Abstract
Objective
Previous studies showed that low-density lipoprotein receptor (LDLR) mRNA 3′ untranslated region (UTR) contains regulatory elements responsible for rapid mRNA turnover in hepatic cells and mediates the mRNA stabilization induced by berberine (BBR). Here, we elucidate the underlying mechanism of BBR’s action by characterizing mRNA-binding proteins that modulate LDLR mRNA decay via 3′ UTR in liver tissue in vivo.
Approach and Results
We generated a transgenic mouse model (Alb-Luc-UTR) that expresses Luc-LDLR3′ UTR reporter gene driven by the albumin promoter to study 3′ UTR function in mediating LDLR mRNA decay in liver tissue. We show that treating Alb-Luc-UTR mice with BBR led to significant increases in hepatic bioluminescence signals, Luc-UTR mRNA, and LDLR mRNA levels as compared with control mice. These effects were accompanied by specific reductions of mRNA decay-promoting factor heterogeneous nuclear ribonucleoprotein D (hnRNP D) in liver of BBR-treated mice. Knockdown and overexpression studies further demonstrated that hnRNP D p37 isoform plays a major role in promoting hepatic LDLR mRNA degradation. In addition, we examined LDLR mRNA half-life, Luc-UTR reporter activity, and hnRNP D expression levels in cell lines derived from extrahepatic tissues. We demonstrated that strengths of 3′ UTR in promoting mRNA degradation correlate with hnRNP D cellular abundances in nonhepatic cell lines, thereby suggesting its involvement in LDLR mRNA degradation beyond liver tissue.
Conclusions
hnRNP D is critically involved in LDLR mRNA degradation in liver tissue in vivo. The inverse relationship of hnRNP D abundance with LDLR mRNA levels after BBR treatment suggests the potential of hnRNP D of being a novel therapeutic target for LDL cholesterol lowering.
Keywords: 3′ untranslated regions, AU rich elements, berberine, hypercholesterolemia, receptors, LDL, RNA stability
Cardiovascular disease remains the leading cause of mortality and morbidity in the United States and other parts of the world. Mounting evidence from population-based data and clinical trials has demonstrated that low-density lipoprotein– associated cholesterol (LDL-c) reduction is an effective strategy to prevent coronary heart disease, to slow down atherosclerotic development, and to reduce mortality.1,2 The liver is the organ responsible for removing >70% to 80% of LDL-c from circulation via LDL receptor (LDLR)–mediated endocytosis. Therefore, the amount of LDLR expressed on the surface of hepatocytes is a major determinant of LDL-c circulating levels.3–5 There is a direct correlation of high expression level of LDLR in liver with low LDL-c level in blood.
It has been well documented that the expression of hepatic LDLR is regulated by 3 distinct mechanisms: transcriptional, post-transcriptional, and post-translational mechanisms. The transcriptional regulation of the LDLR gene is primarily governed by intracellular cholesterol levels through its effect on the processing of active forms of transcription factors, sterol-regulatory element binding proteins (SREBPs), that bind to the SRE-1 site of the LDLR promoter and activates the gene transcription.6,7 The identification of proprotein convertase subtilisin/kexin type 9 (PCSK9) defined a cellular mechanism for controlling LDLR expression at the protein level.8–11 The post-transcriptional regulation of LDLR mRNA stability has been shown to be mediated through the mRNA 3′untranslated region (3′UTR).12–15
Human LDLR mRNA contains a 2.5-kb-long stretch of 3′UTR16 in which 3 AU-rich elements (AREs) were previously identified and shown to mediate the rapid turnover rate of LDLR mRNA in liver cells.12,13 AREs are the best characterized sequence determinants of messenger stability among known RNA cis-regulatory elements.17 The destabilizing functions of ARE sequences are achieved through their interaction with ARE-binding proteins (ARE-BPs).18 Some ARE-BPs are decay-promoting factors, such as KH-type splicing regulatory proteins (KSRPs), that interact with AREs and recruit RNA degradation machinery to the mRNA.19 The ability of LDLR-AREs to target host mRNA toward degradation was first demonstrated 14 years ago in a heterologous system. It was shown that inclusion of the most 5′ ARE (ARE1) of the LDLR3′ UTR into a β-globin fusion mRNA resulted in a 3-fold increase in its turnover rate, whereas inclusion of all 3 AREs to the coding region of β-globin mRNA resulted in further destabilization of the β-globin fusion transcripts.20 Phorbol-12-myristate-13-acetate was the first agent to show a regulatory effect on LDLR expression at the level of mRNA stability through its effect on sequences presence in the distal 3′UTR.13 Other examples of LDLR mRNA stability regulation are bile acids21 and gemfibrozil, a fibrate drug.14 These 2 classes of compounds have been shown to increase mRNA levels of LDLR in HepG2 cells through mechanisms that affect LDLR mRNA turnover rate.
In addition to hepatoma cells, the effect of mRNA stability on the expression and function of LDLR was examined in a mouse model of type III hyperlipoproteinemia.20 It was shown that expression of a human LDLR mRNA transcript with a deletion of ARE1- and ARE2-containing region in these hyperlipidemic mice resulted in a modest increase in the hepatic level of LDLR, which, however, produced significant reducing effects in total cholesterol and serum lipid levels. Although the mRNA stabilizing determinants were not further investigated, these in vitro and in vivo studies provided primary evidence supporting the notion that mRNA stability is an important mechanism to fine tune the expression of the LDLR gene in liver tissue.
The significance of 3′UTR in control of liver LDLR expression was further highlighted by our demonstration that the herbal medicine berberine (BBR), which reduced LDL-c in hyperlipidemic patients,15,22–24 increased LDLR mRNA half-life nearly 3-fold without affecting gene transcription in HepG2.15 We have further mapped the effect of BBR to the 1 kb of 5′ proximal ARE-containing section in the LDLR mRNA 3′UTR.15
To further understand how the decay of LDLR mRNA in liver cells is modulated through the regulatory elements of 3′UTR, we had taken a great effort to identify mRNA-binding proteins that specifically interact with LDLR mRNA 3′UTR.25 By conducting small interfering RNA library construction and screening, reporter gene transfection, biotinylated RNA pull-down, mass spectrometry analysis, and functional assays, we had identified 3 ARE-BPs, heterogeneous nuclear ribonucleoprotein D (hnRNP D), hnRNP I, and KSRP as negative regulators of LDLR mRNA stability in HepG2 cells. Cellular depletion of these ARE-BPs by small interfering RNA transfection, individually or in combination, greatly elevated LDLR expression and activity to uptake LDL particles from the culture medium of HepG2 cells. Our results also suggested that interference with the ability of destabilizing ARE-BPs to interact with LDLR-ARE motifs could be an underlying mechanism for increased LDLR mRNA expression in BBR-treated HepG2 cells.25
Although these mechanistic studies conducted in cell culture models have provided valuable information for understanding the important role of mRNA stability in control of LDLR expression in cultured hepatic cells in vitro, the critical question of which ARE-BP is the key player that modulates LDLR mRNA levels in liver tissue in vivo has not been addressed. Identification of the decay-promoting protein targeting LDLR mRNA 3′ UTR in liver tissue is of clinical importance, and such studies could lead to the discovery of novel therapeutic targets for reducing plasma LDL-c levels in hypercholesterolemic patients.
In this current study, we generated a transgenic mouse model (Alb-Luc-UTR) to study the regulatory role of 3′UTR in LDLR mRNA stability under in vivo conditions. In this model, we engineered transgenic reporter mice that express luciferase-LDLR3′UTR (Luc-UTR) transgene under the control of mouse albumin promoter which directs the transgene expression in liver tissue specifically. By imaging Alb-Luc-UTR mice and examining endogenous genes, our studies identified hnRNP D as an important negative regulator of LDLR mRNA levels in liver tissue and its downregulation by BBR treatment.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
BBR Treatment Elevated LDLR mRNA Levels Exclusively Through 3′UTR in Liver of Alb-Luc-UTR Mice
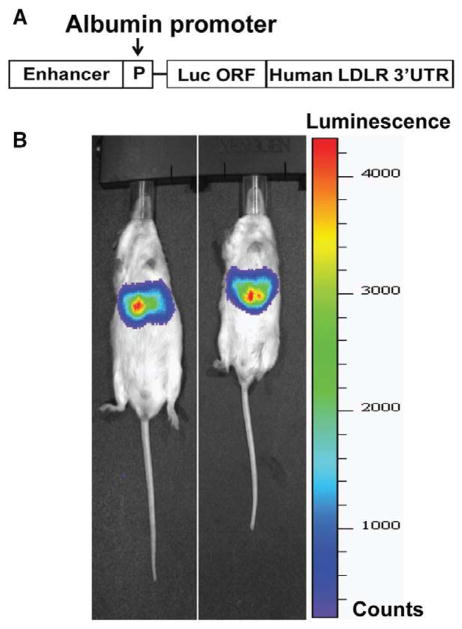
The mouse albumin (Alb) promoter has been widely used to obtain constitutive target gene expression specifically in liver tissue.26–28 To study the effect of BBR on LDLR mRNA expression mediated through 3′UTR in liver tissue, we generated a transgenic mouse model that expresses Luc-LDLR3′UTR reporter gene driven by the albumin promoter. The pAlb-Luc-UTR reporter construct (Figure 1A) was microinjected into fertilized oocytes of FVB mice to establish the transgenic lines. The tissue expression pattern of Luc-UTR transgene was visualized noninvasively in living mice using in vivo bioluminescent imaging. Figure 1B shows that intraperitoneal injection of luciferin into Alb-Luc-UTR mice resulted in strong emissions of photons from the liver region only, which confirmed the liver-specific expression of the transgene.
Figure 1.
Luciferase-LDLR3′UTR (Luc-UTR) transgene is specifically expressed in liver tissue of Alb-Luc-UTR mice. A, Schematic presentation of the albumin promoter driven chimeric luciferase–low-density lipoprotein receptor (LDLR) 3′ untranslated region (UTR) construct. B, Bioluminescence emission from live Alb-Luc-UTR mice. Three-month-old Alb-Luc-UTR mice were anesthetized, and luciferin was administered (50 mg/kg IP). After 10 minutes, mice were imaged with a bioluminescence imaging system for 1 minute. Bioluminescence is presented in pseudo-colored intensity maps superimposed on photographic images of the objects. ORF indicates open reading frame.
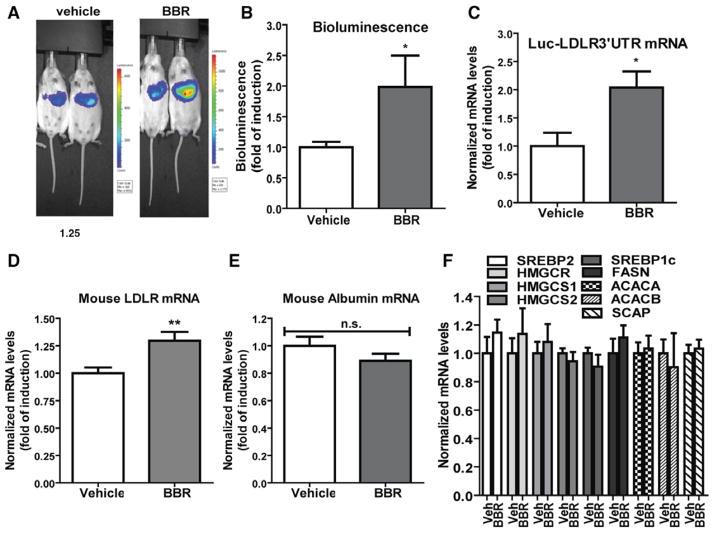
Alb-Luc-UTR mice were treated with BBR (n=10) at a dose of 200 mg/kg per day for 14 days by oral gavage. The control group (n=10) received equal volume of vehicle. The bioluminescence live imaging was conducted before the treatment and at the last day of treatment on all mice. Figure 2A shows representatives of bioluminescent imaging of vehicle-treated and BBR-treated mice. Figure 2B is the summarized imaging results (mean±SEM) showing that BBR treatment increased bioluminescence signal ≈2-fold over the control mice (P<0.05). Using quantitative reverse transcription polymerase chain reaction, we analyzed liver mRNA levels of Luc-UTR transgene (Figure 2C) and the endogenous mouse LDLR mRNA (Figure 2D). BBR treatment increased Luc-UTR mRNA levels by 2-fold (P<0.05) and elevated mouse LDLR mRNA levels by 30% (P<0.01) as compared with vehicle control. To rule out a transcriptional effect of BBR on Luc-UTR, we examined albumin mRNA levels in control and BBR-treated mice. The results confirmed the lack of inducing effect of BBR on albumin mRNA expression (Figure 2E). In addition to LDLR, we assessed mRNA levels of several genes involved in cholesterol biosynthetic pathway including SREBP2, SREBP1, 3-hydroxy-3-methylglutaryl (HMG) coenzyme A reductase (HMGCR), HMG coenzyme A synthetases (HMGCS1/2), fatty acid synthetase, acetyl coenzyme A carboxylase A, acetyl coenzyme A carboxylase B, and SREBP cleavage-activating protein in all liver samples. The results in Figure 2F showed that none of these mRNA levels were altered by BBR treatment. Altogether, these data provided the first in vivo demonstration that BBR upregulates LDLR mRNA expression primarily through the 3′UTR region.
Figure 2.
Effects of berberine (BBR) treatment on luciferase-LDLR3′ UTR (Luc-UTR) transgene and low-density lipoprotein receptor (LDLR) mRNA levels. Alb-Luc-UTR mice were administered either BBR (200 mg/kg per day; n=10) or vehicle (n=10) for 14 days. Bioluminescence imaging was conducted 1 week before the treatment as the baseline and at the last day of treatment. A, Representative bioluminescent emissions from vehicle- and BBR-treated mice before termination. B, Bioluminescence was quantified from each mouse and expressed as fold induction over the baseline values. The fold induction in control mice is expressed as 1. Statistical significance (*) was determined by 2-tailed Student t test (P<0.05). C to F, Hepatic mRNA levels of Luc-UTR transgene and indicated endogenous genes were assessed by quantitative reverse transcription polymerase chain reaction (PCR) using specific PCR primers. After normalization with GAPDH mRNA levels, the relative levels are presented, and the results are means±SE of 10 animals per group with duplicate measurement of each cDNA sample. *P<0.05, **P<0.01 compared with the vehicle control group. ACACA indicates acetyl coenzyme A (CoA) carboxylase A; ACACB, acetyl CoA carboxylase B; FASN, fatty acid synthetase; HMGCR, 3-hydroxy-3-methylglutaryl CoA reductase; HMGCS1/2, HMG CoA synthetases; n.s., no statistical difference; SCAP, SREBP cleavage-activating protein; and SREBP, sterol-regulatory element binding protein.
Identification of mRNA Destabilizing ARE Motifs in Mouse LDLR mRNA 3′UTR
Our previous 3′UTR deletion analysis has confined the BBR-responsive region to the 1-kb 5′ proximal section of the 3′UTR where 3 AREs are located.15 Furthermore, mutation of each ARE site individually elevated Luc-UTR reporter activity.25 To confirm the involvement of these ARE motifs in BBR-enhanced LDLR mRNA stability, wild-type and ARE mutated constructs were examined for their responses to BBR induction in HepG2 cells. Comparisons of BBR activity on wild-type luciferase–UTR construct with ARE mutated reporters showed that the enhancing effect of BBR is reduced by ARE mutations (Figure IA in the online-only Data Supplement). This suggests that these ARE sequences are possibly involved in the mRNA stabilizing process as well as in the rapid degradation process.
Because BBR increased the expression of Luc-UTR transgene that contains human LDLR3′UTR and raised mouse endogenous LDLR mRNA level in Alb-Luc-UTR mice, we hypothesized that BBR may regulate mouse ARE-BPs that could interact with same regulatory motifs present in both human and mouse 3′UTR sequences of LDLR transcripts. We performed a sequence alignment of human and mouse LDLR mRNA 3′UTR sequences, which revealed that 3 ARE motif–containing sections of human 3′UTR sequence are highly conserved in mouse sequence (Figure IB in the online-only Data Supplement). To examine the function of these ARE motifs in mediating LDLR mRNA degradation in mouse hepatic cells, we constructed 3 Luc reporters by insertion of individual ARE-containing sections adjacent to the 3′Luc coding sequence in pCMV-Luc vector (pLuc), yielding pLuc-ARE 1–3 vectors. Activities of the LDLR3′UTR full length and individual AREs in degradation of luciferase mRNA were tested in mouse hepatoma cell line Hepa 1–6 (Figure IC in the online-only Data Supplement). Inclusion of full-length UTR led to an 80% reduction in luciferase activity. Inclusion of a single copy of each ARE segment significantly reduced Luc activity from 37% to 62% (P<0.001). These data demonstrated that 3 ARE motifs residing in 3′UTR mediate the degradation of human and mouse LDLR mRNAs in a similar fashion.
BBR Treatment Lowered the Liver Abundance of mRNA Destabilizing Protein hnRNP D
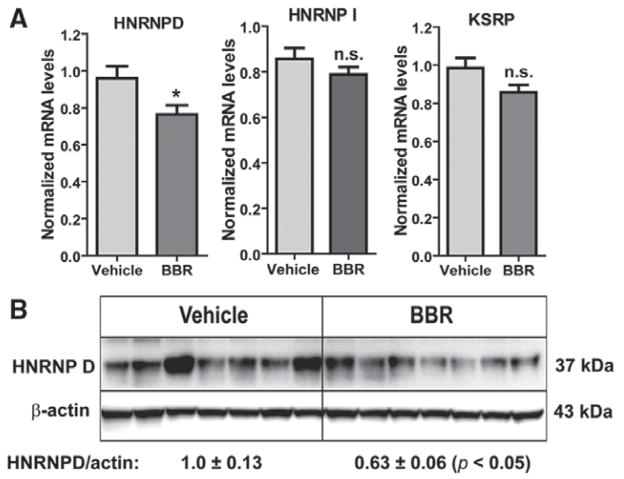
Our previous studies conducted in HepG2 cells identified 3 ARE-BPs (hnRNP D, hnRNP I, and KSRP) that specifically bound to ARE sites of LDLR mRNA 3′UTR sequence and mediated the mRNA degradation.25 To address why BBR increased the mRNA expression of Luc-UTR transgene and endogenous LDLR transcript in mouse liver, we examined mRNA levels of hnRNP D, hnRNP I, and KSRP in vehicle- and BBR-treated liver samples by quantitative reverse transcription polymerase chain reaction. Because hnRNP D mRNA comprises 4 isoforms that arise from alternative splicing of a single genetic locus,29 we designed the primers that are capable of detecting all isoforms.
Figure 3A shows that mRNA levels of hnRNP I and KSRP were not changed by BBR treatment. However, BBR reduced the total mRNA abundance of hnRNP D by 20% (P<0.05). To confirm this observation at the level of protein expression, we examined hnRNP D protein abundance in these liver samples (Figure 3B). hnRNP D has 4 isoforms of p37, p40, p42, and p45.30–32 Western blotting with anti–mouse hnRNP D antibody detected p37 isoform as the prominent immunoreactive hnRNP D band in liver homogenates, and its signal intensity was ≈37% lower in BBR-treated livers (P<0.05) as compared with control samples.
Figure 3.
Reduction of heterogeneous nuclear ribonucleoprotein D (hnRNP D) mRNA and protein levels in liver tissue by berberine (BBR) treatment. A, Quantitative reverse transcription polymerase chain reaction was performed to examine mRNA expressions of AU-rich element–binding proteins in liver samples of BBR-treated (n=10) and vehicle-treated mice (n=10). B, Western blotting with anti–hnRNP D antibody was conducted by analyzing individual liver homogenates of each group (n=7 per group). The membrane was reprobed with anti–β-actin antibody. The protein abundances of hnRNP D p37 were quantified using the Alpha View Software with normalization by signals of β-actin. Values are mean±SEM of 7 samples per group. *P<0.05 compared with the vehicle group. KSRP indicates KH-type splicing regulatory protein; and n.s., no statistical difference.
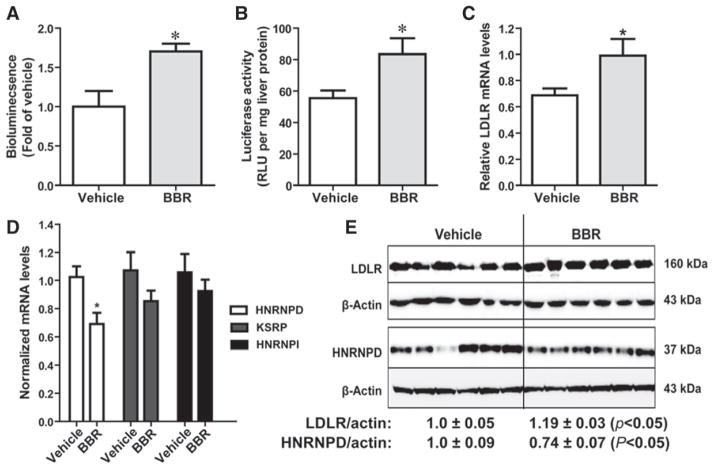
To further demonstrate the role of hnRNP D in BBR-mediated LDLR mRNA stabilization, we conducted another study by treating Alb-Luc-UTR mice with BBR for 3 days to assess the acute effects of BBR on transgene and endogenous LDLR mRNA expression in liver tissue. Examination of bioluminescence signal (Figure 4A) and the luciferase activity of isolated liver tissues (Figure 4B) confirmed the increased expression of Luc-UTR transgene in BBR-treated mice, which was corroborated by a 45% increase (P<0.05) in the endogenous mouse LDLR mRNA level (Figure 4C). Importantly, similar to the chronic treatment, the transient administration of BBR led to ≈25% decrease in hnRNP D mRNA (Figure 4D) and protein levels (Figure 4E) without affecting mRNA expressions of KSRP and hnRNP I. The liver LDLR protein levels in BBR-treated mice were slightly increased with statistic significance. Collectively, these 2 treatment studies consistently demonstrated that rises in LDLR mRNA level occurred concomitantly with declines of cellular abundance of hnRNP D protein, which provide the first in vivo evidence linking this decay-promoting ARE-BP to LDLR mRNA destabilization in liver tissue.
Figure 4.
Acute effects of berberine (BBR) on luciferase-LDLR3′ UTR (Luc-UTR) transgene and low-density lipoprotein receptor (LDLR) mRNA levels. Alb-Luc-UTR mice were treated with BBR (n=10) at a dose of 200 mg/kg per day or equal volume of vehicle (n=10) for 3 days. Bioluminescence imaging was conducted 1 week before the treatment as the baseline and at the last day of treatment. A, Bioluminescence was quantified from each mouse and expressed as fold induction over the baseline values. The fold induction in control mice is expressed as 1. Statistical significance (*) was determined by 2-tailed Student t test (P<0.05). B, Luciferase activity from liver homogenate was measured and normalized to tissue protein. C and D, Hepatic gene expression analysis by quantitative reverse transcription polymerase chain reaction. After normalization with GAPDH mRNA levels, the relative levels are presented, and the results are means±SE of 10 animals per group. E, Six individual liver protein homogenates from vehicle group and BBR cohort were randomly chosen for Western blot analysis of heterogeneous nuclear ribonucleoprotein D (hnRNP D) and LDLR. The protein abundances of hnRNP D p37 and LDLR were quantified using the Alpha View Software with normalization by signals of β-actin. Values are mean±SEM of 6 samples per group. *P<0.05 compared with the vehicle group. KSRP indicates KH-type splicing regulatory protein; RLU, relative light unit; and UTR, untranslated region.
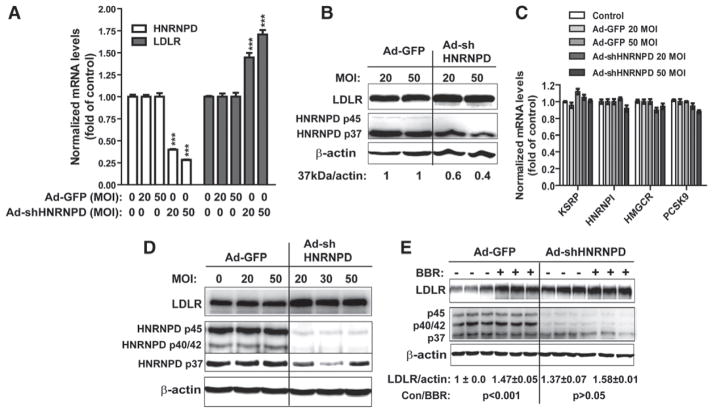
Knockdown of hnRNP D Increased LDLR mRNA Expression in Mouse Primary Hepatocytes
Next, we performed ex vivo experiments to examine the impact of depleting hnRNP D on hepatic LDLR mRNA expression. To this end, first, we compared the expression pattern of hnRNP D isoforms in mouse liver, mouse primary hepatocytes, and mouse hepatoma-derived cells (Hepa 1–6; Figure IIA in the online-only Data Supplement). Mouse liver tissues and primary hepatocytes express the predominant p37 isoform and the minor p45 form. A low signal of p42/40 band was detected in Hepa 1–6 cells. Alignment of cDNA sequences of mouse and human hnRNP D isoforms identified a 29-bp region that is identical in all sequences (Figure IIB in the online-only Data Supplement). Thus, adenovirus expressing a shRNA targeting this region was made and transduced into mouse primary hepatocytes. Ad-shHNRNPD transduction produced dose-dependent decreases in hnRNP D mRNA and protein levels. At 50 multiplicity of infection, hnRNP D mRNA level was reduced by 75% (Figure 5A), the protein amount of p37 isoform was reduced by 60% as compared with cells infected with control virus Ad-GFP, and the p45 isoform was decreased to a level of below detection, which was accompanied by increased LDLR protein levels in Ad-shHNRNPD–infected hepatocytes (Figure 5B). The amount of LDLR mRNA was markedly increased by 50% and 75% at 20 and 50 multiplicity of infection of Ad-shHNRNPD, respectively (Figure 5A). By contrast, mRNA levels of other mRNA-binding proteins (hnRNP I, KSRP) as well as SREBP2 target genes (HMGCR, PCSK9) in mouse primary hepatocytes were not affected by Ad-shHNRNPD infection (Figure 5C), thus confirming the specific targeting effect of the shRNA to hnRNP D. The reduction of hnRNP D by Ad-shHNRNPD transduction with a consequential increase in LDLR protein level was also observed in mouse hepatoma-derived cells (Figure 5D).
Figure 5.
Knockdown of heterogeneous nuclear ribonucleoprotein D (hnRNP D) expression increased low-density lipoprotein receptor (LDLR) mRNA and protein levels in mouse primary hepatocytes and hepatic cell lines. A to C, Mouse primary hepatocytes were transduced with Ad-shHNRNPD virus or control virus (Ad-GFP) at viral doses of 20 and 50 multiplicity of infection (MOI). Total RNA and total cell lysates were isolated 3 days postinfection. Quantitative reverse transcription polymerase chain reaction was conducted to analyze hnRNP D and LDLR mRNA levels in A and other genes in C. Total cell lysates were harvested 3 days postinfection for analyzing hnRNP D and LDLR protein levels in B. D, Mouse hepatoma-derived cells (Hepa 1c–7) were infected with Ad-shHNRNPD or Ad-GFP at indicated MOI for 3 days and analyzed for hnRNP D and LDLR protein levels. E, HepG2 cells were infected with Ad-shHNRNPD or Ad-GFP at 30 MOI. Two days after viral transduction, cells were treated with berberine (BBR) or dimethyl sulfoxide for 24 hours before isolations of cell lysates. Triplicate samples were analyzed in each condition.
We further examined the effects of hnRNP D shRNA expression on BBR-regulated LDLR protein expressions in HepG2 cells. As shown in Figure 5E, hnRNP D protein levels were markedly reduced in Ad-shHNRNPD–infected HepG2 cells, which was accompanied with 37% increase in LDLR protein abundance. Furthermore, the BBR-inducing effects on LDLR protein levels were clearly diminished in Ad-shHNRNPD–infected cells as compared with cells infected with Ad-GFP.
hnRNP D p37 Is the Principal Isoform in Hepatic LDLR mRNA Degradation
It has been shown that the extent of destabilizing effect of hnRNP D varies among the 4 isoforms, depending on the target mRNAs as well as cell types.30,33 To determine which isoform has the most profound effect on LDLR mRNA in liver cells, pLuc-LDLR3′UTR reporter was cotransfected with individual plasmids that encode mouse hnRNP D single isoforms into Hepa 1–6 and HepG2 cells (Figure IIIA in the online-only Data Supplement). The results showed that exogenous expression of p37 or p40 markedly reduced luciferase activity by ≈80% in Hepa 1–6 cells and by 50% to 60% in HepG2 cells. In Hepa 1–6 cells, hnRNP D p42 and p45 isoforms displayed modest activities, whereas p42 did not repress the reporter activity in HepG2 cells. Western blotting using anti-Flag antibody demonstrated similar expression levels among 4 isoforms in transfected cells (Figure IIIB in the online-only Data Supplement). Considering the fact that p37 is the abundant isoform in mouse liver, these results point p37 as the key player in promoting LDLR mRNA decay in liver tissue.
To determine whether overexpression of hnRNP D could affect BBR-mediated LDLR mRNA stability through 3′UTR, we cotransfected pLuc-LDLR3′UTR reporter with p37 or p42 plasmid, and the transfected cells were either treated with BBR or vehicle dimethyl sulfoxide for 24 hours. The results showed that p37 and p42 overexpression not only reduced the luciferase activity in control cells but they also lowered the induction of luciferase activity by BBR treatment (Figure IIIC in the online-only Data Supplement), which provided additional evidence supporting the functional involvement of hnRNP D in BBR-mediated stabilization of LDLR mRNA.
In addition to mRNA-binding proteins, we explored the possibility that microRNA (miRNA) plays a role in the regulation of LDLR mRNA stability by BBR. We treated HepG2 cells with BBR for 4, 8, and 24 hours or dimethyl sulfoxide at each time point. After total RNA isolation, we validated the BBR effects on LDLR mRNA expression in these cells by quantitative reverse transcription polymerase chain reaction. BBR induced a time-dependent elevation of LDLR mRNA levels as shown in Figure IVA in the online-only Data Supplement. Thus, 3 sets of miRNA gene array were conducted. Using a cutoff threshold of 1.5-fold change and a false discovery rate threshold of 0.05, we identified 7 miRNAs that have target sequences in human LDLR mRNA 3′UTR and were downregulated by BBR treatment at ≥1 time point as shown in Table II in the online-only Data Supplement. Two miRNAs, miR-1244 and miR-1979, have no binding sites in LDLR3′UTR but they were substantially downregulated by BBR, thus they were included in our validation studies. We used anti-miRNAs to individually deplete these 9 miRNAs in HepG2 cells and examined the effects of knockdown on LDLR3′UTR reporter activity, LDLR mRNA, and protein expressions. The results presented in Figure IVB to IVD in the online-only Data Supplement showed that none of these anti-miRNAs produced a measurable result in altering LDLR mRNA or protein expression. These negative results of miRNA studies provided additional support indirectly to underscore the important role of mRNA decay-promoting factor hnRNP D in LDLR mRNA degradation in liver tissue.
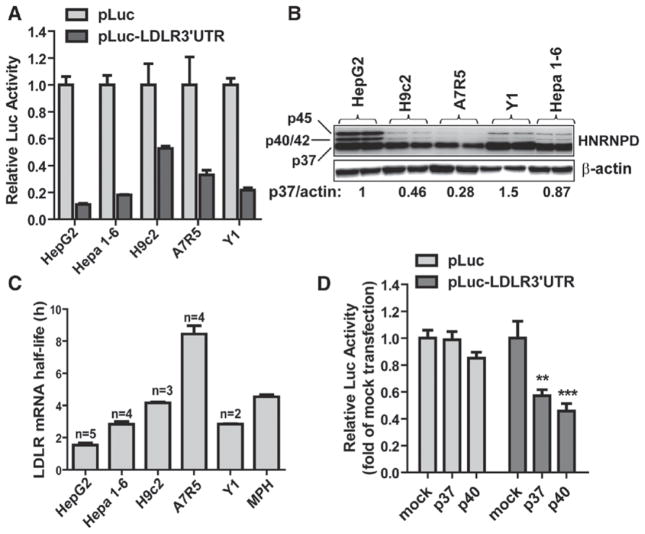
Involvement of hnRNP D in LDLR mRNA Stability in Nonhepatic Cell Lines
Finally, we assessed the function of 3′UTR in modulating LDLR mRNA stability in cell types derived from extra hepatic tissues. To this end, we conducted LDLR3′UTR reporter assays in H9c2 cardiomyocytes,34 A7r5 vascular myocytes,35 and mouse adrenal Y1 cells36 along with hepatic cells (HepG2, Hepa 1–6; Figure 6A). LDLR3′UTR exhibited a stronger activity in reducing luciferase activity in hepatic cells as compared with that in H9c2 and A7r5 cells. A relative strong decay- promoting effect of 3′UTR was also observed in Y1 cells. Western blot analysis of hnRNP D revealed significant lower levels of p37 and p40/42 isoforms in H9c2 and A7r5 cells (Figure 6B) than that in hepatic and Y1 cells. In addition to Luc assay and hnRNP D expression analysis, we measured LDLR mRNA half-lives in these cell lines (Figure 6C). Actinomycin D was added to cells for different times to block new mRNA synthesis, and the decay rate of LDLR mRNA was determined by measuring remaining mRNA levels with quantitative reverse transcription polymerase chain reaction. The results showed that the half-lives of LDLR transcript in A7r5 and H9c2 cells were 8.4±0.5 and 4.1±0.1 hours, which were considerably longer than the half-life of LDLR mRNA in HepG2 cells (1.5±0.1 hours) and Hepa 1–6 cells (2.7±0.2 hours), respectively. We also measured LDLR mRNA half-life in mouse primary hepatocytes which displayed a longer half-life (4.5±0.1 hours) than HepG2 and Hepa 1–6 cells as well as the reported 1-hour half-life of mouse liver.20 Examination of hnRNP D expression in mouse primary hepatocytes (Figure IIA in the online-only Data Supplement) showed that they had substantially lower p37 protein levels as compared with mouse liver and Hepa 1–6 cells, which could explain the longer LDLR mRNA half-life observed. In the mRNA decay experiments conducted in these cell lines and mouse primary hepatocytes, we measured the decay rates of cyclin D1 mRNA, which did not show substantial differences (Figure V in the online-only Data Supplement).
Figure 6.
Involvement of heterogeneous nuclear ribonucleoprotein D (hnRNP D) in 3′ untranslated region (UTR) mediated regulation of low-density lipoprotein receptor (LDLR) mRNA stability in extrahepatic cells. A, pLuc-LDLR3′ UTR or control vector pCMV-Luc (pLuc) was transfected into cells along with pRL-SV40. Dual luciferase activities were measured and expressed as relative Luc activity. B, Duplicate cell lysates from each cell line were analyzed for hnRNP D protein levels by Western blotting using anti–hnRNP D antibody. Signal intensities of hnRNP D p37/p45 were quantified with normalization by signals of β-actin. Values are average of 2 samples. The relative signal intensity of hnRNP D in HepG2 is expressed as 1. C, Cells were treated with actinomycin D (5 μg/mL) for different intervals. Total RNA was isolated and analyzed for the amounts of LDLR mRNAs by quantitative reverse transcription polymerase chain reaction. The LDLR mRNA levels were plotted as the percentage of the mRNA remaining. Decay curves (mean±SEM) were plotted vs time. The T1/2 from each cell line was derived from separate experiments as indicated; the mouse (mean±SEM) was primary hepatocytes T1/2 derived from a single experiment with quadruple RNA samples per time point. D, H9c2 cells were cotransfected with pLuc-LDLR3′ UTR and plasmids encoding hnRNP D isoform p37, p40 or the empty vector (mock). pLuc was included in this assay as negative control.
Next, we examined the effects of exogenous expression of p37 or p40 isoforms on LDLR3′UTR activity in H9c2 cardiomyocytes (Figure 6D). Expressions of either isoform did not affect the pLuc control reporter activity but reduced pLuc-LDLR3′UTR Luc activity to 57% and 45% of control. Collectively, these data obtained from various cell lines suggest that 3′UTR regulates LDLR mRNA stability with cell-type specificity and variable expression levels of hnRNP D in different cell types might be an underlying factor contributing to the strength of 3′UTR in LDLR mRNA degradation in a particular cell type or a tissue.
Discussion
The expression of liver LDLR regulates human plasma LDL-c homeostasis. Increased hepatic LDLR expression results in improved clearance of plasma LDL-c through receptor-mediated endocytosis, which has been strongly associated with a decreased risk of developing cardiovascular disease in humans. The LDL-c lowering effect of the herbal medicine BBR has been demonstrated in multiple clinical studies across the globe.22–24,37,38 Our previous studies, almost a decade ago, had identified the LDLR mRNA stabilization being the underlying mechanism for increased LDLR mRNA levels in HepG2 cells on BBR treatment. It is thought that the same mechanism accounted for the increased LDLR mRNA levels in liver of BBR-treated animals and responsible for lowering cholesterol in hyperlipidemic patients. However, until this study, there is no investigation to validate or challenge this BBR action mechanism under in vivo conditions.
We set out to establish a mouse model (Alb-Luc-UTR) to examine the effect of BBR on liver LDLR mRNA expression through LDLR3′UTR region without the involvement of transcriptional components. Driven by the liver-specific albumin promoter, the Luc-UTR transgene is exclusively expressed in liver tissue. Bioluminescence imaging exhibited the strong emission of photons in liver tissue on luciferin injection. BBR administration in Alb-Luc-UTR mice either chronically (14 days; Figure 2) or acutely (3 days; Figure 4) elevated bioluminescent signals and increased mRNA levels of Luc-UTR transcript and endogenous LDLR mRNA in liver. Importantly, increases of Luc-UTR transgene and LDLR mRNA levels occurred in the absence of changes in albumin mRNA levels or the activation of SREBP pathways. These results, for the first time, validated the action mechanism of BBR in stabilizing LDLR mRNA in hepatic cells under in vivo conditions. In both BBR treatment studies, we observed that the inductive effect of BBR on endogenous LDLR mRNA expression was less potent than that of Luc-UTR transgene expression. The reason for this differential effect was not clear. One possible explanation would be that the transcription of liver LDLR gene is active in mice fed a regular chow diet. The high basal level of LDLR mRNA could diminish the inducing effect of BBR.
Our previous work conducted in HepG2 cells have identified hnRNP D, hnRNP I, and KSRP as LDLR ARE-BPs.25 However, it was unknown whether these ARE-BPs are functionally involved in LDLR mRNA decay in liver tissue. Thus, the second goal of the current study was to identify the mRNA destabilizing proteins that mediate hepatic LDLR mRNA degradation in Alb-Luc-UTR mice. We have now demonstrated that the mRNA and protein levels of hnRNP D were specifically reduced by BBR treatment in Alb-Luc-UTR mice, which correlated with elevated endogenous LDLR mRNA and Luc-UTR mRNA levels. These novel in vivo findings were further supported by our ex vivo experiments conducted in mouse primary hepatocytes in which depletion of this ARE-BP by shRNA resulted in a substantial increase in LDLR mRNA levels. hnRNP D also called AU-binding factor 1 comprises 4 isoforms (p37, p40, p42, p45). One study has shown that the extent of destabilizing effect varied among the 4 isoforms, with p37 and p42 displaying the most profound effect.30 Because we have shown that p37 is the abundant isoform expressed in liver of Alb-Luc-UTR mice and mouse primary hepatocytes, it is important to demonstrate that p37 is highly capable of degrading LDLR mRNA. Indeed, through LDLR3′UTR reporter assays, we showed that exogenous expression of p37 markedly lowered luciferase activity in both mouse hepatic cells and HepG2 cells (Figure IIIA in the online-only Data Supplement). These data together identify hnRNP D p37 isoform as the key player in promoting LDLR mRNA degradation in liver tissue.
Knockdown studies in HepG2 cells provided the initial evidence that hnRNP D is also involved in BBR-induced LDLR mRNA stabilization in this hepatic model cell line without significant reductions of hnRNP D protein levels by BBR treatment (Figure 5E). We have previously demonstrated that the MAP kinase MEK-ERK signaling pathway is critically involved in BBR’s action mechanism.15 A recent study has linked ERK signaling pathway with the nuclear export of p42 isoform and its stabilizing activity to the 3′UTR of COX-2 mRNA in human synovial fibroblast.39 It is possible that BBR treatment affected the phosphorylation status of hnRNP D and consequently reduced its ability to bind LDLR-ARE sequences in HepG2 cells. Further studies are required to fully understand the role of hnRNP D in BBR-mediated LDLR mRNA stabilization in HepG2 cells.
In addition to study the BBR effect on LDLR3′UTR in liver tissue and hepatic cells, we explored a possible role of hnRNP D in regulating LDLR mRNA stability in cell types derived from tissues such as heart in which intracellular levels of cholesterol are not as fluctuated as that in liver. Our results showed that cardiac cells (H9c2 and A7r5) have longer half-life of LDLR mRNA and reduced destabilizing activity of 3′UTR, which were consistent with the observation that hnRNP D expression levels in H9c2 and A7r5 were notably lower than that of hepatic cells. By cotransfection of p37 or p40 isoform with pLuc-LDLR3′UTR construct into H9c2 cells, we further demonstrated that exogenous expression of each isoform accelerated luciferase mRNA degradation in cardiomyocytes. These in vitro studies suggest that 3′UTR regulates LDLR mRNA stability with cell-type/tissue specificity and its activity positively correlates with cellular levels of hnRNP D p37 isoform.
It is worthy to note that in this study the LDLR mRNA half-life in isolated primary hepatocytes was notably longer than the reported half-life of mouse liver tissue20 and hepatoma cell lines (Figure 6A) and this increased stability correlated with the reduced hnRNP D expression. Defining a BBR-independent mechanism that regulates hnRNP D expression will be an important question for future investigation.
In summary, we have generated a Luc-UTR in vivo model to study the regulation of LDLR mRNA stability in liver tissue of living mice. The inverse relationship of hnRNP D abundance and LDLR mRNA levels after BBR treatment of Alb-Luc-UTR mice suggests a critical function of hnRNP D in mediating hepatic LDLR mRNA degradation and the potential of hnRNP D of being a novel therapeutic target for LDL-c lowering.
Supplementary Material
Significance.
The expression of liver low-density lipoprotein receptor (LDLR) regulates human plasma LDL cholesterol homeostasis. Increased hepatic LDLR expression results in improved clearance of plasma LDL cholesterol and reduces the risk of developing cardiovascular disease in humans. Our previous studies have demonstrated that stabilizing LDLR mRNA is a viable means to upregulate hepatic LDLR expression. In this study, we have generated a novel transgenic mouse model (Alb-Luc-UTR) to study the LDLR mRNA stability and regulation by the natural cholesterol-lowering drug berberine in living mice. We further identified an LDLR mRNA-binding protein heterogeneous nuclear ribonucleoprotein D as a critical negative regulator of LDLR mRNA stability in liver tissue. Our studies demonstrated an inverse relationship between decreased heterogeneous nuclear ribonucleoprotein D expression levels and elevated LDLR expression in liver tissue of berberine-treated Alb-Luc-UTR mice. Hence, heterogeneous nuclear ribonucleoprotein D has the potential of being a novel therapeutic target for plasma LDL cholesterol reduction in hypercholesterolemic patients.
Acknowledgments
Sources of Funding
This study was supported by the Department of Veterans Affairs (Office of Research and Development, Medical Research Service) and by grants (1R01AT002543-01A1, 1R21AT003195-01A2, and 1R01AT006336-01A1) from National Center of Complementary and Alternative Medicine.
Nonstandard Abbreviations and Acronyms
- Alb
albumin
- ARE
AU-rich element
- ARE-BP
ARE-binding protein
- BBR
berberine
- HMG
3-hydroxy-3-methylglutaryl
- hnRNP D
heterogeneous nuclear ribonucleoprotein D
- KSRP
KH-type splicing regulatory protein
- LDL
low-density lipoprotein
- LDL-c
LDL cholesterol
- LDLR
LDL receptor
- Luc
luciferase
- Luc-UTR
luciferase-LDLR3′ UTR
- miRNA
microRNA
- PCSK9
proprotein convertase subtilisin/kexin type 9
- SREBP
sterol-regulatory element binding protein
- UTR
untranslated region
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.112.301131/-/DC1.
Disclosures
None.
References
- 1.Nakajima K, Nakajima Y, Takeichi S, Fujita MQ. Plasma remnant-like lipoprotein particles or LDL-C as major pathologic factors in sudden cardiac death cases. Atherosclerosis. 2008;198:237–246. doi: 10.1016/j.atherosclerosis.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Homma Y. Predictors of atherosclerosis. J Atheroscler Thromb. 2004;11:265–270. doi: 10.5551/jat.11.265. [DOI] [PubMed] [Google Scholar]
- 3.Spady DK. Hepatic clearance of plasma low density lipoproteins. Semin Liver Dis. 1992;12:373–385. doi: 10.1055/s-2008-1040407. [DOI] [PubMed] [Google Scholar]
- 4.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 6.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 7.Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci U S A. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A, Chretien M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A. 2003;100:928–933. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abifadel M, Varret M, Rabès JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 10.Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci U S A. 2003;100:12027–12032. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maxwell KN, Soccio RE, Duncan EM, Sehayek E, Breslow JL. Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice. J Lipid Res. 2003;44:2109–2119. doi: 10.1194/jlr.M300203-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Wilson GM, Vasa MZ, Deeley RG. Stabilization and cytoskeletal- association of LDL receptor mRNA are mediated by distinct domains in its 3′ untranslated region. J Lipid Res. 1998;39:1025–1032. [PubMed] [Google Scholar]
- 13.Wilson GM, Roberts EA, Deeley RG. Modulation of LDL receptor mRNA stability by phorbol esters in human liver cell culture models. J Lipid Res. 1997;38:437–446. [PubMed] [Google Scholar]
- 14.Goto D, Okimoto T, Ono M, Shimotsu H, Abe K, Tsujita Y, Kuwano M. Upregulation of low density lipoprotein receptor by gemfibrozil, a hypolipidemic agent, in human hepatoma cells through stabilization of mRNA transcripts. Arterioscler Thromb Vasc Biol. 1997;17:2707–2712. doi: 10.1161/01.atv.17.11.2707. [DOI] [PubMed] [Google Scholar]
- 15.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, Wang S, Wu J, Li Z, Liu J, Jiang J-D. Berberine is a promising novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1352. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto T, Davis CG, Brown MS, Schneider WJ, Casey ML, Goldstein JL, Russell DW. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984;39:27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]
- 17.Zhang T, Kruys V, Huez G, Gueydan C. AU-rich element-mediated translational control: complexity and multiple activities of trans-activating factors. Biochem Soc Trans. 2002;30(pt 6):952–958. doi: 10.1042/bst0300952. [DOI] [PubMed] [Google Scholar]
- 18.Meyer S, Temme C, Wahle E. Messenger RNA turnover in eukaryotes: pathways and enzymes. Crit Rev Biochem Mol Biol. 2004;39:197–216. doi: 10.1080/10409230490513991. [DOI] [PubMed] [Google Scholar]
- 19.Briata P, Chen CY, Giovarelli M, Pasero M, Trabucchi M, Ramos A, Gherzi R. KSRP, many functions for a single protein. Front Biosci (Landmark Ed) 2011;16:1787–1796. doi: 10.2741/3821. [DOI] [PubMed] [Google Scholar]
- 20.Knouff C, Malloy S, Wilder J, Altenburg MK, Maeda N. Doubling expression of the low density lipoprotein receptor by truncation of the 3′-untranslated region sequence ameliorates type iii hyperlipoproteinemia in mice expressing the human apoe2 isoform. J Biol Chem. 2001;276:3856–3862. doi: 10.1074/jbc.M009423200. [DOI] [PubMed] [Google Scholar]
- 21.Nakahara M, Fujii H, Maloney PR, Shimizu M, Sato R. Bile acids enhance low density lipoprotein receptor gene expression via a MAPK cascade-mediated stabilization of mRNA. J Biol Chem. 2002;277:37229–37234. doi: 10.1074/jbc.M206749200. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Li X, Zou D, Liu W, Yang J, Zhu N, Huo L, Wang M, Hong J, Wu P, Ren G, Ning G. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93:2559–2565. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- 23.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–717. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei W, Zhao H, Wang A, Sui M, Liang K, Deng H, Ma Y, Zhang Y, Zhang H, Guan Y. A clinical study on the short-term effect of berberine in comparison to metformin on the metabolic characteristics of women with polycystic ovary syndrome. Eur J Endocrinol. 2012;166:99–105. doi: 10.1530/EJE-11-0616. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Chen W, Zhou Y, Abidi P, Sharpe O, Robinson WH, Liu J. Identification of mRNA-binding proteins that regulate the stability of LDL receptor mRNA through AU-rich elements. J Lipid Res. 2009;50:820–831. doi: 10.1194/jlr.M800375-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinkert CA, Ornitz DM, Brinster RL, Palmiter RD. An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice. Genes Dev. 1987;1:268–276. doi: 10.1101/gad.1.3.268. [DOI] [PubMed] [Google Scholar]
- 27.Izban MG, Papaconstantinou J. Cell-specific expression of mouse albumin promoter. Evidence for cell-specific DNA elements within the proximal promoter region and cis-acting DNA elements upstream of −160. J Biol Chem. 1989;264:9171–9179. [PubMed] [Google Scholar]
- 28.Wu Y, Wang YY, Nakamoto Y, Li YY, Baba T, Kaneko S, Fujii C, Mukaida N. Accelerated hepatocellular carcinoma development in mice expressing the Pim-3 transgene selectively in the liver. Oncogene. 2010;29:2228–2237. doi: 10.1038/onc.2009.504. [DOI] [PubMed] [Google Scholar]
- 29.Raineri I, Wegmueller D, Gross B, Certa U, Moroni C. Roles of AUF1 isoforms, HuR and BRF1 in ARE-dependent mRNA turnover studied by RNA interference. Nucleic Acids Res. 2004;32:1279–1288. doi: 10.1093/nar/gkh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loflin P, Chen CY, Shyu AB. Unraveling a cytoplasmic role for hnRNP D in the in vivo mRNA destabilization directed by the AU-rich element. Genes Dev. 1999;13:1884–1897. doi: 10.1101/gad.13.14.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker A, Epis MR, Porter CJ, Hopkins BR, Wilce MC, Wilce JA, Giles KM, Leedman PJ. Sequence requirements for RNA binding by HuR and AUF1. J Biochem. 2012;151:423–437. doi: 10.1093/jb/mvs010. [DOI] [PubMed] [Google Scholar]
- 32.Mazan-Mamczarz K, Kuwano Y, Zhan M, White EJ, Martindale JL, Lal A, Gorospe M. Identification of a signature motif in target mRNAs of RNA-binding protein AUF1. Nucleic Acids Res. 2009;37:204–214. doi: 10.1093/nar/gkn929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pont AR, Sadri N, Hsiao SJ, Smith S, Schneider RJ. mRNA decay factor AUF1 maintains normal aging, telomere maintenance, and suppression of senescence by activation of telomerase transcription. Mol Cell. 2012;47:5–15. doi: 10.1016/j.molcel.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flores-Muñoz M, Godinho BM, Almalik A, Nicklin SA. Adenoviral delivery of angiotensin-(1–7) or angiotensin-(1–9) inhibits cardiomyocyte hypertrophy via the mas or angiotensin type 2 receptor. PLoS One. 2012;7:e45564. doi: 10.1371/journal.pone.0045564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monet M, Francoeur N, Boulay G. Involvement of phosphoinositide 3-kinase and PTEN protein in mechanism of activation of TRPC6 protein in vascular smooth muscle cells. J Biol Chem. 2012;287:17672–17681. doi: 10.1074/jbc.M112.341354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King SR, Matassa AA, White EK, Walsh LP, Jo Y, Rao RM, Stocco DM, Reyland ME. Oxysterols regulate expression of the steroidogenic acute regulatory protein. J Mol Endocrinol. 2004;32:507–517. doi: 10.1677/jme.0.0320507. [DOI] [PubMed] [Google Scholar]
- 37.Derosa G, D’Angelo A, Bonaventura A, Bianchi L, Romano D, Maffioli P. Effects of berberine on lipid profile in subjects with low cardiovascular risk. Expert Opin Biol Ther. 2013;13:475–482. doi: 10.1517/14712598.2013.776037. [DOI] [PubMed] [Google Scholar]
- 38.Pisciotta L, Bellocchio A, Bertolini S. Nutraceutical pill containing berberine versus ezetimibe on plasma lipid pattern in hypercholesterolemic subjects and its additive effect in patients with familial hypercholesterolemia on stable cholesterol-lowering treatment. Lipids Health Dis. 2012;11:123. doi: 10.1186/1476-511X-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhai B, Yang H, Mancini A, He Q, Antoniou J, Di Battista JA. Leukotriene B(4) BLT receptor signaling regulates the level and stability of cyclooxy-genase-2 (COX-2) mRNA through restricted activation of Ras/Raf/ERK/ p42 AUF1 pathway. J Biol Chem. 2010;285:23568–23580. doi: 10.1074/jbc.M110.107623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.