Abstract
Objectives:
There is growing evidence that pituitary adenylate cyclase–activating polypeptide (PACAP) is associated with Alzheimer disease (AD) pathology in animal models, but human studies are needed.
Methods:
We studied the brains of patients with pathologically confirmed late-onset AD and age-matched cognitively normal (CN) subjects to investigate the expression of PACAP messenger RNA (34 AD and 14 CN) and protein (12 AD and 11 CN) in a case-control study.
Results:
We report that PACAP levels are reduced in multiple brain regions, including the entorhinal cortex, the middle temporal gyrus, the superior frontal gyrus, and the primary visual cortex. This reduction is correlated with higher amyloid burden (CERAD plaque density) in the entorhinal cortex and superior frontal gyrus but not in the primary visual cortex, a region spared in most cases of AD. PACAP expression is lower in advanced Braak stages (V and VI) than in moderate stages (III and IV). Increased PACAP levels are associated with decreased scores on the Dementia Rating Scale, a global cognitive measure. Finally, CSF levels paralleled brain levels in AD but not in Parkinson dementia or frontotemporal dementia brains.
Conclusions:
The close relationship between PACAP reduction and the severity of AD pathology suggests that downregulation of PACAP may contribute to AD pathogenesis.
Pituitary adenylate cyclase–activating polypeptide (PACAP) is a potent neurotrophic and neuroprotective peptide that binds to and activates G protein–coupled receptors.1 In a gene expression survey study using 3 different mouse models of Alzheimer disease (AD), PACAP was one of the 3 downregulated genes.2 Recent studies demonstrate that PACAP can stimulate nonamyloidogenic processing, inhibit amyloid deposition, facilitate β-amyloid clearance, and improve cognitive performance in amyloid precursor protein transgenic mice.3 PACAP can also promote synaptic transmission, long-term potentiation, and memory performance under physiologic conditions.4 However, the relevance of PACAP expression has not been studied in the human AD brain. We hypothesized that PACAP expression is reduced in human AD, and its reduction is associated with AD pathology and cognitive performance.
METHODS
We obtained postmortem human brains from the Banner Sun Health Research Institute Brain and Body Donation Program. Frozen brain tissue was obtained from patients with a clinical and pathologic diagnosis of late-onset AD and from age-matched cognitively normal (CN) subjects. Brain donors all underwent extensive longitudinal clinical and neuropsychological assessment antemortem, as previously described.5 We selected AD cases as being “intermediate” or “high” probability for AD according to National Institute on Aging–Reagan criteria (National Institute on Aging–Alzheimer's Association criteria).6 CN subjects did not meet the criteria for AD or dementia. The AD cases and controls did not differ significantly in their age at death, sex, educational level, or postmortem interval. A neuropathologist (T.G.B.) determined cortical β-amyloid neuritic plaque density (CERAD evaluation) and Braak tangle stage. We obtained postmortem cisternal CSF samples from the same cohort.
We quantified protein sample solution (RIPA buffer containing 1× proteinase inhibitor cocktails, P8340; Sigma-Aldrich, St. Louis, MO) and CSF (AD = 12 and CN = 11) for PACAP expression using a standard ELISA kit (MBS160511; MyBioSource Inc., San Diego, CA) according to the manufacturer's protocol. Briefly, we loaded samples and incubated them with biotin-labeled PACAP antibody at 37°C for 60 minutes. We washed the plate with the washing buffer 5 times, followed by incubation with the chromogen solution at 37°C for 10 minutes. We stopped the reaction by adding a stop solution. We read the final results at OB 450 nm. We compared the ELISA results of PACAP levels in the brain with the Western blot results. The PACAP level measured was in the middle range of the standard curve and correlated closely with the Western blot results (CN: Pearson r = 0.9093, p < 0.01; AD: Pearson r = 0.8243, p < 0.01; data not shown).
We used another set of postmortem brain samples (AD = 34, CN = 14) for transcriptome study as described previously.7 Briefly, we stained brain sections with a combination of thioflavin-S (Sigma-Aldrich, Dallas, TX) and 1% neutral red (Fisher Scientific, Chicago, IL). We identified and laser-captured pyramidal neurons onto Arcturus CapSure Macro LCM Caps and extracted according to the manufacturer's protocol. We isolated total RNA from the neuronal cell lysate with the Arcturus PicoPure RNA Isolation Kit with DNase I treatment using Qiagen's RNase-free DNase Set (Valencia, CA). Isolated total RNA from each sample of approximately 500 neurons was double-round amplified, cleaned, and biotin-labeled with Affymetrix's GeneChip per the manufacturer's protocol. Amplified and labeled complementary RNA was quantified on a spectrophotometer and run on a 1% Tris-acetate-EDTA (TAE) gel to check for an evenly distributed range of transcript sizes.
We used t tests to compare the values of the 2 groups. For comparing values across multiple groups, we used a one-way analysis of variance with post hoc Tukey test. We applied Pearson correlation tests for correlation analyses. We reported all results as mean ± standard error and set p < 0.05 as statistically significant.
Standard protocol approvals, registrations, and patient consents.
We received approval from an ethical standards committee on human experimentation (the Western Institutional Review Board) for any experiments using human subjects. Written informed consent was obtained from all patients (or guardians of patients) participating in the study (consent for research).
RESULTS
PACAP is reduced in multiple areas of human AD brain.
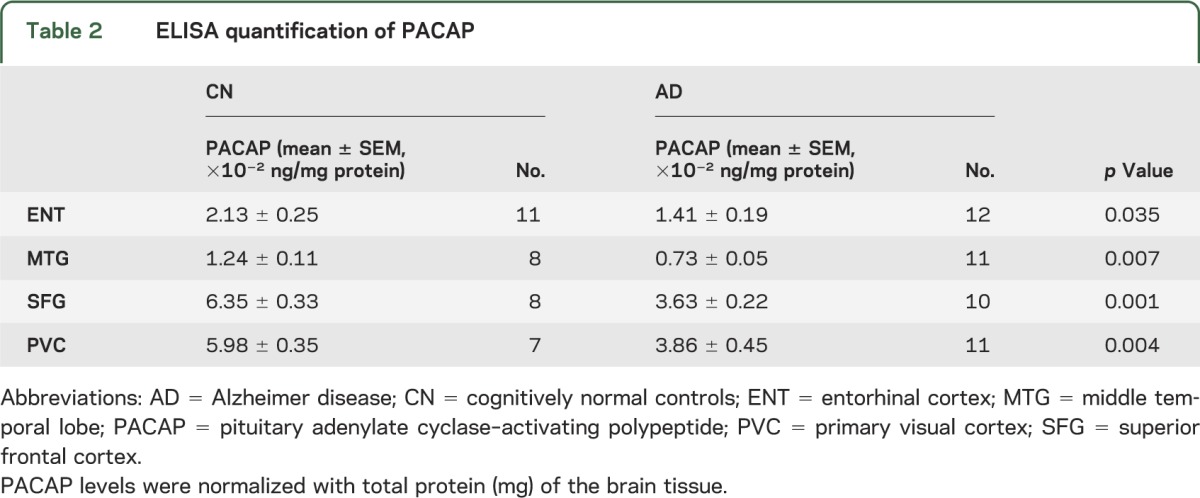
Neurons were laser-captured and microdissected from multiple brain regions of AD and CN subjects. Neuronal expression of ADCYAP1 (the PACAP gene) was significantly reduced in AD brains overall, and regionally in the middle temporal gyrus (MTG), superior frontal gyrus (SFG), and primary visual cortex (PVC) (table 1). To validate this, we selected a different cohort of 12 cases of AD postmortem brain samples and 11 CN cases for neurotranscriptome-based screening. Using ELISA to quantify PACAP protein expression, PACAP protein levels were reduced in AD in the same 3 regions as well as the entorhinal cortex (ENT) (table 2).
Table 1.
Neurotranscriptome of ADCYAP1 gene expression
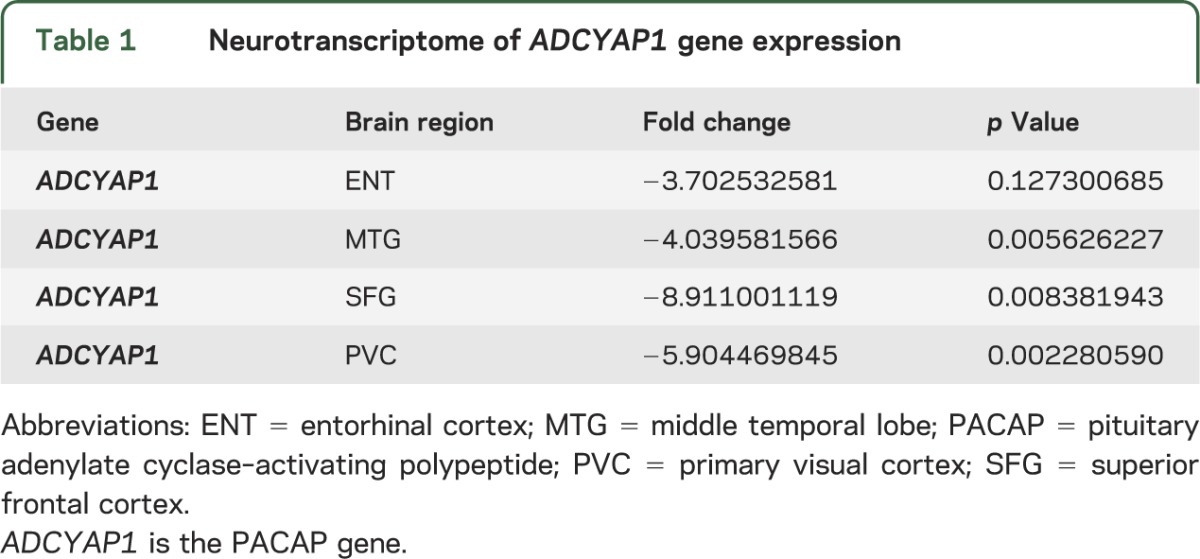
Table 2.
ELISA quantification of PACAP
PACAP reduction is associated with pathologic hallmarks of AD.
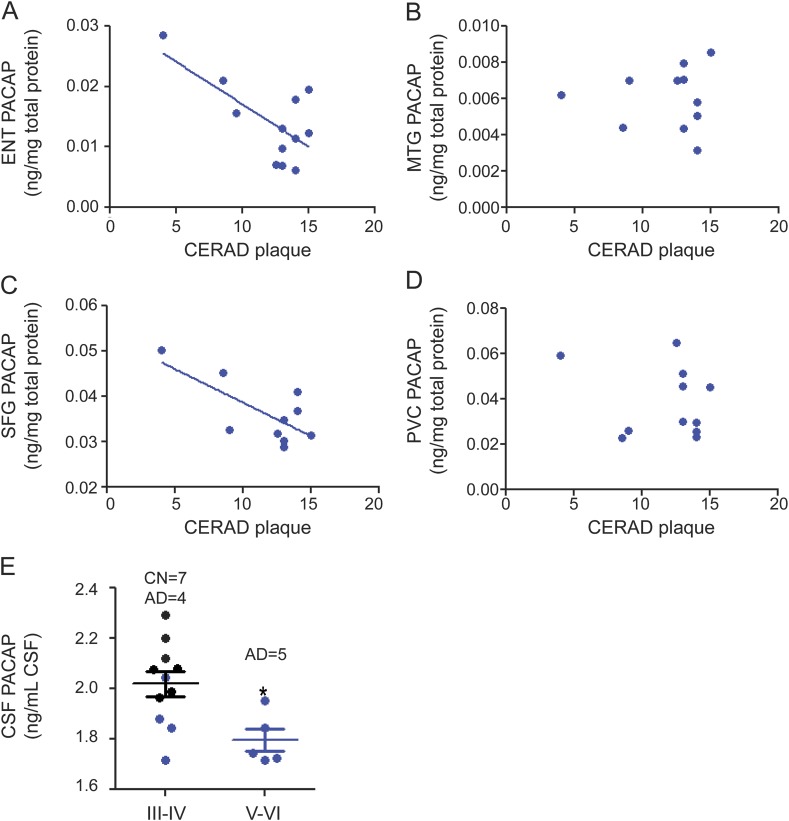
Amyloid plaques and neurofibrillary tangles are the 2 pathologic hallmarks of AD. PACAP protein levels were reduced with higher CERAD amyloid plaque scores in the ENT and SFG but not in the MTG or PVC (figure 1, A–D). Regarding neurofibrillary tangles, the AD cases had Braak stages ranging from IV to VI, whereas the stages in CN samples were III and IV. PACAP levels were reduced in Braak stages V and VI (all AD cases) compared with stages III and IV (figure 1E).
Figure 1. PACAP levels are inversely related to AD pathology.
(A–D) PACAP levels in AD brains were analyzed for correlation with amyloid plaque quantity (indicated by CERAD plaque score). Lower PACAP levels were correlated with higher CERAD in the ENT (Pearson r = −0.6764, p < 0.05, panel A) and SFG (Pearson r = −0.7088, p < 0.05, panel C) but not in the MTG (B) or PVC (D). PACAP level was quantified by ELISA and normalized with total protein (mg) of the brain tissue. (E) PACAP level in CSF was quantified and correlated with tau pathology (indicated by Braak stage). All CN cases were in stage III or IV. Four AD cases were in stage III or IV, while the other 5 AD cases were in stage V or VI. PACAP was lower in advanced Braak stages V and VI than in moderate Braak stages III and IV (p < 0.05). PACAP level was quantified by ELISA and normalized with CSF volume (mL). AD = Alzheimer disease (blue dot); CERAD = Consortium to Establish a Registry for Alzheimer's Disease; CN = cognitively normal controls (black dot); ENT = entorhinal cortex; MTG = middle temporal lobe; PACAP = pituitary adenylate cyclase–activating polypeptide; PVC = primary visual cortex; SFG = superior frontal cortex.
PACAP levels in CSF parallel brain levels.
PACAP concentration in AD CSF was reduced relative to CN CSF (figure 2A). In contrast, CSF PACAP levels from patients with Parkinson disease with dementia (PDD) and frontotemporal lobar degeneration were similar to those of CN subjects (figure 2A). CSF PACAP concentrations in patients with AD strongly correlated with Mattis Dementia Rating Scale–Revised scores, a measure of global cognitive functioning (figure 2B).
Figure 2. Reduction in PACAP levels in CSF is specific to AD.
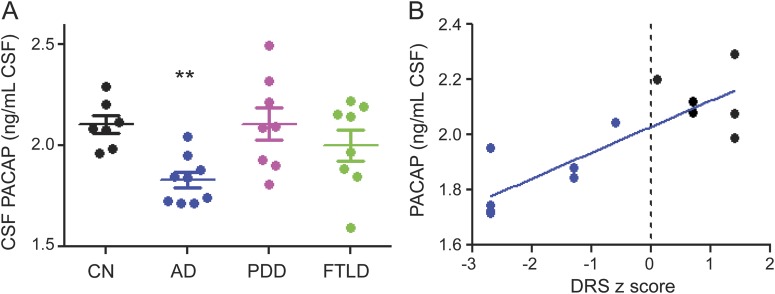
PACAP levels were quantified by ELISA as ng PACAP per mL undiluted CSF. (A) PACAP was reduced in AD but not in PDD or FTLD. (B) PACAP is correlated with the z score of the DRS (Pearson r = 0.8197, p = 0.0001). CN and AD cases were separated by the dotted line based on their DRS-R score. AD = Alzheimer disease (blue dot); CN = cognitively normal controls (black dot); DRS-R = Dementia Rating Scale–Revised, a global cognitive assessment; FTLD = frontotemporal lobe dementia (green dot); PACAP = pituitary adenylate cyclase–activating polypeptide; PDD = Parkinson disease with dementia (purple dot). *p < 0.05; **p < 0.01.
DISCUSSION
In the 4 brain areas we examined, both PACAP messenger RNA transcription and protein expression were significantly reduced in AD brains compared with matched CN controls. PACAP levels in the CSF were also significantly decreased in AD vs controls. Our findings suggest that the reduced neurotrophic effects from PACAP may be an important factor contributing to Alzheimer pathology.
The CERAD amyloid plaque burden was correlated with lower PACAP expression in the ENT and SFG but not the MTG or PVC. Although the lack of correlation with the MTG was surprising, the PVC is often relatively spared (save in the variant syndrome of posterior cortical atrophy). The involvement of neurofibrillary tangles in AD follows the progression from the ENT to the temporal lobe and then to the distal neocortex. AD cases had higher Braak stages but lower PACAP levels, suggesting that PACAP could reflect tau pathology.
PACAP is an abundant neurotrophin in the brain. PACAP levels in the CSF may be a good surrogate for diagnosis and monitoring the disease progression because CSF is more readily available than brain biopsy. PACAP levels in the CSF are reduced in AD but not in PDD or frontotemporal lobar degeneration, suggesting that it may also be specific for AD, consistent with the close relationship between PACAP and pathologic markers of AD.
PACAP is reduced in the cortical regions that typically characterize the topography of AD pathology. In addition to its role as an effective biomarker, PACAP may have potential as a therapeutic target as has been shown in animal models of AD. Translation of PACAP research to human subjects may provide us with not only new early biomarkers but also exciting therapeutic options for AD. Additional studies are needed to clarify the nature of the relationship between PACAP reductions and the neuropathologic features of AD and the extent to which it might provide a target for therapeutic interventions.
ACKNOWLEDGMENT
The authors are deeply grateful for the patients and families who have participated in our brain donation program. The authors thank Dr. Geidy Serrano for preparing the human pathologic tissues.
GLOSSARY
- AD
Alzheimer disease
- CERAD
Consortium to Establish a Registry for Alzheimer's Disease
- CN
cognitively normal
- ENT
entorhinal cortex
- MTG
middle temporal gyrus
- PACAP
pituitary adenylate cyclase–activating polypeptide
- PDD
Parkinson disease with dementia
- PVC
primary visual cortex
- SFG
superior frontal gyrus
AUTHOR CONTRIBUTIONS
Dr. Han: drafting and revising the manuscript, experiment design, and data acquisition and analysis. Dr. Liang: experiment design of the transcriptome part and critical revisions. Dr. Baxter: neuropsychologic data analysis and critical revisions. Dr. Yin and Dr. Tang: data acquisition and revision. Dr. Beach: neuropathologic examination, and data interpretation and revision. Dr. Caselli and Dr. Reiman: data interpretation and critical revision. Dr. Shi: concept and design, data analysis and interpretation, and critical revision.
STUDY FUNDING
Supported by Arizona Alzheimer's Disease Consortium and Barrow Neurological Foundation.
DISCLOSURE
P. Han and W. Liang report no disclosures relevant to the manuscript. L. Baxter receives funding from Arizona Alzheimer's Disease Consortium and Barrow Neurological Foundation. J. Yin and Z. Tang report no disclosures relevant to the manuscript. T. Beach receives grant support from the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson's Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer's Disease Core Center), and the Michael J. Fox Foundation for Parkinson's Research. He performs research services (payments to the institute, not to himself) as part of contractual agreements with Avid Radiopharmaceuticals/Eli Lilly Corporation, Bayer Healthcare, GE Healthcare, and Navidea Healthcare. R. Caselli receives research support from the NIH/National Institute on Aging and the Arizona Alzheimer's Research Consortium. E. Reiman reports serving as a scientific advisor to SYGNIS, AstraZeneca, Bayer, Eisai, Elan, Eli Lilly, GlaxoSmithKline, Intellect, Link Medicine, Novartis, Siemens, and Takeda. He has had research contracts with AstraZeneca and Avid/Eli Lilly; a patent pending for a biomarker strategy to evaluate preclinical AD treatments (through Banner Health); and research grants from NIH (National Institute on Aging), Anonymous Foundation, Nomis Foundation, Banner Alzheimer's Foundation, and the State of Arizona. J. Shi receives funding from Arizona Alzheimer's Disease Consortium and Barrow Neurological Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Vaudry D, Falluel-Morel A, Bourgault S, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 2009;61:283–357 [DOI] [PubMed] [Google Scholar]
- 2.Wu ZL, Ciallella JR, Flood DG, O'Kane TM, Bozyczko-Coyne D, Savage MJ. Comparative analysis of cortical gene expression in mouse models of Alzheimer's disease. Neurobiol Aging 2006;27:377–386 [DOI] [PubMed] [Google Scholar]
- 3.Rat D, Schmitt U, Tippmann F, et al. Neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) slows down Alzheimer's disease-like pathology in amyloid precursor protein-transgenic mice. FASEB J 2011;25:3208–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberto M, Brunelli M. PACAP-38 enhances excitatory synaptic transmission in the rat hippocampal CA1 region. Learn Mem 2000;7:303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caselli RJ, Walker D, Sue L, Sabbagh M, Beach T. Amyloid load in nondemented brains correlates with APOE e4. Neurosci Lett 2010;473:168–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging 1997;18:S1–S2 [PubMed] [Google Scholar]
- 7.Liang WS, Dunckley T, Beach TG, et al. Altered neuronal gene expression in brain regions differentially affected by Alzheimer's disease: a reference data set. Physiol Genomics 2008;33:240–256 [DOI] [PMC free article] [PubMed] [Google Scholar]