Abstract
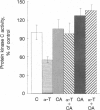
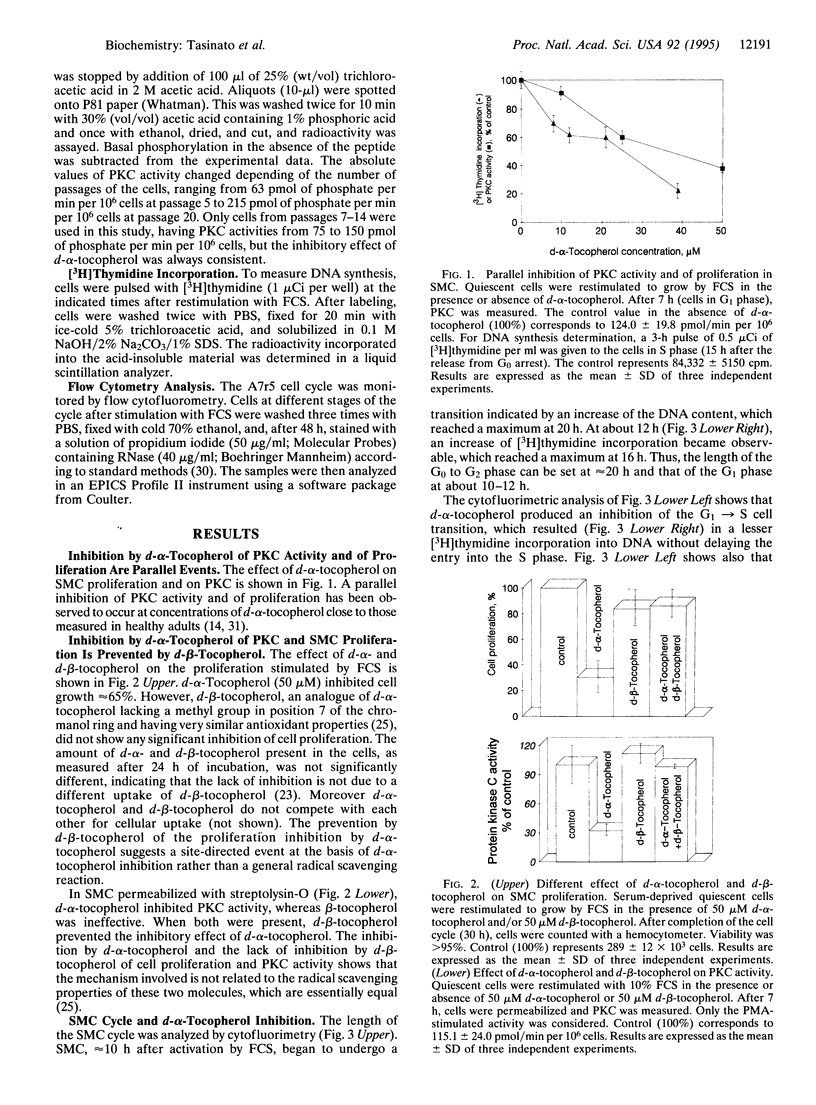
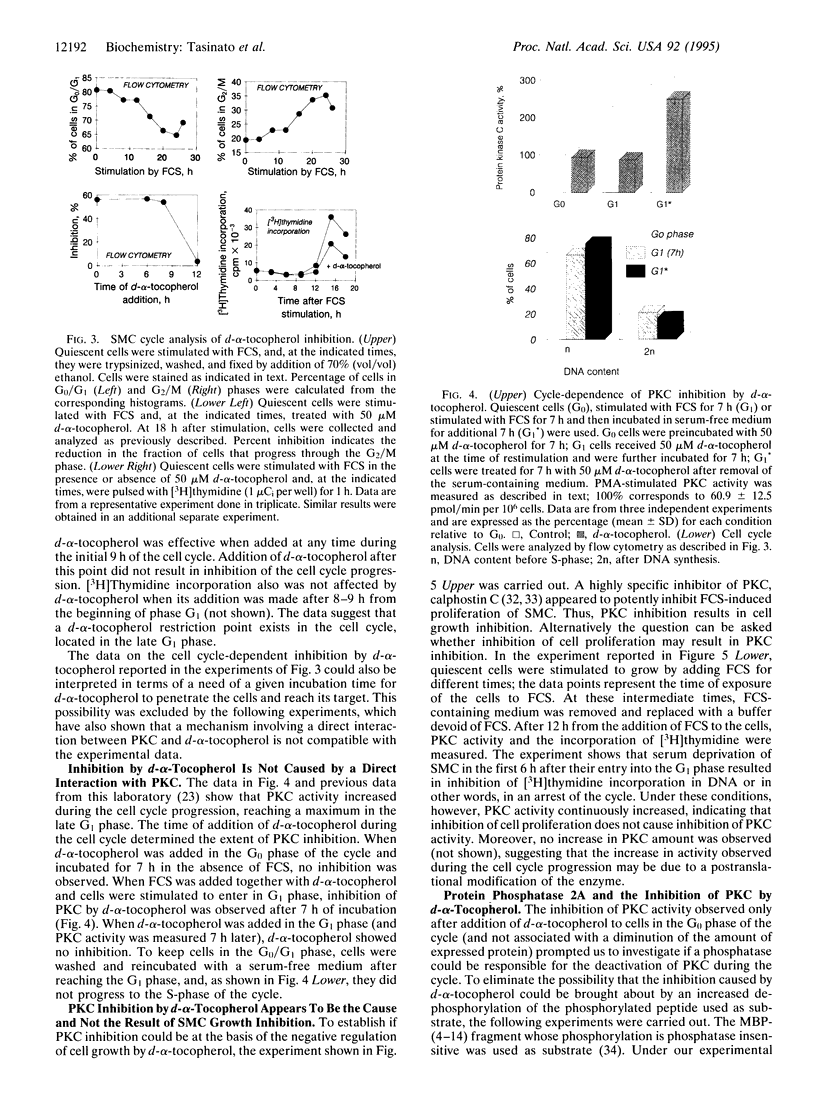
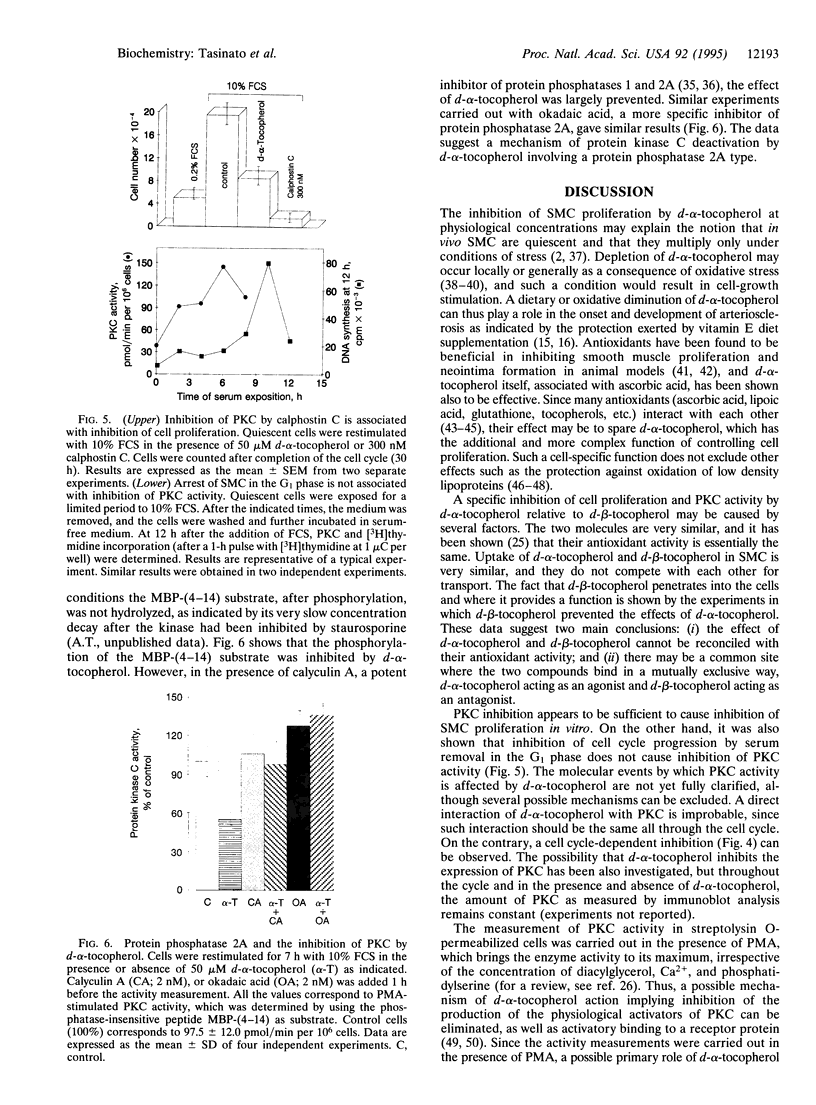
d-alpha-Tocopherol, but not d-beta-tocopherol, negatively regulates proliferation of vascular smooth muscle cells at physiological concentrations. d-alpha-Tocopherol inhibits protein kinase C (PKC) activity, whereas d-beta-tocopherol is ineffective. Furthermore d-beta-tocopherol prevents the inhibition of cell growth and of PKC activity caused by d-alpha-tocopherol. The negative regulation by d-alpha-tocopherol of PKC activity appears to be the cause and not the effect of smooth muscle cell growth inhibition. d-alpha-Tocopherol does not act by binding to PKC directly but presumably by preventing PKC activation. It is concluded that, in vascular smooth muscle cells, d-alpha-tocopherol acts specifically through a nonantioxidant mechanism and exerts a negative control on a signal transduction pathway regulating cell proliferation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. R., Graves J. D., Lucas S. C., Cantrell D. A., Crumpton M. J. A method for measuring protein kinase C activity in permeabilized T lymphocytes by using peptide substrates. Evidence for multiple pathways of kinase activation. Biochem J. 1990 Jun 1;268(2):303–308. doi: 10.1042/bj2680303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi A., Boscoboinik D., Hensey C. The protein kinase C family. Eur J Biochem. 1992 Sep 15;208(3):547–557. doi: 10.1111/j.1432-1033.1992.tb17219.x. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Henriksson-Freyschuss A., Breuer O., Diczfalusy U., Berglund L., Henriksson P. The antioxidant butylated hydroxytoluene protects against atherosclerosis. Arterioscler Thromb. 1991 Jan-Feb;11(1):15–22. doi: 10.1161/01.atv.11.1.15. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Ingold K. U. Vitamin E as an in vitro and in vivo antioxidant. Ann N Y Acad Sci. 1989;570:7–22. doi: 10.1111/j.1749-6632.1989.tb14904.x. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Traber M. G. Vitamin E: antioxidant activity, biokinetics, and bioavailability. Annu Rev Nutr. 1990;10:357–382. doi: 10.1146/annurev.nu.10.070190.002041. [DOI] [PubMed] [Google Scholar]
- Chan A. C., Tran K. The uptake of (R,R,R)alpha-tocopherol by human endothelial cells in culture. Lipids. 1990 Jan;25(1):17–21. doi: 10.1007/BF02562422. [DOI] [PubMed] [Google Scholar]
- Chatelain E., Boscoboinik D. O., Bartoli G. M., Kagan V. E., Gey F. K., Packer L., Azzi A. Inhibition of smooth muscle cell proliferation and protein kinase C activity by tocopherols and tocotrienols. Biochim Biophys Acta. 1993 Mar 10;1176(1-2):83–89. doi: 10.1016/0167-4889(93)90181-n. [DOI] [PubMed] [Google Scholar]
- Clarke P. R., Siddhanti S. R., Cohen P., Blackshear P. J. Okadaic acid-sensitive protein phosphatases dephosphorylate MARCKS, a major protein kinase C substrate. FEBS Lett. 1993 Dec 20;336(1):37–42. doi: 10.1016/0014-5793(93)81604-x. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Schwartz S. M. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res. 1985 Jan;56(1):139–145. doi: 10.1161/01.res.56.1.139. [DOI] [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mascio P., Murphy M. E., Sies H. Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am J Clin Nutr. 1991 Jan;53(1 Suppl):194S–200S. [PubMed] [Google Scholar]
- Esterbauer H., Waeg G., Puhl H., Dieber-Rotheneder M., Tatzber F. Inhibition of LDL oxidation by antioxidants. EXS. 1992;62:145–157. doi: 10.1007/978-3-0348-7460-1_15. [DOI] [PubMed] [Google Scholar]
- Farrar Y. J., Vanaman T. C., Slevin J. T. A phosphatase resistant substrate for the assay of protein kinase C in crude tissue extracts. Biochem Biophys Res Commun. 1991 Oct 31;180(2):694–701. doi: 10.1016/s0006-291x(05)81121-0. [DOI] [PubMed] [Google Scholar]
- Freyschuss A., Stiko-Rahm A., Swedenborg J., Henriksson P., Björkhem I., Berglund L., Nilsson J. Antioxidant treatment inhibits the development of intimal thickening after balloon injury of the aorta in hypercholesterolemic rabbits. J Clin Invest. 1993 Apr;91(4):1282–1288. doi: 10.1172/JCI116326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster V., Badimon L., Badimon J. J., Chesebro J. H. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992 Jan 23;326(4):242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- Fuster V., Badimon L., Badimon J. J., Chesebro J. H. The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N Engl J Med. 1992 Jan 30;326(5):310–318. doi: 10.1056/NEJM199201303260506. [DOI] [PubMed] [Google Scholar]
- Gavino V. C., Miller J. S., Ikharebha S. O., Milo G. E., Cornwell D. G. Effect of polyunsaturated fatty acids and antioxidants on lipid peroxidation in tissue cultures. J Lipid Res. 1981 Jul;22(5):763–769. [PubMed] [Google Scholar]
- Gey K. F. The antioxidant hypothesis of cardiovascular disease: epidemiology and mechanisms. Biochem Soc Trans. 1990 Dec;18(6):1041–1045. doi: 10.1042/bst0181041. [DOI] [PubMed] [Google Scholar]
- Hess D., Keller H. E., Oberlin B., Bonfanti R., Schüep W. Simultaneous determination of retinol, tocopherols, carotenes and lycopene in plasma by means of high-performance liquid chromatography on reversed phase. Int J Vitam Nutr Res. 1991;61(3):232–238. [PubMed] [Google Scholar]
- Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989 Mar 31;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Kagan V. E., Serbinova E. A., Packer L. Recycling and antioxidant activity of tocopherol homologs of differing hydrocarbon chain lengths in liver microsomes. Arch Biochem Biophys. 1990 Nov 1;282(2):221–225. doi: 10.1016/0003-9861(90)90108-b. [DOI] [PubMed] [Google Scholar]
- Kita T., Nagano Y., Yokode M., Ishii K., Kume N., Ooshima A., Yoshida H., Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E., Ando K., Nakano H., Iida T., Ohno H., Morimoto M., Tamaoki T. Calphostins (UCN-1028), novel and specific inhibitors of protein kinase C. I. Fermentation, isolation, physico-chemical properties and biological activities. J Antibiot (Tokyo) 1989 Oct;42(10):1470–1474. doi: 10.7164/antibiotics.42.1470. [DOI] [PubMed] [Google Scholar]
- Kobayashi E., Nakano H., Morimoto M., Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989 Mar 15;159(2):548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B., Cooper H. L., Sando J. J. Decrease in cytosolic calcium/phospholipid-dependent protein kinase activity following phorbol ester treatment of EL4 thymoma cells. J Biol Chem. 1982 Nov 25;257(22):13193–13196. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Kuzuya M., Naito M., Funaki C., Hayashi T., Yamada K., Asai K., Kuzuya F. Antioxidants stimulate endothelial cell proliferation in culture. Artery. 1991;18(3):115–124. [PubMed] [Google Scholar]
- Liebler D. C., Kling D. S., Reed D. J. Antioxidant protection of phospholipid bilayers by alpha-tocopherol. Control of alpha-tocopherol status and lipid peroxidation by ascorbic acid and glutathione. J Biol Chem. 1986 Sep 15;261(26):12114–12119. [PubMed] [Google Scholar]
- Lindsey J. A., Zhang H. F., Kaseki H., Morisaki N., Sato T., Cornwell D. G. Fatty acid metabolism and cell proliferation. VII. Antioxidant effects of tocopherols and their quinones. Lipids. 1985 Mar;20(3):151–157. doi: 10.1007/BF02534247. [DOI] [PubMed] [Google Scholar]
- Meyer M., Pahl H. L., Baeuerle P. A. Regulation of the transcription factors NF-kappa B and AP-1 by redox changes. Chem Biol Interact. 1994 Jun;91(2-3):91–100. doi: 10.1016/0009-2797(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Minoves M., Garcia A., Magriña J., Pavia J., Herranz R., Setoain J. Evaluation of myocardial perfusion defects by means of "bull's eye" images. Clin Cardiol. 1993 Jan;16(1):16–22. doi: 10.1002/clc.4960160104. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D., Khaner H., Lopez J. Identification of intracellular receptor proteins for activated protein kinase C. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3997–4000. doi: 10.1073/pnas.88.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D., Khaner H., Lopez J., Smith B. L. Intracellular receptors for activated protein kinase C. Identification of a binding site for the enzyme. J Biol Chem. 1991 Aug 15;266(23):14866–14868. [PubMed] [Google Scholar]
- Morisaki N., Lindsey J. A., Stitts J. M., Zhang H., Cornwell D. G. Fatty acid metabolism and cell proliferation. V. Evaluation of pathways for the generation of lipid peroxides. Lipids. 1984 Jun;19(6):381–394. doi: 10.1007/BF02537399. [DOI] [PubMed] [Google Scholar]
- Morisaki N., Sprecher H., Milo G. E., Cornwell D. G. Fatty acid specificity in the inhibition of cell proliferation and its relationship to lipid peroxidation and prostaglandin biosynthesis. Lipids. 1982 Dec;17(12):893–899. doi: 10.1007/BF02534584. [DOI] [PubMed] [Google Scholar]
- Morisaki N., Yokote K., Saito Y. Atherosclerosis from a viewpoint of arterial wall cell function: relation to vitamin E. J Nutr Sci Vitaminol (Tokyo) 1992;Spec No:196–199. doi: 10.3177/jnsv.38.special_196. [DOI] [PubMed] [Google Scholar]
- Olivier A. R., Parker P. J. Bombesin, platelet-derived growth factor, and diacylglycerol induce selective membrane association and down-regulation of protein kinase C isotypes in Swiss 3T3 cells. J Biol Chem. 1994 Jan 28;269(4):2758–2763. [PubMed] [Google Scholar]
- Ozer N. K., Palozza P., Boscoboinik D., Azzi A. d-alpha-Tocopherol inhibits low density lipoprotein induced proliferation and protein kinase C activity in vascular smooth muscle cells. FEBS Lett. 1993 May 17;322(3):307–310. doi: 10.1016/0014-5793(93)81592-n. [DOI] [PubMed] [Google Scholar]
- Pears C., Stabel S., Cazaubon S., Parker P. J. Studies on the phosphorylation of protein kinase C-alpha. Biochem J. 1992 Apr 15;283(Pt 2):515–518. doi: 10.1042/bj2830515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Smooth muscle cells and the pathogenesis of the lesions of atherosclerosis. Br Heart J. 1993 Jan;69(1 Suppl):S30–S37. doi: 10.1136/hrt.69.1_suppl.s30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao G. N., Berk B. C. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res. 1992 Mar;70(3):593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- Rao G. N., Lasségue B., Griendling K. K., Alexander R. W., Berk B. C. Hydrogen peroxide-induced c-fos expression is mediated by arachidonic acid release: role of protein kinase C. Nucleic Acids Res. 1993 Mar 11;21(5):1259–1263. doi: 10.1093/nar/21.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemersma R. A., Wood D. A., Macintyre C. C., Elton R. A., Gey K. F., Oliver M. F. Risk of angina pectoris and plasma concentrations of vitamins A, C, and E and carotene. Lancet. 1991 Jan 5;337(8732):1–5. doi: 10.1016/0140-6736(91)93327-6. [DOI] [PubMed] [Google Scholar]
- Rimm E. B., Stampfer M. J., Ascherio A., Giovannucci E., Colditz G. A., Willett W. C. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med. 1993 May 20;328(20):1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Berk B. C., Gravanis M. B., Santoian E. C., Cipolla G. D., Tarazona N., Lassegue B., King S. B., 3rd Probucol decreases neointimal formation in a swine model of coronary artery balloon injury. A possible role for antioxidants in restenosis. Circulation. 1993 Aug;88(2):628–637. doi: 10.1161/01.cir.88.2.628. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Heimark R. L., Majesky M. W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990 Oct;70(4):1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- Stampfer M. J., Hennekens C. H., Manson J. E., Colditz G. A., Rosner B., Willett W. C. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993 May 20;328(20):1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- Stocker R., Bowry V. W., Frei B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does alpha-tocopherol. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1646–1650. doi: 10.1073/pnas.88.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber M. G., Rudel L. L., Burton G. W., Hughes L., Ingold K. U., Kayden H. J. Nascent VLDL from liver perfusions of cynomolgus monkeys are preferentially enriched in RRR- compared with SRR-alpha-tocopherol: studies using deuterated tocopherols. J Lipid Res. 1990 Apr;31(4):687–694. [PubMed] [Google Scholar]
- Walter G., Mumby M. Protein serine/threonine phosphatases and cell transformation. Biochim Biophys Acta. 1993 Aug 23;1155(2):207–226. doi: 10.1016/0304-419x(93)90005-w. [DOI] [PubMed] [Google Scholar]