Abstract
Objective:
To investigate whether impaired consciousness in partial seizures can usually be attributed to specific deficits in the content of consciousness or to a more general decrease in the overall level of consciousness.
Methods:
Prospective testing during partial seizures was performed in patients with epilepsy using the Responsiveness in Epilepsy Scale (n = 83 partial seizures, 30 patients). Results were compared with responsiveness scores in a cohort of patients with severe traumatic brain injury evaluated with the JFK Coma Recovery Scale–Revised (n = 552 test administrations, 184 patients).
Results:
Standardized testing during partial seizures reveals a bimodal scoring distribution, such that most patients were either fully impaired or relatively spared in their ability to respond on multiple cognitive tests. Seizures with impaired performance on initial test items remained consistently impaired on subsequent items, while other seizures showed spared performance throughout. In the comparison group, we found that scores of patients with brain injury were more evenly distributed across the full range in severity of impairment.
Conclusions:
Partial seizures can often be cleanly separated into those with vs without overall impaired responsiveness. Results from similar testing in a comparison group of patients with brain injury suggest that the bimodal nature of Responsiveness in Epilepsy Scale scores is not a result of scale bias but may be a finding unique to partial seizures. These findings support a model in which seizures either propagate or do not propagate to key structures that regulate overall arousal and thalamocortical function. Future investigations are needed to relate these behavioral findings to the physiology underlying impaired consciousness in partial seizures.
Impaired consciousness is a major cause of disability in people with epilepsy; however, the mechanisms for loss of consciousness remain incompletely understood. In other disorders of consciousness, patients often show deficits in both the overall level of consciousness and the specific cognitive functions comprising the content of consciousness.1–4 In partial seizures, recently termed focal seizures,5 it is not clear whether loss of responsiveness is usually attributable to selective deficits in contents of consciousness such as language, vision, or memory,6 or to a more general decrease in overall level of consciousness.7,8
Prior studies concerning responsiveness in partial epilepsy have relied mainly on retrospective and subjective methods.9–16 We recently developed the Responsiveness in Epilepsy Scale (RES), a prospective, standardized tool to assess patients during seizures, including tests of verbal, nonverbal, sensorimotor functions, and memory.17–19 Of note, initial results using the RES suggested that most partial seizures cause either severe global impairment of cognition or overall sparing and that intermediate or selective deficits are relatively uncommon.17,18
The goal of this study was to further investigate the precise nature of impaired consciousness in partial seizures. We examined the distribution of impairment scores during partial seizures compared with similar standardized testing in traumatic brain injury (TBI). Common domains of cognition were tested, allowing for direct comparison of performance outcomes of the 2 groups. We found a striking bimodal distribution in the severity of deficits in partial seizures but not in TBI, suggesting that this finding may reflect underlying physiology unique to partial seizures.
METHODS
Standard protocol approvals, registrations, and patient consents.
All procedures were approved by the Yale University Human Investigations Committee. Written informed consent was obtained for all participants.
Participants.
Patients with epilepsy were recruited with the following inclusion criteria: (1) admission to the inpatient Yale Epilepsy Video/EEG Monitoring Unit between June 2009 and November 2012; (2) age 7 years or older; and (3) ability and willingness to participate in behavioral testing during seizures. Exclusion criteria were as follows: (1) nonepileptic (psychogenic) events; (2) severe cognitive or motor impairment at baseline that prevented performance of the tasks (in practice; because RES items are relatively simple, this only led to exclusion of 1 or 2 patients per year); and (3) pregnancy. Video/EEG recordings were reviewed by neurologists specializing in epilepsy, and seizures were classified by established criteria.5,20 Only partial (focal) seizures were included (table e-1 on the Neurology® Web site at Neurology.org); primary or secondarily generalized seizures were excluded. Of note, the current cohort included 60 seizures from 26 patients that were previously analyzed through similar but distinct methods.17–19 Patients with structural brain abnormalities were included in the seizure cohort, including 8 patients with hippocampal atrophy or increased signal, 7 with cortical gliosis or poststroke encephalomalacia, 5 malformations of cortical development, 4 previous resections, and 1 brain tumor (table e-1).
We also analyzed responsiveness testing in a comparison group with disordered consciousness of a known etiology distinct from epilepsy. Patients with severe TBI were recruited as described previously,21 with the following inclusion criteria: (1) age 16 to 65 years; (2) receiving inpatient rehabilitation for nonpenetrating TBI sustained 4 to 16 weeks before enrollment; (3) vegetative or minimally conscious state, as indicated by a Disability Rating Scale score greater than 11; and (4) inability to follow commands consistently and engage in functional communication as assessed by the score on the JFK Coma Recovery Scale–Revised (CRS-R). Exclusion criteria were as follows: (1) disability related to CNS that predated TBI; (2) medical instability; (3) pregnancy; (4) more than one seizure in the previous month; and (5) prior treatment with or allergy to amantadine; or (6) serious renal injury. Neurobehavioral function was assessed for all patients at the time of enrollment (week 0) and again at week 4 and week 6. Patients were randomized to amantadine-treated and placebo groups as described previously21; however, for this study, we did not analyze treatment effects because the purpose in this study was only to obtain a group of patients with impaired or recovering consciousness after brain injury.
Standardized testing batteries.
The RES is a standardized prospective battery used to assess responsiveness during epileptic seizures.17,18 Test items evaluate orientation, receptive and expressive language, visual processing, and sensorimotor function (table e-2). RES test items were derived from the JFK CRS-R, a comprehensive, validated tool for the evaluation of patients with chronic disorders of consciousness.21,22 The JFK CRS-R consists of 6 subscales to evaluate auditory, visual, motor, oromotor, communication, and arousal functions.
RES testing was initiated by a trained bedside examiner at the clinical onset of each seizure event, and patients were given 7 to 9 seconds to respond to each item administered in a standard sequence.17,18 CRS-R items were also administered by a trained examiner, and patients with TBI were similarly allowed up to 10 seconds to respond to each item. In both RES and JFK CRS-R, subject responses are graded and assigned a score based on level of responsiveness.
Three closely related versions of the RES were used during this study. The original RES18 was used during the period from June 2009 to September 2011; RES-II17 was used from September 2011 to September 2012. The 2 versions are generally similar, including many of the same test items, but are administered in a different order to improve efficiency of testing and minimize administration errors.17 The most recent RES version used from September 2012 through November 2012 contains only minor further revisions consisting of an alternate version of the “Ear” command and interchange of the order of the “Mirror” and “Ball” items in the battery (table e-2).
Data analysis.
As described previously,17,18 all seizures were scored within 24 hours of testing based on video review and agreement of 2 reviewers. In the original RES, a few items (Memory encoding, Name, Place, Year) were scored from 0 to 5, others (Ball, Mirror) were scored from 0 to 3, and the remainder, as with RES-II, were scored from 0 to 4. To simplify analysis, all items were converted to the 0 to 4 scale using the same criteria as RES-II so that all items were scored consistently (table e-2).
RES testing commenced as soon as possible after seizure onset, with dedicated personnel sitting at bedside during inpatient monitoring, and the sequence of test items was repeated through seizure termination and into the postictal period.17,18 For consistency across seizures, we analyzed RES items only from the initial cycle of ictal testing. Items administered after seizure offset were excluded. Seizures often terminated before the completion of the first cycle, resulting in low numbers for some items, so items with insufficient data (n ≤ 5 ictal scores) were excluded. Therefore, scores from only 13 of the total 16 possible RES items were included in the final analysis.
For the comparison group, CRS-R scores were analyzed for each subject at weeks 0, 4, and 6 after enrollment, as described previously.21,22 We analyzed data from 3 of the 6 subscales for which item scoring was as similar as possible to RES, namely, the CRS-R oromotor/verbal, auditory, and motor scales. These scales tested, respectively, verbal responses, motor responses to verbal command, and nonverbal motor responses (table e-2).
Statistics were performed with JMP 9 for Windows (SAS Institute Inc., Cary, NC, 1989–2013). Bonferroni correction was applied using a 2-tailed t test at the p < 0.05 level. To determine the relationship between scores on different test items within test administrations in patients with partial seizure and patients with TBI, we analyzed the association frequency between item pairs using MATLAB (R2012a; The MathWorks Inc., Natick, MA) and displayed the results as 3-dimensional histograms. For example, if during an administration of RES a patient scored a 4 on the memory encoding task and a 3 on the pen naming task, then a point would be added at position 4, 3 on the plot for this pair of items. We then calculated frequency by dividing by all points obtained this way for all item pairs and test administrations and multiplying by 100%.
RESULTS
Eighty-three partial seizures were evaluated in 30 patients (16 male and 14 female; mean age 35.2, range 15–68 years) using the RES. Demographic and clinical information can be found in table e-1. Onset of RES testing began on average 31 ± 6.7 seconds (mean ± SEM) after seizure onset. Mean seizure duration was 115 ± 20.5 seconds, and mean duration of the first cycle of RES testing was 106 ± 13.4 seconds so that testing included most of the middle period of seizures. Nineteen patients had more than one seizure, and in this subgroup, there were 3.79 ± 0.55 seizures per patient (mean ± SEM). Level of impairment was relatively consistent for patients tested on multiple seizures; average RES scores within patients tended to vary by less than 1 point (on a 5-point scale) with an SD mean of 0.71 points. Test items administered in the postictal period were not included in the analysis.
We also obtained data from 184 patients with brain injury (133 male, 51 female; mean age 36.4, range 16–65 years) using the JFK CRS-R. Patients were in a vegetative (n = 64) or minimally conscious (n = 120) state at the time of enrollment, 4 to 16 weeks after injury.21
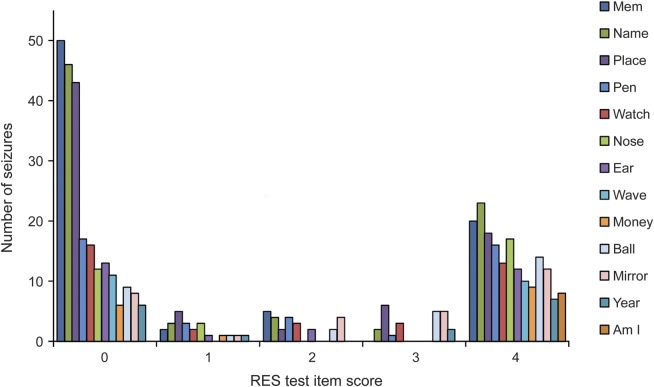
Partial seizure behavioral test scores are bimodally distributed.
During partial seizures, there was a striking bimodal distribution of behavioral performance (figure 1). A large proportion of ictal scores for all RES items showed values of either 0 (no response) or 4 (unimpaired response), with very few intermediate values. The severity of impairment thus appeared bimodal rather than graded. The bimodal distribution is also evident when results are analyzed by smaller subgroups of patients classified by temporal (n = 9) vs extratemporal (n = 4) or by right (n = 9) vs left (n = 6) hemisphere onset (figure e-1). In addition, this separate subgroup analysis reveals that patients with temporal lobe seizures or left hemisphere onset tended to have more severe impairment, as shown in prior studies.23 Of note, the RES includes both verbal and nonverbal test items (see table e-2).
Figure 1. Bimodal distribution of RES scores in partial seizures.
Ictal scores from the initial cycle of testing are shown for all partial seizures (n = 83). Scores show a bimodal distribution such that scores of 0 (no response) and 4 (unimpaired response) occur most frequently for all RES items. Names of RES test items are shown on the right and included both verbal and nonverbal items (see table e-2 for details). RES = Responsiveness in Epilepsy Scale.
Performance tends to be consistently impaired or spared in partial seizures.
A bimodal distribution could be attributed to consistently good or bad performance within each seizure, or potentially to behavior switching repeatedly between extremes of good and bad performance from item to item within each seizure. To distinguish between these possibilities, we plotted the time course of scores on consecutive items during seizures (figure 2). We sorted seizures into those with initially good (score 4) or bad (score 0) performance on the first test item. Seizures showing initial impairment tended to remain consistently impaired throughout the ictal period, and those with spared initial performance usually sustained good performance. Average scores for sequential test items were significantly better for the initially spared group compared with the initially impaired group at most time points during the seizure (figure 2) (p < 0.05, t test with Bonferroni correction).
Figure 2. Behavior on different test times remains consistently impaired or spared in partial seizures.
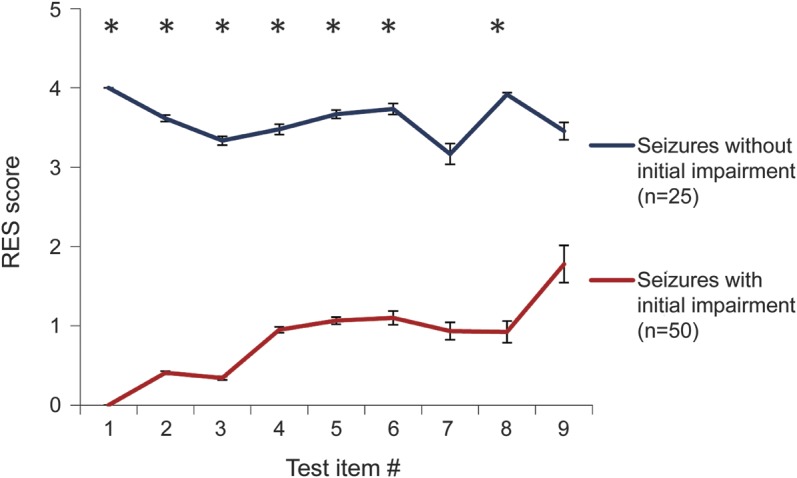
The majority of partial seizures tested received a score of 0 (no response) or 4 (unimpaired response) on the first RES question administered (n = 75). Only 8 seizures received an intermediate score of 1, 2, or 3 on the initial item and were therefore not included in this analysis. Initial impairment predicted impairment on subsequent items. The average scores on most questions differed significantly for the 2 groups (*p < 0.05, t test with Bonferroni correction). Note that “test item #” refers to the number of items administered from the onset of testing. Only ictal data points were included. Items beyond the ninth item had sample size ≤5 in one or both groups and were thus excluded from this analysis. RES = Responsiveness in Epilepsy Scale.
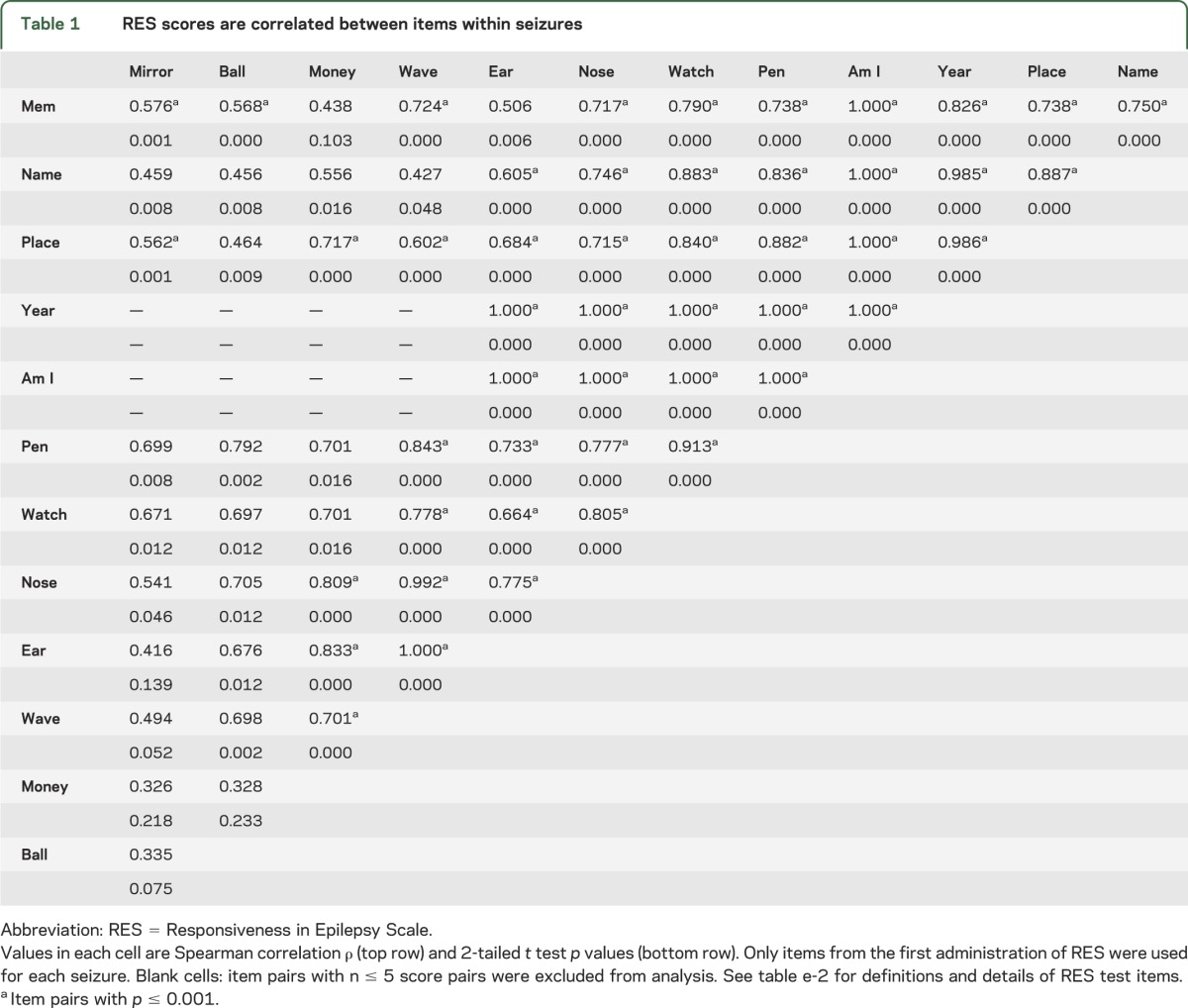
To further examine the similarity between scores on different test items within a single seizure, we performed a correlation analysis (table 1). The pairwise relationship between RES item scores within each seizure was analyzed across all seizures. Most item pairs were highly correlated (p ≤ 0.001), again suggesting that overall impaired or spared function is consistent across items within a given seizure.
Table 1.
RES scores are correlated between items within seizures
Comparison to JFK CRS-R scores in patients with brain injury.
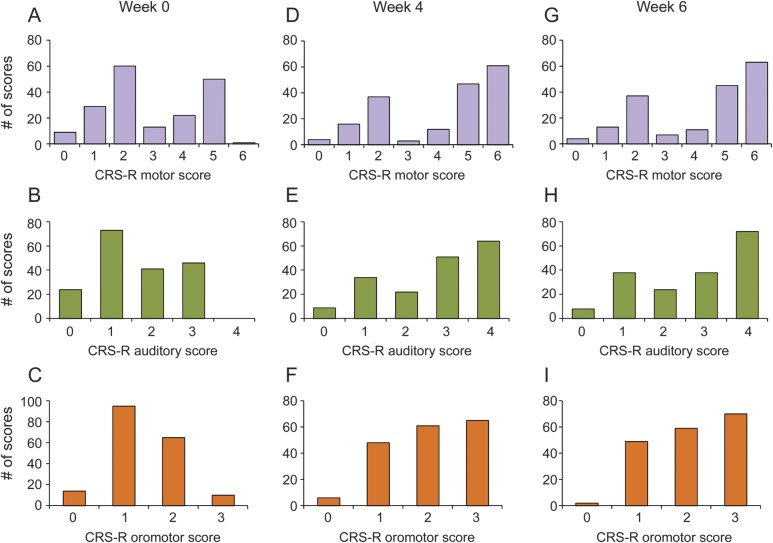
To determine whether the bimodal distribution of RES scores might be related to bias intrinsic to the scale, we examined the data distributions in patients with TBI for CRS-R subscales similar to RES (figure 3). In contrast to the bimodal distribution of ictal RES scores (see figure 1), we did not find a bimodal distribution of CRS-R scores in patients with TBI who had impaired consciousness. The distribution of scores at weeks 4 and 6 after enrollment showed some improvement (figure 3, D–I), with more patients showing maximal scores as they recovered, but unlike partial seizures, intermediate scores were common at all time points.
Figure 3. Distribution of CRS-R scores in the patients with brain injury.
Testing using the CRS-R testing battery of patients in the cohort with brain injury (n = 184) is shown for subscales that closely resemble RES testing items21 at week 0 (A–C), week 4 (D–F), and week 6 (G–I) after enrollment. Traumatic brain injury occurred in these patients 4 to 16 weeks before enrollment. In contrast to the bimodal distribution of ictal RES scores (see figure 1), we find a more even distribution of scores in patients with brain injury. For details of CRS-R test items and scoring, see table e-2. CRS-R = Coma Recovery Scale–Revised; RES = Responsiveness in Epilepsy Scale.
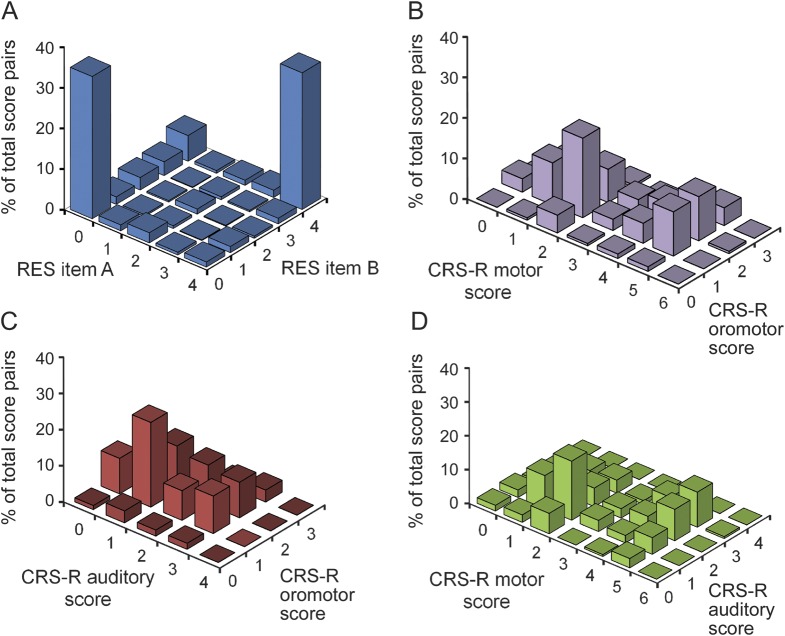
To further compare score distributions in partial seizures with TBI, we generated association frequency plots for both datasets (figure 4). These plots show the pairwise distribution of item scores for seizures (figure 4A) compared with traumatic brain injury (figure 4, B–D) (see the methods section). For a given administration of the scale, each item is paired to each other item (similar to table 1) and represented by a vertical point, so that score pairs occurring more frequently have greater vertical height. The bimodal distribution of partial seizure scores (figure 4A) is evident given that 4-4 and 0-0 are the most frequent RES item score pairs within seizures. In contrast, the associations between scores in patients with brain injury (figure 4, B–D) are more evenly distributed across different item score pairs. Data for patients with brain injury are shown only for week 0, but similar relatively even distribution plots were seen at weeks 4 and 6 as well (not shown).
Figure 4. Distribution of RES and CRS-R item pairs.
Pairwise distribution of item scores for the RES testing in all partial seizures (n = 83) and the CRS-R testing in patients with brain injury (n = 184) is shown. These association frequency plots show how often any given score on one item is associated with every possible score on a second item within the same test administration. (A) “RES item A” represents the first RES item in the pair and “RES item B” the second item (see table e-2 for details of RES test items). All possible item score pairs were plotted for each seizure. (B–D) CRS-R subscale scores at week 0 are paired in 3 combinations. Each vertical point represents the scores for a single pair comparison, and the percent of total score pairs is given on the y-axis. We found that the most frequent RES item pairs are 0-0 and 4-4 within seizures, while testing in patients with brain injury yields a wide range of subscale score pairs within test administrations. Because all RES items were scored on a scale of 0 to 4, the pairs between different items are displayed on a single plot (A). CRS-R items were scored on different scales (0–3, 0–4, and 0–6), so 3 plots are shown (B–D), one for each pair of CRS-R items. CRS-R = Coma Recovery Scale–Revised; RES = Responsiveness in Epilepsy Scale.
DISCUSSION
We found that standardized behavioral testing during partial seizures reveals a striking bimodal distribution of impairment, such that most partial seizures either fully spare or severely impair diverse cognitive functions. Furthermore, RES performance was consistent across different verbal and nonverbal tasks within a given seizure, and pairwise analysis of RES items revealed that scores were highly correlated within seizures. These data suggest that most partial seizures can be cleanly separated into those with broadly impaired or relatively spared responsiveness.
Similar behavioral testing in patients with impaired consciousness after TBI reveals a more even distribution of scores across multiple cognitive domains, consistent with prior studies.22,24,25 The cohort with brain injury serves as a reasonable comparison group, because prior studies suggest that complex partial seizures resemble the minimally conscious or vegetative states more closely than coma.8,19,26,27 We expect a similar pattern of graded impairment scores in other populations, such as poststroke patients or patients with encephalitis who should be investigated in further studies. Our current results suggest that the bimodal nature of ictal impairment is not the result of scale bias, but rather may be a finding unique to partial seizures and distinct from other disorders of consciousness.
Both the observed bimodal severity and global pervasiveness of deficits could be explained by physiologic properties of seizure propagation to key subcortical arousal structures. Findings from human neuroimaging,10 intracranial EEG,23,28 and animal models29,30 have led us to propose the network inhibition hypothesis,8,31 in which partial seizures propagate to subcortical structures and inhibit arousal. Decreased arousal and altered thalamocortical functioning then produces sleep-like slow waves and reduced metabolism in multiple cortical regions, resulting in a unique state of depressed consciousness.10,14,15,23,26,30,32 Our present behavioral results in which most partial seizures exhibit either global impaired or spared function are consistent with a model in which seizures either do or do not propagate to critical subcortical structures. As a result, partial seizures often involve a relatively abrupt and global transition between levels of consciousness,33 whereas other disorders of consciousness tend to impair arousal across a continuum. Of note, there are partial seizures that cause relatively isolated cognitive deficits, such as impaired language or visual processing34–36; in these seizures, impairment can be attributed to localized discharges that do not propagate to key arousal structures.
Although we found that partial seizures can cause broad deficits, it is important to note that these deficits mainly affect higher functions, whereas more rudimentary automatisms are often preserved.9 For example, visual tracking, blink to visual threat, or grasping movements are preserved in approximately half of complex partial seizures, but not in generalized tonic-clonic seizures.9,19
We acknowledge that there are limitations to our study. It is important to note that the duration of seizures is brief compared with other disorders of consciousness, such that slower test administration in TBI might allow more gradations in impairment to be detected. Nevertheless, it is unlikely that the bimodal distribution was entirely due to the transient nature of seizures for the following reasons: (1) the allowed response time interval of 10 seconds for items on the CRS-R for patients with TBI22 was similar to the typical response interval for items on RES of 7 to 9 seconds17,18; (2) scores on even single items, which can easily be administered within the time frame of a seizure, were bimodally distributed (figure 1); and (3) the bimodal spread of scores on successive items was not transient but sustained during the course of seizures (figure 2). Use of different testing batteries including the CRS-R and 3 versions of RES is an additional potential limitation. Although similar items and scoring were used, ideally an identical scale would be used in all cases for future comparative studies. Another potential concern is that RES testing often missed the first approximately 30 seconds of seizures, during which time ictal impairments might have occurred in a more graded rather than bimodal manner. Although this is certainly possible, a minimum of approximately 30 seconds' delay is a clinically realistic time before most patients are evaluated during seizures, making the present data clinically relevant for the majority of patients. An additional limitation includes the heterogeneity of the seizure cohort regarding anatomical localization and etiology, which were not accounted for in this study. Future behavioral testing in a larger population is needed to further characterize impairment by subgroups, including seizure localization and etiology types.
We have demonstrated that standardized and prospective behavioral testing during partial seizures reveals bimodal deficits across multiple cognitive domains, suggesting that partial seizures tend to cause fully impaired or mostly spared responsiveness. These results suggest that it is the level of consciousness that is most often affected during partial seizures. This hints that in partial seizures with impaired responsiveness, it is the disruption of arousal (vs specific cognitive deficits) that usually accounts for the patient's inability to attend to the surrounding environment. Together with recent advances in the physiologic understanding of impaired consciousness in epilepsy,10,14,16,23,29,32 these findings support the traditional clinical division between complex partial and simple partial seizures.20,37 We believe that this is an important distinction with implications both for patient safety and for improved treatments aimed at preventing impairment in partial seizures.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all of the study participants who made this research possible.
GLOSSARY
- CRS-R
Coma Recovery Scale–Revised
- RES
Responsiveness in Epilepsy Scale
- TBI
traumatic brain injury
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Courtney Cunningham acquired the data, performed statistical analysis, and wrote the manuscript. William C. Chen, Andrew Shorten, Michael McClurkin, and Tenzin Choezom acquired the data and performed statistical analysis. Christian P. Schmidt, Victoria Chu, Anne Bozik, Cameron Best, and Melissa Chapman acquired the data and participated in data analysis. Moran Furman and Kamil Detyniecki participated in data analysis. Joseph T. Giacino and Hal Blumenfeld designed the study and helped write the manuscript.
STUDY FUNDING
Supported by NIH R01 NS055829, Clinical and Translational Science Award (CTSA) UL1 RR0249139, National Institute on Disability and Rehabilitation Research (NIDRR) H133A031713, the Patrick and Catherine Weldon Donaghue Medical Research Foundation, and by the Betsy and Jonathan Blattmachr Family.
DISCLOSURE
C. Cunningham reports no disclosures relevant to the manuscript. W. Chen has received research support from NIH. A. Shorten, M. McClurkin, T. Choezom, C. Schmidt, V. Chu, A. Bozik, C. Best, and M. Chapman report no disclosures relevant to the manuscript. M. Furman has received research support from the Epilepsy Foundation of America. K. Detyniecki reports no disclosures relevant to the manuscript. J. Giacino has received research support from NIDRR. H. Blumenfeld has received research support from NIH, CTSA, the Patrick and Catherine Weldon Donaghue Medical Research Foundation, and the Betsy and Jonathan Blattmachr Family. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Laureys S, Tononi G. The Neurology of Consciousness: Cognitive Neuroscience and Neuropathology. New York: Academic Press; 2008 [Google Scholar]
- 2.Plum F, Posner JB. The Diagnosis of Stupor and Coma, 3rd ed Philadelphia: Davis; 1982 [Google Scholar]
- 3.Laureys S, Schiff ND. Disorders of Consciousness. Annals of the New York Academy of Sciences. Hoboken, NJ: Wiley-Blackwell; 2009 [DOI] [PubMed] [Google Scholar]
- 4.Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol 2004;3:537–546 [DOI] [PubMed] [Google Scholar]
- 5.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010;51:676–685 [DOI] [PubMed] [Google Scholar]
- 6.Blume WT, Luders HO, Mizrahi E, Tassinari C, van Emde Boas W, Engel J., Jr Glossary of descriptive terminology for ictal semiology: report of the ILAE Task Force on Classification and Terminology. Epilepsia 2001;42:1212–1218 [DOI] [PubMed] [Google Scholar]
- 7.Ali F, Rickards H, Cavanna AE. The assessment of consciousness during partial seizures. Epilepsy Behav 2012;23:98–102 [DOI] [PubMed] [Google Scholar]
- 8.Blumenfeld H. Impaired consciousness in epilepsy. Lancet Neurol 2012;11:814–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escueta AV, Bacsal FE, Treiman DM. Complex partial seizures on closed-circuit television and EEG: a study of 691 attacks in 79 patients. Ann Neurol 1982;11:292–300 [DOI] [PubMed] [Google Scholar]
- 10.Blumenfeld H, McNally KA, Vanderhill SD, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex 2004;14:892–902 [DOI] [PubMed] [Google Scholar]
- 11.Johanson M, Valli K, Revonsuo A. How to assess ictal consciousness? Behav Neurol 2011;24:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavanna AE, Mula M, Servo S, et al. Measuring the level and content of consciousness during epileptic seizures: the Ictal Consciousness Inventory. Epilepsy Behav 2008;13:184–188 [DOI] [PubMed] [Google Scholar]
- 13.Lee KH, Meador KJ, Park YD, et al. Pathophysiology of altered consciousness during seizures: subtraction SPECT study. Neurology 2002;59:841–846 [DOI] [PubMed] [Google Scholar]
- 14.Arthuis M, Valton L, Regis J, et al. Impaired consciousness during temporal lobe seizures is related to increased long-distance cortical-subcortical synchronization. Brain 2009;132:2091–2101 [DOI] [PubMed] [Google Scholar]
- 15.Guye M, Regis J, Tamura M, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain 2006;129:1917–1928 [DOI] [PubMed] [Google Scholar]
- 16.Lambert I, Arthuis M, McGonigal A, Wendling F, Bartolomei F. Alteration of global workspace during loss of consciousness: a study of parietal seizures. Epilepsia 2012;53:2104–2110 [DOI] [PubMed] [Google Scholar]
- 17.Bauerschmidt A, Koshkelashvili N, Ezeani CC, et al. Prospective assessment of ictal behavior using the revised Responsiveness in Epilepsy Scale (RES-II). Epilepsy Behav 2013;26:25–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L, Shklyar I, Lee HW, et al. Impaired consciousness in epilepsy investigated by a prospective Responsiveness in Epilepsy Scale (RES). Epilepsia 2012;53:437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McPherson A, Rojas L, Bauerschmidt A, et al. Testing for minimal consciousness in complex partial and generalized tonic-clonic seizures. Epilepsia 2012;53:e180–e183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International League Against Epilepsy. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1981;22:489–501 [DOI] [PubMed] [Google Scholar]
- 21.Giacino JT, Whyte J, Bagiella E, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med 2012;366:819–826 [DOI] [PubMed] [Google Scholar]
- 22.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale–Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004;85:2020–2029 [DOI] [PubMed] [Google Scholar]
- 23.Englot DJ, Yang L, Hamid H, et al. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain 2010;133:3764–3777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovstad M, Froslie KF, Giacino JT, Skandsen T, Anke A, Schanke AK. Reliability and diagnostic characteristics of the JFK Coma Recovery Scale–Revised: exploring the influence of rater's level of experience. J Head Trauma Rehabil 2010;25:349–356 [DOI] [PubMed] [Google Scholar]
- 25.Kalmar K, Giacino JT. The JFK Coma Recovery Scale–Revised. Neuropsychol Rehabil 2005;15:454–460 [DOI] [PubMed] [Google Scholar]
- 26.Blumenfeld H. Epilepsy and the consciousness system: transient vegetative state? Neurol Clin 2011;29:801–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology 2002;58:349–353 [DOI] [PubMed] [Google Scholar]
- 28.Blumenfeld H, Rivera M, McNally KA, Davis K, Spencer DD, Spencer SS. Ictal neocortical slowing in temporal lobe epilepsy. Neurology 2004;63:1015–1021 [DOI] [PubMed] [Google Scholar]
- 29.Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci 2009;29:13006–13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci 2008;28:9066–9081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norden AD, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav 2002;3:219–231 [DOI] [PubMed] [Google Scholar]
- 32.Motelow JE, Gummadavelli A, Zayyad Z, et al. Brainstem cholinergic and thalamic dysfunction during limbic seizures: possible mechanism for cortical slow oscillations and impaired consciousness. Soc Neurosci Abs. 2012. Available at: http://www.sfn.org. Accessed April 6, 2014
- 33.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature 2006;441:589–594 [DOI] [PubMed] [Google Scholar]
- 34.Herskovitz M, Schiller Y. Prolonged ictal aphasia: a diagnosis to consider. J Clin Neurosci 2012;19:1605–1606 [DOI] [PubMed] [Google Scholar]
- 35.Cavanna AE, Rickards H, Ali F. What makes a simple partial seizure complex? Epilepsy Behav 2011;22:651–658 [DOI] [PubMed] [Google Scholar]
- 36.Laff R, Mesad S, Devinsky O. Epileptic kinetopsia: ictal illusory motion perception. Neurology 2003;61:1262–1264 [DOI] [PubMed] [Google Scholar]
- 37.Blumenfeld H, Jackson GD. Should consciousness be considered in the classification of focal (partial) seizures? Epilepsia 2013;54:1125–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.