Abstract
Humans in a negative emotional state are more likely to judge ambiguous stimuli as negative. In recent years, similar judgement biases have been found in some non-human animals that were exposed to long-term or short-term treatments aimed at influencing their affective states. Here we tested pet dogs in the presence and absence of their owners in a judgement bias test with an established go/no-go procedure. Even though owner absence is thought to induce a state of anxiety in dogs that have formed an attachment bond with their primary care-takers, we found no difference between the dogs’ responses to ambiguous stimuli in the presence or absence of their owners. This result may be explained by absence of anxiety in dogs that are accustomed to brief periods of separation from their owners, or by a sensitivity limit of the customary judgement bias tests in non-human animals when only a moderate, short-term state of anxiety is induced. In addition, we found significant differences between individuals and populations in the responses to ambiguous stimuli, which give impetus for further research.
Keywords: domestic dog, Canis familiaris, cognitive bias
Introduction
Humans in negative emotional states commonly exhibit a negative judgement bias when confronted with ambiguous stimuli (e.g. Eysenck et al. 1991). Recently, this link between emotional state and cognitive biases has been adopted to develop a tool to detect affective states in animals. In a seminal study, Harding et al. (2004) found that rats kept under unpredictable housing conditions were less likely to judge ambiguous stimuli as positive than rats kept under predictable housing conditions. Since then, a number of studies have found evidence for judgement biases elicited by both positive and negative affective states in a wide range of species, including mammals (Mendl et al. 2010; Doyle et al. 2010), birds (Matheson et al. 2008; Salmeto et al. 2011) and insects (Bateson et al. 2011). Judgement biases were not only elicited by long-term treatments lasting for several days or weeks (Harding et al. 2004; Burman et al. 2008; Matheson et al. 2008), but also by short-term treatments lasting for no more than a few minutes (Burman et al. 2009; Bateson et al. 2011; Salmeto et al. 2011). While long-term treatments typically manipulated housing conditions, short-term treatments resorted to acute aversive stimuli such as a change from low to high light levels (in rats, Burman et al. 2009), or vigorous shaking simulating a predator attack (in bees, Bateson et al. 2011). Some studies did not find the predicted effects, which may be explained by confounding effects (e.g. Burman et al. 2011) or small sample sizes (e.g. Brilot et al. 2010).
In two recent studies, cognitive bias tests have been successfully applied to two dog populations, shelter dogs at UK re-homing centres (Mendl et al. 2010) and laboratory dogs at the Swedish University of Agricultural Sciences (Burman et al. 2011). Mendl et al. found that dogs showing high levels of separation related behaviours (such as barking/howling, toileting or destructive behaviours) judged ambiguous stimuli more negatively than dogs showing less separation anxiety. Burman et al. aimed to induce a positive affective state in the dogs by letting them search for (and find) food immediately prior to the cognitive bias test. However, they found that this treatment elicited a negative judgement bias compared to the baseline, rather than the predicted positive bias, which may be explained by confounding effects such as satiation or termination of a positive event (Burman et al. 2011). To our knowledge, no study to date has tested whether dogs living in their most common environment, as companion animals for humans, exhibit judgement biases when experiencing short periods of anxiety in their everyday lives.
Here we tested pet dogs in a classic judgement bias task in the presence and absence of their owners. Previous research has shown that dogs form attachment bonds with their owners and respond anxiously to brief separations (Topál et al. 1998; Prato-Previde et al. 2003), though short-term owner absence induces only moderate anxiety in well-socialized dogs (i.e. dogs that are habituated to short periods of owner absence: Lindsay 2001). Dogs showing signs of strong separation anxiety were not tested in this study (see below). We tested all dogs in the owner-present as well as in the owner-absent condition (in balanced order) and predicted that the dogs would be less likely to judge ambiguous stimuli as positive in the absence of their owners.
Methods
Subjects
We recruited 32 pet dogs with private owners, 8 herding dogs, 10 retrievers, 10 mongrels and 4 companion dogs ranging in age from 1 to 7 years (see table S1 for details). All subjects lived as companion animals in an intact “natural” (human) environment for domestic dogs, unlike the dogs tested in previous cognitive bias studies (Mendl et al. 2010: shelter dogs; Burman et al. 2011: laboratory beagles). None of the subjects were specifically bred or trained to work independently of humans. Eight of the dogs did not complete the experiments, either because they did not reach the initial training criterion (6 dogs, 5 of them showed signs of strong separation anxiety, staying and occasionally scratching at the door during owner-absent trials) or because they repeatedly failed to reach the criterion in the refreshers on the testing days (2 dogs), resulting in a final dataset of 24 dogs. None of these dogs exhibited strong signs of separation anxiety during short-term absence of the owner, such as barking/howling, toileting or destructive behaviour (as shown by some of the shelter dogs tested by Mendl et al. 2010). The owners were asked not to feed their dogs for at least 3 hours before their visits.
Procedures
We adopted the spatial task of Mendl et al. (2010) with some modifications. As in the Mendl et al. study, the dogs were initially trained to differentiate between a positive location (P, reinforced with a food reward) and a negative location (N, no food). The positive location was randomly allocated to the right side, for half of the subjects, or the left side for the other half. Training was completed when the dogs reached a pre-set criterion, that is, when the longest latency to approach the positive location was shorter than the shortest latency to approach the negative location for the last ten trials (Mann-Whitney U test: p<0.01). The dogs were then tested with unreinforced probe trials of three ambiguous locations (NP: near positive, M: middle, NN: near negative; 4 trials per location) interspersed within 40 standard N and P trials. All five possible locations were marked with a cross on the floor in a semicircle 3 m from the dog’s starting position with distance between neighbouring locations at 60 cm.
Unlike Mendl et al., we used a within-subjects design: All dogs were tested in the Owner-absent as well as in the Owner-present condition in balanced order. The training session and the two test sessions took place on separate days, with intervals between training day and first test day ranging between 3 and 14 days, and intervals between first and second test day between 4 and 9 days.
On the training day, the dogs were given sets of 10 trials (5 N and 5 P in random order), half of them with the owner present and half with the owner absent (outside the room). For each trial, the dog was held loosely by its collar in a position 3m from the N and P location either by the blindfolded owner or by a blindfolded experimenter (in the Owner-absent condition) seated on a chair. Experimenter 1 started from behind the dog to place either a bowl with a piece of food (1 cm3 of sausage or cheese, depending on the dog’s preference) on the P location or an empty bowl on the N location. Two visually identical bowls were used for rewarded and non-rewarded locations and both bowls had a piece of sausage (or cheese if applicable) taped to their bottom side and thus inaccessible to the dog to control for odour cues. After placing the bowl on its location, experimenter 1 returned to the position behind dog and the owner/experimenter 2 and, as soon as the dog was facing forward, placed a hand on the owner/experimenter 2’s shoulder as a signal to release the dog. The owner/experimenter 2 then released the dog, giving a “go” command. If the dog did not leave, the command was repeated a second time. After the dog had reached the bowl, or after a maximum of 30s, experimenter 1 signalled to the owner/experimenter 2 to take the dog back to its starting position and a new trial started. Training was terminated once the above-mentioned criterion was met (checked after every set of 10 trials) or after a maximum of 120 trials. For dogs that did not reach the criterion within 60 trials, the training was continued on a separate day.
Testing days started with a refresher of no more than 20 standard trials to ensure that the dogs still remembered the distinction of N and P locations learnt several days previously. Of the 24 subjects in the final dataset, 23 reached the criterion again within these 20 trials and proceeded to testing. The one dog who did not reach the criterion in the refresher was given a second complete training session and the tests were conducted on two subsequent days. After completing the refresher and a break of 5 min, the dogs were given two blocks of 26 trials (six probe trials - two per probe location - interspersed within 20 standard trials). The two blocks lasted about 20 min each and were separated by a break of 15 min. For the (non-reinforced) probe locations the same bowl was used as for the N location. When the bowl was placed in the middle location, experimenter 1 approached from the P side for half of the trials (2 trials per dog) and from the N side for the other half. For NN and NP trials, experimenter 1 always used the shortest route to place the bowl.
Analysis
Approach latencies for all trials were determined from video recordings using Solomon Coder (©András Péter) as the time from the dog’s release (hand movement of owner/experimenter 2) until the dog’s nose was within 30cm of the bowl (from this position the dog could see whether the bowl was empty nor not). One randomly chosen test block for each dog (24 × 26 trials) was coded independently by two coders (CR and JS) and interobserver reliability was high (Pearson’s r = 0.99). Latency scores for the probe locations were calculated (following Mendl et al. 2010) as
Mean latencies to reach N and P locations were taken for the 20 standard trials within test sessions. A latency score of 0 thus corresponds to an approach latency equal to the one for the positive location, a latency score of 100 corresponds to an approach latency equal to the one for the negative location. These adjusted scores account for differences in running speed (e.g. due to variable motivation or body size) between individuals and between testing days.
Latency scores were log-transformed to attain homogeneity of variances and analysed in R 2.13.1 (R Development Core Team 2011) using linear mixed effects models (LMMs, Pinheiro and Bates 2000) with treatment (Owner present vs. Owner absent), location (NN, M, NP) and experiment order (first vs. second testing day) as fixed effects, and dog identity as a random factor. Additionally, the potentially confounding variables breed group, dog sex and dog age were included as fixed effects. We further included the two-way interaction between treatment and experiment order to test whether any treatment effect may be restricted to the first testing day due to learning effects, and the two-way interaction between treatment and location to test whether any treatment effect might be restricted to some of the ambiguous stimuli, as found in previous cognitive bias studies (Doyle et al. 2010; Harding et al. 2004; Burman et al. 2008; Mendl et al. 2010; Salmeto et al. 2011).
Results
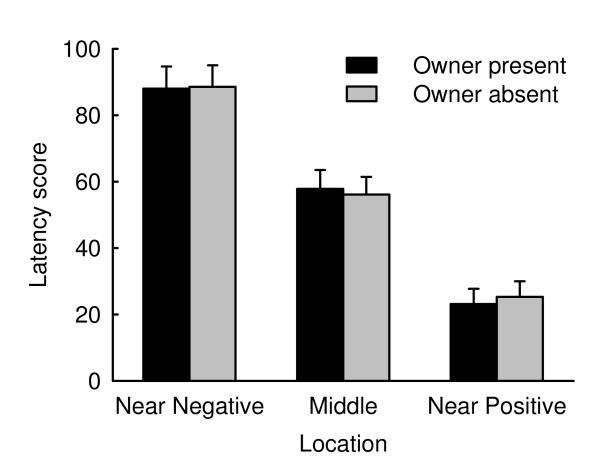
The 24 dogs reached the training criterion after a mean of 42 trials (range 30 – 90 trials). In the test phase, the dogs’ responses differed significantly between the three ambiguous locations (LMM, F2, 537=78.1, p<0.001) with latency scores being lowest for the near-positive location and highest for the near-negative location (Fig. 1). Furthermore, latency scores for the middle location were lower when the experimenter had placed the bowl there coming from the side of the positive location (LMM, F1, 164=6.01, p=0.015). Approach latencies were consistently longer on the second test day compared to the first (LMM, F1, 537=25.5 p<0.001). Owner absence did not have an influence on the latency scores (LMM, F1, 537=0.04, p=0.84; Fig. 1) irrespective of whether the Owner-absent condition was tested on the first or on the second testing day (treatment*order interaction: F1, 537=0.27, p=0.60), and irrespective of the bowl location (treatment*location interaction: F2, 537=0.05, p=0.95).
Fig. 1. Latency scores in response to three ambiguous stimuli for Owner-present and Owner-absent conditions.
Data are shown as mean and standard error.
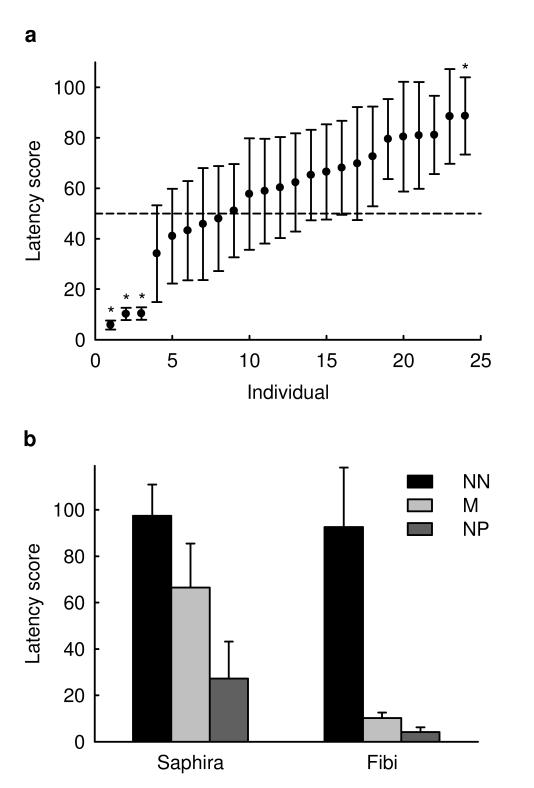
We found no sex difference or breed-group differences for the latency scores (LMM, sex: F1, 19=1.07, p=0.31; breed groups: F3, 19=0.25, p=0.86). However, significant individual differences were found (likelihood-ratio test for random effect: p=0.004, Fig. 2a). In addition to overall shorter or longer latency scores, response patterns to the three ambiguous stimuli varied markedly between individuals: While for some individuals, latency scores increased more or less continuously from the near-negative to the middle and the near positive location, for others, responses were indistinguishable for two locations and markedly different for the third (Fig. 2b).
Fig. 2. Individual differences in responses to ambiguous stimuli.
a Latency scores in response to the ambiguous Middle stimulus for the 24 subjects. Data of Owner-present and Owner-absent condition are pooled. Stars designate significant deviation from intermediate value of 50 (one-sample t test, p<0.05). b Latency scores in response to the three ambiguous stimuli Near Negative (NN), Middle (M) and Near Positive (NP) for two exemplary individuals. Fibi showed a continuous decrease in latency (9 dogs in our sample of 24 showed a pattern like this) whereas Saphira showed an indistinguishable response to two of the stimuli and a markedly different response to the third (10 dogs in our sample showed a pattern like this, with the sharp drop in latency either between the NN and M location as shown or between the M and NP location; the remaining 5 dogs showed intermediate patterns). All data are shown as mean and standard error.
Discussion
Short-term owner absence did not induce a negative cognitive bias in our sample of pet dogs. The following alternative explanations may account for this lack of an effect: 1) Our sample may have provided insufficient statistical power to detect an effect. This seems unlikely since our sample of 24 dogs tested in a within-subjects design provides better statistical power than most previous animal cognitive bias studies, most of which included no more than 12 replicates. 2) Our treatment may not have elicited any anxiety in our subjects. This also seems unlikely given the strong attachment bonds pet dogs form with their owners (Topál et al. 1998; Prato-Previde et al. 2003), even though habituation to brief owner absence during training may have led to reduced anxiety during the test sessions (note that all subjects were used to periods of owner absence in their everyday lives). Furthermore, confounding effects like satiation (as in Burman et al. 2011) or motivation cannot explain our results since the dogs were not fed before the experiment and received equal amounts of food in both conditions, and since the latency scores we used for the analysis correct for differences in motivation between testing days. 3) Our results may reflect a sensitivity limit of the commonly used cognitive bias tests when short-term treatments (here owner absence of less than half an hour) inducing only mild anxiety are applied (but see Burman et al 2009 for a study showing that a short-term treatment inducing moderate anxiety affected judgement bias in rats). 4) Based on findings in humans, it has been predicted that anxiety only induces a judgement bias for “near negative” stimuli, whereas depression would induce a judgement bias also for “near positive” stimuli (Salmeto et al. 2011). In our study, like in others before, the negative reinforcer was merely absence of food, which might be interpreted as a neutral rather than as an aversive stimulus. Based on this, one might expect an effect only when truly aversive stimuli are used. However, published studies provide mixed support for this prediction: While the results of Salmeto et al. (2011) support the anxiety/depression model, Harding et al. (2004), using noise as a negative reinforcer, found a judgement bias for the middle and near positive stimuli, but not for the near negative stimulus as would have been predicted by the anxiety/depression model. Furthermore, Mendl et al. (2010) found a judgement bias for the middle location (and a tendency for the near-negative location) despite using a neutral reinforcer (no food) for the negative location.
Most of our subjects responded to the intermediate stimuli with intermediate response latencies, whereas only a few individuals showed evidence of a judgement bias. This stands in contrast to previous cognitive bias studies with dogs (Mendl et al. 2010; Burman et al. 2011), where the baseline was a strong positive judgement bias, i.e. responses to the ambiguous middle stimulus were as if the stimulus was positive. This discrepancy may be explained by us using normal pet dogs living in an intact social environment, which may be less likely to exhibit extreme behaviours than dogs living in shelters (Mendl et al. 2010) or laboratory animals (Burman et al. 2011). Shelter and laboratory dogs, which spend most of their time with little interaction with humans, may quickly reach a very positive affective state when somebody finally engages them. This notion is supported by the finding that none of the studies on cognitive bias in rats, sheep, starlings, chicks and honey bees found a comparable positive bias in the baseline condition. In any case, the contrast between baseline judgement biases within (and between) species warrants further investigation.
Marked individual differences appeared in the responses to the ambiguous stimuli. While only few individuals deviated clearly from an intermediate response to the middle stimulus (exhibiting a positive or a negative bias), two distinct response patterns to the ambiguous stimuli became apparent: about half of the subjects showed a continuous increase in response latency from the near-positive to the middle and the near-negative location; the other half showed very similar response latencies to two neighbouring locations and a marked jump in response latency to the third. Such different response patterns may be attributable to personality differences.
In summary, our study found that brief owner separation has no detectable cognitive effect in pet dogs when a commonly used judgement bias test is applied. In addition, our results stimulate further research to fathom the basis of distinct response patterns in cognitive bias tests and of differences in baseline biases.
Supplementary Material
Acknowledgements
This study was funded by the Austrian Science Fund (P21418 to L.H.). The Clever Dog Lab is supported by Royal Canin, the University of Vienna and a private sponsor. We thank Mike Mendl and Oliver Burman for discussions about cognitive bias tests, the reviewers for comments on the manuscript, and András Péter for the behaviour coding software SOLOMON.
References
- Bateson M, Desire S, Gartside SE, Wright GA. Agitated honeybees exhibit pessimistic cognitive biases. Curr Biol. 2011;21:1070–1073. doi: 10.1016/j.cub.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilot BO, Asher L, Bateson M. Stereotyping starlings are more ‘pessimistic’. Anim Cogn. 2010;13:721–731. doi: 10.1007/s10071-010-0323-z. [DOI] [PubMed] [Google Scholar]
- Burman O, McGowan R, Mendl M, Norling Y, Paul E, Rehn T, Keeling L. Using judgement bias to measure positive affective state in dogs. Appl Anim Behav Sci. 2011;132:160–168. [Google Scholar]
- Burman OHP, Parker R, Paul ES, Mendl M. A spatial judgement task to determine background emotional state in laboratory rats, Rattus norvegicus. Anim Behav. 2008;76:801–809. [Google Scholar]
- Burman OHP, Parker RMA, Paul ES, Mendl MT. Anxiety-induced cognitive bias in non-human animals. Physiol Behav. 2009;98:345–350. doi: 10.1016/j.physbeh.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Doyle RE, Fisher AD, Hinch GN, Boissy A, Lee C. Release from restraint generates a positive judgement bias in sheep. Appl Anim Behav Sci. 2010;122:28–34. [Google Scholar]
- Eysenck MW, Mogg K, May J, Richards A, Mathews A. Bias in interpretation of ambiguous sentences related to threat in anxiety. J Abnorm Psychol. 1991;100:144–150. doi: 10.1037//0021-843x.100.2.144. [DOI] [PubMed] [Google Scholar]
- Harding EJ, Paul ES, Mendl M. Cognitive bias and affective state. Nature. 2004;427:312–312. doi: 10.1038/427312a. [DOI] [PubMed] [Google Scholar]
- Lindsay SR. Handbook of applied dog behavior and training. Vol. 2. Iowa State Press; Ames, IA: 2001. [Google Scholar]
- Matheson SM, Asher L, Bateson M. Larger, enriched cages are associated with ‘optimistic’ response biases in captive European starlings (Sturnus vulgaris) Appl Anim Behav Sci. 2008;109:374–383. [Google Scholar]
- Mendl M, Brooks J, Basse C, Burman O, Paul E, Blackwell E, Casey R. Dogs showing separation-related behaviour exhibit a ‘pessimistic’ cognitive bias. Curr Biol. 2010;20:R839–R840. doi: 10.1016/j.cub.2010.08.030. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-Plus. Springer; New York: 2000. [Google Scholar]
- Prato-Previde E, Custance DM, Spiezio C, Sabatini F. Is the dog-human relationship an attachment bond? An observational study using Ainsworth’s strange situation. Behaviour. 2003;140:225–254. [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. http://www.R-project.org. [Google Scholar]
- Salmeto AL, Hymel KA, Carpenter EC, Brilot BO, Bateson M, Sufka KJ. Cognitive bias in the chick anxiety-depression model. Brain Res. 2011;1373:124–130. doi: 10.1016/j.brainres.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Topál J, Miklósi A, Csányi V, Dóka A. Attachment behavior in dogs (Canis familiaris): A new application of Ainsworth’s (1969) Strange Situation Test. J Comp Psychol. 1998;112:219–229. doi: 10.1037/0735-7036.112.3.219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.