Abstract
Interactions between the central nervous system and the immune system have been studied primarily in the context of pathology, popularizing the view that interplay between these two systems is inherently detrimental. However, recent experimental data have demonstrated productive neuroimmune interactions that occur under normal physiological conditions. In this Essay, we outline our current understanding of contemporary neuroimmunology, describe a working model of T cell function in support of learning and memory, and offer ideas regarding the selective advantages of immune-mediated effects on brain function.
The discipline of neuroimmunology has grown out of the clinical field of neuropathology. Thus, its focus has been largely on central nervous system (CNS) inflammatory diseases, such as multiple sclerosis1–6. In contrast to most peripheral organs, the access of blood-borne cells to the brain is largely restricted. The blood–brain barrier acts as a physical obstacle, preventing the entrance of leukocytes into the brain parenchyma in the steady state7. Accordingly, it was long believed that the detection of peripheral immune cells within the CNS was a hallmark of neuropathology, and that any disruption of the blood–brain barrier would allow unwanted immune cells to infiltrate delicate brain tissue, resulting in neuroinflammation and neuronal degeneration. But why would the immune system, which is crucial for defending other tissues in the body, be restricted from perhaps the most important organ of all? The answer generally given is that the danger of catastrophic inflammation in neural tissue is too great.
In reality there is abundant communication between the immune system and the CNS. For example, intraperitoneal injection of pro-inflammatory cytokines was shown to generate CNS-mediated sickness behaviour, which could be blocked by vagus nerve transection8. Similarly, direct stimulation of the peripheral vagus nerve was demonstrated to downregulate systemic inflammation9 and, most recently, macrophages and T cells were shown to be relay points in cholinergic signalling through the parasympathetic nervous system from the brain to the spleen10.
Immune cell function in the CNS has now been shown to extend beyond pathological conditions. Indeed, recent data have suggested key roles for immune cells in healthy brain functions, including psychological stress responses11, spatial learning and memory12,13, and adult neurogenesis14. In this Essay, we summarize current literature suggesting a role for T cells (and other immune cells) in regulating physiological aspects of brain function and discuss possible mechanisms underlying the beneficial effects mediated by T cells on learning. We speculate on the unique anatomical location at which these effects are mediated and discuss the antigenic specificity (or lack thereof) of these ‘pro-cognitive’ T cells.
T cells protect neurons from degeneration
Acutely injured neurons in the CNS inevitably die, triggering the death of neighbouring neurons that were uninjured by the initial insult. This spread of damage is termed secondary degeneration15. Animals that are devoid of an adaptive immune system have accelerated secondary degeneration compared with wild-type counterparts, resulting in decreased neuronal survival after CNS injury. Repopulation of immune-deficient animals with T cells from wild-type donors reduces secondary degeneration and thus improves neuronal survival16,17. Moreover, neuronal survival after CNS injury can be improved in wild-type mice by an intravenous injection of exogenous T cells specific for CNS-restricted self proteins, such as myelin basic protein (MBP) and myelin oligodendrocyte glycoprotein18. By contrast, neuronal survival in the injured mice was not affected by an intravenous injection of T cells specific for non-CNS-restricted self antigens (such as heat shock protein-derived peptides) or non-self proteins (such as ovalbumin)18. The beneficial effect of T cells specific for CNS-restricted self antigens has been observed in models of optic nerve injury18,19, spinal cord contusion16,20,21 and stroke22, as well as in other models of acute and chronic neurodegenerative conditions.
T cells have been proposed to mediate their neuroprotective effect via the production of neurotrophins23,24, the modulation of glutamate release by astrocytes and microglia25,26, the regulation of innate immunity at the site of injury27 and other, as yet unexplored, mechanisms. These data suggest that there is a link between the neuroprotective function of T cells and their recognition of self antigens. However, it is still unclear whether neuroprotective T cells that are spontaneously induced in vivo in response to injury are indeed autoreactive and, if so, whether their antigen specificity is restricted to CNS antigens.
T cells make mice smart(er)
As a feature of life in the wild, stress is a prominent part of day-to-day existence that can be associated with securing food and shelter, finding a mate, or almost any other evolutionarily driven requirement. As an organism that deals appropriately with stress is at an advantage in terms of survival, this feature is likely to be evolutionarily ‘selected’ for, with the organisms that are most resilient to stress being the fittest to survive.
A role for immune cells in stress resilience has been demonstrated in a mouse model of post-traumatic stress disorder (PTSD), in which mice are exposed to a predator odour that induces a long-lasting stress response reminiscent of PTSD in humans. In this model, it was shown that severe combined immunodeficient (SCID) mice and nude (T cell-deficient) mice were more likely to develop PTSD than their wild-type counterparts11,28,29. Reconstitution of SCID mice with CD3+ T cells isolated from wild-type donors ameliorated the overactive stress response. Moreover, when the T cell response was boosted in wild-type mice by vaccination with a myelin-derived peptide, the long-term pathological response to stress was further diminished11,28,29. These results suggest that T cells can actively mediate an improved response to stress.
Most learning experiences in either experimental settings or in daily life contain a component of stress. Although acute stress has been suggested by some to improve task acquisition if the particular stress is relevant to the task, task-irrelevant acute stress and, in particular, chronic stress have been largely shown to be detrimental, as measured by assays of both memory and neurogenesis30,31. Therefore, we hypothesized that immune-deficient mice with a maladaptive response to stress would also exhibit learning impairments
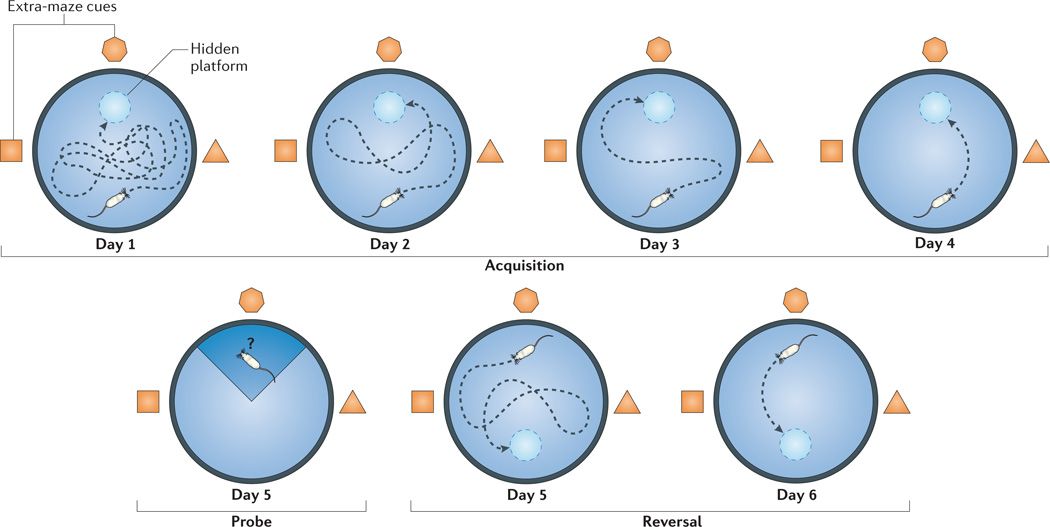
Using the Morris water maze — a classic assay of spatial learning and memory in which animals must find an underwater platform on the basis of extra-maze cues (FIG. 1) — initial studies indeed demonstrated that SCID mice are severely impaired in the acquisition of this task compared with wild-type controls12,13. These results were recapitulated using different strains of immunocompromised mice, as well as using biological (antibody-mediated) and pharmacological methods to deplete T cells (see below).
Figure 1. A schematic representation of the Morris water maze.
The Morris water maze is a hippocampus-dependent spatial learning task. Mice are introduced individually into a pool that is 1 m in diameter and filled with opaque water (non-toxic paint). There is a hidden platform just beneath the surface of the water, and extra-maze cues are spread throughout the room to allow the mouse to learn the platform location with respect to visuospatial cues. The ‘acquisition’ portion of the task (that is, the portion in which learning is measured) consists of four trials per day of 60 second duration (or until the platform is found) with 5 minute intervals. On day 1 (usually after 60 seconds of failure to find the platform), mice are placed on the platform and are allowed to stay there for 20 seconds. During subsequent trials and days of acquisition, the distance travelled by the mice to reach the hidden platform and the time taken to reach the platform (that is, the latency) are measured with computerized equipment. After 4 days of acquisition, the platform is removed, and the mice are introduced to a single ‘probe’ trial (measuring memory) in which the time spent in the original platform quadrant of the pool is measured. During the next 2 days (the ‘reversal’ portion of the task), the platform is returned to the pool, but to a location opposite to the original one. Four trials per day are performed to measure the ability of the mice to relearn the modified task; this ability is an indication of memory plasticity. After 2 days of reversal, a ‘visible’ trial is performed, wherein the platform is clearly visible, to ensure that basic behaviour in the water is comparable between strains (not shown).
Importantly, restoration of the immune compartment of nude or SCID mice through the adoptive transfer of splenocytes from wild-type donors resulted in improved learning behaviour, whereas splenocyte populations that were depleted of T cells did not have this effect12–14,32. Furthermore, chimeric mice that were generated using bone marrow from SCID mice (and therefore lacked functional T cells) were markedly impaired in the Morris water maze test compared with control mice. This learning deficit was reversed 2 weeks after injection with splenocytes from wild-type mice12–14,32. Similar results were obtained following the depletion of T cells using CD4-specific antibodies, but not when CD8-specific antibodies were used33,34. These results indicate that CD4+ T cells are involved (directly or indirectly) in learning behaviour.
Pro-cognitive T cells: the location
From the time that T cells were first suggested to have a positive effect on learning behaviour12, one of the principal questions has been to determine where their functional effects occur. T cells are rarely detected in the brain parenchyma of naive or trained mice. However, it is possible that T cells do penetrate the CNS parenchyma, but only in very small numbers and for short time periods, rendering them virtually undetectable by available technology. The effect of T cells on the CNS might also be mediated via soluble cytokines that are released into the circulation. This raises the issue of the variability of blood–brain barrier permeability and how this influences the possibility of a peripheral (systemic) T cell effect.
So, if the T cell effect is not mediated in the parenchyma, what other options exist? An alternative location to consider as the site of a T cell-mediated effect on learning behaviour is at the ‘boundaries’ of the brain, namely the multipartite meningeal structures35. These structures comprise the leptomeninges, the choroid plexus and the perivascular spaces, all of which are bathed in cerebrospinal fluid (CSF) (FIG. 2). Human CSF — by some estimates — contains as many as 5 × 105 T cells36,37 and, in mice, T cells can be routinely retrieved from thoroughly perfused meningeal preparations32,35. Most CSF T cells in humans are CD45RO+ memory T cells, and these cells are proposed to patrol the brain boundaries for pathogens38 while retaining the ability to return to the lymph nodes, as suggested by their expression of CC-chemokine receptor 7 (CCR7) and L-selectin36,39. Separated from the parenchyma by the pia mater, these T cells are uniquely positioned to affect and be affected by the brain.
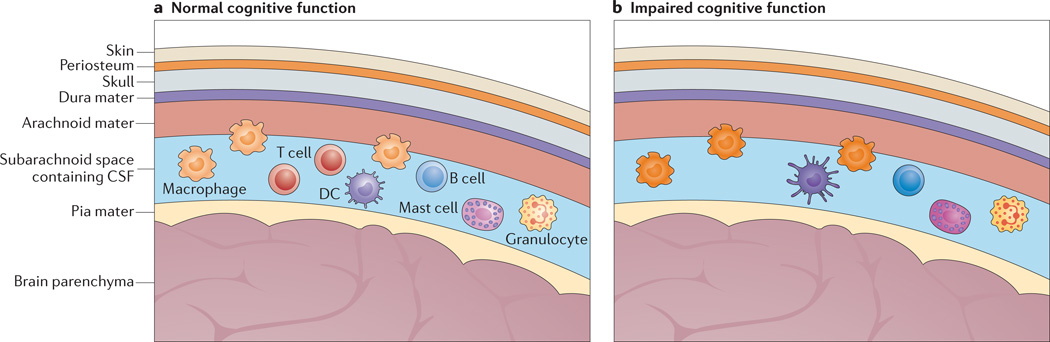
Figure 2. T cell-competent and T cell-deficient meningeal spaces and their effects on learning behaviour.
a | The meninges are a multipartite membrane structure composed of the dura mater, which is in contact with the skull, the arachnoid mater and the pia mater, which is in contact with the brain parenchyma. Cerebrospinal fluid (CSF), within which the majority of meningeal immune cells reside, flows between the arachnoid mater and the pia mater in the subarachnoid space. Meningeal immune cells — including B cells, T cells, dendritic cells (DCs), macrophages, mast cells and granulocytes — are found within the subarachnoid space. Access from blood vessels to the meningeal spaces requires cells to penetrate through the blood–meningeal barrier (not shown). In the pr esence of meningeal T cells, the phenotype of meningeal myeloid cells is kept ‘in check’, and normal cognitive function is ensured. b | In the absence of T cells, the meningeal myeloid cells acquire a pro-inflammatory phenotype, which interferes with learning behaviour.
Although it is not well understood how T cells migrate into and out of the CSF in the healthy brain, the migration of immune cells across brain boundaries has been intensively studied in the inflamed CNS35,40–42, for example in experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis35,38,43,44. Several routes have been described for T cell migration into the CNS38, all of which might also be involved in the ability of T cells to patrol the healthy brain.
We have recently demonstrated that the performance of cognitive tasks by mice is accompanied by a sustained increase in T cell numbers in the meningeal spaces, and that these T cells exhibit an activated phenotype32,45. Treatment of mice with an antibody specific for the integrin VLA4 (which blocks T cell transmigration) or with FTY720 (which traps T cells in the lymph nodes) resulted in a substantial decrease in the numbers of T cells in the meningeal spaces and impaired Morris water maze performance32,45. The localized reduction in T cell numbers as a result of such treatments and concomitant impaired learning suggest that T cells mediate their effects on learning via the CSF and meningeal spaces (FIG. 2); however, further evidence is needed to substantiate this.
Pro-cognitive T cells: mechanism of action
In an attempt to understand the contribution of T cells to learning behaviour, we asked whether T cells actively benefit the brain or whether their presence is necessary to arrest processes that are detrimental to brain function. In other words, is the T cell-mediated beneficial effect on learning behaviour direct or indirect?
Numerous types of immune cell — including B and T cells, granulocytes, macrophages, mast cells and dendritic cells — reside within the meningeal structures of the brain32,35,46–48 (FIG. 2), and therefore the loss of T cells in immunocompromised mice would be likely to affect other immune cells that are resident in the meninges. Indeed, in the absence of T cells it was recently shown that meningeal myeloid cells acquire a pro-inflammatory phenotype. These pro-inflammatory myeloid cells produce cytokines such as interleukin-1β (IL-1β), IL-12 and tumour necrosis factor (TNF), all of which have been previously shown to negatively affect brain function when provided peripherally or intracerebroventricularly49,50. What leads to this pro-inflammatory skew in the phenotype of meningeal myeloid cells is unknown, but one possibility is that endogenous molecules associated with stress underlie this response.
The danger model proposed by Matzinger in 1994 (REF. 51) posits that trauma- or pathogen-mediated tissue damage results in the release of innate signals that direct a strong immune response. Extending this model, we propose that learning-associated stress and brain activity may release mediators that direct the immune response to assume a ‘protective’ role. To this end, we propose that there is a brain-derived set of molecular cues that is analogous to the set of damage-associated molecular patterns (DAMPs). These cues would be released not as a result of overt damage, but rather during any condition wherein the fine physiological balance of the CNS is altered by salient learning- or stress-associated stimuli. Such stimuli could be either ‘positive’, as in the case of appetitive or psychological rewards, or ‘negative’, as in the case of fear-based conditioning using aversive stimuli such as foot shocks. The cues induced by these stimuli could include molecular patterns shed by neurons and/or glial cells — for example, myelin debris — and/or canonical neuron-derived signalling molecules, such as neurotransmitters and neuropeptides. When released from the brain into CSF or blood, these molecular patterns and neurotransmitters could serve as a trigger for meningeal myeloid cells, lymphocytes, mast cells and dendritic cells to assume a pro-cognitive agenda (FIG. 3).
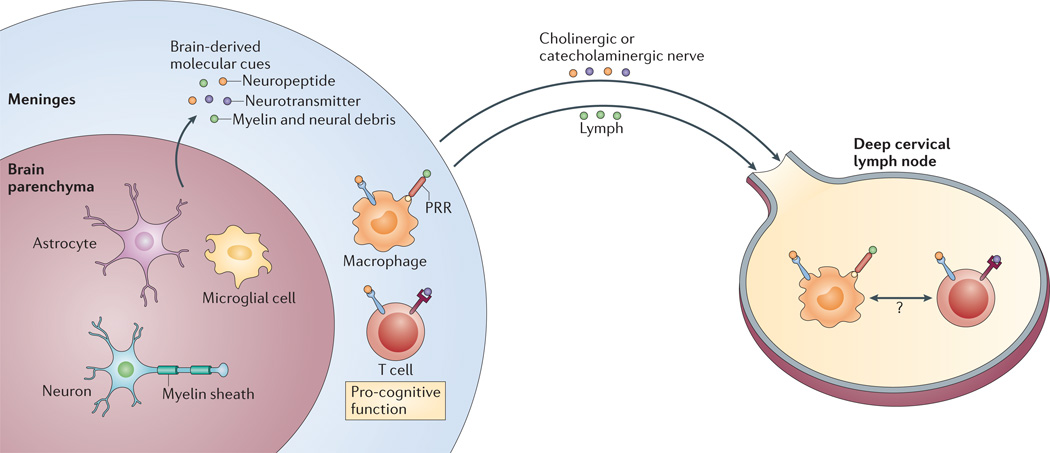
Figure 3. Brain-derived molecular cues and their targets.
A brain that is ‘alert’ as a result of performing cognitive tasks or undergoing minor stress produces numerous molecular mediators that signal to meningeal immune cells, the draining lymph nodes and possibly also lymph node-resident neural cells. These molecular mediators include myelin and neural debris, neurotransmitters and neuropeptides. Neurotransmitters and neuropeptides can interact with different immune cells directly through their specific receptors expressed on the immune cells. Molecular patterns such as myelin and neuronal debris possibly activate meningeal myeloid cells via pattern-recognition receptors (PRRs), as well as being processed by antigen-presenting cells (in the meninges or in the draining lymph nodes), leading to the activation of antigen-specific T cells.
Several neurotransmitters — including acetylcholine and the catecholamine family members adrenaline, noradrenaline and dopamine — are well-described players in both the propagation and resolution of immune responses (reviewed in REF. 52). The thymus, lymph nodes, bone marrow and spleen are all innervated by cholinergic and catecholaminergic fibres53. Although neurotransmitter signalling is typically thought of in terms of the synapses found in the CNS and peripheral nervous system, it has also been established that neurotransmitters released by neurons into the non-synaptic extracellular space propagate signals over considerable distances via high-affinity neurotransmitter receptors54,55. Immune cells express a wide range of receptors for adrenaline, noradrenaline and dopamine. Stress, in particular, is associated with spikes in the circulating levels of these catecholamines.
It has been shown that, following injury, vagus nerve activity leads to the release of the immunomodulatory neurotransmitter acetylcholine in the spleen, thereby inhibiting the release of pro-inflammatory cytokines by splenic myeloid cells expressing the appropriate receptors10,52. Although this phenomenon has been well studied, it remained a paradox that the nerves terminating in the spleen did not themselves produce acetylcholine, but rather the catecholamine adrenaline. Surprisingly, a small but significant population of splenic memory T cells was shown to be the key local producer of the acetylcholine, suggesting that T cells are the final link in this neuroimmune chain, termed the ‘inflammatory reflex’ (REFS 10,52).
The neurotransmitter dopamine has also received attention for having a substantial immunomodulatory function. Analogous to the example involving acetylcholine, it was shown that lymphocytes produce dopamine themselves, which suggests the possibility of dopaminergic autocrine regulation56. Dopamine signalling through the D1 and D5 receptors in regulatory T cells was shown to attenuate the suppressive properties of these cells, and thus possibly functions as an immune ‘emergency brake release’ in the case of stress or tissue injury57. Intriguingly, dopamine was also shown to directly induce the selective secretion of either TNF or IL-10 by human T cells, depending on the specific dopamine receptors that were engaged58. The finding that dopaminergic signalling through β-adrenoceptors in dendritic cells suppresses the production of IL-12 (REF. 59) suggests that T cells activated by these dendritic cells would be skewed towards a T helper 2 (TH2)-type phenotype and IL-4 production.
In addition to the antigen-independent activation of T cells by signalling molecules such as neurotransmitters and neuropeptides, molecular cues may drain into the deep cervical lymph nodes and be processed by lymph node antigen-presenting cells and presented to resident T cells (FIG. 4). The phenotype of the T cells that respond to these molecular cues will, based on our hypothesis, dictate the phenotype of the meningeal innate immune response.
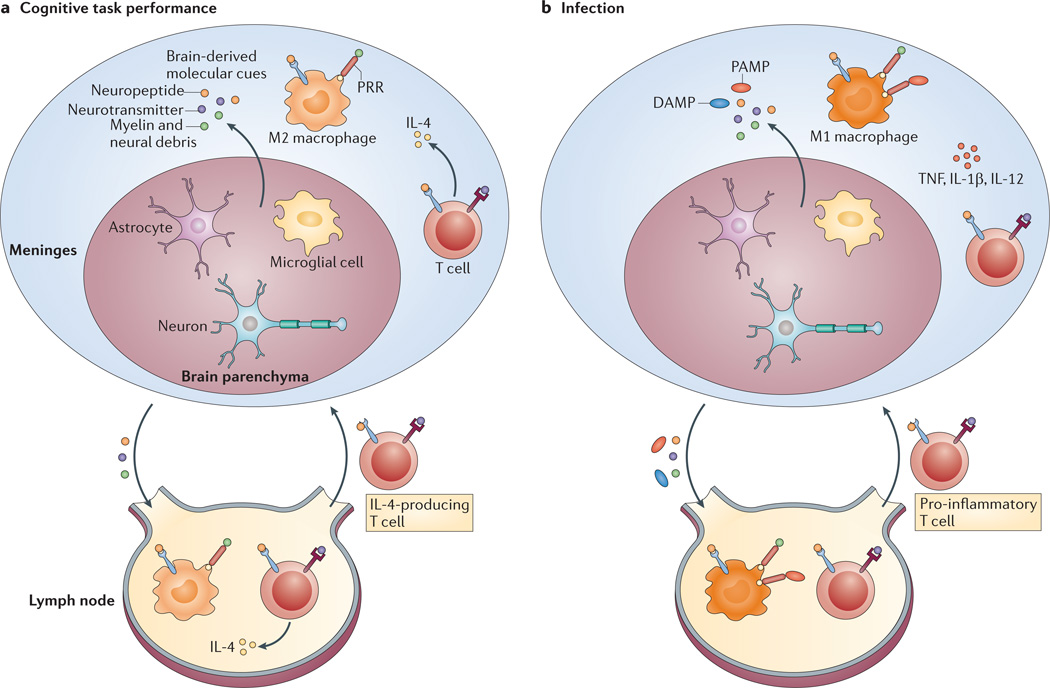
Figure 4. A model for the physiological recall of T cells to support learning behaviour versus a response to a pathogen.
a | We propose that, under physiological conditions, cognitive task performance or minor stress results in the release of brain-derived molecular cues from the ‘alert’ brain that trigger a specific T cell response, predominantly resulting in the production of interleukin-4 (IL-4). IL-4-producing T cells are also recalled from the draining deep cervical lymph nodes to the meningeal spaces and maintain meningeal myeloid cells (depicted as macrophages) in an M2, anti-inflammatory state. b | In the presence of a pathogen, the draining lymph nodes receive signals from pathogen-associated molecular patterns (PAMPs) and/or damage-associated molecular patterns (DAMPs), which dominate the T cell response regardless of the presence of the brainderived molecular cues. Consequently, pro-inflammatory T cells are recalled to the meninges to fight off pathogens, meningeal myeloid cells adopt an M1, pro-inflammatory state and cognitive function is impaired. PRR, pattern-recognition receptor; TNF, tumour necrosis factor.
A pro-cognitive cytokine: IL-4
After mice perform learning and memory tasks, the activated T cells found in their meningeal spaces express high levels of IL-4 (REF. 32). Furthermore, the learning behaviour of Il4−/− mice is substantially impaired compared with that of wild-type mice, and this effect can be reversed by the injection of wild-type T cells but not Il4−/− T cells32. Both wild-type and Il4−/− T cells that were transferred to SCID mice were shown to reach and populate meningeal spaces within 2–3 weeks. In fact, SCID mice that received Il4−/− T cells had higher numbers of T cells in the meningeal spaces after 2–3 weeks compared with SCID mice that received wild-type T cells32. After these mice performed the Morris water maze task, meningeal myeloid cells in SCID mice that received Il4−/− T cells exhibited a skewed, pro-inflammatory phenotype (that is, an M1 phenotype), whereas a balanced cytokine response by meningeal myeloid cells was observed in SCID mice injected with wild-type T cells32. Furthermore, the injection of SCID mice with autologous bone marrow-derived macrophages that were skewed towards an M2 phenotype improved learning behaviour45.
These results support an indirect mode of T cell-mediated support of learning behaviour. Indeed, they suggest that, in the absence of T cells (or IL-4), meningeal myeloid cells respond to molecular cues from the alerted brain in a pro-inflammatory manner and that the resulting meningeal environment contributes to the impairment of learning.
However, IL-4 may also have a direct beneficial effect on learning behaviour, as IL-4 has been shown to induce astrocytic expression of brain-derived neurotrophic factor (BDNF)32, which is crucial for cognitive task acquisition60–63. Thus, in addition to maintaining the M2 phenotype of meningeal myeloid cells, IL-4 could directly mediate an improvement in learning behaviour via the upregulation of BDNF expression by neural cells. The contributions of these individual pathways to learning, as well as the contributions of other possible targets of IL-4, still need to be fully addressed.
Pro-cognitive T cells: antigenic specificity
To begin tackling the issue of the antigen specificity of pro-cognitive T cells, T cell receptor (TCR)-transgenic mice were examined for learning and memory defects. Mice bearing CD4+ T cells that express a TCR specific for ovalbumin exhibit learning impairments, whereas mice bearing CD4+ T cells specific for the CNS autoantigen MBP exhibit a learning ability equal to or even superior to that of wild-type controls14. These data could be interpreted to suggest that autoreactive T cells similar to those shown to be protective following CNS injury (see above) are necessary for normal learning and memory. However, this interpretation could be an oversimplification, as the data represent only two transgenic strains of mice. The activation status of the T cells, rather than their antigenic specificity, might instead be key. Indeed, activated T cells are undetectable in mice with ovalbumin-specific CD4+ T cells, as the epitope that they recognize is not present in mice, whereas activated T cells are constantly present in mice with MBP-specific T cells, because their cognate antigen is constitutively expressed in the CNS.
Further experiments are needed to examine learning in TCR-transgenic mice bearing T cells specific for a non-CNS self antigen or in mice with ovalbumin-specific CD4+ T cells after vaccination with ovalbumin (with and without ovalbumin presentation in the meningeal spaces).
Pro-cognitive T cells: a working hypothesis
Our working model proposes that stress or any other salient CNS stimulation causes the release of molecular cues that result in the activation of meningeal myeloid cells. In addition, memory T cells are activated either in an antigen-specific manner in the cervical lymph nodes or by cytokines (in an antigen-independent manner) in the case of resident meningeal T cells. These T cells ensure that meningeal myeloid cells have the appropriate phenotype. It is still not clear, however, why either of these activation paradigms result in the induction of IL-4-producing T cells or whether these T cells are classical or unconventional TH2 cells. However, the nature of an immune response is influenced by tissue type64. For example, adiponectins, which are secreted by adipocytes, have been shown to affect Toll-like receptor signalling and to modulate the expression of both anti-inflammatory and pro-inflammatory cytokines by myeloid cells65. Similarly, it is possible that the CNS normally directs local immune cells towards a ‘pro-cognitive’ response by inducing T cells to produce IL-4, a cytokine that can have both direct effects and indirect effects (via myeloid cells) on learning behaviour.
As proposed above, T cells that are present within the boundaries of the brain are capable of responding directly to molecular signals associated with brain activity. In this case, the salient molecular signals would be considered to be ‘self ’ but also benign in nature. Therefore, the resulting T cell response would be IL-4 dominated, preventing the skewing of meningeal myeloid cells towards a pro-inflammatory phenotype (FIG. 4).
Molecular signals associated with bacterial or viral infection in the CSF would be perceived as non-self and would induce a different type of immune response by T cells. In this case, the response would be driven by antigens or by molecular patterns that are recognized by Toll-like receptors or other pattern-recognition receptors (FIG. 4). An infection-associated non-self response would probably take precedence over a ‘benign-self ’ response and could offer an explanation for the cognitive impairment associated with infection49. Based on this hypothesis, injection of lipopolysaccharide in conjunction with a cognitive task or stress would indeed result in impaired learning behaviour49, simply because T cells normally associated with learning and memory would acquire a phenotype better focused on fighting bacterial infection, thus potentiating the pro-inflammatory skewing of meningeal myeloid cells.
A concluding perspective
In this article we have presented a model describing how and where T cells can affect learning behaviour. We freely acknowledge that our interpretation of the paucity of available data in this field could be well off the mark, but nevertheless we hope that it is worth recording in the interests of stimulating curiosity and debate concerning the fascinating interactions between the immune and nervous systems. It is possible that responses similar to the T cell-mediated maintenance of brain function we have described here could be taking place in other parts of the body66. Each organ might possess patrolling T cells that respond to unique organ-specific molecular cues (such as hormones, soluble molecules and molecular patterns) that are associated with a stress to that organ. By alerting T cells, such cues might trigger an immune response directed towards tissue maintenance.
Acknowledgements
We thank S. Smith and N. Watson for editing the manuscript. We thank the members of the Kipnis laboratory for their valuable comments during multiple discussions of this work. N.C.D. is the recipient of a Hartwell Foundation postdoctoral fellowship. This work was primarily supported by a grant from the US National Institute on Aging, National Institutes of Health (award AG034113 to J.K.)
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Jonathan Kipnis’s homepage: http://www.medicine.virginia.edu/basic-science/departments/neurosci/faculty/kipnis
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Hohlfeld R, Wekerle H. Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: from pipe dreams to (therapeutic) pipelines. Proc. Natl Acad. Sci. USA. 2004;101(Suppl. 2):14599–14606. doi: 10.1073/pnas.0404874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by TH1 and TH17 cells. Nature Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huseby ES, Sather B, Huseby PG, Goverman J. Age-dependent T cell tolerance and autoimmunity to myelin basic protein. Immunity. 2001;14:471–481. doi: 10.1016/s1074-7613(01)00127-3. [DOI] [PubMed] [Google Scholar]
- 4.Korn T, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nature Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α–PU.1 pathway. Nature Med. 2011;17:64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanz TV, et al. Angiotensin II sustains brain inflammation in mice via TGF-β. J. Clin. Invest. 2010;120:2782–2794. doi: 10.1172/JCI41709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Goehler LE, et al. Interleukin-1β in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J. Neurosci. 1999;19:2799–2806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 10.Rosas-Ballina M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen H, et al. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J. Neurobiol. 2006;66:552–563. doi: 10.1002/neu.20249. [DOI] [PubMed] [Google Scholar]
- 12.Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc. Natl Acad. Sci. USA. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav. Immun. 2008;22:861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Ziv Y, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nature Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 15.Yoles E, Schwartz M. Degeneration of spared axons following partial white matter lesion: implications for optic nerve neuropathies. Exp. Neurol. 1998;153:1–7. doi: 10.1006/exnr.1998.6811. [DOI] [PubMed] [Google Scholar]
- 16.Yoles E, et al. Protective autoimmunity is a physiological response to CNS trauma. J. Neurosci. 2001;21:3740–3748. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kipnis J, et al. Neuronal survival after CNS insult is determined by a genetically encoded autoimmune response. J. Neurosci. 2001;21:4564–4571. doi: 10.1523/JNEUROSCI.21-13-04564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moalem G, et al. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nature Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 19.Moalem G, et al. Autoimmune T cells retard the loss of function in injured rat optic nerves. J. Neuroimmunol. 2000;106:189–197. doi: 10.1016/s0165-5728(00)00240-x. [DOI] [PubMed] [Google Scholar]
- 20.Hauben E, et al. Posttraumatic therapeutic vaccination with modified myelin self-antigen prevents complete paralysis while avoiding autoimmune disease. J. Clin. Invest. 2001;108:591–599. doi: 10.1172/JCI12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauben E, et al. Vaccination with dendritic cells pulsed with peptides of myelin basic protein promotes functional recovery from spinal cord injury. J. Neurosci. 2003;23:8808–8819. doi: 10.1523/JNEUROSCI.23-25-08808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frenkel D, et al. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J. Neurol. Sci. 2005;233:125–132. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Moalem G, et al. Production of neurotrophins by activated T cells: implications for neuroprotective autoimmunity. J. Autoimmun. 2000;20:6421–6430. doi: 10.1006/jaut.2000.0441. [DOI] [PubMed] [Google Scholar]
- 24.Serpe CJ, Byram SC, Sanders VM, Jones KJ. Brain-derived neurotrophic factor supports facial motoneuron survival after facial nerve transection in immunodeficient mice. Brain Behav. Immun. 2005;19:173–180. doi: 10.1016/j.bbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Shaked I, et al. Protective autoimmunity: interferon-gamma enables microglia to remove glutamate without evoking inflammatory mediators. J. Neurochem. 2005;92:997–1009. doi: 10.1111/j.1471-4159.2004.02954.x. [DOI] [PubMed] [Google Scholar]
- 26.Garg SK, Banerjee R, Kipnis J. Neuroprotective immunity: T cell-derived glutamate endows astrocytes with a neuroprotective phenotype. J. Immunol. 2008;180:3866–3873. doi: 10.4049/jimmunol.180.6.3866. [DOI] [PubMed] [Google Scholar]
- 27.Shechter R, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewitus GM, et al. Vaccination as a novel approach for treating depressive behavior. Biol. Psychiatry. 2009;65:283–288. doi: 10.1016/j.biopsych.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Lewitus GM, Cohen H, Schwartz M. Reducing post-traumatic anxiety by immunization. Brain Behav. Immun. 2008;22:1108–1114. doi: 10.1016/j.bbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:742–755. doi: 10.1016/j.pnpbp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Goshen I, et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- 32.Derecki NC, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf SA, et al. Adaptive peripheral immune response increases proliferation of neural precursor cells in the adult hippocampus. FASEB J. 2009;23:3121–3128. doi: 10.1096/fj.08-113944. [DOI] [PubMed] [Google Scholar]
- 34.Wolf SA, et al. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J. Immunol. 2009;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- 35.Kivisakk P, et al. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann. Neurol. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kivisakk P, et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc. Natl Acad. Sci. USA. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kivisakk P, Tucky B, Wei T, Campbell JJ, Ransohoff RM. Human cerebrospinal fluid contains CD4+ memory T cells expressing gut- or skin-specific trafficking determinants: relevance for immunotherapy. BMC Immunol. 2006;7:14. doi: 10.1186/1471-2172-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Kivisakk P, et al. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann. Neurol. 2004;55:627–638. doi: 10.1002/ana.20049. [DOI] [PubMed] [Google Scholar]
- 40.Trettel F, Di Angelantonio S, Limatola C, Ransohoff RM. Chemokines and chemokine receptors in the nervous system: Rome, 27/28 October, 2007. J. Neuroimmunol. 2008;198:1–8. doi: 10.1016/j.jneuroim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog. Neurobiol. 2008;84:116–131. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kivisakk P, et al. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann. Neurol. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulze-Topphoff U, et al. Activation of kinin receptor B1 limits encephalitogenic T lymphocyte recruitment to the central nervous system. Nature Med. 2009;15:788–793. doi: 10.1038/nm.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cayrol R, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nature Immunol. 2008;9:137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- 45.Derecki NC, Quinnies KM, Kipnis J. Alternatively activated myeloid (M2) cells enhance cognitive function in immune compromised mice. Brain Behav. Immun. 2011;25:379–385. doi: 10.1016/j.bbi.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nautiyal KM, Liu C, Dong X, Silver R. Blood-borne donor mast cell precursors migrate to mast cell-rich brain regions in the adult mouse. J. Neuroimmunol. 2011;240–241:142–146. doi: 10.1016/j.jneuroim.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nautiyal KM, Ribeiro AC, Pfaff DW, Silver R. Brain mast cells link the immune system to anxiety-like behavior. Proc. Natl Acad. Sci. USA. 2008;105:18053–18057. doi: 10.1073/pnas.0809479105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anandasabapathy N, et al. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J. Exp. Med. 2011;208:1695–1705. doi: 10.1084/jem.20102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelley KW, et al. Cytokine-induced sickness behavior. Brain Behav. Immun. 2003;17:S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 51.Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 52.Tracey KJ. Reflex control of immunity. Nature Rev. Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 54.Vizi ES. Role of high-affinity receptors and membrane transporters in nonsynaptic communication and drug action in the central nervous system. Pharmacol. Rev. 2000;52:63–89. [PubMed] [Google Scholar]
- 55.Vizi ES, Kiss JP. Neurochemistry and pharmacology of the major hippocampal transmitter systems: synaptic and nonsynaptic interactions. Hippocampus. 1998;8:566–607. doi: 10.1002/(SICI)1098-1063(1998)8:6<566::AID-HIPO2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 56.Alaniz RC, et al. Dopamine β-hydroxylase deficiency impairs cellular immunity. Proc. Natl Acad. Sci. USA. 1999;96:2274–2278. doi: 10.1073/pnas.96.5.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kipnis J, et al. Dopamine, through the extracellular signal-regulated kinase pathway, downregulates CD4+CD25+ regulatory T-cell activity: implications for neurodegeneration. J. Neurosci. 2004;24:6133–6143. doi: 10.1523/JNEUROSCI.0600-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNFα or both. J. Neuroimmunol. 2005;169:161–171. doi: 10.1016/j.jneuroim.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 59.Hasko G, Szabo C, Nemeth ZH, Deitch EA. Dopamine suppresses IL-12 p40 production by lipopolysaccharide-stimulated macrophages via a β-adrenoceptor-mediated mechanism. J. Neuroimmunol. 2002;122:34–39. doi: 10.1016/s0165-5728(01)00459-3. [DOI] [PubMed] [Google Scholar]
- 60.Tokuyama W, Okuno H, Hashimoto T, Xin Li Y, Miyashita Y. BDNF upregulation during declarative memory formation in monkey inferior temporal cortex. Nature Neurosci. 2000;3:1134–1142. doi: 10.1038/80655. [DOI] [PubMed] [Google Scholar]
- 61.Kemppainen S, et al. Impaired TrkB receptor signaling contributes to memory impairment in APP/PS1 mice. Neurobiol. Aging. 2012;33:1122.e23–1122.e39. doi: 10.1016/j.neurobiolaging.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Meis S, Endres T, Lessmann V. Postsynaptic BDNF signalling regulates long-term potentiation at thalamo-amygdala afferents. J. Physiol. 2012;590:193–208. doi: 10.1113/jphysiol.2011.220434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu YF, et al. Upregulation of hippocampal TrkB and synaptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol. Learn. Memory. 2008;90:81–89. doi: 10.1016/j.nlm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 64.Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nature Rev. Immunol. 2011;11:221–230. doi: 10.1038/nri2940. [DOI] [PubMed] [Google Scholar]
- 65.Yamaguchi N, et al. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005;579:6821–6826. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 66.Tumanov AV, et al. T cell-derived lymphotoxin regulates liver regeneration. Gastroenterology. 2009;136:694–704. doi: 10.1053/j.gastro.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]