Abstract
Background
Experimentally upregulating compliant titins has been suggested as a therapeutic for lowering pathological diastolic stiffness levels. However, how increasing titin compliance impacts global cardiac function requires in-depth study. We investigate the effect of upregulating compliant titins in a novel mouse model with a genetically altered titin splicing factor; integrative approaches were used from intact cardiomyocyte mechanics to pressure(P)-volume(V) analysis and Doppler echocardiography.
Methods and Results
Compliant titins were upregulated through deletion of the RNA Recognition Motif of the splicing factor RBM20 (Rbm20ΔRRM mice). A genome-wide exon expression analysis and a candidate approach revealed that the phenotype is likely to be dominated by greatly increased lengths of titin’s spring-elements. At both cardiomyocyte and left ventricular (LV)chamber levels diastolic stiffness was reduced in heterozygous (+/−) Rbm20ΔRRM mice with a further reduction in homozygous (−/−) mice at only the intact myocyte level. Fibrosis was present in only −/− Rbm20ΔRRM hearts. The Frank-Starling Mechanism was reduced in a graded fashion in Rbm20ΔRRM mice, at both the cardiomyocyte and LV chamber levels. Exercise tests revealed an increase in exercise capacity in +/− mice.
Conclusions
Titin is not only important in diastolic but also in systolic cardiac function. Upregulating compliant titins reduces diastolic chamber stiffness due to increased compliance of myocytes but depresses end-systolic elastance; under conditions of exercise the beneficial effects on diastolic function dominate. Therapeutic manipulation of the RBM20-based splicing system might be able to minimize effects on fibrosis and systolic function while improving diastolic function of heart failure patients.
Keywords: diastole, titin, cardiomyocyte, contractility, physiology
Introduction
The giant myofilament titin has an I-band spanning segment that functions as a molecular spring that develops force when sarcomeres are stretched1. This molecular spring has a complex makeup that can be restructured through post-transcriptional mechanisms2 with an important role played by the recently discovered titin splicing factor RBM20 (RNA Binding Motif protein 20)3. Fetal cardiac titin has a very long spring segment; the adult cardiac N2B titin isoform has a short molecular spring and the adult N2BA isoform an intermediate length spring4, 5. It is well-established that passive stiffness of cardiomyocytes varies inversely with the length of titin’s spring segment. However, it is less well understood how this manifests at the level of the heart where chamber geometry and the extracellular matrix are also determinants of diastolic stiffness. Titin isoform switching will not only alter titin’s stiffness but could also impact other important properties of the heart. An example is the Frank-Starling Mechanism (FSM), the ability of the heart to increase contractile force in response to increased venous return6. At the cellular level the FSM manifests at a given submaximal Ca2+ level as an increase in length-dependent activation (LDA). Studies in which increased titin stiffness enhanced LDA suggest that titin plays a role in the FSM6. However, these studies were based on skinned myocyte/muscle experiments and integrative studies are needed to critically test whether the experimental findings are significant in-vivo.
In heart failure patients expression of the compliant N2BA titin isoforms is increased7–10 and this is considered a beneficial adaptation to counter fibrosis, but no experimental studies have addressed in detail the functional consequences of expressing titins with increased compliance on diastolic and systolic performance. These studies are important as it has been suggested that upregulating compliant titins might be a therapeutic approach for lowering diastolic stiffness in HFpEF (Heart Failure with preserved Ejection Fraction) patients7, 11. Hence, integrative studies on how increasing titin compliance affects global cardiac function are needed.
We engineered the Rbm20ΔRRM mouse model that expresses giant titins that are largest in homozygous (−/−) mice and intermediate in heterozygous (+/−) mice. This unique model was used to study how titin compliance affects cardiac function using innovative and integrative approaches. Experiments on loaded intact cardiac myocytes revealed that crossbridges contribute to diastolic stiffness but that titin is dominant with a graded stiffness reduction in Rbm20ΔRRM +/− and −/− mice. Diastolic stiffness of the LV chamber was reduced in +/− mice but with no additional reduction in −/− mice. The FSM was reduced in Rbm20ΔRRM mice, both at the intact myocyte and the LV chamber levels. Exercise tests in a cardiac-specific Rbm20ΔRRM +/− mouse suggest that up-regulating large titins and increasing diastolic compliance has beneficial and dominant effects on global cardiac function. Our results indicate that manipulating titin splicing and improving diastolic compliance should be explored as a therapeutic approach for lowering pathological stiffness levels in patients with HFpEF.
Methods
An expanded Methods section is available in the online Supplement Materials.
Generation of Rbm20ΔRRM Mice
Exons 6 and 7 were deleted from the Rbm20 mouse gene, causing an in-frame deletion of the RNA Recognition Motif (RRM), the Rbm20ΔRRM model, see Figs. S1A–C. We also made a cardiac-specific RRM deletion model in which exons 6 and 7 were flanked by LoxP sites (cRbm20ΔRRMflox model) Mice were on a C57BL/6 background and were 4 months old and male, unless indicated otherwise. All animal experiments were approved by the Institutional Animal Care and Use Committee and the NIH “Using Animals in Intramural Research“ guidelines. See Supplement Materials for details.
PROTEIN EXPRESSION, PHOSPHORYLATION AND EXPRESSION ANALYSIS
Protein expression analysis was performed using standard gel electrophoresis methods12. Phosphorylation was studied using ProQ staining and phospho-specific antibodies12. Titin exon expression, gene expression and quantitative RT-PCR (qPCR) were with routine methods (online Supplement).
HISTOLOGY
Histology with Picrosirius red (PSR) staining measured the collagen volume fraction in LV cross-sections.
CELL AND TISSUE MECHANICS
Skinned cardiac myocyte mechanics and intact myocyte mechanics were as in13. LDA was studied in skinned papillary muscle14 and ktr and tension cost were measured as in15.
IN-VIVO CHARACTERIZATION
In-vivo pressure-volume measurements used an admittance-based system in anesthetized mice16. Echocardiography was performed on anesthetized and conscious mice (online Supplemental Materials). Exercise tolerance was evaluated by measuring the maximal running speed in a treadmill running test12.
STATISTICS
Statistical analysis was performed in Graphpad Prism (GraphPad Software, Inc). A one-way ANOVA with a Bonferroni post-hoc analysis that calculates p-values corrected for multiple comparisons was performed to assess differences between genotypes. When non-normally distributed data were analyzed, a Mann-Whitney test was used when comparing two groups and a Kruskal-Wallis test when comparing three groups, in both cases data were also plotted in dot plots (appended as Supplemental Figures in the online Supplemental Materials). A repeated measure two-way ANOVA with a Tukey’s multiple comparison was used to assess differences in the stress test echocardiographic study and a t-test in the exercise study that compared two groups only. Results are shown as mean ± SEM. p<0.05 was taken as significant
Results
Rbm20ΔRRM model characterization
Both +/− and −/− Rbm20ΔRRM mice are viable, appear to have a normal life span, and +/− breeders have normal litter sizes (8–10) and produce genotypes at Mendelian ratios. Echocardiography did not show significant differences amongst the genotypes when studying anaesthetized 4 months (mo) old male or female mice (Table 1) or 20 mo old female mice (Tables S1a); blood pressures measured by tail-cuff were also not different (Table S1b). Morphometry revealed no differences (Table S2a), except for a significant reduction in heart weight and LV weight in female 4 mo old mice (Table S2a, right columns) but a significant difference was absent when these parameters were normalized to body weight (BW) and, furthermore, the findings were not present in 20 mo old female mice (Table S2b). LV chamber geometry was assessed by the eccentricity index (LV diameter in diastole/LV wall thickness in diastole); this index was not significantly different amongst the genotypes in 4 mo old mice anesthetized (Table 1) nor in mice that we followed out to 20 mo of age (Table S1a). An echo study on conscious mice also found no significant differences in LV chamber dimension or in other parameters, including heart rate (Table S1c). An exception was that in conscious −/− mice significant reductions in fractional shortening and stroke volume were detected (Table S1c). It is likely that absence of this effect in anesthetized mice is due to the reduction in contractility of +/+ mice induced by the anesthetic (highlighting the importance of including conscious echo in phenotyping studies). In summary, no consistent differences were found in chamber geometry and dimensions, contractility is not significantly different in +/− mice but is depressed in −/− mice.
Table 1.
Echocardiography of anesthetized male and female Rbm20ΔRRM mice.
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| +/+ (n=13) | +/− (n=14) | −/− (n=25) | +/+ (n=15) | +/− (n=14) | −/− (n=15) | |||
| Age (days) | 135.9±5.7 | 119.9±6.0 | 129.1±3.3 | ns | 117.2±4.4 | 121.7±3.1 | 118.8±6.5 | ns |
| BW (g) | 29.2±1.0 | 29.1±0.9 | 29.2±0.9 | ns | 21.8±0.8 | 21.5±0.6 | 22.3±0.9 | ns |
| HR (BPM) | 481±11 | 500±14 | 479±7 | ns | 457±10 | 482±14 | 485±17 | ns |
| LV-M mode protocol: | ||||||||
| LVID;d (mm) | 3.87±0.09 | 3.76±0.12 | 3.70±0.08 | ns | 3.35±0.07 | 3.27±0.07 | 3.36±0.05 | ns |
| WT;d (mm) | 0.78±0.03 | 0.76±0.03 | 0.79±0.02 | ns | 0.77±0.03 | 0.77±0.02 | 0.77±0.02 | ns |
| LVID;s (mm) | 2.50±0.12 | 2.45±0.16 | 2.43±0.11 | ns | 2.04±0.07 | 2.09±0.09 | 2.23±0.08 | ns |
| WT; s (mm) | 1.15±0.05 | 1.08±0.03 | 1.07±0.04 | ns | 1.12±0.03 | 1.07±0.04 | 1.08±0.03 | ns |
| Eccentricity | 5.05±0.23 | 4.99±0.24 | 4.81±0.22 | ns | 4.22±0.18 | 4.32±0.16 | 4.4±0.14 | ns |
| LA (mm) | 1.98±0.07 | 1.90±0.03 | 1.98±0.06 | ns | 1.68±0.04 | 1.77±0.06 | 1.83±0.05 | ns |
| LV Vol;d (ul) | 65.1±3.5 | 61.1±4.6 | 59.0±2.8 | ns | 46.4±2.2 | 43.8±2.4 | 46.3.±1.8 | ns |
| LV Vol;s (ul) | 23.4±2.6 | 22.7±3.8 | 22.5±2.1 | ns | 14.1±1.3 | 15.0±1.5 | 17.2±1.6 | ns |
| EF (%) | 64.8±3.1 | 63.5±4.1 | 63.6±2.3 | ns | 69.8±2.1 | 66.6±2.3 | 63.2±2.6 | ns |
| FS (%) | 35.6±2.3 | 34.8±3.2 | 34.9±2.0 | ns | 38.7±1.6 | 36.3±1.9 | 33.9±1.9 | ns |
| LVW (mg) | 86.1±4.1 | 80.4±5.6 | 79.9±2.3 | ns | 68.0±4.1 | 64.5±3.5 | 67.0±2.6 | ns |
Abbreviations: HR: heart rate; BPM: beats per minute; LV: left ventricle; LVIDd: left ventricular internal diastolic diameter; WTd: diastolic wall thickness; LVIDs; left ventricular internal systolic diameter; WTs: systolic wall thickness; Eccentricity: LVIDd/WTd; LA: left atrium; LV Vold: left ventricular diastolic volume; LV Vols: left ventricular systolic volume; EF: ejection fraction; FS: fractional shortening; LVW: left ventricular weight; NS no significant differences amongst genotypes (one-way ANOVA with a Bonferroni post-hoc analysis, p-values corrected for multiple comparisons).
Titin protein expression
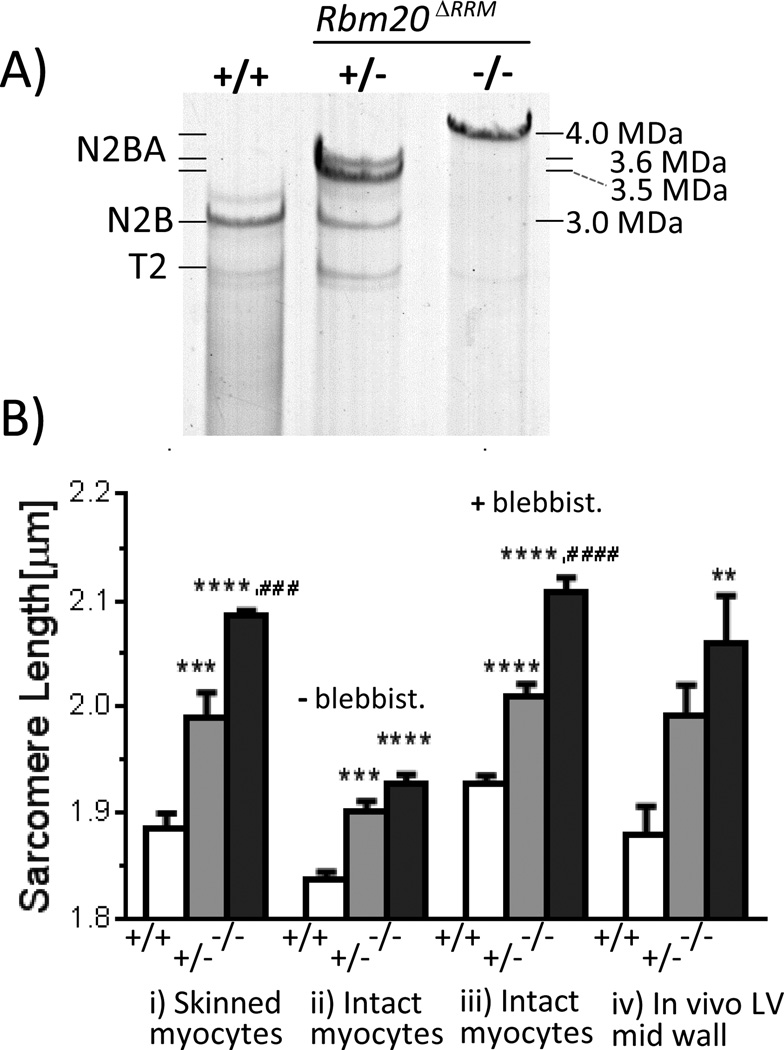
The LV of −/− mice expresses a single giant titin isoform with molecular mass estimated (as in17) at ~4.0 MDa (Fig. 1A). The +/− mice express a low level of the N2B isoform (this ~3.0 MDa isoform dominates in wild-type (+/+) mice) and two large isoforms estimated at ~3.5 and ~3.6 MDa(Fig. 1A). A titin exon expression analysis using a custom microarray17 found massive upregulation of exons that contribute to two titin spring elements: the serially linked immunoglobulin(Ig)-containing tandem Ig element (consisting of proximal, middle, and distal segments) and the proline(P), glutamate(E), valine(V), and lysine(K) rich PEVK element (Fig. S1D). Western blots show that the large titins in Rbm20ΔRRM mice contain many additional PEVK sequences and belong to the N2BA type (Figs. S1E–G).
Figure 1.
Titin expression in LV myocardium (A) and slack sarcomere lengths in LV cardiac myocytes (B). A) Rbm20ΔRRM mice express giant titin isoforms. Bi) Skinned cardiomyocytes in relaxing solution; Intact cardiomyocytes in absence (ii) and presence (iii) of blebbistatin. iv) In vivo LV mid-wall SL. Symbols: **p<0.01, ***p<0.001, ****p<0.0001 vs. wild-type (+/+); ####p<0.001, ####p<0.0001 vs. Het (+/−). See text for details. Bi: 114 cells/6 mice +/+, 145 cells/5 mice +/− and 149 cell/7 mice −/−; Bii: 71 cells/7 mice +/+, 74 cells/6 mice +/− and 91 cell/8 mice −/−; Biii: 20 cells/5 mice +/+, 22 cells/7 mice +/− and 27 cell/7 mice −/−; Biv: 138 muscle strips/6 mice +/+, 145 muscle strips/7 mice +/− and 148 muscle strips/7mice −/−.
Genome-wide exon expression analysis
To examine all genes that are alternatively spliced in Rbm20ΔRRM mice, a genome-wide exon expression analysis was performed using the mouse Exon 1.0ST Array (Affymetrix) platform, with results analyzed with the Exon Array Analyzer server and PLIER (See Methods). We found that exons 6 and 7 of RBM20 were differentially expressed (as expected) and that Ttn exons greatly dominated the differentially expressed exons, as indicated by the pie charts of exons affected in +/− or −/− Rbm20ΔRRM mice(Fig. S2a and Supplemental spreadsheets). Additionally we performed a PCR study with a candidate approach based on genes that have been reported to be differentially spliced in both the Rbm20 rat model and in patients with Rbm20 mutations3. Two additional genes were alternatively spliced, Ldb3/Cypher and CaMKIIδ(Fig.S2b). Western blot analysis with an antibody that recognizes all cardiac Cypher isoforms revealed two Cypher long isoforms (72 and 78 kDa) and one short (32 kDa) isoform18. Expression levels of the long isoforms were unaltered in Rbm20ΔRRM mice (Fig. S3-panel A) whereas the short isoform was significantly reduced with the largest reduction in −/− mice (Fig. S3-panel B). For CaMKIIδ we examined its expression level and studied the following CaMKIIδ downstream targets, cMyBP-C, PLB, and p53 and we measured Ca2+ transients in beating cardiocytes because they can be altered by changes in expression of CaMKIIδ19. None of the tested CaMKIIδ targets were altered in the Rbm20ΔRRM model (Fig. S2c) and neither were the Ca2+ transients (Figs. S4a and S4b).
Diastolic stiffness--Cardiac myocytes
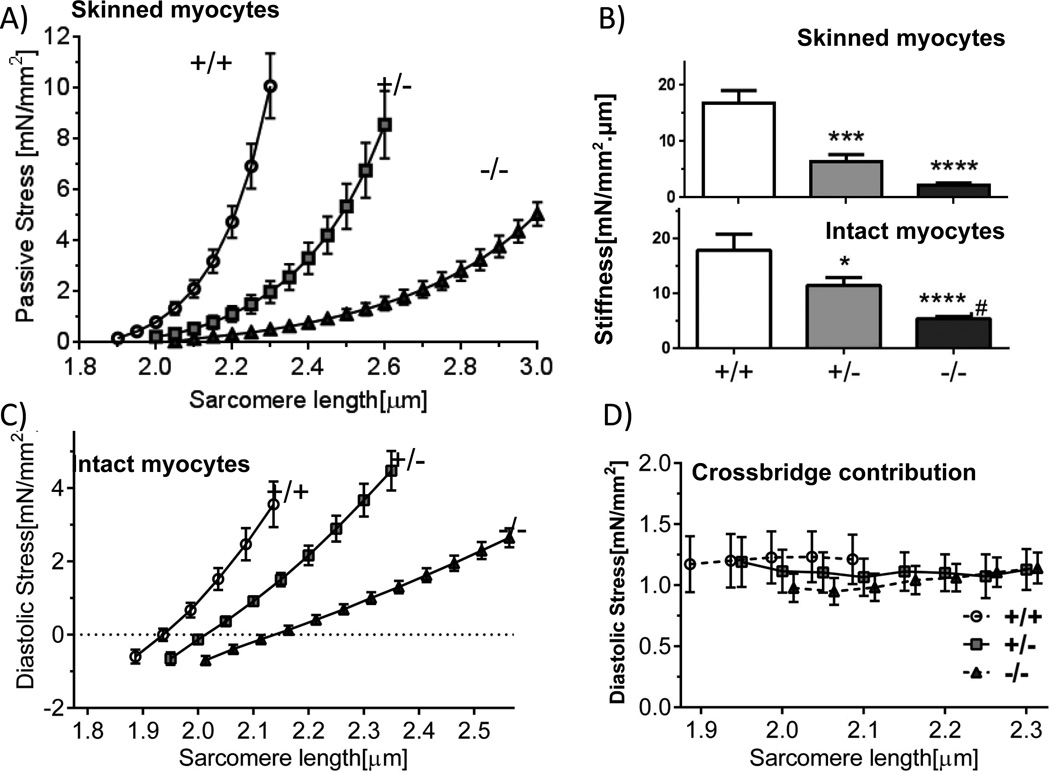
LV skinned cardiac myocytes in relaxing solution had longer slack sarcomere length (SL) in Rbm20ΔRRM cells than in +/+ cells, with the largest difference in −/− cells (Fig. 1Bi). When cells were stretched, passive stress was much less in Rbm20ΔRRM mice with the lowest levels in −/− mice (Fig. 2A). Passive stiffness (slope of stress-SL relation for the first 10% SL increases) was 87% less in −/− mice (relative to +/+) and 61% less in +/− mice (Fig. 2B, top).
Figure 2.
Effect of expressing large titin isoforms on cardiomyocyte stiffness. Passive stress (A) and passive stiffness (B, top) of skinned cells. Titin based diastolic stress (C) and stiffness (B, bottom) of intact cells in presence of blebbistatin. D) Magnitude of actomyosin-based diastolic stress (stress reduction in blebbistatin). Symbols: *p<0.05, ***p<0.001, ****p<0.0001 vs. wild-type (+/+); #p<0.05 vs. Het (+/−). A and B top: 12 cells/6 mice +/+, 10 cells/7mice +/− and 15 cells/7mice −/−; C, B bottom and D: 20 cells/5 mice +/+, 22 cells/7 mice +/− and 27 cells/7 mice −/−.
The slack SL of intact cardiac myoctes was less than that of skinned cells (Fig. 1Bii). This is likely due to residual actomyosin interaction13 as adding the actomyosin inhibitor blebbistatin increased the slack SL to that of skinned cells(Fig. 1Biii). Cardiomyocytes at their base length were attached to a force-measurement system13, cells were paced at 2Hz and diastolic and twitch stresses were measured (Fig. 4A, Methods). Cells were stretched during the diastolic interval to determine diastolic stress and blebbistatin was added to ensure measurement of titin-based diastolic stress. Titin-based diastolic stress was much less in Rbm20ΔRRM cells (Fig. 2C) and titin-based diastolic stiffness showed, relative to +/+ cells, a 67% reduction in −/− and 36% reduction in +/− cells (Fig. 2B, bottom). When intact cells held by carbon fibers at their base length were perfused with blebbistatin, actomyosin-based force that pulls on the Z-disks was abolished but the restoring stress remained and pushed outward on the carbon fibers, registering as a negative stress (negative values in Fig. 2C). Actomyosin-based diastolic stress was measured from the blebbistatin-based diastolic stress reduction. Actomyosin interaction contributed ~ 1.0 mN/mm2 to cellular diastolic stress and was SL- and genotype-independent (Fig. 2D). Overall these studies show that diastolic stress of cardiac myocytes has a low actomyosin contribution, that the main diastolic stress contributor is titin, and that diastolic stress is much reduced when large titin isoforms are expressed.
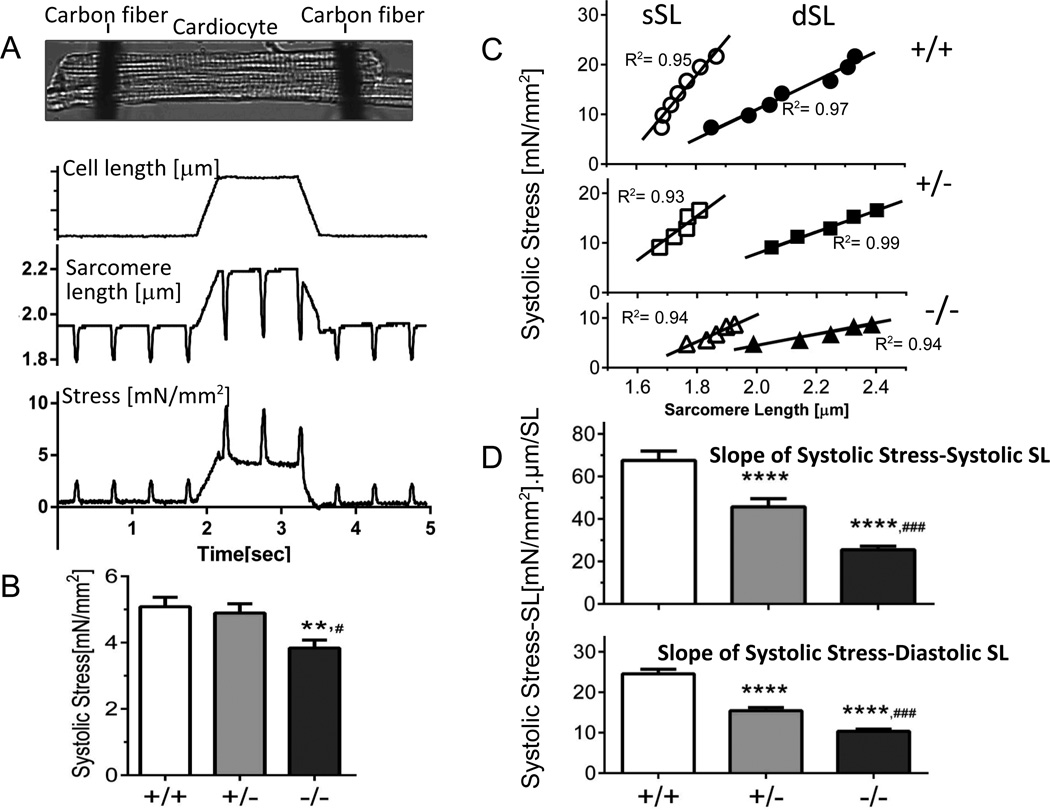
Figure 4.
Systolic function of intact cardiocytes. A) Protocol: Cells were attached at their base-length to carbon fibers, activated at 2 Hz, and a stretch was imposed during the diastolic interval. Cell Length (top), SL (middle) and stress (bottom). B) Systolic stress of cells held at their base-length. C) Examples showing systolic stress vs. diastolic SL (dSL) and vs. systolic SL (sSL). The slopes of the relations were determined and used as a measurement of the Frank-Starling mechanism. D) Frank-Starling effect calculated from slope of systolic stress-systolic SL (top) and systolic stress-diastolic SL (bottom). Symbols: **p<0.01, ****p<0.0001 vs. wild-type (+/+); #p<0.05, ###p<0.0001 vs. Het (+/−). B–D: 81 cells/13 mice +/+, 85 cells/15 mice +/− and 82 cell/13 mice −/−.
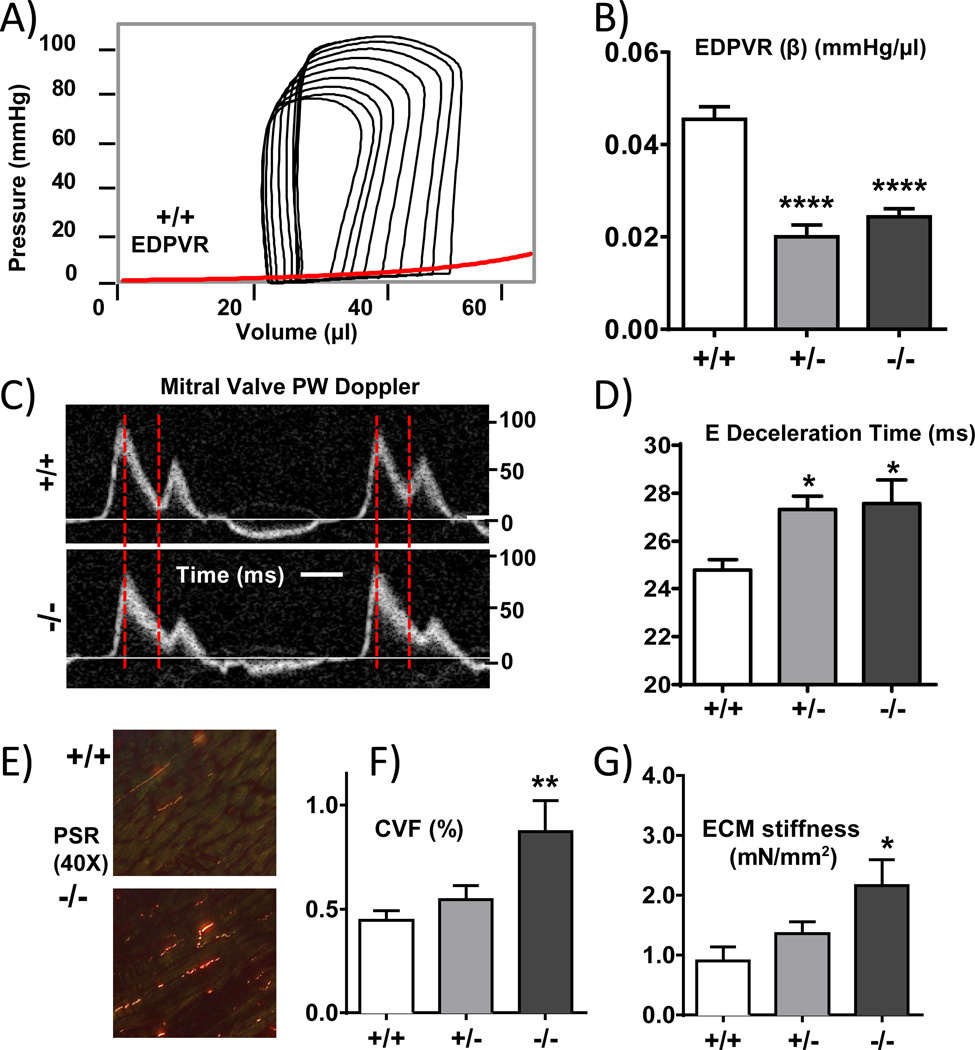
Diastolic stiffness--LV chamber
A pressure (P)-volume (V) analysis was performed in closed chest anesthetized mice. By using a transient inferior vena caval (IVC) occlusion, PV loops were generated at a range of filling volumes and from the end-diastolic pressure-volume relation (EDPVR) we determined the coefficient of diastolic stiffness, (see Fig. 3A). Compared to +/+ mice, diastolic chamber stiffness was significantly reduced in Rbm20ΔRRM mice (Fig. 3B, Table S3). However, there was no significant difference between +/− and −/− mice, unlike in the above described results for single cardiomyocytes. We also estimated in-vivo diastolic stiffness by pulse-wave Doppler echocardiography and measured the deceleration time of the early (E) filling wave(Fig. 3C shows examples), a parameter that varies inversely with LV diastolic stiffness20. The E deceleration time was significantly increased in Rbm20ΔRRM mice (Fig. 3D), supporting reduced stiffness in these genotypes. However, there was again no difference between +/− and −/− genotypes, mimicking the PV results. To explore whether there might be differences in the extracellular matrix we measured the LV collagen-volume fraction (CVF), using picrosirus red staining of collagen fibers. Examples in Fig. 3E and summarized results in Fig. 3F show that in +/− Rbm20ΔRRM mice CVF is unaltered whereas in −/− mice CVF is significantly increased. The absence of hypertrophy in the Rbm20ΔRRM mice makes it unlikely that fibrosis is induced by hypertrophic signaling. Instead the results suggest that the presence of hyper-compliant titin in −/− mice is causative for the development of fibrosis. We also measured ECM-based stiffness and consistent with the fibrosis found it to be significantly increased in −/− mice (Fig. 3 panel G). Thus the fibrosis-based increase in ECM stiffness of −/− mice might be compensatory for the lower titin-based cellular stiffness.
Figure 3.
In vivo characterization of LV diastolic compliance. A) Representative example of PV loops of an inferior vena caval (IVC) occlusion experiment; the end-diastolic PV relationship (EDPVR) is shown as a red line. B) Diastolic stiffness coefficient β of EDPVR is significantly decreased in +/− and −/− mice. C) Pulsed-wave Doppler to visualize mitral valve flow patterns. D) E-wave deceleration time is increased in +/− and −/− mice. E) Representative LV sections with picrosirus red (PSR) staining for collagen. (F) Collagen volume fraction (CVF). G) ECM stiffness (elastic coefficient). Symbols: *p<0.05, **p<0.01, ****p<0.0001 vs. wild-type (+/+). A–B: 11 +/+ mice, 12 +/− mice and 10 −/− mice; C–D: 9 +/+ mice, 10 +/− mice and 8 −/− mice; E-F: 9 +/+ mice, 9 +/− mice and 7 −/− mice; G: 6 +/+ mice, 6 +/− mice and 6 −/− mice.
Systolic function--Cardiac myocytes
The steady-state systolic stress at base length was the same in +/+ and +/− Rbm20ΔRRM cells: 5.1 ± 0.3 mN/ mm2 and 4.9 ± 0.3 mN/ mm2 respectively. Considering the 37°C experimental temperature and the 2Hz beat frequency, these are normal stress levels21. However, the −/− cells produced significantly less stress, 3.8± 0.2 mN/ mm2, a 25% reduction from +/+ levels (Fig. 4B). To evaluate its cause we measured Ca2+ transients with Fura-2. No differences were found in the transient parameters (Figs. S4a and S4b). This is consistent with the absence of differences in expression levels of the Ca2+ handling proteins (Fig. S4a-panel C). To determine whether the low systolic stress of −/− cells might be due to a deficiency in maximal active stress we skinned cardiac myocytes and measured the steady-state stress developed in a pCa 4.5 activating solution. Results revealed no difference between +/+ and +/− cells, but a 27% reduction in −/− cells(Table S4), indicating that it is likely that the reduced twitch stress of intact −/− cells is due to a reduced maximal active stress.
To characterize length-dependence of systolic stress, cells were stretched during the diastolic interval, after three beats the cell was returned back to base length, and 60 sec later the protocol was repeated but with a different stretch amplitude (Fig. 4A further explains the protocol). The first twitch following stretch was studied and its diastolic SL, systolic SL, and diastolic and systolic stresses were measured. Examples of results for individual cells are shown in Fig. 4C. The results could be fit well with linear regression lines (r2 >0.9) with slopes that reflect the Frank-Starling mechanism (FSM). The +/+ cells showed a robust FSM (large slope) but the FSM was reduced in +/− and −/− cells, both when plotting systolic stress vs. diastolic SL (Fig. 4D bottom) or vs. systolic SL (Fig. 4D top). It is interesting to note that when plotting systolic stress versus diastolic stress an identical relation was obtained in all 3 genotypes (Table S4) consistent with the notion that diastolic stress is an important determinant of systolic stress. Finally, we studied whether differences in beta-adrenergic tone amongst the genotypes might have affected the FSM and carried out studies in propranolol (Supplement). Identical results were found, i.e. a reduced FSM in Rbm20ΔRRM cells. Thus under all conditions the FSM is attenuated in Rbm20ΔRRM mice with an intermediate reduction in +/− cells and the largest effect in −/− cells.
Several possible mechanisms were investigated as a cause of reduced FSM in Rbm20ΔRRM mice. First we evaluated calcium transients of unloaded cells but found no differences (Figs. S4a and S4b) and we tested whether in Rbm20ΔRRM mice Ca2+ release is affected by stretch and compared the Ca2+ transient of the first post-stretch beat with that of the last pre-stretch beat. Stretch did not affect Ca2+ release in +/+ (consistent with results of others22) nor in the Rbm20ΔRRM myocytes(Fig. S5). Thus the attenuated FSM in Rbm20ΔRRM mice is unlikely due to changes in Ca2+ handling. It has been recently suggested that the size of titin’s spring element is a factor that controls thin filament length (TFL)23. If this were to be the case, differences in TFL could exist in Rbm20ΔRRM mice that affect the FSM24. In studies that we will report elsewhere, we determined average TFL at ~ 0.95 µm in all 3 genotypes, indicating that the attenuated FSM in Rbm20ΔRRM mice is unlikely due to differences in TFLs.
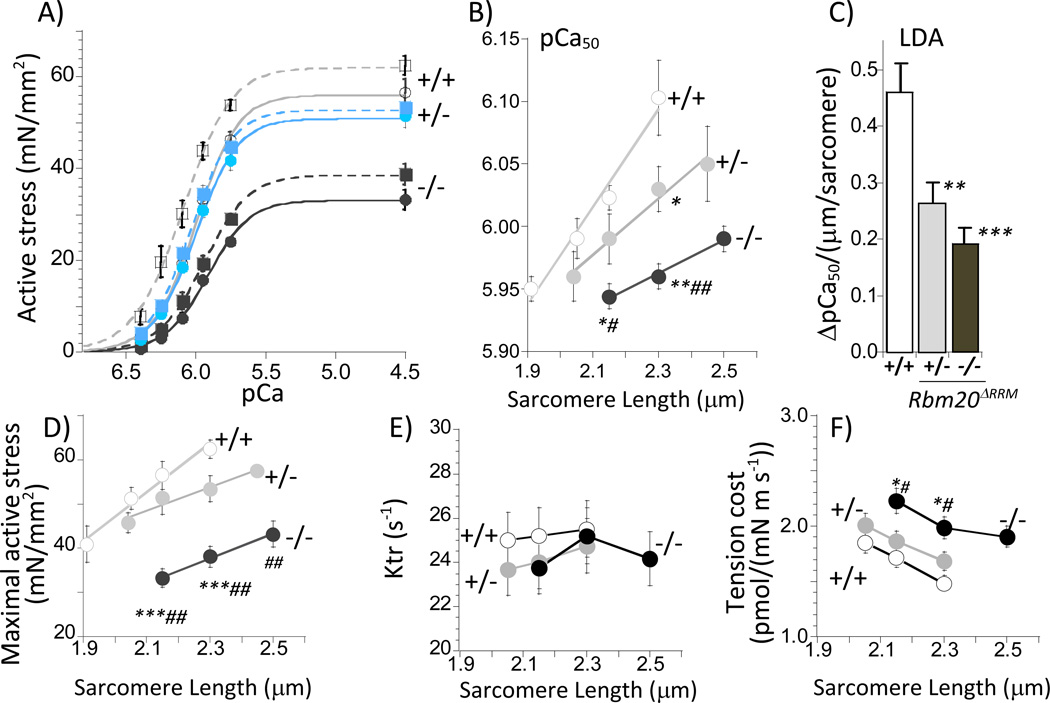
We also investigated length-dependence of activation (LDA) as a cause of the attenuated FSM in Rbm20ΔRRM mice and show results in Fig. 5 and Table S5. We used skinned papillary muscle for this work because of the robustness of the preparation that makes it possible to measure active stress –pCa relations at 3 different SLs with negligible rundown (<5%). Fig. 5A shows the active stress-pCa curves at two sarcomere length, 5B the pCa50– SL relations, and 5C the slope of the pCa50– SL relations as a measure of LDA. LDA was significantly reduced in Rbm20ΔRRM mice with the largest reduction in −/− mice. The experiments also revealed that maximal active stress was reduced in −/− muscle but not in +/− muscle (Fig. 5D), consistent with the skinned myocyte results (Table S4). To determine whether differences existed in myofilament proteins that might explain the LDA result we investigated thin and thick filament proteins, including their phosphorylation status, but found no differences (Fig. S6). To establish whether titin-based stiffness affects crossbridge cycling kinetics we measured the rate constant of tension redevelopment (Ktr) as well as tension cost. No significant differences were found amongst the genotypes for Ktr (Fig. 5E) whereas tension cost was increased in −/− Rbm20ΔRRM mice (Fig. 5 F and Table S5).
Figure 5.
Length dependence of active stress(A-D), Ktr (E), and tension cost (F) in RBM20ΔRRM skinned papillary muscle. A) Average stress–pCa curves at SL 2.15 (solid curves) and 2.3 µm (broken curves). Increasing sarcomere length results in left-shift of the stress–pCa curves. B) pCa50 vs. SL. C) ΔpCa50 is an index of LDA and was determined from the slope of the fits in B. D) Maximal active stress (pCa 4.5) vs SL. E) Ktr vs. SL and F) Tension cost vs SL. Symbols: *p<0.05, **p<0.01, ***p<0.001 vs. wild-type (+/+); #p<0.05, ##p<0.01 vs. Het (+/−). A–C: 12 muscle strips in each group (6 mice); D–F: 11 muscle strips in each group (6 mice).
Systolic function--LV chamber
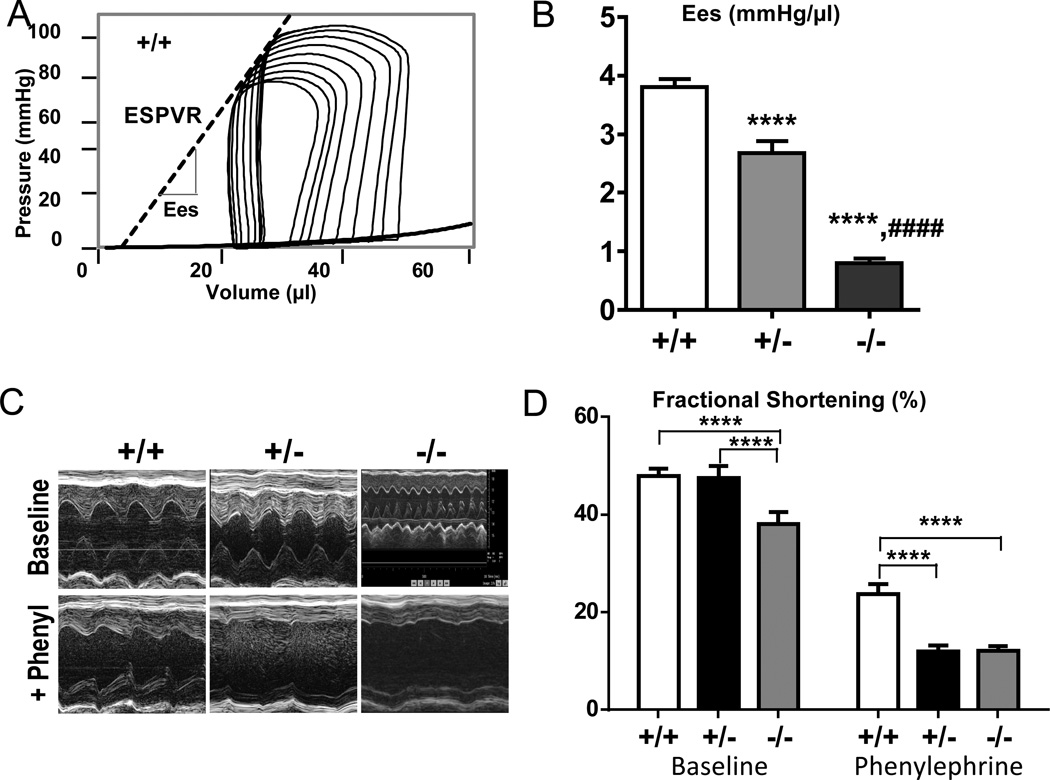
PV analysis showed that under baseline conditions the end-systolic pressure (ESP) was unaltered in +/− mice but reduced by 20% in −/− mice (Table S3). In order to be able to compare these results to those in cardiac myocytes we evaluated SL in the LV. SL was determined in hearts that were arrested by perfusion with KCL/BDM, vented by puncturing the LV apex (to ensure that hearts were at zero cavity pressure/diastasis during fixation) and were then perfusion-fixed followed by SL measurement in the LV free wall (see Methods). Obtained SL values (Fig. 1Biv) show significant SL differences amongst the genotypes that are in line with those measured on myocytes. Because of this similarity it is justified to compare myocyte results with LV chamber results, leading to the conclusion that the differences in twitch stresses in intact cardiac myocytes under baseline conditions(Fig.4B) translate to difference in LV pressures (i.e., no change in +/− but a reduction in −/− mice). We also measured the end-systolic pressure volume relation (ESPVR) of the LV chamber (Fig. 6A) and determined the end-systolic elastance (Ees), a parameter that reflects the FSM. Ees was reduced in the Rbm20ΔRRM mice with the largest reduction in −/− mice (Fig. 6B), again consistent with results of cardiac myocytes.
Figure 6.
In vivo characterization of LV systolic function. A) Representative example of an IVC occlusion PV loop series; End-systolic elastance (Ees) (broken line) is depicted and (B) quantitative data show a significant decrease in Ees in Rbm20ΔRRM mice. C) Representative +/+, +/−, and −/− echocardiographic M-mode images and (D) quantitative analysis of the phenylephrine stress test performed in conscious mice, indicating a decrease in systolic reserve in Rbm20ΔRRM mice. Symbols: ****p<0.0001 vs. wild-type (+/+); ####p<0.0001 vs. Het (+/−). A–B: 12 +/+ mice, 12 +/− mice and 13 −/− mice; C–D: 13 +/+ mice, 12 +/− mice and 12 −/− mice.
Functional implications--Response to increased afterload
Conscious mice were under echo observation before and after an i.p. injection of the α1-adrenergic agonist phenylephrine, PE (Methods). PE is well-known to increase systemic vascular resistance and increase thereby the afterload on the LV chamber, but to not affect Ees. M-mode echocardiography revealed that in all genotypes PE increased LV chamber dimensions (Fig. 6C) and reduced fractional shortening (FS) (Fig. 6D). The reduction in FS was significantly larger in Rbm20ΔRRM mice (Fig. 6C, bottom row, Fig. 6D). Thus, under baseline conditions the FS of Rbm20ΔRRM mice is indistinguishable (+/−) or reduced to a limited degree (−/−) compared that of +/+ mice and when challenged with an increase in afterload a significant and large FS deficit becomes apparent in both +/− and −/− mice.
Functional implications--Exercise capacity
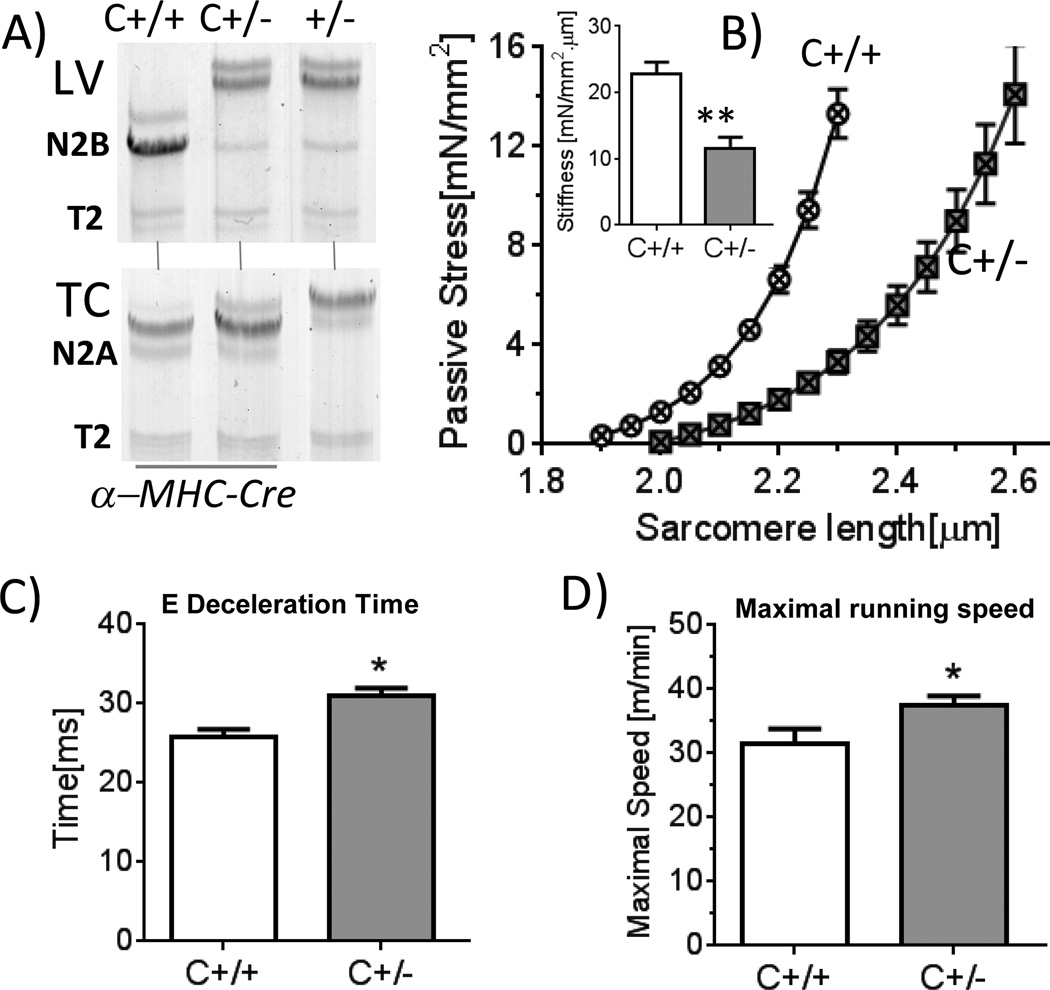
We studied the maximal running speed in treadmill exercise experiments. Considering that in Rbm20ΔRRM mice titin is increased in size not only in cardiac muscle but also in skeletal muscle, we used for these experiments cardiac-specific α-MHC-Cre; Rbm20ΔRRM flox/+ mice (Supplement) and as controls α-MHC-Cre; +/+ mice; we refer to these mice as C+/− and C+/+, respectively (C for cardiac-specific). Protein analysis showed that titin in LV of the C+/− mouse was identical to the conventional +/− mouse (Fig. 7A, top) but skeletal muscle of the C+/− mouse expressed wild-type titin(Fig. 7A, bottom). Thus the C+/− is a cardiac-specific model that is well-suited to study the effect of exercise on the cardiovascular system in RRM deficient mice. Skinned LV cardiocytes had a reduced stiffness in C+/− cells (Fig. 7B) similar to observed in the conventional +/− Rbm20ΔRRM mice. Anesthetized and conscious echocardiography(Tables S1d and S1e) and tissue morphometry (Table S2c) revealed no differences between C+/+ and C+/− mice, except for the prolonged deceleration time of the E-wave(Fig. 7C) that suggests a reduced diastolic LV chamber stiffness. A free running wheel protocol showed that after mice were acclimatized, the mean running distance per night of C+/− and C+/+ mice was 8.9 ± 0.9 km vs. 7.7± 1.4 km but the difference was not significant (p=0.1). We also measured the maximal running speed in a treadmill exercise protocol (Supplement). The maximal running speed was determined for 4 days in a row and regression analysis revealed a significantly (p=0.004) increased maximal running speed in C+/− mice; results of an individual running day are shown in Fig. 7D.
Figure 7.
Exercise capacity of cardiac specific α-MHC-Cre; Rbm20ΔRRM flox/+ (C+/−) and α-MHC-Cre; + /+ (C+/+) mice. A) Titin gels of C+/+, C+/− and Rbm20ΔRRM +/− LV (Top) and Tibialis Cranialis (TC) skeletal muscle (bottom). Titin expression in LV of C+/− is as in Rbm20ΔRRM Hets (+/−) but titin expression in TC of C+/− is as in C+/+, mice. Thus the C+/− mouse is cardiac-specific. B) Stress and stiffness of passive skinned LV cardiocytes is reduced in C+/− mice compared to WT (C+/+) mice. C) Pulsed-wave Doppler reveals an increased E-wave deceleration time in C+/− mice. D) Maximal running speed in treadmill exercise test. C+/− mice have a higher maximal running speed. Symbols: *p<0.05, **p<0.01, vs. wild-type (C+/+). B: 5 C +/+ mice and 5 C+/− mice; C: 7 C +/+ mice and 6 C+/− mice; D: 7 C +/+ mice and 6 C+/− mice.
Discussion
Rbm20ΔRRM mouse model
RBM20 is a splicing factor that contains a centrally located RNA-recognition motif (RRM) that provides RNA-binding specificity and a C-terminal RS domain (rich in arginine and serine) that promotes protein–protein interactions and recruits RBM20 to the spliceosome3. Recent studies with a rat that carries a spontaneous mutation that deletes most of the Rbm20 gene have shown that RBM20 is an important titin splicing factor3. Our goal was to abolish the splicing function of RBM20 and avoid altering the other functional domains within RBM20 and we made an in-frame RRM deletion (exons 6 and 7); to avoid a skeletal muscle phenotype in exercise studies we made a cardiac-specific version for these experiments. Rbm20ΔRRM mice express two large ~3.5 and 3.6 MDa titins in +/− mice and a single ~4.0 MDa isoform in the −/− mice (Fig. 1A). These titin expression patterns are similar to those of a rat with a spontaneous mutation in the Rbm20 gene3. The expression pattern in +/− hearts is indistinguishable from that of fetal cardiac titins that are present in late embryonic hearts of wild-type mice4, suggesting that splicing patterns of fetal cardiac titin are due to the presence of an intermediate level of RBM20 protein(relative to pre-mRNA levels). Finally, exon expression analysis showed that Rbm20ΔRRM mice upregulate titin exons in the tandem Ig segment and the PEVK region and that Ig exons are more sensitive to RBM20 dose than the PEVK exons (Fig. S1, panel D, Fig. S2a). No changes were found in or near the Z-disk or in the A-band regions of titin, and splicing of novex-3 titin is similarly unaffected (Fig. S1, panel G). In summary, RBM20 exclusively splices the spring region of full-length titins and the intermediate level of wild-type RBM20 protein in +/− Rbm20ΔRRM mice (Fig. S1, panel C) results in adult mice that express fetal cardiac titin isoforms.
The genome-wide exon expression analysis of the Rbm20ΔRRM mouse and the candidate approach consisting of genes that have been reported to be differentially spliced in both the Rbm20 rat model and in patients with Rbm20 mutations3 revealed only Ttn, Rbm20, Ldb3/Cypher and CaMKIIδ to be differentially spliced in the mouse whereas other candidate genes were unaltered (see Fig. S2b). Possibly this is due to the RS domain that remains in the mouse which may keep the spliceosome and some of RBM20’s functionality intact. It is also possible that the genes that are only alternatively spliced in the Rbm20 mutant rat and in patients are not primary RBM20 targets and that their alternative splicing is secondary to disease processes that only occur in the rat and the patients. Patients with Rbm20 mutations have severe dilation25 and the Rbm20 rat model also has dilation (although their ejection fraction is normal)3. The Rbm20ΔRRM mouse model has normal chamber dimensions (Tables 1 and S1a–e), findings that are independent of gender, age (up to 20 months), the use of anesthetized vs. conscious mice, and model type (conventional or cardiac-specific). Whatever its cause, high specificity of the Rbm20ΔRRM mouse model for titin splicing is fortuitous because it lends confidence that the phenotype that we found is more likely due to alterations in titin splicing. Considering that Ldb3/Cypher and CaMKIIδ are also alternatively spliced they might also contribute to the phenotype of the Rbm20ΔRRM mouse. Expression levels of the short isoform of Ldb3/Cypher was significantly reduced in Rbm20ΔRRM mice with the largest reduction in −/− mice (Fig. S3). It is unknown what the function of this short Ldb3/Cypher isoform is and a study in which it was selectively deleted did not produce a phenotype18. Thus it is unlikely that alternative splicing of Cypher plays a major role in the phenotype of the Rbm20ΔRRM mouse. For CaMKIIδ, its expression level was unaltered and none of the tested CaMKIIδ targets were different in the Rbm20ΔRRM model (Fig. S2c) and neither were the Ca2+ transients (Fig. S4). Overall our analysis shows that titin is a major target of RBM20 and that this induces major changes in the mechanically critical spring elements of titin. It is likely therefore that titin plays a dominant role in the phenotype of the Rbm20ΔRRM model.
Diastolic Function
Experiments with the actomyosin inhibitor blebbistatin show that crossbridges contribute to diastolic stiffness of the intact myocyte, consistent with earlier work13. An identical diastolic crossbridge contribution to diastolic stiffness was found in the different genotypes (Fig. 2D), revealing that diastolic crossbridge interaction is not affected by titin’s passive stress. Comparison of diastolic stiffness of intact cells in blebbistatin with skinned cells in relaxing solution shows that passive stiffness in the 3 studied genotypes is similarly reduced. The studies also establish that diastolic stiffness of cardiac myocytes is inversely related to the size of titin, i.e., stiffness is largest in +/+ cells, intermediate in +/− cells and smallest in −/− cells. Titin-based force is entropic in nature, with force increasing in proportion with the spring segment’s fractional extension (end-to-end length divided by the contour length)26. Considering that expressing large titin isoforms is due solely to an increase in the contour length of titin’s spring segment, large isoforms will at a given SL have a reduced fractional extension of their spring segment and, thus, an inverse relationship between size of titin and titin stiffness is expected. The increased slack SL of cardiac myocytes of Rbm20ΔRRM is also expected as the mean square end-to-end distance of a flexible chain at zero external force (titin in slack sarcomeres) is a function of the square root of the chain’s contour length27. It is interesting that the SLs measured in the LV of hearts arrested and fixed at diastasis is comparable to that of isolated slack cardiomyocytes and follows the same genotype dependence, i.e., short in +/+, intermediate in +/− and longest in −/− mice (Fig. 1Biv). This indicates that the SL at which titin’s force is zero determines diastasis. The similarity of SLs between hearts and cells also validates extending findings at the single cell level to the LV level. In +/− Rbm20ΔRRM mice diastolic stiffness of intact cells and the LV are similarly reduced (relative to +/+ mice, by 36% and 48%, respectively) which supports that LV diastolic stiffness is dominated by cellular stiffness. However, in −/− Rbm20ΔRRM mice diastolic stiffness was further reduced in intact cells but not in the LV chamber. A likely explanation is provided by increased collagen expression in the −/− LV (Fig. 3F) that increases chamber stiffness (Fig. 3G) and compensates for the reduced stiffness of the cardiac myocytes. Cross-talk between titin and ECM has previously been proposed to take place in DCM (dilated cardiomyopathy) patients9 and the present work suggests that when titin stiffness falls below a certain level (as in −/− mice) adaptive changes in ECM are triggered. Elucidating the sensing and signaling mechanisms that are responsible for titin-ECM crosstalk is an important area for future research. In summary, the studies with the Rbm20ΔRRM model support the high importance of titin in diastolic function and show that increases in titin-based compliance manifest in vivo as increased chamber compliance.
Systolic Function
It has been shown in multiple studies on skinned cardiomyocytes and skinned muscle that titin-based passive stress correlates with Ca2+ sensitivity of actomyosin-based stress3, 6 and the present study supports these findings (Fig. 5). Additionally we found that maximal active stress is reduced in −/− but not in +/− mice (Fig. 5, panel D). The reduction in maximal active stress of −/− mice is consistent with that reported in the mutant Rbm20 rat model3 (+/− rats were not studied) and is likely to underlie the reduction in end-systolic pressure of our PV studies (Table S3) and the reduction in fractional shortening that our echo study on conscious mice revealed (Table S1C). Protein analysis did not support that the active stress reduction is caused by a change in MHC isoform (Fig. S6) or MHC content (results not shown). Instead we speculate that the reduced maximal active stress of −/− mice is due to a reduction in the fraction of crossbridges that are in force-generating states and that this occurs when titin-based passive stress falls below a certain level (intermediate between passive stress in −/− and +/− mice). This notion is supported by the increased tension cost of −/− mice (Fig. 5F) that suggests an increase in the apparent rate constant of detachment of force-generating crossbridges (gapp)28. Ktr reflects the sum of gapp and fapp (rate constant of crossbridge attachment)28 and the finding that Ktr is unchanged in the −/− mice (Fig. 5E) combined with its increased tension cost suggests a reduced fapp. Titin’s multiple interactions with the thin and thick filaments and its effect on thick filament strain29 provide possible pathways by which titin might modulate actomyosin interaction. Future X-ray diffraction work will be needed to establish the possible underlying mechanisms.
An important focus was on the FSM of intact myocytes. Our work shows that the FSM of intact myocytes is reduced when titin compliance is increased (Fig. 4), a conclusion consistent with the work of others (see Cazorla and Lacampagne30 and references therein) and supported by measurements at the level of the LV chamber where Ees was reduced proportional to the increase in titin compliance (Fig. 6B). The functional importance of the reduced Ees was addressed in stress tests with phenylephrine, a α1-adrenergic agonist which increases systemic vascular resistance and causes an increase in end-diastolic volume. This condition results in a deficit in fractional shortening that is significantly larger in Rbm20ΔRRM mice (Fig. 6D), supporting that increasing titin compliance impacts systolic function. Thus, our work supports that titin not only plays a role in diastole, but that it is also important for systole.
Exercise Capacity
Although Rbm20ΔRRM mice have alterations in diastolic and systolic function they do not display a phenotype under sedentary conditions. To study whether abnormalities arise in exercise tests we used a cardiac-specific α-MHC-Cre; Rbm20ΔRRM flox/+(C+/−) mice to avoid a possible skeletal muscle phenotype. We focused on only C+/− mice in order to eliminate an effect from the reduced systolic pressure of −/− mice under baseline conditions (Table S3) and to keep our workload manageable. Surprisingly, an improvement in function was detected as C+/− mice reached a higher maximal speed in the involuntary treadmill running test (Fig. 7D). To explain this result, recent studies on instrumented mice are relevant as they showed that exercise increases cardiac output in the mouse by increasing heart rate (in a treadmill exercise test from ~600 to ~800 bpm31) with no known increases in end-diastolic volume (a slight reduction was measured in vivo as heart rate was increased with isoproterenol32). Thus the depressed FSM in Rbm20ΔRRM mice might not play a dominant role during changes that occur in exercise. Additionally, the increased contractile state that exists during exercise might result in systolic sarcomere lengths sufficiently short for titin-based restoring force to play a role33 and we hypothesize that the lower restoring force of C +/− mice might enhance LV systolic emptying during exercise. This proposal requires critical testing. Furthermore, another recent study on instrumented wild-type mice during treadmill exercise showed large increases in LV diastolic pressure34, presumably due to incomplete relaxation The increased titin-based LV compliance of Rbm20ΔRRM mice is expected to be beneficial as it will counter the elevated diastolic pressure and enhance diastolic filling. In summary, the depressed FSM of C +/− mice might have a limited functional role during exercise whereas the reduced restoring force of titin in short sarcomeres (systole) and the reduced passive force in long sarcomeres (diastole) are beneficial and play dominant roles. We conclude that the enhanced exercise capacity of C +/− mice supports that increased titin compliance has a beneficial effect on global cardiac function.
Clinical implications
The N2BA/N2B isoform expression ratio is ~0.4 in normal human adults but this ratio is increased in multiple cardiac diseases8–10. For example in DCM the ratio has been reported to be as high as ~1.29. Comparison with the results of +/− and −/− Rbm20ΔRRM mice suggests that the level of N2BA upregulation in patients might be insufficiently large to depress maximal active stress or to cause an increase in collagen expression. A detailed study on a group of DCM patients with variable dysfunction levels revealed a positive correlation between N2BA expression and echo-derived improvements in filling, and a positive correlation between N2BA expression and exercise capacity9. Thus, studies on DCM patients and +/− Rbm20ΔRRM mice both support that upregulating compliant titins has beneficial effects on global cardiac function by improving diastolic filling.
In addition to alternative splicing, titin-based stiffness can be varied through phosphorylation of titin’s spring segment with an important pathway being PKA/PKG phosphorylation that lowers titin’s stiffness35, 36. Studies have shown that in heart failure with preserved ejection fraction (HFpEF), titin’s PKA/PKG sites are hypo-phosphorylated and that this increases passive stiffness of cardiomyocytes far beyond that of control cells10. This important finding led to the recent clinical RELAX trial that evaluated whether elevating PKG activity through blocking cGMP breakdown might relieve symptoms in HFpEF patients, but unfortunately this trial was unsuccessful37. An alternative approach might be the manipulation of titin splicing by using small molecule inhibition of RBM20 binding to titin pre-mRNA, thereby lowering passive stiffness in HFpEF patients7. Our work shows that full inhibition must be avoided as it can depress maximal systolic stress and increase collagen expression. However, through careful dosing an intermediate inhibition level might be achievable with an increase in compliance that balances out the increased stiffness due to hypo-phosphorylation of titin, thereby normalizing diastolic and systolic function of the cardiac myocyte. Considering the high prevalence of the HFpEF syndrome and the current lack of effective therapies for lowering pathological stiffness levels, new insights provided by our study should be extended with as goal to relieve HFpEF symptoms by manipulating titin splicing and improving diastolic compliance.
Supplementary Material
Acknowledgements
We are grateful to our lab members and acknowledge UA’s GEMM and Genomics Cores.
Funding Sources: This work was supported by an AHA pre-doctoral fellowship (MM), T32HL 07249 (KH) and NIH HL062881 and HL115988 (HG).
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.LeWinter MM, Granzier H. Cardiac titin: A multifunctional giant. Circulation. 2010;121:2137–2145. doi: 10.1161/CIRCULATIONAHA.109.860171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S. The complete gene sequence of titin, expression of an unusual approximately 700-kda titin isoform, and its interaction with obscurin identify a novel z-line to i-band linking system. Circ Res. 2001;89:1065–1072. doi: 10.1161/hh2301.100981. [DOI] [PubMed] [Google Scholar]
- 3.Guo W, Schafer S, Greaser ML, Radke MH, Liss M, Govindarajan T, Maatz H, Schulz H, Li S, Parrish AM, Dauksaite V, Vakeel P, Klaassen S, Gerull B, Thierfelder L, Regitz-Zagrosek V, Hacker TA, Saupe KW, Dec GW, Ellinor PT, MacRae CA, Spallek B, Fischer R, Perrot A, Ozcelik C, Saar K, Hubner N, Gotthardt M. Rbm20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat Med. 2012;18:766–773. doi: 10.1038/nm.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lahmers S, Wu Y, Call DR, Labeit S, Granzier H. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ Res. 2004;94:505–513. doi: 10.1161/01.RES.0000115522.52554.86. [DOI] [PubMed] [Google Scholar]
- 5.Opitz CA, Leake MC, Makarenko I, Benes V, Linke WA. Developmentally regulated switching of titin size alters myofibrillar stiffness in the perinatal heart. Circ Res. 2004;94:967–975. doi: 10.1161/01.RES.0000124301.48193.E1. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda N, Granzier HL, Ishiwata S, Kurihara S. Physiological functions of the giant elastic protein titin in mammalian striated muscle. J Physiol Sci. 2008;58:151–159. doi: 10.2170/physiolsci.RV005408. [DOI] [PubMed] [Google Scholar]
- 7.Lewinter MM, Granzier HL. Titin is a major human disease gene. Circulation. 2013;127:938–944. doi: 10.1161/CIRCULATIONAHA.112.139717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makarenko I, Opitz CA, Leake MC, Neagoe C, Kulke M, Gwathmey JK, del Monte F, Hajjar RJ, Linke WA. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res. 2004;95:708–716. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- 9.Nagueh SF, Shah G, Wu Y, Torre-Amione G, King NM, Lahmers S, Witt CC, Becker K, Labeit S, Granzier HL. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110:155–162. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- 10.Borbely A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, Stienen GJ, Paulus WJ. Hypophosphorylation of the stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 11.van Heerebeek L, Franssen CP, Hamdani N, Verheugt FW, Somsen GA, Paulus WJ. Molecular and cellular basis for diastolic dysfunction. Curr Heart Fail Rep. 2012;9:293–302. doi: 10.1007/s11897-012-0109-5. [DOI] [PubMed] [Google Scholar]
- 12.Chung CS, Hutchinson KR, Methawasin M, Saripalli C, Smith JE, 3rd, Hidalgo CG, Luo X, Labeit S, Guo C, Granzier HL. Shortening of the elastic tandem immunoglobulin segment of titin leads to diastolic dysfunction. Circulation. 2013;128:19–28. doi: 10.1161/CIRCULATIONAHA.112.001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King NM, Methawasin M, Nedrud J, Harrell N, Chung CS, Helmes M, Granzier H. Mouse intact cardiac myocyte mechanics: Cross-bridge and titin-based stress in unactivated cells. J Gen Physiol. 2011;137:81–91. doi: 10.1085/jgp.201010499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee EJ, Nedrud J, Schemmel P, Gotthardt M, Irving TC, Granzier HL. Calcium sensitivity and myofilament lattice structure in titin N2B ko mice. Arch Biochem Biophys. 2013;535:76–83. doi: 10.1016/j.abb.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stienen GJ, Zaremba R, Elzinga G. Atp utilization for calcium uptake and force production in skinned muscle fibres of xenopus laevis. J Physiol. 1995;482(Pt 1):109–122. doi: 10.1113/jphysiol.1995.sp020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granzier HL, Radke MH, Peng J, Westermann D, Nelson OL, Rost K, King NM, Yu Q, Tschope C, McNabb M, Larson DF, Labeit S, Gotthardt M. Truncation of titin's elastic pevk region leads to cardiomyopathy with diastolic dysfunction. Circ Res. 2009;105:557–564. doi: 10.1161/CIRCRESAHA.109.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ottenheijm CA, Knottnerus AM, Buck D, Luo X, Greer K, Hoying A, Labeit S, Granzier H. Tuning passive mechanics through differential splicing of titin during skeletal muscle development. Biophys J. 2009;97:2277–2286. doi: 10.1016/j.bpj.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng H, Zheng M, Peter AK, Kimura K, Li X, Ouyang K, Shen T, Cui L, Frank D, Dalton ND, Gu Y, Frey N, Peterson KL, Evans SM, Knowlton KU, Sheikh F, Chen J. Selective deletion of long but not short cypher isoforms leads to late-onset dilated cardiomyopathy. Hum Mol Genet. 2011;20:1751–1762. doi: 10.1093/hmg/ddr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson ME, Brown JH, Bers DM. CamkII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohno M, Cheng CP, Little WC. Mechanism of altered patterns of left ventricular filling during the development of congestive heart failure. Circulation. 1994;89:2241–2250. doi: 10.1161/01.cir.89.5.2241. [DOI] [PubMed] [Google Scholar]
- 21.Layland J, Kentish JC. Positive force- and [ca2+]i-frequency relationships in rat ventricular trabeculae at physiological frequencies. Am J Physiol. 1999;276:H9–H18. doi: 10.1152/ajpheart.1999.276.1.H9. [DOI] [PubMed] [Google Scholar]
- 22.Allen DG, Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1982;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castillo A, Nowak R, Littlefield KP, Fowler VM, Littlefield RS. A nebulin ruler does not dictate thin filament lengths. Biophys J. 2009;96:1856–1865. doi: 10.1016/j.bpj.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granzier HL, Akster HA, Ter Keurs HE. Effect of thin filament length on the force-sarcomere length relation of skeletal muscle. Am J Physiol. 1991;260:C1060–C1070. doi: 10.1152/ajpcell.1991.260.5.C1060. [DOI] [PubMed] [Google Scholar]
- 25.Li D, Morales A, Gonzalez-Quintana J, Norton N, Siegfried JD, Hofmeyer M, Hershberger RE. Identification of novel mutations in rbm20 in patients with dilated cardiomyopathy. Clin Transl Sci. 2010;3:90–97. doi: 10.1111/j.1752-8062.2010.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe K, Nair P, Labeit D, Kellermayer MS, Greaser M, Labeit S, Granzier H. Molecular mechanics of cardiac titin's pevk and N2B spring elements. J Biol Chem. 2002;277:11549–11558. doi: 10.1074/jbc.M200356200. [DOI] [PubMed] [Google Scholar]
- 27.Radke MH, Peng J, Wu Y, McNabb M, Nelson OL, Granzier H, Gotthardt M. Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. Proc Natl Acad Sci U S A. 2007;104:3444–3449. doi: 10.1073/pnas.0608543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner B. The cross-bridge cycle in muscle. Mechanical, biochemical, and structural studies on single skinned rabbit psoas fibers to characterize cross-bridge kinetics in muscle for correlation with the actomyosin-atpase in solution. Basic Res Cardiol. 1986;81(Suppl 1):1–15. doi: 10.1007/978-3-662-11374-5_1. [DOI] [PubMed] [Google Scholar]
- 29.Irving T, Wu Y, Bekyarova T, Farman GP, Fukuda N, Granzier H. Thick-filament strain and interfilament spacing in passive muscle: Effect of titin-based passive tension. Biophys J. 2011;100:1499–1508. doi: 10.1016/j.bpj.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cazorla O, Lacampagne A. Regional variation in myofilament length-dependent activation. Pflugers Arch. 2011;462:15–28. doi: 10.1007/s00424-011-0933-6. [DOI] [PubMed] [Google Scholar]
- 31.Lujan HL, Janbaih H, Feng HZ, Jin JP, DiCarlo SE. Myocardial ischemia, reperfusion, and infarction in chronically instrumented, intact, conscious, and unrestrained mice. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1384–R1400. doi: 10.1152/ajpregu.00095.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joho S, Ishizaka S, Sievers R, Foster E, Simpson PC, Grossman W. Left ventricular pressure-volume relationship in conscious mice. Am J Physiol Heart Circ Physiol. 2007;292:H369–H377. doi: 10.1152/ajpheart.00704.2006. [DOI] [PubMed] [Google Scholar]
- 33.Helmes M, Trombitas K, Granzier H. Titin develops restoring force in rat cardiac myocytes. Circ Res. 1996;79:619–626. doi: 10.1161/01.res.79.3.619. [DOI] [PubMed] [Google Scholar]
- 34.Lujan HL, Janbaih H, Feng HZ, Jin JP, DiCarlo SE. Ventricular function during exercise in mice and rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:R68–R74. doi: 10.1152/ajpregu.00340.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kruger M, Kotter S, Grutzner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG, Linke WA. Protein kinase g modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 36.Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase a phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res. 2002;90:1181–1188. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 37.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, Trial R. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.