Abstract
Objective:
Few data describe antiretroviral treatment (ART)-related adverse events when treatment is initiated at CD4+ cell counts more than 350 cells/μl. We compared rates of laboratory-defined adverse events (LDAEs) according to CD4+ cell count at ART initiation.
Design:
Analysis of on-going cohort study.
Methods:
ART-naive persons initiating ART from 2000 to 2010 were included. Chi-square, analysis of variance (ANOVA) and Kruskal–Wallis tests compared characteristics among those starting ART with a CD4+ cell count of 350 or less, 351–499 and at least 500 cells/μl. Time-updated Poisson regression compared rates of LDAE in the three CD4+ cell strata. Cox proportional hazard models compared risk of ART discontinuation.
Results:
Nine thousand, four hundred and six individuals were included: median age 37 years, 61% white, 80% men, median viral load 4.8 log copies/ml. Four hundred and forty-seven (4.9%) and 1099 (11.7%) started ART with a CD4+ cell count at least 500 and 351–499 cells/μl, respectively. One thousand, two hundred and eighty-three (13.6%) patients experienced at least one LDAE. The rate of LDAE did not differ between those starting ART with a CD4+ cell count 351–499 and less than 350 cells/μl [relative rate 0.90, 95% confidence interval (CI) 0.74–1.09)], but an increased risk of ART discontinuation was observed (hazard ratio 1.58, 95% CI 1.10–2.27). Those starting ART at CD4+ cell count at least 500 cells/μl had an increased rate of LDAE (relative rate 1.44, 95% CI 1.13–1.82) but were not more likely to discontinue ART (hazard ratio 1.15, 95% CI 0.64–2.09).
Conclusion:
This study demonstrates the need to consider ART-related toxicities when initiating therapy at CD4+ cell counts at least 500 cells/μl. Whilst evidence from randomized controlled trials is awaited, the timing of ART initiation in terms of benefits and risks of ART remains an important question.
Keywords: biological markers, CD4+ lymphocyte count, drug toxicity, HAART, HIV
Introduction
The impact of antiretroviral therapy (ART) on viral transmission [1] and pragmatic programmatic recommendations have led to several HIV treatment guidelines committees now recommending ART at CD4+ cell counts more than 350 cells/μl [2–4]. Although public health benefits of earlier ART have recently been reported by the HPTN 052 study [1,5] and from observational studies within HIV endemic communities in Kwa-Zulu Natal [6], there remains insufficient randomized evidence on the risk:benefit ratio for the individual to support a shift to earlier ART initiation [7]. Currently, randomized controlled trial (RCT) evidence demonstrates a survival benefit of initiating ART at CD4+ cell count of 350 cells/μl or less compared with deferring to lower CD4+ cell count levels [8]. Well designed cohort studies have also shown morbidity and mortality benefits of ART at CD4+ cell counts more than 350 cells/μl with these effects appearing enhanced in the presence of comorbidities, coinfections and advancing age [9,10]. Other analyses, however, have not demonstrated clear survival benefits for asymptomatic HIV-positive individuals with CD4+ cell counts more than 500 cells/μl [11], and as such, possible survival benefits must be balanced against potential ART toxicities. It is hoped that the START and TEMPRANO trials (RCTs to investigate the risks and benefits to an individual of early ART initiation) will definitively address this particular question, but these will not report until 2015 [12,13]. In the meantime, data from observational cohorts provide valuable direction.
The UK Collaborative HIV Cohort (CHIC) Study is an observational study collecting data from many of the UK's largest HIV treatment centres [14]. We sought to determine the relative risk of developing severe (grade 3/4) [15] laboratory-defined adverse events (LDAEs) and rates of ART discontinuation among individuals with CD4+ cell counts of 350 or less, 351–499 and at least 500 cells/μl at ART initiation.
Materials and methods
Individuals were included in this study if they initiated ART between 2000 and 2010 at a UK CHIC centre that provided laboratory data. Those excluded were pregnant women, those with a viral load less than 50 copies HIV RNA/ml at ART initiation and those without at least one CD4+ cell count, viral load or laboratory measurement during follow-up. Pregnancy was established through linkage between the UK CHIC and National Study of HIV in Pregnancy and Childhood (NSHPC) databases [16]. Eligible individuals were followed until the date last seen at a UK CHIC centre or the earliest of drop-out of follow-up for at least 18 months; discontinuation of ART for at least 1 month; transfer to a centre not providing laboratory data; pregnancy.
LDAEs were defined as grade 3/4 adverse events [15] for any of alanine transaminase (ALT); aspartate transaminase (AST); alkaline phosphatase (ALP); albumin; creatinine; haemoglobin; platelets; amylase; cholesterol; glucose. Bilirubin was not considered a LDAE so as to exclude atazanavir-induced hyperbilirubinaemia. ART discontinuation was defined as stopping all ART for at least 1 month.
Statistical analysis
Baseline characteristics according to CD4+ cell count at ART start were compared using Chi-square and analysis of variance (ANOVA) or Kruskal–Wallis tests. Poisson regression compared LDAE rates according to CD4+ cell count at ART initiation, censoring follow-up at first LDAE. In sensitivity analyses, we excluded those with hepatitis B virus (HBV) or hepatitis C virus (HCV) coinfection and those who commenced nevirapine at CD4+ cell counts more than 350 cells/μl, as this is now contraindicated [17]. Further Poisson regression models considered rates of LDAE that were likely to be related to liver (ALT, AST, ALP, albumin), kidney (creatinine), blood (haemoglobin, platelets) and other body systems (amylase, cholesterol, glucose) separately. Individuals’ follow-up was split into 1-month intervals to create time-updated covariates for viral load, ART regimen, HBV/HCV status and time on ART. Time-independent covariates were sex, exposure category, ethnicity, age and previous experience of a LDAE at ART start. Time-updated Cox proportional hazards models investigated associations between CD4+ cell count and LDAE and the risk of discontinuing ART considering the same covariates as listed above as well as LDAE as a time-updated covariate. To avoid including those who discontinued treatment at high CD4+ cell counts as part of the SPARTAC study [18], we first only considered discontinuations more than 1 year after the start of ART, excluding those who attended main recruiting centres for the SPARTAC study. In addition, the analysis was performed considering all discontinuations occurring after ART initiation (including those in the first year) but restricting to the few centres that had no involvement in the SPARTAC study. All analyses were performed in SAS (version 9.3; SAS Institute Inc., Cary, North Carolina, USA).
Results
A total of 14 022 individuals commenced ART after 2000 in a centre that provided laboratory data. Of these, 3068 were excluded with viral load less than 50 copies/ml at ART start, 534 had no follow-up, 363 had no CD4+ cell count, 432 were pregnant and 219 had no laboratory measures. Excluded individuals were more likely to be female, of black or unknown ethnicity, and less likely to have acquired HIV through sex between men.
Of 9406 patients, 7860 (83.6%), 1099 (11.7%) and 447 (4.8%) started ART with a CD4+ cell count of 350 or less, 351–499 and at least 500 cells/μl, respectively. Those who started therapy with a CD4+ cell count more than 350 cells/μl were more likely to be white men who acquired HIV through sex with men. HBV coinfection was observed in 5.8% of those with a CD4+ cell count at least 500 cells/μl compared with 2.6 and 3% in the 351–499 and 350 cells/μl or less groups, respectively (P < 0.0001), whilst HCV coinfection was found in 4.5, 6.1 and 4.0%, respectively. Initial ART regimens differed across CD4+ cell strata, with those in the at least 500 cells/μl group more likely to begin a protease inhibitor-based regimen (P < 0.0001) (Table 1).
Table 1.
Patient characteristics at start of antiretroviral therapy, stratified by CD4+ cell count at start of antiretroviral therapy.
| CD4+ cell count at start of ART (cells/μl) | P | ||||
| All | <350 | 351–499 | >500 | ||
| N | 9406 | 7860 | 1099 | 447 | |
| Age, median (IQR) | |||||
| years | 37 (32–43) | 37 (32–43) | 38 (32–44) | 35 (30–42) | 0.016a |
| Sex, n (%) | |||||
| Male | 7511 (79.9) | 6147 (78.2) | 958 (87.2) | 406 (90.8) | <0.0001b |
| Ethnicity, n (%) | |||||
| White | 5707 (60.7) | 4586 (58.4) | 787 (71.6) | 334 (74.7) | <0.0001b |
| Black African | 2034 (21.6) | 1861 (23.7) | 131 (11.9) | 42 (9.4) | |
| Black other | 473 (5.0) | 404 (5.1) | 52 (4.7) | 17 (3.8) | |
| Other/unknown | 1192 (12.7) | 1009 (12.8) | 129 (11.7) | 54 (12.1) | |
| Mode of HIV acquisition, n (%) | |||||
| Homosexual/bisexual | 5666 (60.2) | 4518 (57.5) | 801 (72.9) | 347 (77.6) | <0.0001b |
| Heterosexual | 3019 (32.1) | 2739 (34.9) | 212 (19.3) | 68 (15.2) | |
| Other/unknown | 721 (7.7) | 603 (7.7) | 86 (7.8) | 32 (7.2) | |
| Hepatitis B coinfection, n (%) | |||||
| No | 5986 (63.6) | 4913 (62.5) | 775 (70.5) | 298 (66.7) | <0.0001b |
| Yes | 290 (3.1) | 235 (3.0) | 29 (2.6) | 26 (5.8) | |
| Not tested | 3130 (33.3) | 2712 (34.5) | 295 (26.8) | 123 (27.5) | |
| Hepatitis C coinfection, n (%) | |||||
| No | 6209 (66.0) | 5107 (65.0) | 784 (71.3) | 318 (71.1) | <0.0001b |
| Yes | 398 (4.2) | 311 (4.0) | 67 (6.1) | 20 (4.5) | |
| Not tested | 2799 (29.8) | 2442 (31.1) | 248 (22.6) | 109 (24.4) | |
| Viral load, median (IQR) | |||||
| log10 copies/ml | 4.8 (3.9–5.3) | 4.8 (4.1–5.3) | 4.4 (3.3–5.0) | 4.3 (3.0–5.0) | <0.0001c |
| Year of starting ART, n (%) | |||||
| 2000–2003 | 2325 (24.7) | 2035 (25.9) | 160 (14.6) | 130 (29.1) | <0.0001b |
| 2004–2007 | 3734 (39.7) | 3257 (41.4) | 339 (30.9) | 138 (30.9) | |
| 2008–2010 | 3347 (35.6) | 2568 (32.7) | 600 (54.6) | 179 (40.0) | |
| Regimen type, n (%) | |||||
| 2 NRTI and PI (/r) | 2221 (23.7) | 1752 (22.3) | 295 (26.8) | 174 (38.9) | <0.0001b |
| 2 NRTI and PI | 169 (1.8) | 141 (1.8) | 16 (1.5) | 12 (2.7) | |
| 2 NRTI and NNRTI | 6513 (69.2) | 5559 (70.7) | 718 (65.3) | 236 (52.8) | |
| ≥3 NRTI | 203 (2.2) | 173 (2.2) | 22 (2.0) | 8 (1.8) | |
| Other combination | 300 (3.2) | 235 (3.0) | 48 (4.4) | 17 (3.8) | |
| Previous adverse event | |||||
| Yes | 825 (8.8) | 679 (8.6) | 98 (8.9) | 48 (10.7) | 0.31b |
ART, antiretroviral therapy; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI (/r), protease inhibitor (with/without ritonavir). P = p-value of test of an overall difference between the 3 CD4+ cell count groups.
aAnalysis of variance (ANOVA) test.
bChi-square test.
cKruskal–Wallis test.
Rates of laboratory-defined adverse events
In 27 861 person-years of follow up, 1283 (13.6%) individuals experienced a LDAE. There were 1094, 113 and 76 individuals with a LDAE in those starting ART with a CD4+ cell count of 350 or less, 351–499 and at least 500 cells/μl giving incidence rates [95% confidence interval (95% CI)] of 4.6 (4.4–4.7), 4.9 (4.0–5.8) and 8.2 (6.4–10.1) per 100 person-years, respectively. Those who started ART with a CD4+ cell count at least 500 cells/μl had a higher rate of LDAE in both unadjusted and adjusted analyses (relative rate, 95% CI 1.44, 1.13–1.82) than in those commencing ART below 350 cells/μl (Fig. 1a). No differences in rates of LDAE were seen between those starting ART at CD4+ cell count 351–499 or 350 cells/μl or less (relative rate, 95% CI 0.90 (0.74–1.09). Excluding those with HBV/HCV coinfection or those who started a nevirapine regimen with a CD4+ cell count greater than 350 cells/μl did not alter our conclusions (results not shown).
Fig. 1.
Rate of laboratory-defined adverse events according to CD4+ cell count group at start of combination antiretroviral therapy.
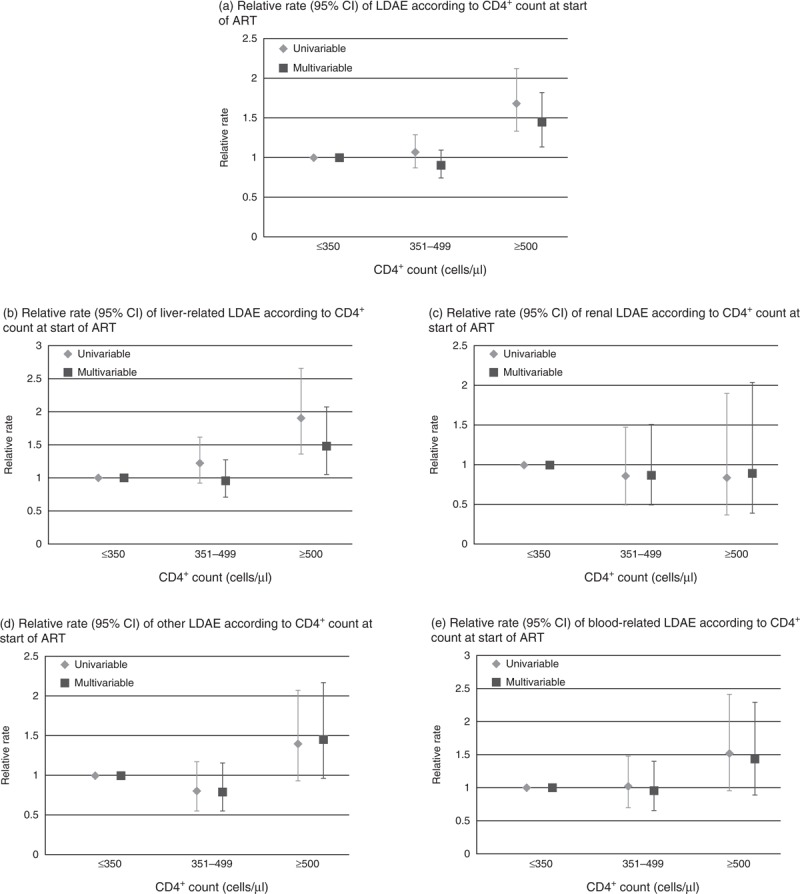
All univariable estimates adjusted for centre due to differences in testing practices. (a) Multivariable model adjusted for viral load, ART regimen, HBV coinfection, HCV coinfection, age, exposure, time on ART, centre. (b) Multivariable model adjusted for viral load, ART regimen, HBV coinfection, HCV coinfection, sex, ethnicity, time on ART, centre. (c) Multivariable model adjusted for viral load, antiretroviral regimen, ethnicity, age, centre. (d) Multivariable model adjusted for viral load, ART regimen, time on ART, exposure, age, centre. (e) Multivariable model adjusted for viral load, ART regimen, HBV coinfection, HCV coinfection, exposure, time on ART, centre. ART, antiretroviral therapy; CI, confidence interval; LDAE, laboratory-defined adverse event.
Those with a CD4+ cell count at least 500 cells/μl had a greater risk of liver-related abnormalities after adjustment for other factors (relative rate, 95% CI 1.45, 1.03–2.04). There was no evidence of more liver, kidney, blood or other LDAEs in the 351–499 cells/μl CD4+ cell count group compared to the 350 cells/μl or less CD4+ cell count group, in either unadjusted or adjusted analyses (Fig. 1).
Discontinuation of antiretroviral therapy
Of 4583 individuals included in the analysis of ART discontinuation after 1 year, 15.2, 14.2 and 18.6% of the 350 or less, 351–499 and at least 500 cells/μl CD4+ cell count groups discontinued ART, respectively. Those excluded from this analysis were more likely to be of unknown ethnicity, to have started ART since 2008 and to have a CD4+ cell count greater than 350 cells/μl. Neither CD4+ cell count nor LDAEs were associated with discontinuation of ART after 1 year (results not shown).
In 1746 individuals attending centres not involved in the SPARTAC study, 27.8, 28.6 and 31.3% of those who started ART with a CD4+ cell count 350 or less, 351–499 and at least 500 cells/μl discontinued ART. After adjustment for viral load, ART regimen, centre and exposure group, an increased risk of discontinuation was seen in those initiating ART with a CD4+ cell count 351–499 cells/μl (hazard ratio, 95% CI 1.59, 1.11–2.29) but not in those with a CD4+ cell count at least 500 cells/μl (1.16, 0.64–2.09). LDAE was not associated with ART discontinuation (0.77, 0.54–1.10) and did not explain the increased risk of discontinuation in those initiating ART with a CD4+ cell count 351–499 cells/μl, as the CD4+ cell effect estimates remained unchanged with the inclusion of LDAE in the model (hazard ratio, 95% CI 1.58, 1.10–2.27 351–499 cells/μl; 1.15, 0.64–2.09 ≥500 cells/μl).
Discussion
Individuals starting ART with a CD4+ cell count at least 500 cells/μl had a 44% increased risk of grade 3/4 LDAE compared with those initiating ART with a CD4+ cell count 350 cells/μl or less. This increased risk was largely explained by liver-test abnormalities. Higher rates of discontinuation were observed in those starting ART with a CD4+ cell count between 351 and 499 cells/μl in a small group of centres, but presence of a LDAE was not associated with ART discontinuation.
Despite adjusting for possible confounders, limitations persist in our analysis. Firstly, the indication for starting ART in those with a CD4+ cell count more than 350 cells/μl was not known. It is likely that many individuals starting ART with CD4+ cell counts at least 500 cells/μl did so due to the presence of comorbidities, which may independently cause laboratory abnormalities that would have been classified as LDAEs in our analyses. Although we were unable to capture much information on the presence of comorbidities at ART initiation, we did observe higher rates of HBV coinfection in those with CD4+ cell counts at least 500 cells/μl; however, it must be acknowledged that information on coinfection with HBV or HCV was unavailable for a large proportion of people. Adjustment for HBV/HCV coinfection and LDAE prior to ART initiation, to try and account for comorbidity, had no impact on the association between CD4+ cell count and LDAE. A possible explanation for the increased rate of liver-related LDAE is that HIV seroconverters may be overrepresented in the group initiating ART with a CD4+ cell count at least 500 cells/μl when elevated transaminases is common [19,20]. Exclusion of those who started a nevirapine-containing regimen at a CD4+ cell count more than 350 cells/μl did not alter our conclusions.
One concern of earlier ART initiation is the assumed ambivalence of asymptomatic HIV-positive individuals who start ART with high CD4+ cell counts who may be less inclined to remain adherent to ART in the long term. We saw no evidence that starting ART with a CD4+ cell count more than 350 cells/μl was associated with more treatment discontinuation after 1 year, but observed an increased risk of discontinuation at any time with a CD4+ cell count between 351 and 499 cells/μl in a small selection of centres. Reasons for ART discontinuation are not well reported in the UK CHIC study; however, this association did not appear to be explained by presence of an LDAE. The occurrence of grade 3/4 LDAEs was not independently associated with increased discontinuation rates, an observation in agreement with the recent publication of the UK Register of HIV Seroconverters [21].
ART guidelines may be moving towards universal treatment irrespective of CD4+ cell count. The individual risk of ART-related adverse events must be evaluated in the context of the potential benefits for that individual, considering comorbidities, age, sexual practices and long-term prognosis. Although we await evidence from ongoing RCTs regarding the potential risks and benefits of ART initiation with CD4+ cell counts at least 500 cells/μl, the potential for added adverse events in starting ART at high CD4+ cell counts must remain a key discussion with patients.
Acknowledgements
The UK CHIC Study is funded by the Medical Research Council, UK (Grant numbers G0000199, G0600337 and G0900274). The views expressed in this manuscript are those of the researchers and not necessarily those of the MRC.
UK CHIC Steering Committee includes Jonathan Ainsworth, Jane Anderson, Abdel Babiker, David Chadwick, Valerie Delpech, David Dunn, Martin Fisher, Brian Gazzard, Richard Gilson, Mark Gompels, Phillip Hay, Teresa Hill, Margaret Johnson, Stephen Kegg, Clifford Leen, Fabiola Martin, Mark Nelson, Chloe Orkin, Adrian Palfreeman, Andrew Phillips, Deenan Pillay, Frank Post, Caroline Sabin (PI), Memory Sachikonye, Achim Schwenk, John Walsh, Nicky Mackie, Alan Winston.
Central Co-ordination includes UCL Research Department of Infection & Population Health, Royal Free Campus, London (Teresa Hill, Susie Huntington, Sophie Jose, Andrew Phillips, Caroline Sabin, Alicia Thornton); Medical Research Council Clinical Trials Unit (MRC CTU), London (David Dunn, Adam Glabay).
Participating Centres include Barts and The London NHS Trust, London (C. Orkin, N. Garrett, J. Lynch, J. Hand, C. de Souza); Brighton and Sussex University Hospitals NHS Trust (M. Fisher, N. Perry, S. Tilbury, D. Churchill); Chelsea and Westminster Hospital NHS Trust, London (B. Gazzard, M. Nelson, M. Waxman, D. Asboe, S. Mandalia); Health Protection Agency – Centre for Infections London (HPA) (V. Delpech); Homerton University Hospital NHS Trust, London (J. Anderson, S. Munshi); King's College Hospital NHS Foundation Trust, London (H. Korat, M. Poulton, C. Taylor, Z. Gleisner, L. Campbell); Mortimer Market Centre, London (R. Gilson, N. Brima, I. Williams); North Middlesex University Hospital NHS Trust, London (A. Schwenk, J. Ainsworth, C. Wood, S. Miller); Royal Free NHS Trust and UCL Medical School, London (M. Johnson, M. Youle, F. Lampe, C. Smith, H. Grabowska, C. Chaloner, D. Puradiredja); St. Mary's Hospital, London (J. Walsh, J. Weber, F. Ramzan, N. Mackie, A. Winston); The Lothian University Hospitals NHS Trust, Edinburgh (C. Leen, A. Wilson); North Bristol NHS Trust (M. Gompels, S. Allan); University of Leicester NHS Trust (A. Palfreeman, A. Moore); South Tees Hospitals NHS Foundation Trust (D. Chadwick, K. Wakeman); St. George's NHS Trust (P. Hay, M. Dhillon); York (F. Martin, S. Douglas).
S.J. performed the analysis and helped prepare the manuscript. C.A.S. and S.F. designed the study and were involved in analysis and preparation of the manuscript. K.Q. was involved in discussions around study design and analysis and helped prepare the manuscript. T.H. prepared the data used for the study. As steering committee members for participating centres, P.H., F.A.P., R.G., M.N., M.G., M.F., M.J., D.C., C.L. advised on study concept and critically reviewed the analysis.
Conflicts of interest
S.J., T.H., K.Q. and C.L. have no conflicts of interest to declare. J.W. has received funds from Gilead sciences in connection with conference attendance. P.H. has received funding from Bristol Myers Squibb (BMS), Viiv, Gilead, Boehringer-Ingelheim, Merck and Johnson and Johnson in connection with conference attendance, speakers fees, consultancy, project grants and preparation of educational materials. M.F. has received funding from Gilead, BMS, Viiv, Abbott, Janssen and Merck in connection with project grants, speakers fees and conference attendance. FAP has received funding from BMS, Gilead, Viiv, Janssen in connection with speakers fees, project grants, conference attendance and preparation of educational materials. M.N. has received funding from Merck, Viiv, Gilead, Viiv and Abbott in connection with project grants, consultancy, speakers fees, conference attendance and preparation of educational materials. M.G. has received speakers fees from Janssen, BMS, Gilead, Viiv, Micropharma, CSL Behring. D.C. has received fees from BMS, Viiv and Gilead in connection with conference attendance and preparation of educational materials. R.G. has received funding from BMS, Gilead, Pfizer, Janssen, Roche and Merck in connection with project grants, conference attendance, speakers fees and membership of advisory boards. C.A.S. has received funding from Glaxo-Smith Klein, Janssen, Abbott, Gilead, BMS and Viiv in connection with speakers fees, membership of advisory boards and preparation of educational materials. S.F. has received grants from NIH, Gates, BRC and MRC. M.J. has received honoraria and research funding from VIIV Healthcare, Janssen-Cilag, Abbvie, Gilead Sciences, BMS and MSD.
References
- 1.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA 2012; 308:387–402 [DOI] [PubMed] [Google Scholar]
- 3.Back DJ, Khoo SH, Gibbons SE, Merry C. The role of therapeutic drug monitoring in treatment of HIV infection. Br J Clin Pharmacol 2001; 52 Suppl 1:89S–96S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html [Accessed 7 August 2013] [PubMed] [Google Scholar]
- 5.Grinsztejn B, Hosseinipour MC, Swindells S, Ribaudo H, Eron J, Chen YQ, et al. Effect of early versus delayed initiation of antiretroviral therapy (ART) on clinical outcomes in the HPTN 052 randomized clinical trial. XIX International AIDS Conference (AIDS 2012); 2012; Washington, DC [Google Scholar]
- 6.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science 2013; 339:966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabin CAC, David A. Collins, Simon; Schechter, Mauro Rating evidence in treatment guidelines: a case example of when to initiate combination antiretroviral therapy (cART) in HIV-positive asymptomatic persons. AIDS 2013; 27:1839–1846 [DOI] [PubMed] [Google Scholar]
- 8.El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283–2296 [DOI] [PubMed] [Google Scholar]
- 9.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 2009; 360:1815–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, Harris R, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet 2009; 373:1352–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cain LE, Logan R, Robins JM, Sterne JA, Sabin C, Bansi L, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med 2011; 154:509–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babiker AG, Emery S, Fatkenheuer G, Gordin FM, Grund B, Lundgren JD, et al. Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials 2013; 10:S5–S36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouattara E, Danel C, Moh R, Gabillard D, Peytavin G, Konan R, et al. Early upper digestive tract side effects of zidovudine with tenofovir plus emtricitabine in West African adults with high CD4 counts. J Int AIDS Soc 2013; 16:18059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UK Collaborative HIC Cohort Steering Committee The creation of a large UK-based multicentre cohort of HIV-infected individuals: The UK Collaborative HIV Cohort (UK CHIC) Study. HIV Med 2004; 5:115–124 [DOI] [PubMed] [Google Scholar]
- 15.National Institute of Allergy and Infectious Diseases (NIAID) Division of AIDS table for grading the severity of adult and pediatric adverse events. 2004; Bethesda, MD:National Institute of Allergy and Infectious Diseases, http://www3.niaid.nih.gov/LabsAndResources/resources/DAIDSClinRsrch/PDF/DAIDSAEGradingTable.pdfhttp://www3.niaid.nih.gov/LabsAndResources/resources/DAIDSClinRsrch/PDF/DAIDSAEGradingTable.pdf (Accessed 9 July 2012) [Google Scholar]
- 16.Townsend CL, Cortina-Borja M, Peckham CS, Tookey PA. Trends in management and outcome of pregnancies in HIV-infected women in the UK and Ireland, 1990–2006. BJOG 2008; 115:1078–1086 [DOI] [PubMed] [Google Scholar]
- 17.Stern JO, Robinson PA, Love J, Lanes S, Imperiale MS, Mayers DL. A comprehensive hepatic safety analysis of nevirapine in different populations of HIV infected patients. J Acquir Immune Defic Syndr 2003; 34 Suppl 1:S21–S33 [DOI] [PubMed] [Google Scholar]
- 18.Fidler S, Porter K, Ewings F, Frater J, Ramjee G, Cooper D, et al. Short-course antiretroviral therapy in primary HIV infection. N Engl J Med 2013; 368:207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lodi S, Phillips A, Touloumi G, Geskus R, Meyer L, Thiebaut R, et al. Time from human immunodeficiency virus seroconversion to reaching CD4+ cell count thresholds <200, <350, and <500 cells/mm(3): assessment of need following changes in treatment guidelines. Clin Infect Dis 2011; 53:817–825 [DOI] [PubMed] [Google Scholar]
- 20.Kahn JO, Walker BD. Acute human immunodeficiency virus type 1 infection. N Engl J Med 1998; 339:33–39 [DOI] [PubMed] [Google Scholar]
- 21.Lodi S, Phillips A, Fidler S, Hawkins D, Gilson R, McLean K, et al. on behalf of the UK Register of HIV Seroconverters Role of HIV infection duration and CD4 cell level at initiation of combination antiretroviral therapy on risk of virological failure. PLoS One 2013; 8:e75608. [DOI] [PMC free article] [PubMed] [Google Scholar]


