjects responded correctly (i.e. indicated the correct digit). The stimulus intensity rated for vibrotactile digit stimulation during the functional MRI scan was contrast between groups with a Student’s t-test. In order to clarify whether hand dominance, and its relation to the tested hand, affected any differences in sensory discrimination between groups, we also performed an additional subgroup analysis with CTS and healthy control subjects who were both right hand dominant and were tested on their right hand.
Functional MRI data were first registered to each subject’s structural MRI data [bbregistration (Greve and Fischl, 2009), Freesurfer: v.5.1]. Slice timing correction, motion correction, high pass filtering (cut-off period = 90 s) and slight spatial Gaussian smoothing (full-width at half-maximum = 1 mm, i.e. below voxel resolution, FSL: v.4.1) were performed on the volumetric space data during preprocessing. Spatial smoothing in volume space was minimal to limit inadvertent cross-sulcus spread in functional MRI signal, but was still used as (i) volume to surface mapping was completed using an intersection plane defined as the grey/white matter boundary surface (mri_vol2surf, Freesurfer), which may be slightly offset from the peak activation voxel; and (ii) our region of interest analysis extracted values from individual subject maps (i.e. before more robust surface smoothing for group analyses, see below), and smoothing is known to enhance signal-to-noise ratio [spatial smoothing acts as a low pass spatial frequency filter (Petersson et al., 1999)]. Preprocessed functional MRI data from each brain voxel were then analysed using a general linear model (GLM). This GLM was univariate and the event timing design, convolved with a canonical double-gamma haemodynamic response function, served as explanatory variable (Feat, FSL).
We then performed region of interest analyses using subjects’ digit stimulation maps and an anatomical post-central gyrus label for Brodmann area 3b (BA3b label, from aparc.annot.2009, Freesurfer) intersected with an unbiased (across all CTS and healthy control subjects) group map cluster corrected for multiple comparisons (z = 2.3, P < 0.05) for each digit. For each subject’s digit statistical map, the location of the peak vertex of the most significant activation cluster within this region of interest was extracted (mri_surfcluster, Freesurfer). The surface distance between pairs of peak vertices for these D2, D3, and D5 statistical maps (fsaverage, Freesurfer) was calculated on each subject’s Freesurfer brain surface using an edge cost and Dijkstra’s algorithm for surface distance (mris_pmake, Freesurfer). Hence, distance measurement respected surface topography (i.e. not Euclidean distance between two voxels). These distances were then contrasted between CTS and healthy control subjects with a Student’s t-test.
Correlations between clinical assessment, psychomotor performance, sensory discrimination, and functional MRI testing metrics which differed between groups were also calculated (SPSS version 20).
To calculate group functional MRI response maps, the resultant parameter estimates and variances for each digit on the single subject level were projected on to the average surface brain (fsaverage, Freesurfer) and smoothed on the spherical cortical surface (full-width at half-maximum = 5 mm) (mri_vol2surf, mri_surf2surf, Freesurfer). Subjects with CTS whose more affected hand was the left and, thus, experienced finger stimulation on the left hand, had their functional and structural data flipped across the mid-sagittal plane to perform group analyses with right hand-affected subjects. Accurate registration was ensured by visualization (tkmedit, tkregister, Freesurfer). Group maps were created for CTS and healthy control subjects and cluster-corrected for multiple comparisons (z = 2.3, P < 0.05).
Results
Demographic and clinical assessments
A total of 63 subjects with CTS (48.9 ± 9.7 years old, mean ± SD, 52 females) and 28 age-matched healthy control subjects (48.4 ± 9.9 years old, 21 females) were enrolled. Although median nerve sensory velocity was significantly slower in CTS compared to healthy control subjects (CTS: 37.3 ± 7.2 m/s, healthy control subjects: 53.6 ± 5.3 m/s, mean ± SD P < 0.001), ulnar nerve sensory velocity was not significantly different (CTS: 55.7 ± 6.7 m/s, healthy control subjects: 55.6 ± 5.3 m/s) (Table 1). Further, although median nerve motor latency was significantly longer in CTS compared to healthy control subjects (CTS: 5.0 ± 1.3 ms, healthy control subjects: 3.3 ± 0.4 ms, mean ± SD P < 0.001), ulnar nerve motor latency was not significantly different (CTS: 2.9 ± 0.3 ms, healthy control subjects: 3.0 ± 0.3 ms) (Table 1). The overall BCTSQ symptom severity scale and functional status scale scores were 2.7 ± 0.7, and 2.1 ± 0.8, respectively (Table 1). Within the BCTSQ symptom severity scale, subjects reported moderate pain and paraesthesia (2.6 ± 0.9 and 3.0 ± 0.7, mean ± SD, respectively on the BCTSQ scale of 1 to 5). Self-reported symptom duration was 8.7 ± 8.7 years (mean ± SD, Table 1).
Table 1.
Demographics and clinical assessments
| Healthy control (n = 28, 21 female) | Carpal tunnel syndrome (n = 63, 52 female) | P-value | |
|---|---|---|---|
| Age (years) | 48.4 ± 9.9 | 48.9 ± 9.7 | n.s. |
| Symptom duration (years) | n/a | 8.7 ± 8.7 | n/a |
| Nerve conduction study | |||
| Median nerve sensory velocity (m/s) | 53.6 ± 5.3 | 37.3 ± 7.2 | <0.001 |
| Ulnar nerve sensory velocity (m/s) | 55.6 ± 5.3 | 55.7 ± 6.7 | n.s. |
| Median nerve motor latency (ms) | 3.3 ± 0.4 | 5.0 ± 1.3 | <0.001 |
| Ulnar nerve motor latency (ms) | 3.0 ± 0.3 | 2.9 ± 0.3 | n.s. |
| Boston carpal tunnel syndrome questionnaire | |||
| Symptom severity score (1–5) | n/a | 2.7 ± 0.7 | n/a |
| Function status score (1–5) | n/a | 2.1 ± 0.8 | n/a |
Data is shown as mean ± SD. n.s. = not significant; n/a = not applicable.
BCTSQ pain and paraesthesia ratings were positively correlated (r = 0.43, P < 0.001). Thus, subjects with greater pain also reported greater paraesthesia. The BCTSQ symptom severity score was significantly correlated with the BCTSQ functional status score (r = 0.57, P < 0.001).
In order to clarify whether hand dominance, and its relation to the tested hand, affected any sensory discrimination or psychomotor performance differences between groups, we also performed an additional subgroup analysis with CTS and healthy control subjects who were both right hand-dominant and were tested on their right hand (CTS: n = 38, 34 females, healthy control subjects: n = 27, 21 females, sex distribution was not significantly different between these subgroups, chi-square test) (Supplementary Table 1). The subgroups still differed significantly in terms of median nerve sensory velocity (CTS: 37.3 ± 7.4 m/s, healthy control subjects: 53.4 ± 5.4 m/s, mean ± SD, P < 0.001) and median motor latency (CTS: 5.1 ± 1.4 ms, healthy control subjects: 3.3 ± 0.4 ms, P < 0.001, Mann Whitney U-test) (Supplementary Table 1).
Psychomotor performance testing
Due to some data loss, we analysed psychomotor performance data from 58 subjects with CTS and 24 healthy control subjects. Psychomotor performance data were lost to device failure (CTS: n = 5, healthy control subjects: n = 4) (Supplementary Table 2). Pinch maximum voluntary contraction was significantly reduced in CTS compared to healthy control subjects (CTS: 53.6 ± 18.2 N, healthy control subjects: 71.7 ± 24.1 N, mean ± SD, P < 0.01, Table 2). The number of successful pinch and release manoeuvres during testing was significantly reduced in CTS compared to healthy control subjects [CTS: 3.3 ± 1.2 times/s (Hz), healthy control subjects: 4.0 ± 1.6 Hz, mean ± SD, P < 0.05, Fig. 2A and Table 2]. We also evaluated accuracy in completing this task. The pinch overshoot and release undershoot were not significantly different between groups (overshoot; CTS: 15.6 ± 9.7 N, healthy control subjects: 13.4 ± 7.4 N; undershoot: CTS: −5.2 ± 6.7 N, healthy control subjects: −3.0 ± 3.0 N mean ± SD, Table 2). Additionally, the percentage of successful pinch and release manoeuvres during testing wasalso not significantly different between groups (CTS: 98.5 ± 3.8%, healthy control subjects: 97.3 ± 4.7%, mean ± SD, Table 2), suggesting that group differences in the number of successful pinch and release manoeuvres could, in fact, be attributed to task speed.
Table 2.
Psychomotor performance testing
| Healthy control (n = 24, 17 female) | Carpal tunnel syndrome (n = 58, 48 female) | P-value | |
|---|---|---|---|
| Maximum voluntary contraction (N)a | 71.7 ± 24.1 | 53.6 ± 18.2 | <0.01 |
| Number of pinch and release (Hz)a | 4.0 ± 1.6 | 3.3 ± 1.2 | <0.05 |
| Overshoot (N)a | 13.4 ± 7.4 | 15.6 ± 9.7 | n.s. |
| Undershoot (N)a | −3.0 ± 3.0 | −5.2 ± 6.7 | n.s. |
| Rate of successful pinch & release (%)a | 97.3 ± 4.7 | 98.5 ± 3.8 | n.s. |
Data is shown as mean ± SD. n.s. = not significant. aMann Whitney U-test.
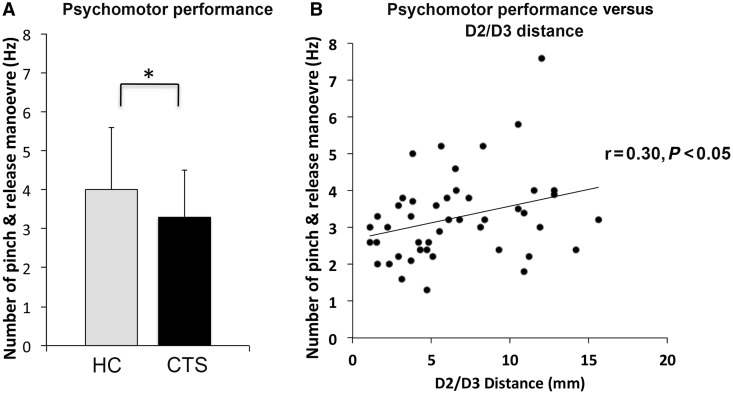
Figure 2.
Psychomotor performance deficits in CTS. (A) The number of pinch and release manoeuvres per second was significantly reduced in CTS compared to healthy control subjects (HC) [CTS: 3.3 ± 1.2 times/s (Hz), healthy control subjects: 4.0 ± 1.6 Hz, mean ± SD P < 0.05]. (B) The number of pinch and release manoeuvres was positively correlated with D2/D3 separation distance in contralateral S1 of the brain (r = 0.30, P < 0.05). Thus, closer D2/D3 separation distance was associated with reduced fine motor skill performance during psychomotor performance testing.
To examine whether handedness impacted these results, we repeated these analyses on a matched subset of subjects who were tested on their right hand and were right hand-dominant. We again found significant differences between CTS and healthy control subjects in terms of maximum voluntary contraction (CTS: 55.0 ± 17.3 N, healthy control subjects: 70.6 ± 24.0 N, mean ± SD, P < 0.01, Supplementary Table 1) and the number of pinch and release manoeuvres [CTS: 3.2 ± 1.3 times/s (Hz), healthy control subjects: 3.8 ± 1.2 Hz, mean ± SD, P < 0.05, Supplementary Table 1]. There were again no significant differences in overshoot, undershoot, or percentage of successful pinch and release manoeuvres between CTS and healthy control subject subgroups.
For subjects with CTS, the number of successful pinch and release manoeuvres was significantly correlated with the median nerve (averaged D2 and D3) sensory velocity (r = 0.31, P < 0.05) and median motor latency (r = −0.27, P < 0.05). Thus slower velocities and longer latencies were associated with reduced ability to pinch and release during testing. No other significant correlations between psychomotor performance and clinical metrics were noted.
Sensory discrimination testing
Data were lost from a few subjects. We analysed sensory discrimination data from 54 patients with CTS and 24 healthy control subjects (Supplementary Table 2). Sensory discrimination data were lost because of time constraints (CTS: n = 6, healthy control subjects: n = 3), lack of compliance (CTS: n = 1, healthy control subjects: n = 0), drop out (CTS: n = 1, healthy control subjects: n = 1), or device failure (CTS: n = 1). For the four-finger forced choice protocol, subjects with CTS demonstrated increased response time for median nerve (averaged D2 and D3, CTS: 0.92 ± 0.10 s, healthy control subjects: 0.85 ± 0.12 s, mean ± SD, P < 0.05, Table 3), but not ulnar nerve (D5: CTS: 0.89 ± 0.10 s, healthy control subjects: 0.86 ± 0.12 s, mean ± SD) innervated digits. Discrimination accuracy (percentage of correct trials) for median nerve innervated digits (averaged D2 and D3 trials) was significantly lower in CTS compared to healthy control subjects (CTS: 90.4 ± 12.4%, healthy control subjects: 98.4 ± 4.2%, mean ± SD, P < 0.001, Mann-Whitney U-test, Fig. 3A and Table 3). Accuracy for D5 did not differ between groups (CTS: 98.2 ± 8.2%, healthy control subjects: 100.0 ± 0.0%, mean ± SD, Mann-Whitney U-test, Fig. 3A and Table 3). To examine whether handedness impacted these results, we repeated these analyses on a matched subset of subjects who were tested on their right hand and were right hand-dominant. We again found a significant difference between these subgroups of CTS and healthy control subjects in terms of discrimination accuracy (P < 0.05, Mann Whitney U-test) and a trend in response time (P = 0.07) for median nerve innervated digits (averaged D2 and D3, see Supplementary Table 1).
Table 3.
Sensory discrimination testing
| Healthy control (n = 24, 19 female) | Carpal tunnel syndrome (n = 54, 45 female) | P-value | |
|---|---|---|---|
| Response time: four-finger forced choice | |||
| Average D2 and D3 (s) | 0.85 ± 0.12 | 0.92 ± 0.10 | <0.05 |
| D5 (s) | 0.86 ± 0.12 | 0.89 ± 0.10 | n.s. |
| Accuracy: four-finger forced choice | |||
| Average D2 and D3 (%)a | 98.4 ± 4.2 | 90.4 ± 12.4 | <0.001 |
| D5 (%)a | 100.0 ± 0.0 | 98.2 ± 9.2 | n.s. |
n.s. = not significant. aMann Whitney U-test.
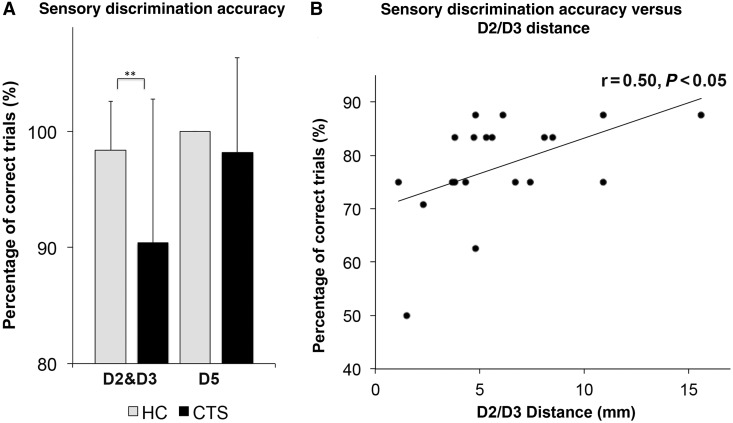
Figure 3.
Sensory discrimination deficits in CTS. (A) Discrimination accuracy (percentage of correct trials) for median nerve innervated digits (D2 and D3) was significantly lower in CTS compared to healthy control subjects (HC) (CTS: 90.4 ± 12.4%, healthy control subjects: 98.4 ± 4.2%, mean ± SD P < 0.001, Mann-Whitney U-test). Accuracy for D5 did not differ between groups (CTS: 98.2 ± 8.2%, healthy control subjects: 100.0 ± 0.0%, mean ± SD Mann-Whitney U-test). (B) For subjects with CTS who did make errors, D2 and D3 accuracy was positively correlated with D2/D3 separation distance in contralateral S1 of the brain (r = 0.50 P < 0.05). Thus, closer D2/D3 separation distance was associated with reduced accuracy in discriminating median nerve innervated digits (D2 and D3) in four-finger forced choice sensory discrimination testing.
The averaged D2 and D3 response time in the four-finger forced choice protocol was significantly correlated with BCTSQ paraesthesia (r = 0.33, P < 0.05). No other correlations between sensory discrimination testing metrics and psychomotor performance or clinical metrics were noted.
Somatosensory cortical mapping with functional magnetic resonance imaging
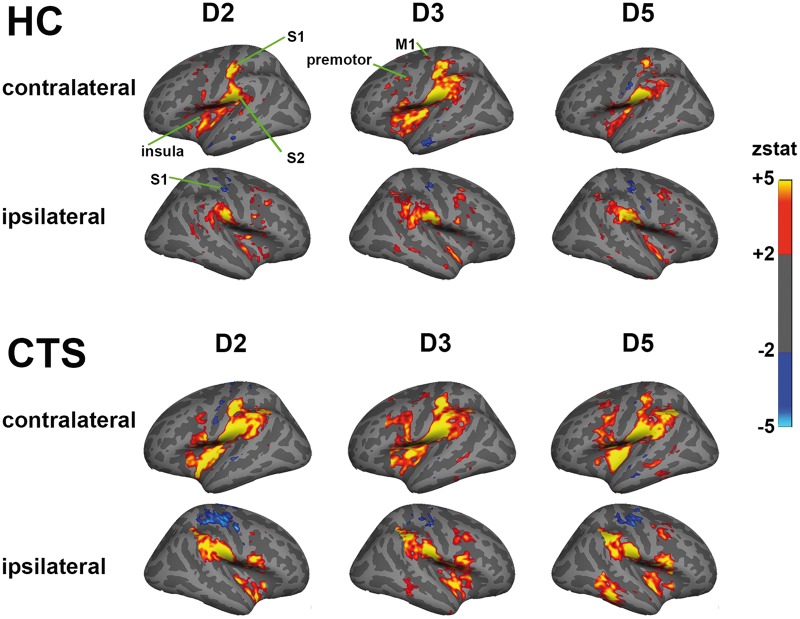
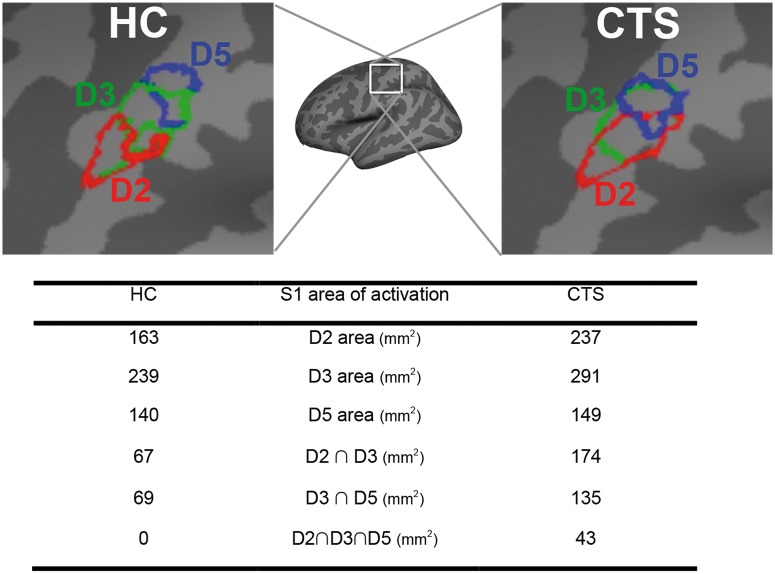
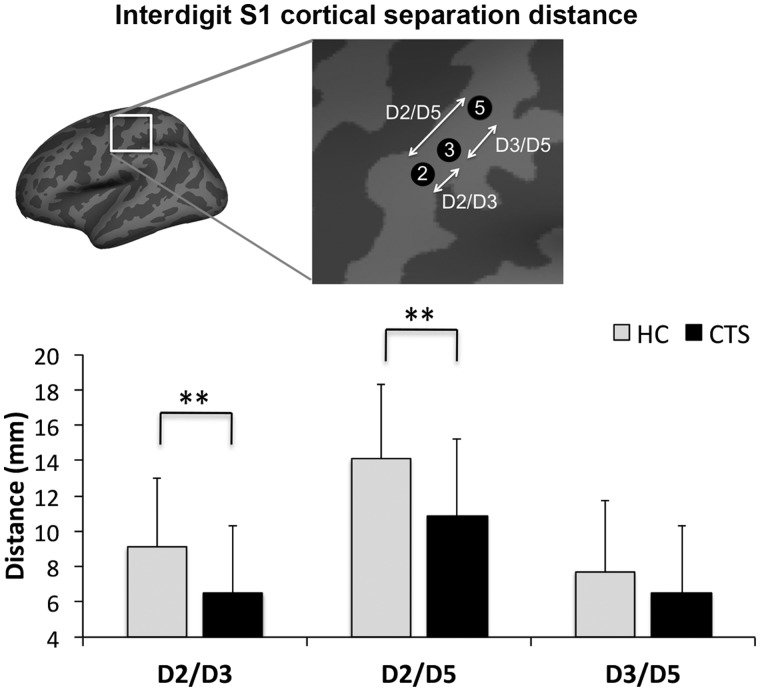
Due to data loss, we analysed functional MRI data from a total of 57 subjects with CTS and 26 healthy control subjects (Supplementary Table 2). Some functional MRI data were lost due to claustrophobia (CTS: n = 1), sleep during scan (CTS: n = 1), excessive head motion (>2 mm) during scanning (CTS: n = 2), or subject drop out (healthy control subjects: n = 2). For a few subjects, we noted paradoxical blood oxygen level-dependent signal reduction in contralateral S1 hand area in response to finger stimulation (CTS: n = 2). These data were analysed and discussed separately (Supplementary Fig. 1). Vibrotactile stimulation produced similar sensation intensity for CTS and healthy control subjects (5.0 ± 1.2, 4.3 ± 1.7 on a scale of 0–10, respectively). Group functional MRI maps demonstrated that vibrotactile stimulation produced robust activation in contralateral primary somatosensory cortex (S1) and bilateral secondary somatosensory (S2), primary motor, premotor, and insular cortices (Fig. 4). Deactivation was noted in ipsilateral S1 for both CTS and healthy control subjects. Within contralateral S1, there appeared to be more overlapped activation in the group maps for CTS compared to healthy control subjects (Fig. 5). In fact, individual analysis found that cortical separation distance was significantly lower in CTS compared to healthy control subjects for D2/D3 (CTS: 6.5 ± 3.8 mm, healthy control subjects: 9.1 ± 3.9 mm, mean ± SD, P < 0.01) and D2/D5 (CTS: 10.9 ± 4.3 mm, healthy control subjects: 14.1 ± 4.2 mm, mean ± SD, P < 0.01; Fig. 6 and Table 4). However, the D3/D5 separation distance was not significantly different between groups (CTS: 6.5 ± 3.8 mm, healthy control subjects: 7.7 ± 4.0 mm, mean ± SD, Fig. 6 and Table 4).
Figure 4.
Group functional MRI maps demonstrated that vibrotactile stimulation produced robust activation in contralateral primary somatosensory cortex (S1) and bilateral secondary somatosensory (S2), primary motor, premotor, and insular cortices.
Figure 5.
Group-level activation clusters in contralateral S1 for vibrotactile stimulation. Larger and more overlapped activation clusters were noted for CTS compared to healthy control subjects (HC).
Figure 6.
Subject-level evaluation of somatotopy in contralateral S1. Cortical separation distance was significantly lower in CTS compared to healthy control subjects (HC) for D2/D3 (CTS: 6.5 ± 3.8 mm, healthy control subjects: 9.1 ± 3.9 mm, mean ± SD P < 0.01) and D2/D5 (CTS: 10.9 ± 4.3 mm, healthy control subjects: 14.1 ± 4.2 mm, mean ± SD P < 0.01). However, the D3/D5 separation distance was not significantly different between groups (CTS: 6.5 ± 3.8 mm, healthy control subjects: 7.7 ± 4.0 mm, mean ± SD).
Table 4.
Interdigit cortical representation distance in primary somatosensory cortex
| Healthy control (n = 25, 18 female) | Carpal tunnel syndrome (n = 50, 42 female) | P-value | |
|---|---|---|---|
| D2/D3 (mm) | 9.1 ± 3.9 | 6.5 ± 3.8 | <0.01 |
| D2/D5 (mm) | 14.1 ± 4.2 | 10.9 ± 4.3 | <0.01 |
| D3/D5 (mm) | 7.7 ± 4.0 | 6.5 ± 3.8 | n.s. |
Data is shown as mean ± SD. n.s. = not significant; n/a = not applicable.
The D2/D3 cortical separation distance was negatively correlated with the BCTSQ symptom severity score (r = −0.30, P < 0.05) and the BCTSQ paraesthesia subscore (r = −0.31, P < 0.05). Thus, reduced separation between D2 and D3 cortical representations was associated with greater symptom severity, particularly paraesthesia. The D2/D3 cortical separation distance was positively correlated with the number of pinch and release manoeuvres (r = 0.30, P < 0.05, Fig. 2B). Thus, closer D2/D3 separation distance was associated with reduced ability to pinch and release during psychomotor performance testing. A trending negative correlation was also found between D2/D3 cortical separation distance and response time in the sensory discrimination testing for D2 and D3 (r = −0.26, P = 0.08).
Although in general, errors were unusual in the sensory discrimination protocol (∼90% accuracy rate), for subjects with CTS who made errors, the average D2 and D3 accuracy was positively correlated with the D2/D3 cortical separation distance (r = 0.50, P < 0.05, Fig. 3B). Thus, smaller D2/D3 separation distance was associated with lower accuracy in discriminating median nerve innervated digits (D2 and D3) in the sensory discrimination testing. No other significant correlations between functional MRI and sensory discrimination, psychomotor performance, or clinical metrics were noted.
Discussion
This well-powered cross-sectional study evaluated the clinical symptomatology, functional deficits, and brain neuroplasticity in subjects with CTS. We were able to show that the cortical representations in contralateral S1 for median nerve innervated digits were overlapped in CTS, leading to reduced D2/D3 separation distance compared to healthy control subjects. This result corroborated our previous preliminary multi-modal neuroimaging findings (Napadow et al., 2006; Dhond et al., 2012), but with a much larger sample size. In addition, we have now linked the cortical remodelling with symptomatology and functional deficits in CTS. Greater symptom, and particularly paraesthesia severity was associated with reduced separation distance between the D2 and D3 cortical representations in contralateral S1. Also, both fine motor skill and sensory discrimination performance, which were reduced in CTS compared to healthy control subjects, were correlated with the D2/D3 somatotopic separation distance. Specifically D2/D3 distance was correlated with (i) the number of pinch/release manoeuvres performed in a pinch grip task using median nerve innervated digits; and (ii) sensory discrimination accuracy for D2 and D3. In summary, reduced D2/D3 cortical separation distance was associated with worse symptomatology (particularly paraesthesia), reduced fine motor skill performance, and worse sensory discrimination accuracy for median nerve innervated digits. Importantly, many of these differences and correlations were specific to median nerve innervated digits and were not significant for functional and functional MRI metrics involving ulnar nerve innervated D5. Thus, our study demonstrated that S1 neuroplasticity for median nerve innervated digits is indeed maladaptive and underlies the functional deficits seen in CTS.
Contracted D2/D3 distance is likely due to persistent multi-digit paraesthesia localized to median nerve innervated digits (D1 to D4) in CTS. Such paraesthesia represent afferent impulses with greater temporal coherence than is normally experienced from these anatomically distinct digits, and this temporal synchrony leads to Hebbian mechanisms of synaptic strengthening and cortical reorganization (Hebb, 1949). The close association between paraesthesia and the blurring of cortical representation for affected digits was underscored by the significant correlation between paraesthesia intensity and D2/D3 separation distance. This afferent driven reorganization is similar to that seen for experimental manipulations such as syndactyly, or the skin fold fusion of adjacent digits (Clark et al., 1988; Allard et al., 1991), and multi-digit synchronous co-activation (Wang et al., 1995; Godde et al., 1996; Pilz et al., 2004).
Our pilot functional MRI study also found that the separation distance between contralateral S1 cortical representations for D2 and D3 was reduced in CTS (Napadow et al., 2006). That study was performed with 13 subjects with CTS, whereas our current study, performed with 63 subjects with CTS, used improved functional MRI spatial resolution and more automated representation allocation for each subject’s digit activation map. Thus, reduced D2/D3 cortical separation has now been corroborated in a larger study sample and in a separate magneto-encephalography study (Napadow et al., 2006; Dhond et al., 2012), underscoring the veracity of this brain-based neuroimaging metric for characterization of individuals suspected of CTS. Moreover, our pilot functional MRI study found that although D2 was shifted closer to D3, the location of the D3 and D5 cortical representations did not differ between CTS and healthy control subjects (Napadow et al., 2006). Similarly, our current study found that although D2/D3 and D2/D5 separation was contracted in subjects with CTS compared to healthy control subjects, the D3/D5 separation distance did not differ between groups, also confirming our prior results.
Additionally, we sought to evaluate how S1 remodelling relates to functional deficits in CTS. We evaluated several functional measures, including somatosensory discrimination and psychomotor performance capacity. Sensory discrimination relates directly to somatotopy in that subjects are able to distinguish tactile stimuli on different body locations due to the separation in cortical representations evident in contralateral S1. Alteration in somatotopic organization occurs in response to a number of different states of altered afferent input. Syndactyly, digit amputation and synchronous digit stimulation have been demonstrated to lead to altered digit representations in S1 of non-human primates (Clark et al., 1988; Allard et al., 1991). It appears that similar cortical remodelling occurs as a result of CTS. Such blurred cortical representations for median nerve innervated digits are maladaptive, as blurring compromises the ability to discriminate inputs projecting to adjacent digits. This, in turn, could impact sensorimotor integration and functional performance. In the four-finger forced choice discrimination task used in this study, we found that subjects with CTS demonstrated increased response time and diminished accuracy for median nerve, but not ulnar nerve innervated digits.
Aberrant sensory discrimination has been previously reported in subjects with CTS, including impaired gap discrimination (Jeng and Radwin, 1995). Although finger agnosia is one of the signs of Gerstmann’s syndrome, which has been ascribed to angular gyrus lesions (Rusconi et al., 2010), or, more recently, parietal white matter damage (Rusconi et al., 2009), subjects with CTS demonstrate evidence of finger agnosia without other aspects of this syndrome (e.g. agraphia, acalculia). We also found that discrimination accuracy for median nerve innervated digits was associated with subjects’ D2/D3 separation distance, suggesting that primary finger agnosia may be localized to the parietal lobe and is a result of maladaptive S1 reorganization. Interestingly, in healthy adults stimulated by tactile stimuli at sensory threshold, localization errors were distributed preferentially to fingers adjacent to the stimulated finger (Braun et al., 2005), suggesting that some overlap in S1 cortical representations for adjacent fingers exists even in healthy adults. In fact, synchronous stimulation of adjacent digits in healthy adults can lead to closer cortical representations, and mislocalizations on a behavioural task (Pilz et al., 2004). Such synchronous stimulation may be analogous to diffuse multi-finger paraesthesia in CTS and, ultimately, lead to blurring of D2/D3 S1 representations, which likely supports these subjects’ worsened sensory discrimination performance. Additionally, maladaptive somatotopic reorganization and somatosensory mislocalization appears to also occur in other pain subjects. Mislocalization has recently been reported for patients with chronic low back pain (Wand et al., 2013). Interestingly, this chronic pain condition has previously been reported to manifest in altered S1 somatotopy, specifically by a medial shift of the low back representation (Flor et al., 1997; Lloyd et al., 2008). Although low back pain is not typically characterized by paraesthesia, the diffuse nature of pain in these patients may also supply the necessary synchronous afferent inputs to shift or remodel S1 cortical representations.
Psychomotor performance reflects sensorimotor integration, and previous studies have found that subjects with CTS demonstrated impaired pinch grip strength and pinch/release speed in a visually guided task (Jeng et al., 1994, 1997). In our study, we replicated these results, finding reduced pinch strength and reduced number of pinch/release movements per unit time performed by subjects with CTS. The percentage of accurately performed pinch/release cycles, as well as overshoot and undershoot, did not differ between groups, suggesting that it was, in fact, speed and not accuracy that was affected in the subjects with CTS. On the other hand, accuracy may be disrupted when fine motor tasks need to occur within a fixed time, and more sophisticated, multi-finger protocols have been shown to detect sensorimotor integration deficits in accuracy for subjects with CTS (Zhang et al., 2011, 2013). Importantly, the number of pinch/release movements was correlated with D2/D3 separation distance, suggesting that S1 neuroplasticity also impacts sensorimotor integration, and ultimately functional deficits in fine motor skill performance in subjects with CTS (Fernandez-de-Las-Penas et al., 2009b; de la Llave-Rincon et al., 2011). Thus, these deficits may be due to the ambiguity of signal localization for afference coming from receptors on median nerve innervated digits.
Although our finding of contracted D2/D3 cortical separation distance in CTS corroborated our previous pilot functional MRI study, some differences with our prior study were also noted. For instance, the pilot study found that median nerve innervated digits had larger areas of activation in contralateral S1 for CTS compared to healthy control subjects whereas our current study did not find the activation area difference to be statistically significant. This discrepancy might be due to differences in the mode of somatosensory stimulation and stimulus design used in both studies. Our pilot study used 100 Hz electrical stimulation with a 1 minute duty cycle stimulus block design, whereas the current study used 30 Hz vibrotactile stimulation with a 2 s duration event-related design. Activation area (i.e. spread of activation around the peak response location) may be influenced by adaptation, which would be more prominent in a block design, whereas somatotopy (i.e. the location of peak activation) may be less sensitive to stimulus duration. Additionally, electrical stimulation in our pilot study produced a relatively strong stimulus, compared to the moderately (∼5/10) strong ratings attributed by our subjects to the vibrotactile stimulus. Importantly, there was no difference in sensation intensity between CTS and healthy control subjects; thus, neither attention level nor stimulus intensity were likely to have influenced differences between groups.
Several limitations of our study should also be noted. First, due to time constraints we did not evaluate somatotopy or activation area for all digits of the hand. Evaluation of the ring finger (D4), for example, may also prove interesting as this finger is both median and ulnar nerve innervated. Another potential limitation was the asymmetric design (i.e. unequal sample size in the two groups), which stemmed from the anticipated greater variances in the CTS population. We tried to mitigate any statistical shortcomings of this design by incorporating non-parametric tests when non-normal distributions (Shapiro-Wilk test) or unequal variances (Levene’s test) were found.
In conclusion, S1 neuroplasticity is indeed maladaptive in subjects with CTS, and it seems that this maladaptive neuroplasticity underlies the functional deficits seen in CTS. Reduced D2/D3 cortical separation distance was associated with worse symptomatology (particularly paraesthesia), reduced fine motor control performance, and worse sensory discrimination accuracy for median nerve innervated digits.
Funding
This work was supported by National Center for Complementary and Alternative Medicine (NCCAM), National Institutes of Health [R01-AT004714, R01-AT004714-02S1, P01-AT002048], as well as the National Centre for Research Resources (NCRR) [P41RR14075, S10RR021110].
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- BCTSQ
Boston Carpal Tunnel Syndrome Questionnaire
- CTS
carpal tunnel syndrome
- D2/3/5
second/third/fifth digit
- S1/2
primary/secondary somatosensory cortex
References
- Allard T, Clark SA, Jenkins WM, Merzenich MM. Reorganization of somatosensory area 3b representations in adult owl monkeys after digital syndactyly. J Neurophysiol. 1991;66:1048–58. doi: 10.1152/jn.1991.66.3.1048. [DOI] [PubMed] [Google Scholar]
- Braun C, Ladda J, Burkhardt M, Wiech K, Preissl H, Roberts LE. Objective measurement of tactile mislocalization. IEEE Trans Biomed Eng. 2005;52:728–35. doi: 10.1109/TBME.2005.845147. [DOI] [PubMed] [Google Scholar]
- Clark SA, Allard T, Jenkins WM, Merzenich MM. Receptive fields in the body-surface map in adult cortex defined by temporally correlated inputs. Nature. 1988;332:444–5. doi: 10.1038/332444a0. [DOI] [PubMed] [Google Scholar]
- de la Llave-Rincon AI, Fernandez-de-Las-Penas C, Perez-de-Heredia-Torres M, Martinez-Perez A, Valenza MC, Pareja JA. Bilateral deficits in fine motor control and pinch grip force are not associated with electrodiagnostic findings in women with carpal tunnel syndrome. Am J Phys Med Rehabil. 2011;90:443–51. doi: 10.1097/PHM.0b013e31821a7170. [DOI] [PubMed] [Google Scholar]
- Dhond R, Ruzich E, Witzel T, Maeda Y, Malatesta C, Morse L, et al. Spatiotemporal mapping cortical neuroplasticity in carpal tunnel syndrome. Brain. 2012;135(Pt 10):3062–73. doi: 10.1093/brain/aws233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druschky K, Kaltenhauser M, Hummel C, Druschky A, Huk WJ, Stefan H, et al. Alteration of the somatosensory cortical map in peripheral mononeuropathy due to carpal tunnel syndrome. Neuroreport. 2000;11:3925–30. doi: 10.1097/00001756-200011270-00063. [DOI] [PubMed] [Google Scholar]
- Durkan JA. A new diagnostic test for carpal tunnel syndrome. J Bone Joint Surg Am. 1991;73:535–8. [PubMed] [Google Scholar]
- Fernandez-de-las-Penas C, de la Llave-Rincon AI, Fernandez-Carnero J, Cuadrado ML, Arendt-Nielsen L, Pareja JA. Bilateral widespread mechanical pain sensitivity in carpal tunnel syndrome: evidence of central processing in unilateral neuropathy. Brain. 2009a;132(Pt 6):1472–9. doi: 10.1093/brain/awp050. [DOI] [PubMed] [Google Scholar]
- Fernandez-de-Las-Penas C, Perez-de-Heredia-Torres M, Martinez-Piedrola R, de la Llave-Rincon AI, Cleland JA. Bilateral deficits in fine motor control and pinch grip force in patients with unilateral carpal tunnel syndrome. Exp Brain Res. 2009b;194:29–37. doi: 10.1007/s00221-008-1666-4. [DOI] [PubMed] [Google Scholar]
- Flor H, Braun C, Elbert T, Birbaumer N. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett. 1997;224:5–8. doi: 10.1016/s0304-3940(97)13441-3. [DOI] [PubMed] [Google Scholar]
- Florence SL, Kaas JH. Large-scale reorganization at multiple levels of the somatosensory pathway follows therapeutic amputation of the hand in monkeys. J Neurosci. 1995;15:8083–95. doi: 10.1523/JNEUROSCI.15-12-08083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco E, Tannan V, Zhang Z, Holden J, Tommerdahl M. Vibrotactile amplitude discrimination capacity parallels magnitude changes in somatosensory cortex and follows Weber's Law. Exp Brain Res. 2008;191:49–56. doi: 10.1007/s00221-008-1494-6. [DOI] [PubMed] [Google Scholar]
- Godde B, Spengler F, Dinse HR. Associative pairing of tactile stimulation induces somatosensory cortical reorganization in rats and humans. Neuroreport. 1996;8:281–5. doi: 10.1097/00001756-199612200-00056. [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D. The organization of behavior. New York: Wiley and Sons; 1949. [Google Scholar]
- Holden JK, Nguyen RH, Francisco EM, Zhang Z, Dennis RG, Tommerdahl M. A novel device for the study of somatosensory information processing. J Neurosci Methods. 2012;204:215–20. doi: 10.1016/j.jneumeth.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng OJ, Radwin RG. A gap detection tactility test for sensory deficits associated with carpal tunnel syndrome. Ergonomics. 1995;38:2588–601. doi: 10.1080/00140139508925288. [DOI] [PubMed] [Google Scholar]
- Jeng OJ, Radwin RG, Fryback DG. Preliminary evaluation of a sensory and psychomotor functional test battery for carpal tunnel syndrome: Part 1—confirmed cases and normal subjects. Am Ind Hyg Assoc J. 1997;58:852–60. doi: 10.1080/15428119791012180. [DOI] [PubMed] [Google Scholar]
- Jeng OJ, Radwin RG, Rodriquez AA. Functional psychomotor deficits associated with carpal tunnel syndrome. Ergonomics. 1994;37:1055–69. doi: 10.1080/00140139408963718. [DOI] [PubMed] [Google Scholar]
- Lee KG, Jacobs MF, Asmussen MJ, Zapallow CM, Tommerdahl M, Nelson AJ. Continuous theta-burst stimulation modulates tactile synchronization. BMC Neurosci. 2013;14:89. doi: 10.1186/1471-2202-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75:1585–92. doi: 10.2106/00004623-199311000-00002. [DOI] [PubMed] [Google Scholar]
- Lissek S, Wilimzig C, Stude P, Pleger B, Kalisch T, Maier C, et al. Immobilization impairs tactile perception and shrinks somatosensory cortical maps. Curr Biol. 2009;19:837–42. doi: 10.1016/j.cub.2009.03.065. [DOI] [PubMed] [Google Scholar]
- Lloyd D, Findlay G, Roberts N, Nurmikko T. Differences in low back pain behavior are reflected in the cerebral response to tactile stimulation of the lower back. Spine (Phila Pa 1976) 2008;33:1372–7. doi: 10.1097/BRS.0b013e3181734a8a. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Kettner N, Sheehan J, Kim J, Cina S, Malatesta C, et al. Altered brain morphometry in carpal tunnel syndrome is associated with median nerve pathology. Neuroimage Clin. 2013;2:313–9. doi: 10.1016/j.nicl.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Napadow V, Kettner N, Ryan A, Kwong KK, Audette J, Hui KK. Somatosensory cortical plasticity in carpal tunnel syndrome—a cross-sectional fMRI evaluation. Neuroimage. 2006;31:520–30. doi: 10.1016/j.neuroimage.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Nguyen RH, Gillen C, Garbutt JC, Kampov-Polevoi A, Holden JK, Francisco EM, et al. Centrally-mediated sensory information processing is impacted with increased alcohol consumption in college-aged individuals. Brain Res. 2013;1492:53–62. doi: 10.1016/j.brainres.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Petersson KM, Nichols TE, Poline JB, Holmes AP. Statistical limitations in functional neuroimaging. II. Signal detection and statistical inference. Philos Trans R Soc Lond B Biol Sci. 1999;354:1261–81. doi: 10.1098/rstb.1999.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalen GS. The carpal-tunnel syndrome. Seventeen years' experience in diagnosis and treatment of six hundred fifty-four hands. J Bone Joint Surg Am. 1966;48:211–28. [PubMed] [Google Scholar]
- Pilz K, Veit R, Braun C, Godde B. Effects of co-activation on cortical organization and discrimination performance. Neuroreport. 2004;15:2669–72. doi: 10.1097/00001756-200412030-00023. [DOI] [PubMed] [Google Scholar]
- Radwin RG, Sesto ME, Zachary SV. Functional tests to quantify recovery following carpal tunnel release. J Bone Joint Surg Am. 2004;86-A:2614–20. doi: 10.2106/00004623-200412000-00005. [DOI] [PubMed] [Google Scholar]
- Rai N, Premji A, Tommerdahl M, Nelson AJ. Continuous theta-burst rTMS over primary somatosensory cortex modulates tactile perception on the hand. Clin Neurophysiol. 2012;123:1226–33. doi: 10.1016/j.clinph.2011.09.026. [DOI] [PubMed] [Google Scholar]
- Rusconi E, Pinel P, Dehaene S, Kleinschmidt A. The enigma of Gerstmann's syndrome revisited: a telling tale of the vicissitudes of neuropsychology. Brain. 2010;133(Pt 2):320–32. doi: 10.1093/brain/awp281. [DOI] [PubMed] [Google Scholar]
- Rusconi E, Pinel P, Eger E, LeBihan D, Thirion B, Dehaene S, et al. A disconnection account of Gerstmann syndrome: functional neuroanatomy evidence. Ann Neurol. 2009;66:654–62. doi: 10.1002/ana.21776. [DOI] [PubMed] [Google Scholar]
- Rusconi E, Walsh V, Butterworth B. Dexterity with numbers: rTMS over left angular gyrus disrupts finger gnosis and number processing. Neuropsychologia. 2005;43:1609–24. doi: 10.1016/j.neuropsychologia.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Suzuki Y, Yamada R, Kodama T, Takahashi M, Yagi K. Fibers connecting the primary motor and sensory areas play a role in grasp stability of the hand. Neuroimage. 2005;25:936–41. doi: 10.1016/j.neuroimage.2004.12.060. [DOI] [PubMed] [Google Scholar]
- Tannan V, Simons S, Dennis RG, Tommerdahl M. Effects of adaptation on the capacity to differentiate simultaneously delivered dual-site vibrotactile stimuli. Brain Res. 2007;1186:164–70. doi: 10.1016/j.brainres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannan V, Whitsel BL, Tommerdahl MA. Vibrotactile adaptation enhances spatial localization. Brain Res. 2006;1102:109–16. doi: 10.1016/j.brainres.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Tecchio F, Padua L, Aprile I, Rossini PM. Carpal tunnel syndrome modifies sensory hand cortical somatotopy: a MEG study. Hum Brain Mapp. 2002;17:28–36. doi: 10.1002/hbm.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonnard J, Saels P, Van den Bergh P, Lejeune T. Effects of chronic median nerve compression at the wrist on sensation and manual skills. Exp Brain Res. 1999;128:61–4. doi: 10.1007/s002210050817. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Whitsel BL, Vierck CJ, Jr, Favorov O, Juliano S, Cooper B, et al. Effects of spinal dorsal column transection on the response of monkey anterior parietal cortex to repetitive skin stimulation. Cereb Cortex. 1996;6:131–55. doi: 10.1093/cercor/6.2.131. [DOI] [PubMed] [Google Scholar]
- Tucker AT, White PD, Kosek E, Pearson RM, Henderson M, Coldrick AR, et al. Comparison of vibration perception thresholds in individuals with diffuse upper limb pain and carpal tunnel syndrome. Pain. 2007;127:263–9. doi: 10.1016/j.pain.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Wall JT, Huerta MF, Kaas JH. Changes in the cortical map of the hand following postnatal ulnar and radial nerve injury in monkeys: organization and modification of nerve dominance aggregates. J Neurosci. 1992;12:3456–65. doi: 10.1523/JNEUROSCI.12-09-03456.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall JT, Kaas JH, Sur M, Nelson RJ, Felleman DJ, Merzenich MM. Functional reorganization in somatosensory cortical areas 3b and 1 of adult monkeys after median nerve repair: possible relationships to sensory recovery in humans. J Neurosci. 1986;6:218–33. doi: 10.1523/JNEUROSCI.06-01-00218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand BM, Keeves J, Bourgoin C, George PJ, Smith AJ, O'Connell NE, et al. Mislocalization of sensory information in people with chronic low back pain: a preliminary investigation. Clin J Pain. 2013;29:737–43. doi: 10.1097/AJP.0b013e318274b320. [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature. 1995;378:71–5. doi: 10.1038/378071a0. [DOI] [PubMed] [Google Scholar]
- Weibull A, Flondell M, Rosen B, Bjorkman A. Cerebral and clinical effectsof short-term hand immobilisation. Eur J Neurosci. 2011;33:699–704. doi: 10.1111/j.1460-9568.2010.07551.x. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Mortensen J, Van Boven RW, Zeuner KE, Cohen LG. Enhanced tactile spatial acuity and cortical processing during acute hand deafferentation. Nat Neurosci. 2002;5:936–8. doi: 10.1038/nn917. [DOI] [PubMed] [Google Scholar]
- Zhang W, Johnston JA, Ross MA, Sanniec K, Gleason EA, Dueck AC, et al. Effects of carpal tunnel syndrome on dexterous manipulation are grip type-dependent. PLoS One. 2013;8:e53751. doi: 10.1371/journal.pone.0053751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Johnston JA, Ross MA, Smith AA, Coakley BJ, Gleason EA, et al. Effects of carpal tunnel syndrome on adaptation of multi-digit forces to object weight for whole-hand manipulation. PLoS One. 2011;6:e27715. doi: 10.1371/journal.pone.0027715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Francisco EM, Holden JK, Dennis RG, Tommerdahl M. Somatosensory information processing in the aging population. Front Aging Neurosci. 2011a;3:18. doi: 10.3389/fnagi.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Tannan V, Holden JK, Dennis RG, Tommerdahl M. A quantitative method for determining spatial discriminative capacity. Biomed Eng Online. 2008;7:12. doi: 10.1186/1475-925X-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zolnoun DA, Francisco EM, Holden JK, Dennis RG, Tommerdahl M. Altered central sensitization in subgroups of women with vulvodynia. Clin J Pain. 2011b;27:755–63. doi: 10.1097/AJP.0b013e31821c98ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.