Abstract
Autoantibodies to citrullinated protein antigens are specific markers of rheumatoid arthritis (RA). Although protein citrullination can be activated by numerous stimuli in cells, it remains unclear which of these produce the prominent citrullinated autoantigens targeted in RA. In these studies, we show that RA synovial fluid cells have an unusual pattern of citrullination with marked citrullination of proteins across the broad range of molecular weights, which we term cellular hypercitrullination. Although histone citrullination is a common event during neutrophil activation and death induced by different pathways including apoptosis, NETosis, and necroptosis/autophagy, hypercitrullination is not induced by these stimuli. However, marked hypercitrullination is induced by two immune-mediated membranolytic pathways, mediated by perforin and the membrane attack complex (MAC), which are active in the RA joint and of importance in RA pathogenesis. We further demonstrate that perforin and MAC activity on neutrophils generate the profile of citrullinated autoantigens characteristic of RA. These data suggest that activation of peptidylarginine deiminases during complement and perforin activity may be at the core of citrullinated autoantigen production in RA. These pathways may be amenable to monitoring and therapeutic modulation.
Introduction
Protein citrullination, the enzymatic conversion of peptidyl-arginine residues to citrulline, is a posttranslational modification mediated by the family of calcium-dependent peptidylarginine deiminases (PADs). To date, 5 human PAD isoenzymes have been identified and designated PAD1–4 and PAD6. Protein citrullination has been implicated in several physiological and biochemical processes including moisturizing of the skin, hair follicle formation and gene regulation (1, 2). Citrullination is also an important modulator of immune effector functions including chemokine regulation (3) and the formation of neutrophil extracellular traps (NETs) (4).
Abnormal protein citrullination has been suggested to play a pathogenic role in RA. Citrullinated proteins are one of the most specific targets of autoantibodies in RA, and the targets of these antibodies are abnormally expressed and highly enriched in synovial tissue and fluid of RA patients (5–8). Although numerous mechanisms (e.g. cell death and various inflammatory stimuli like LPS, TNFα, and f-MLP) activate PADs in cells (2, 9), the contribution of these processes to the production of citrullinated autoantigens in RA is still unknown. Additionally, since PADs require millimolar concentrations of calcium to citrullinate protein substrates in vitro (10), while intracellular concentrations of calcium typically do not rise above micromolar levels (11–14), it has been suggested that citrullination of intracellular autoantigens may occur extracellularly after release from dying cells (6).
In these studies, we demonstrate that citrullination in the RA joint is cell-associated, and that it is characterized by prominent citrullination of a broad range of proteins. We term this pattern ‘cellular hypercitrullination’. Interestingly, pathways which induce histone citrullination, such as cell activation (e.g. cytokines) and cell death (including apoptosis, NETosis and autophagy/necroptosis), are unable to reproduce the hypercitrullination observed in the RA joint. Instead, hypercitrullination is prominently induced by immune-mediated membranolytic pathways (via perforin and MAC), which are active in the RA joint. Furthermore, examining the whole cell citrullinome shows that perforin and MAC induce the citrullination of numerous autoantigens described to date in RA. Together, these studies focus attention on previously unappreciated mechanistic connections between immune-mediated membranolytic pathways and the activation of the PAD enzymes in RA, and suggest amplification mechanisms potentially amenable to therapy.
Results
Cells from RA synovial fluid show hypercitrullination and activation of the extrinsic apoptotic cell death pathway
Studies of protein citrullination in the rheumatoid joint have focused on synovial tissue and the soluble phase of synovial fluid (SF) (5, 6, 8), but not on the cells contained in the SF. These are largely neutrophils and monocytes (15), which are the major sources of PADs in the rheumatoid joint (7). We initially examined protein citrullination in SF cell pellets from 12 individuals with RA (Fig. 1A and table S1). In one patient, serial samples obtained ~1 month apart were also available (Fig. 1A, lanes 4 to 6). Cellular hypercitrullination (protein citrullination spanning the entire range of molecular weights) was prominent, with variation in the intensity among patients (Fig. 1A) and among the serial samples obtained from the same individual (Fig. 1A, lanes 4 to 6). Heterogeneity and dynamic changes therefore appear to be features of cellular hypercitrullination in RA SF.
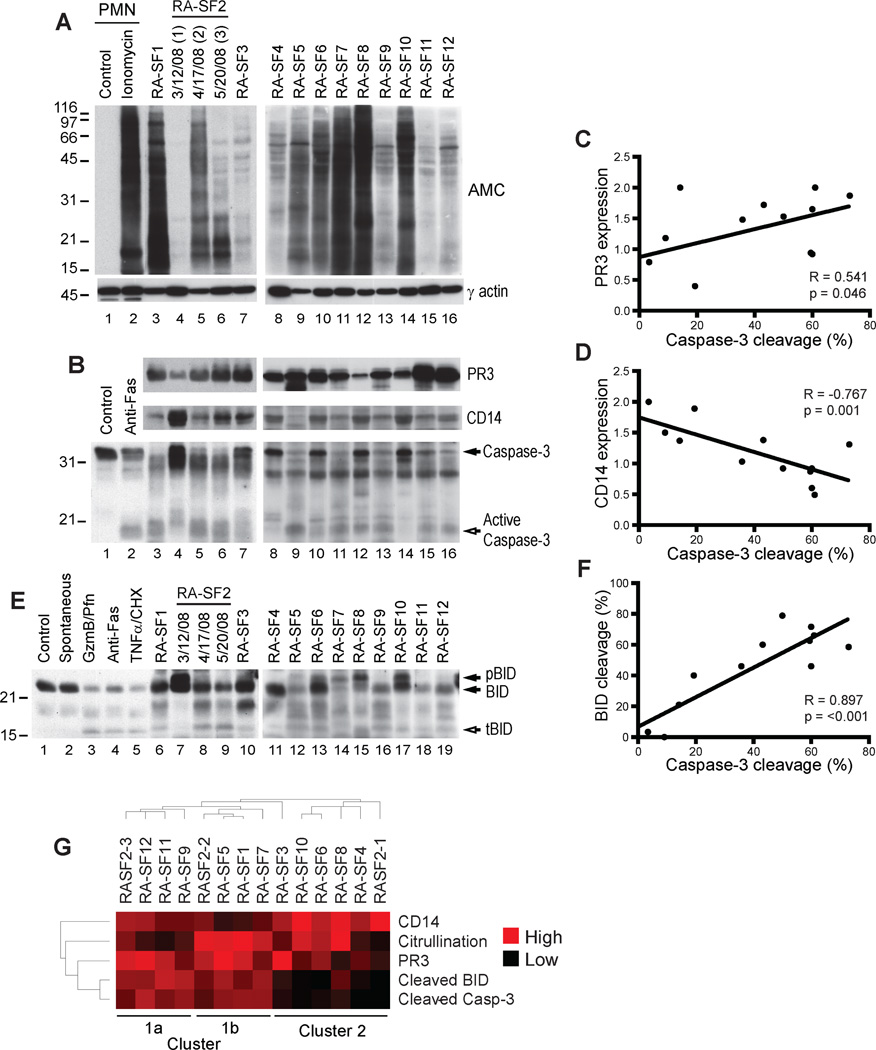
Fig. 1. RA SF cells show hypercitrullination and extrinsic apoptotic cell death.
(A) Protein citrullination in SF cells from 12 RA patients was analyzed by immunoblotting electrophoresed cell lysate using AMC and γ actin (loading control) antibodies. For RA-SF2, data from three samples collected on different dates is shown. Ionomycin was used as a positive control for citrullination. Intervening lanes containing irrelevant data between lanes 2 and 3 were spliced out. PMN, polymorphonuclear cells.(B and E) SF cell lysate was immunoblotted using PR3, CD14, caspase-3 (B), and BID (E) antibodies. Neutrophilsdying by anti-Fas (B and E) or spontaneous apoptosis (E) were positive controls for caspase-3activation, and granzyme B/perforin (GzmB/Pfn) and TNFα/cycloheximide (CHX) (E) were positive controls for BID activation. Filled arrows denote intact proteins, and the unfilled arrows mark active products. pBID, phosphorylated BID; tBID, truncated BID. (C, D, and F) Expression of PR3 and CD14 and cleavage of caspase-3and BID were quantified by densitometry and normalized to actin. Linear correlations were analyzed using Pearson’s correlation coefficient using a two-tailed α of 0.05. GraphPad Prism 5.0 was used to perform analysis and generate figures. (G) Citrullination, expression of PR3 and CD14, and caspase-3 and BID cleavage were quantified by densitometry and normalized to actin. The normalized values were distributed from 0 (low) to 2 (high) and subjected to unsupervised hierarchical clustering using the Cluster and TreeView software programs (71).
To gain insight into the origin and heterogeneity of cellular hypercitrullination in RA SF, we initially analyzed the expression of neutrophil and monocyte markers (that is, proteinase-3 (PR3) and CD14, respectively) (Fig. 1B, upper and middle panels), as well as the presence of apoptotic cell death (determined by cleavage of caspase-3) (Fig. 1B, lower panel). While expression of PR3 was abundant in the majority of RA SF samples (Fig. 1B, upper panel), CD14 showed more heterogeneity, with a tendency to be higher in samples with lower PR3 expression (Fig. 1B, middle panel). Cellular hypercitrullination was found in both groups, independently of PR3 or CD14 expression. Interestingly, caspase-3 cleavage was significantly associated with the expression of PR3 (Fig. 1C, R = 0.541, p= 0.046), while samples containing a more prominent expression of CD14 had a negative correlation with apoptotic cell death (Fig. 1D, R = −0.767, p=0.001). The strong association between caspase-3 cleavage and PR3 expression in hypercitrullinated samples strongly suggested that in a subgroup of RA SF samples, neutrophil apoptosis and hypercitrullination may be related. This focused our attention on defining the mechanism(s) mediating cell death in RA SF.
BID (BH3-interacting domain death agonist), a pro-apoptotic BH3-only Bcl-2 homologue was used as a marker of ‘extrinsic’ apoptosis, as it is activated by cleavage only in response to death receptor activation or by cytotoxic cells via granzyme B (16). Peripheral blood neutrophils dying spontaneously by apoptosis (that is, intrinsic apoptosis) showed no BID cleavage (Fig. 1E, lane 2) despite efficient caspase-3 activation (Fig. 2A, lane 3). In contrast, neutrophils exposed to granzyme B/perforin or death receptor activation (anti-Fas or TNFα/cycloheximide) showed prominent BID cleavage (Fig. 1E, lanes 3 to 5). In RA SF, BID cleavage was strikingly associated with the cleavage of caspase-3 (Fig. 1F, R = 0.897, p< 0.001), particularly in samples where PR3 expression was high. Indeed, when the data was subjected to unsupervised hierarchical clustering, samples clustered into three groups – cluster 1a, 1b and 2 (Fig. 1G). Cluster 1 was neutrophil predominant, with high PR3 expression and clear activation of caspase-3 and BID. Cluster 1b had higher levels of citrullination than cluster 1a. The data in Cluster 1strongly implicate extrinsic apoptosis pathways in neutrophil death in the RA joint. Samples in Cluster 2 were more CD14-rich. Although cleaved caspase-3 and BID levels were lower in these samples, 5 of 6 cell pellets showed hypercitrullination, suggesting the existence of an alternative hypercitrullination pathway not related to apoptotic cell death (Fig. 1G, cluster 2).
Fig. 2. The granzyme B/perforin pathway efficiently induces hypercitrullination in neutrophils compared to other death and activation stimuli.
(A and B) Apoptosis was induced in purified neutrophils by overnight incubation (spontaneous apoptosis), ultraviolet radiation (UVR), granzyme B/perforin (GzmB/Pfn), anti-Fas, and TNFα/CHX. NETosis was induced using phorbol 12-myristate 13-acetate (PMA) or LPS, and autophagy/necroptosis was induced by incubation with granulocyte-macrophage colony-stimulating factor (GM-CSF) and anti-CD44. Cell stimulation with TNFα was also studied. Neutrophils incubated for 0 or 4 hours were used as negative controls. (C) Neutrophils were incubated for 4 hours at 37°C in the absence (Control-4 hr) or presence of granzyme B/perforin, interleukin 6 (IL6), IL8 endothelial-derived (IL8e), IL8 monocyte-derived (IL8m), G-CSF, GM-CSF, fMLP, or ligands for Toll-like receptor 2 (TLR2) [heat-killed Listeria monocytogenes (HKLM)], TLR5 (flagellin), TLR7/8 (CL075), and TLR9 (CpG-C). Neutrophils without incubation (Control-0′) were also included as a negative control. In (A), samples were analyzed by immunoblotting using anti–caspase-3 antibodies. In (B) and (C), general protein citrullination was visualized by AMC immunoblotting (upper panel) and histone H3 citrullination (Cit-H3) using antibodies against citrullinated histone H3 (citrulline 2 + 8 + 17) (middle panel). The piece of the membrane-containing histone H3 was stripped and reprobed using anti–histone H3 antibodies as loading control (lower panel). The experiments were performed on at least three separate occasions with similar results.
The granzyme B/perforin pathway is a potent inducer of cellular hypercitrullination
We next addressed directly whether extrinsic apoptotic pathways could induce neutrophil hypercitrullination. Extrinsic apoptosis was induced by granzyme B/perforin, Fas ligation, and TNFα/CHX. Although all 3 stimuli induce caspase-3 and BID cleavage (Fig. 2A and 1E, respectively), hypercitrullination was only induced in response to granzyme B/perforin (Fig. 2B, upper panel). To compare these stimuli to other modes of cell death with potential relevance in RA, we also studied (i) neutrophils dying by spontaneous apoptosis or ultraviolet radiation (UVR, which is also a potent inducer of reactive oxygen species (17)), (ii) NETosis (induced by PMA or LPS) (18), and (iii) autophagy/necroptosis (induced with GM-CSF and CD44 ligation) (19). TNFα alone was also included in these studies. Caspase-3 was efficiently cleaved during apoptosis (Fig. 2A, lanes 3 and 4, mean±SD cleavage 63.3 ± 19.4%), whereas NETosis, autophagy/necroptosis and TNFα showed minimal processing of caspase-3 (Fig. 2A, lanes 8 to 11, mean±SD cleavage 11.8 ± 8.9%), confirming previous observations that these conditions do not activate the caspase cascade (19, 20). As described previously (4, 9), NETotic stimuli (that is, PMA and LPS) and TNFα induced citrullination of histone H3 (Cit-H3) (Fig. 2B, middle panel, lanes 8, 9 and 11, respectively). In addition, Cit-H3 was induced during apoptosis and autophagy/necroptosis (Fig. 2B, middle panel, lanes 3–7 and 10, respectively), and to some degree in non-activated cells (Fig. 2B and C, lane 2). However, the granzyme B/perforin pathway was the only death stimulus able to induce hypercitrullination (Fig. 2B, upper panel), highlighting that cellular hypercitrullination and H3 citrullination are quite different in terms of the stimuli which induce them.
To further address whether neutrophil-activating stimuli could induce hypercitrullination, we studied cellular citrullination in neutrophils after exposure to IL6, IL8 endothelial-derived (IL8e), IL8 monocyte-derived (IL8m), G-CSF, GM-CSF, fMLP, and ligands for TLR2, TLR5, TLR7/8, and TLR9. Among these conditions, IL8, TLR2, TLR5, TLR7, TLR9 and G-CSF have been associated with the induction of NETs and fMLP is known to induce Cit-H3 (9, 21–23). While all stimuli induced some Cit-H3 (Fig. 2C, middle panel), none induced cellular hypercitrullination in neutrophils (Fig. 2C, upper panel). Taken together, these data suggest that the striking hypercitrullination observed in apoptotic RA SF cells is a marker of the activity of the granule cytotoxicity pathway in RA. Moreover, the data also stress that quantifying histone citrullination alone is not a precise way to define the citrullination pathways most relevant to RA pathogenesis.
Purified human perforin induces hypercitrullination in target cells
The finding that hypercitrullination is not induced by multiple apoptotic stimuli demonstrated that cell death alone was not sufficient to induce hypercitrullination. The hypercitrullination induced by granzyme B/perforin is therefore likely related to a specific function of this pathway that is distinct from the granzyme B ability to induce cell death. Interestingly, during the process of granzyme delivery, perforin triggers a prominent influx of calcium (24). Since PAD enzymatic activity is dependent on calcium (2), we addressed whether perforin could mediate hypercitrullination in cells in the absence of granzyme B. Indeed, sublytic amounts of perforin alone were sufficient to induce hypercitrullination in neutrophils (Fig. 3A).
Fig. 3. Purified perforin induces hypercitrullination in target cells.
(A and B) Purified neutrophils from 2 different healthy donors (A) or 293T cells expressing PAD2, PAD3 or PAD4 (B), were incubated in the absence or presence of sublytic amounts of perforin for 4 hr at 37°C. After terminating the reactions, samples were analyzed by electrophoresis and proteins visualized by immunoblotting using antibodies against AMC (A and B), PAD2, PAD3 or PAD4 (B), and β-actin as a loading control (A and B). The experiments were performed on at least 4 separate occasions, with similar results.
Neutrophils express three PAD isoenzymes (that is, PAD2, PAD3 and PAD4), but histone H3 citrullination is catalyzed preferentially by PAD4 (25). Since H3 citrullination occurs in the absence of hypercitrullination during many forms of neutrophil activation or apoptosis, it is possible that these stimuli only activate PAD4, and that perforin activates additional PADs which are responsible for hypercitrullination. To define the independent role of PAD isoenzymes in perforin-induced hypercitrullination, the PAD-negative cell line 293T was used to express individual enzymatically active PADs by transient transfection (Fig. S1). In the presence of perforin, hypercitrullination was observed in 293T cells expressing PAD2, PAD3 or PAD4. The most prominent effect was seen with PAD2, followed by PAD4 and PAD3 (Fig. 3B), with each PAD isoenzyme generating a distinct pattern of protein citrullination. The observation that PADs 2, 3 or 4 can each induce hypercitrullination in cells upon exposure to perforin demonstrates that it is the perforin stimulus and not activation of a specific PAD which accounts for this striking citrullination pattern.
Cytotoxic cells (through perforin) are potent inducers of hypercitrullination in target cells
To confirm whether perforin delivered during cytotoxic killing can induce cellular hypercitrullination in target cells, primary neutrophils sensitized with anti-CD16 were killed by antibody-dependent cell-mediated cytotoxicity (ADCC) using autologous lymphokine-activated killer (LAK) cells (effector:target ratios of 5:1). Since LAK cells are 5 times more abundant than neutrophils in the killing assays (interfering with the detection of apoptotic markers in neutrophils), specific target cell killing was determined using the apoptotic substrate gelsolin (26), which is absent in LAK cells but abundant in neutrophils (Fig. 4, upper panel, lanes 1 and 2, respectively). In the presence of LAK cells, neutrophils die by apoptosis (evidenced by the cleavage of gelsolin; Fig. 4, upper panel, lane 3) and generate hypercitrullination (Fig. 4, AMC panel, lane 3).
Fig. 4. Cytotoxic cells induce perforin-dependent and apoptosis independent hypercitrullination of human primary neutrophils.
Purified neutrophils sensitized with anti-CD16 were preincubated in the absence or presence of CMA (lane 5), z-DEVD-FMK (lane 6) or z-VAD-FMK (lane 7), followed by incubation in the absence or presence of LAK cells (killer:target ratio 5:1). Killing assays were also performed in the presence of 8mM EGTA (lane 4). After terminating the reactions, the samples were electrophoresed and immunoblotted using anti-gelsolin, anti-granzyme B (GzmB) (LAK loading control), anti-PR3 (neutrophil loading control), or AMC antibodies. Filled and unfilled arrows denote intact gelsolin and its apoptotic fragments, respectively. The experiments were performed on at least four separate occasions with similar results.
To confirm that hypercitrullination in target cells is calcium and perforin-dependent, target cell killing was induced in the presence of EGTA (which blocks calcium-dependent perforin oligomerization and PAD activity) or the perforin inhibitor concanamycin A (CMA), which only affects the granule exocytosis pathway, but not death receptor (e.g. Fas) mediated death (27). EGTA and CMA abolished cytotoxic cell-induced cleavage of gelsolin and neutrophil hypercitrullination (Fig. 4, upper panel, lane 4 and 5, respectively). To further confirm that the induction of hypercitrullination in target cells is independent of apoptosis, target cell killing was induced in the presence of the potent cell permeable pan-caspase inhibitor zVAD-FMK and the effector caspase inhibitor zDEVD-FMK, which blocks apoptotic cell death. Indeed, caspase inhibitor treatment abolished cytotoxic cell-induced cleavage of gelsolin (Fig. 4, upper panel, lane 6 and 7, respectively), but had no effect on protein citrullination (Fig. 4, AMC panel, lane 6 and 7, respectively). Importantly, although perforin is the only cytotoxic component required for inducing hypercitrullination in target cells, the coupling of perforin release and granzyme entry during cytotoxic lymphocyte killing likely explains the striking co-occurrence of hypercitrullination and extrinsic apoptosis in Cluster 1 in RA SF cells (Fig. 1G).
The rheumatoid joint is enriched with numerous types of killer cells expressing perforin, including CD56brightCD94bright NK cells, NK-like cells, CD8+ cells, CD3+CD57+ cells, CD4+CD161+CD28negCD94neg cells (also expressing CD8-αα homodimer), and NK-22 (NKp44+) cells (28–33). In pilot studies where RNA samples were available, markers of these cell populations were not informative in defining whether specific subtypes of killer cells dominate in RA SF samples with evidence of cytotoxic cell-mediated killing (Fig. S2).
Activation of the complement pathway triggers hypercitrullination
The finding that cell activation and death pathways associated with histone citrullination failed to induce cellular hypercitrullination, demonstrate that these pathways are not responsible for the hypercitrullination seen in RA SF cells which lack prominent evidence of apoptosis. Since perforin and MAC share important properties in terms of membranolytic activity and calcium flux (34, 35), and complement activation appears to be highly relevant to RA pathogenesis (36, 37), we addressed whether MAC formation could induce cellular hypercitrullination, and might be responsible for this particularly in SF cells where activity of extrinsic apoptotic pathways was not prominent. To induce antibody-mediated complement activation on neutrophils, trinitrobenzene sulfonate (TNBS)-treated neutrophils (which generates trinitrophenylated surface proteins) were sensitized with anti-dinitrophenyl (DNP) antibodies and incubated with increasing amounts of autologous serum as a source of complement. In the absence of serum or in the presence of sublytic amounts of complement (1:32 diluted serum), anti-DNP antibodies had no effect on cellular citrullination (Fig. 5A, upper panel, lane 1 and 2, respectively). In contrast, lytic amounts of complement (1:16 and 1:8 dilutions) induced prominent hypercitrullination in sensitized neutrophils (Fig. 5A, upper panel, lane 3 and 4, respectively). The lack of gelsolin cleavage during this process further demonstrates that this pathway is not linked to apoptotic cell death (Fig. 5A, middle panel).
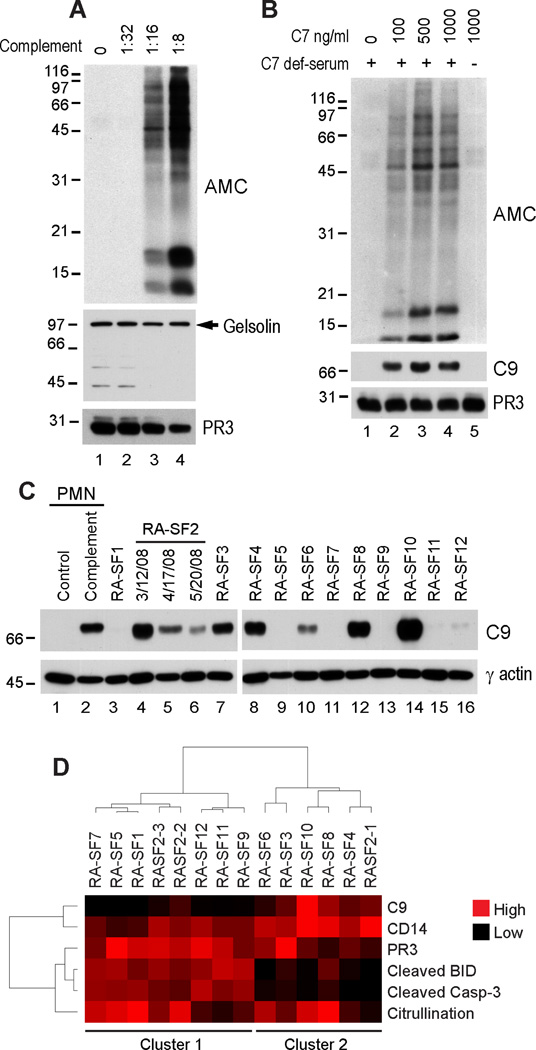
Fig. 5. MAC is a potent inducer of hypercitrullination in neutrophils.
(A and B) TNBS-treated neutrophils sensitized with anti-DNP antibodies were incubated in the absence or presence of increasing amounts of human serum (A) or in the absence (−) or presence (+) of complement C7-deficient serum without or with increasing amounts of purified human C7 (B). After terminating the reactions, the samples were electrophoresed and immunoblotted using anti-gelsolin, anti-C9, anti-PR3 (as loading control), or AMC antibodies. The filled arrow denotes intact gelsolin. The experiments were performed on at least three separate occasions with similar results.(C) RA SF cells were electrophoresed and immunoblotted using anti-C9 or anti-γ-actin (loading control) antibodies. Control (lane 1) and complement treated sensitized neutrophils (lane 2) were used as negative and positive controls for C9 deposition, respectively. (D) C9 levels in C were quantified by densitometry and normalized to actin. The data was then subjected to unsupervised hierarchical clustering (using the Cluster and TreeView software programs) (71) together with the data of citrullination, PR3, CD14, caspase-3 and BID cleavage from Fig. 1G.
To confirm that this process was MAC-dependent, similar assays were performed using serum deficient in complement C7, a component of the terminal pathway of complement that is necessary for MAC formation (38). Strikingly, no hypercitrullination was observed in anti-DNP sensitized neutrophils exposed to complement C7-deficient serum (Fig. 5B, upper panel, lane 1). However, hypercitrullination was restored when increasing amounts of purified C7 were added to C7-deficient serum (Fig. 5B, upper panel, lanes 2 to 4), but not when C7 was used alone (Fig. 5B, upper panel, lane 5).
Complement activation, be it via the classical, mannose-binding lectin, or alternative pathways, ends with the polymerization of C9 into a transmembrane pore-forming structure known as the MAC. Indeed, C9 deposition was detected by immunoblotting in sensitized cells where the MAC was generated (Fig. 5B, middle panel, lanes 2 to 4), but not when MAC formation was impeded by the absence of the terminal complement component C7 (Fig. 5B, middle panel, lane 1). Strikingly, immunoblotting of C9 was prominent in RA SF cell samples, although there was variation among patients (Fig. 5C). When the data was quantified by densitometry and subjected to unsupervised hierarchical clustering together with the additional data quantifying PR3, CD14, citrullination, caspase-3 and BID cleavage (from Fig. 1G), extrinsic apoptosis markers and C9 deposition clustered separately (Fig. 5D), strongly suggesting that complement activation and perforin represent major and potentially independent pathways that induce hypercitrullination in the RA joint.
The MAC/perforin (MACPF) pathway generates citrullinated RA autoantigens
To define whether activity of the MACPF pathway generates citrullinated autoantigens targeted in RA, we defined the perforin- and MAC-induced “citrullinome” by mass spectrometry, and compared it with the citrullinome found in RA SF cells. RA SF cells and cells treated with perforin or complement shared numerous citrullinated proteins (Table S2). In control neutrophils (incubated without perforin or complement for 4 hrs at 37°C), very few citrullinated peptides were identified (Table S2). Since the autoantibody response in RA is directed against a limited number of specificities within the RA synovial citrullinome (8), we focused particular attention on 16 intracellular citrullinated proteins described to date, which have been confirmed to be targeted by autoantibodies in RA (Table 1) (8, 18, 25, 39–45). These included: (i) well-characterized specificities (that is, α-enolase, vimentin, collagen and histones), (ii) less well-characterized specificities (that is, actin, myeloid cell nuclear differentiation antigen, protein disulfide-isomerase, and heat shock 90kDa protein α/β) (8, 25, 43, 44), and (iii) antigens identified using granulocytes that have yet to be confirmed in the rheumatoid joint (that is, adenylyl cyclase-associated protein, aldolase, calreticulin, elongation factor 1a,60kDa heat shock protein, phosphoglycerate kinase 1, and PAD4) (40, 42, 45). Of these 16 citrullinated autoantigens, 13 were identified in RA SF cells, 14 in perforin-treated and 14 in MAC-treated cells (Table 1). Citrullinated autoantigens found in RA SF cells overlapped completely with those found in perforin-treated cells and with 12 autoantigens in the MAC samples. Cellular hypercitrullination and citrullinated autoantigen production are therefore common features that unify RA SF cells and PAD activation induced by immune-mediated pore-forming pathways.
Table 1. Citrullinated autoantigens induced by the MACPF pathway.
+, citrullinated peptides were identified; −, no citrullinated peptides were identified; +/−, potentially citrullinated (see fig. S2 for explanation).
| Citrullinated Autoantigen | Reference | Perforin | Complement | RA SF cells* |
|---|---|---|---|---|
| Actin, cytoplasmic 1/2 | (25) | + | + | + |
| Adenyl cyclase-associated protein | (40) | + | − | − |
| Aldolase | (42) | + | + | + |
| Calreticulin | (42) | + | + | + |
| Collagen type I | (41) | − | + | − |
| Elongation factor 1a | (40) | + | +/− | + |
| α-Enolase | (40) | + | + | + |
| Heat shock 60kDa protein | (42) | + | + | + |
| Heat shock 90kDa protein α/β | (44) | + | + | + |
| Histone H2A | (18) | + | + | + |
| Histone H4 | (18) | + | + | + |
| Myeloid cell nuclear differentiation antigen | (43) | + | + | + |
| Phosphoglycerate kinase 1 | (42) | + | − | + |
| PAD4 | (45) | +/− | +/− | +/− |
| Protein disulfide-isomerase | (8) | − | + | − |
| Vimentin | (39) | + | + | + |
Citrullination was confirmed in at least one RA SF sample
Discussion
The mechanisms underlying the activation of PADs, which generate citrullinated autoantigens in RA, remain unclear. When we examined citrullination in cells isolated from the RA joint, we were struck by the broad spectrum of citrullination across the entire range of molecular weights - a pattern we term cellular hypercitrullination. This was in striking contrast to the much more narrowly focused citrullination described in neutrophils in response to numerous different stimuli such as cytokines, TLR ligands, and N-formylated peptides (9, 21, 22), and during several forms of cell death including NETosis (18, 21, 23), apoptosis and autophagy/necroptosis (Fig. 2), where histone citrullination is prominent. The latter pattern is similar to the limited protein citrullination observed during other physiologic processes, such as cornification of the skin, where citrullination is also restricted to a limited number of substrates (e.g. keratins K1 and K10, filaggrin and trichohyalin), as well as in cells in response to hormones (1, 46). Defining the potential mechanisms underlying the cellular hypercitrullination that characterizes RA SF cells was therefore a major focus of these studies. By characterizing the cells in RA SF based on hypercitrullination, PR3, CD14, caspase-3 and BID activation, and complement C9, we demonstrate that the perforin and MAC pathways are active in the RA joint, and represent two immune-mediated membranolytic pathways with the capacity to citrullinate autoantigens in RA. Unlike other death and activation stimuli tested, the perforin and MAC pathways activate intracellular PADs and induce the pattern of hypercitrullination present in the RA target tissue.
Perforin and complement mediate membranolytic pathways of the cellular and humoral immune response, respectively, and use a common 'MACPF' domain to form pores in membranes to exert their potent anti-pathogen activity (34). Cytotoxic cells and complement are highly expressed and pathologically activated in the RA joint (28–30, 30–33, 36, 37), and their activation products correlate with disease activity and severity in RA (36, 37, 47–50). The mechanisms by which these pathways contribute to joint damage remain unclear. Cytotoxic cells have been implicated in the induction of chondrocyte apoptosis and extracellular matrix degradation (51, 52). Complement activation plays a role in the induction of chemotaxis (e.g. C5a-mediated) and in antibody-mediated tissue damage (e.g. via immune complexes, rheumatoid factor and ACPAs) (36, 37). Although these important pathways have had no obvious connection with citrullination or provision of autoantigens in RA, we show here that their common effector function (for example, the formation of pores in membranes) activates the PAD enzymes and generates cellular hypercitrullination. The observation that the two immune-mediated membranolytic pathways, which are activated in response to a wide variety of pathogens, can induce cellular hypercitrullination is consistent with a preferred model of RA, in which infectious or inflammatory events play a role in initiating immune responses to citrullinated proteins (2).
The mechanisms directing perforin and MAC pathways onto PAD-expressing cells in the RA joint remain uncertain, but numerous potential mediators are expressed in the target tissue. Studies have demonstrated decreased levels of MAC inhibitors (e.g. clusterin, vitronectin and CD59) in SF and tissue in RA, which might contribute to lytic or sublytic attacks on local cells (37). Several mechanisms of complement activation have been proposed in RA, including immune complexes, rheumatoid factors, C-reactive protein, and ACPAs (which activate the classical and/or alternative pathways), and changes in IgG glycosylation which activate complement via the mannose binding protein (37). The rheumatoid joint is also enriched with numerous types of killer cells expressing perforin, including cells with an NK phenotype (e.g. CD56brightCD94bright NK cells, NK-like cells, CD3+CD57+ cells and NK-22 cells) (28, 29, 31, 33, 53), and cytotoxic CD8+ and CD4+ cells (30, 32). Interestingly, anti-neutrophil effects of autoantibodies and killer cells has been demonstrated in RA, and appears to account for the prominent neutrophil destruction observed in Felty’s syndrome, where anti-neutrophil antibodies, immune complexes and cytotoxic cells have all been demonstrated (54–59). Moreover, ACPAs can bind to the surface of monocyte/macrophages (60), suggesting that these cells may be a target for complement activation (37). Although we cannot exclude that cellular hypercitrullination in the rheumatoid joint may reflect bystander membranolysis of cells expressing PADs (while the actual targets of the MACPF pathways are other cells in the RA joint), we propose that PAD-expressing myelomonocytic cells are important targets of direct cell-mediated cytotoxicity (via ADCC) and complement activation in RA. In our in vitro studies, antibodies binding to the cell surface of neutrophils could effectively direct the granule cytotoxicity and MAC pathways and induce cellular hypercitrullination. In a similar process, RA autoantibodies that recognize targets directly on the membrane and/or extracellular targets that bind to the cell surface could drive the abnormal activation of membranolytic pathways on PAD-expressing cells in the rheumatoid joint.
It is also of interest that the synovial fluids with evidence of extrinsic apoptotic pathways are largely distinct from those with complement C9 deposition, suggesting that one or another membranolytic pathway may be dominant in a particular individual or at a particular time, and possibly that different upstream mechanisms may lead to the preferential activation of one of these pathways. The ability of immune-mediated membranolytic pathways to generate autoantigens in RA may engage important feedforward loops, where autoantibody-mediated immune effector pathways drive autoantigen production, driving additional autoantibodies. The excellent therapeutic responses to Rituximab in RA underscore the roles of B cells in driving disease. Although elucidating the mechanisms which drive cellular hypercitrullination in specific patients and defining any associations with phenotype or specific autoantibodies remains a high priority, it is important to consider that independently of the trigger and the pore-forming mechanism, the functional and structural similarity in the MAC and perforin pathways may offer an advantage to target both pathways simultaneously. The early success of chelation therapies that targeted calcium in RA (which is equally required for complement and perforin activation) may support this notion (61, 62).
In our studies, numerous stimuli could induce histone citrullination, but were unable to reproduce the cellular hypercitrullination pattern that is prominent in the RA joint, suggesting that ‘physiological’ and ‘pathological’ citrullination may differ, both qualitatively and quantitatively. The high calcium requirement of PADs has been difficult to reconcile with the calcium concentrations which exist inside cells (11–14), which are >4 orders of magnitude lower than the in vitro calcium concentration needed for optimal PAD activity (10). In a recent study, we demonstrated that, with histone citrullination, the sensitivity of PAD4 to calcium can be dramatically decreased through interactions with an antibody (10), and proposed that PAD4 may be similarly regulated by as yet undefined partners intracellularly. In this case, PAD4 activation may not depend on achieving high calcium concentrations, but rather on promoting the interaction with a binding partner which decreases calcium requirement to the low micromolar range achieved during neutrophil activation (that is, 0.2–0.8 µM) (11–14). This might restrict citrullination to a small group of physiologic targets (e.g. histones), but not the broad group of bystander substrates citrullinated in the RA SF cells. In this model, more focused histone citrullination would be induced by pathways that activate the neutrophil (with calcium flux in the low micromolar range), but cellular hypercitrullination would be induced by pore-forming proteins that similar to calcium ionophores (24), augment intracellular calcium concentrations to a high micromolar range (~100 µM) (12, 24), inducing hyperactivation of the PAD enzymes.
Considering the growing idea that NETosis plays critical role in RA by multiple mechanisms (including abnormal citrullination) (63), it was surprising that this process was unable to reproduce the hypercitrullination observed in the RA joint. The initial idea that NET formation requires rupture of the cell membrane in a process marked by increased cell permeability (20) supports the theory that free calcium access in permeabilized cells suffering NETosis should hyperactivate PADs. However, recent evidence has demonstrated that in vivo, NETs are formed from viable cells, rendering neutrophils anuclear, but without lysis or features of cell death (23). In this scenario, it not surprising that in contrast to membranolytic pathways, NETosis is not associated with cellular hypercitrullination. Despite the view that NETosis is a dangerous form of death, it is important to consider that this process is physiologic and highly regulated (64). In this context, our data supports that citrullination in NETs is not an accidental process in which PADs are abnormally activated, but rather a controlled process directed toward functional substrates (e.g. histones) that are required for the efficient process of NETosis. Besides histones, only one study has reported that vimentin is citrullinated in NETs (63). Although this study did not look per se for the presence of hypercitrullination in NETs, it is noteworthy that citrullinated peptides were not reported when the protein cargo in NETs was analyzed by mass spectrometry. Moreover, none of the PADs were found in the cargo, excluding the possibility that citrullination may occur extracellularly in NETs (in a rich calcium environment). NETosis may certainly play a role in RA by generating citrullinated histones and potentially other citrullinated antigens (vimentin), as well as by inducing cytokine production, chemokines, and adhesion molecules (63). However, our study strongly suggests that NETs are not a source of the cellular hypercitrullination found in the RA joint.
Although we have identified two major pathways involved in the induction of cellular hypercitrullination in RA, the studies have some limitations. First, while we studied multiple stimuli to search for relevant inducers of cellular hypercitrullination in RA, and identified the perforin and MAC pathways, we cannot exclude the possibility that other mechanisms may be involved in this process. Second, direct demonstration of the mechanism(s) by which killer cells or complement attack target cells in vivo is still needed. Third, it would be of great interest to examine the longitudinal association between disease activity, levels of ACPAs and specific forms of cellular hypercitrullination (that is, driven by perforin or MAC) in the rheumatoid joint. Taken together, these studies demonstrate that the cellular hypercitrullination present in SF cells in RA likely represents the result of immune-mediated membranolytic pathways, which activate PADs in inflammatory cells in the joint. This interface of reinforcing pathogenic pathways may offer additional opportunities for prediction, monitoring and therapy in RA.
Materials and Methods
Study design
The objective of the study was to determine the presence and mechanism(s) of protein citrullination in SF cells from patients with RA. Synovial fluids from RA patients were obtained as discarded material after clinically indicated arthrocentesis under a Partners Healthcare IRB approved protocol. SF cells were pelleted and snap frozen for storage prior to biochemical analysis. Fourteen samples from 12 patients with RA were available for analysis. Cellular citrullination was determined by anti–modified citrulline (AMC) immunoblotting as described (65) using commercial AMC antibodies. PR3, CD14, caspase-3, BID and complement C9 were detected by immunoblotting, quantified by densitometry and normalized to actin (loading control).
Antibodies and reagents
Anti-human PAD2, PAD4 and granzyme B were generated in rabbit (Covance). The following reagents were purchased: Anti-modified citrulline (AMC) antibodies (Millipore), purified human perforin (Enzo Life Sciences), z-DEVD-FMK and z-VAD-FMK (EMD Chemicals), anti-human PAD3 and anti-Cit-H3 (citrulline 2 + 8 + 17) antibodies (Abcam); anti-Fas antibodies (CH11) (MBL); human recombinant TNFα, IL6, IL8e, IL8m, G-CSF and GM-CSF (PeproTec); HKLM (heat killed Listeria monocytogenes), LPS, flagellin and CL075 (Invivogen); CpG-C (Coley); anti-caspase-3 antibodies (clone 31A1067) (eBioscience); SuperScript VILO, TaqMan assays, AIM-V medium and rabbit anti-DNP-KLH antibody (Invitrogen); TNBS, fMLP, veronal gelatin buffer, complement C7 deficient serum, purified human C7, anti-human β and γ-actin, anti-histone H3 and anti-human CD44 (clone A3D8) (Sigma); CMA, rat anti-human CD16 (YFC 120.5), mouse anti-human CD14 (5A3B11B5), mouse anti-human PR3 (D-1) and mouse anti-human C9 (E-3) (Santa Cruz Biotech); rabbit anti-human gelsolin (Cell signaling). Human granzyme B was a gift from Nancy Thornberry (Merck Research Laboratories).
Induction of neutrophil activation and death
Heparinized venous blood was obtained from healthy controls after IRB approval and informed consent. Peripheral blood neutrophils were isolated as previously described (25). To induce apoptosis, neutrophils at 5 × 106 cells/ml (RPMI/10% fetal calf serum/1 mM CaCl2) were incubated in the presence of 50 ng/ml of TNFα plus 10 µg/ml of CHX for 4 hrs at 37°C, or with anti-Fas antibodies at 1 µg/ml for 8 hrs at 37°C. In addition, neutrophils were UVB irradiated as previously described (66) and further incubated for 8 hrs at 37°C. Spontaneous apoptosis was induced by incubating neutrophils for 24 hrs at 37°C. To induce NETosis, neutrophils were incubated with 50 nM PMA or LPS (200 ng/ml) for 4 hrs at 37°C. Autophagy/necroptosis was induced by incubating neutrophils with GM-CSF (25 ng/ml) for 30 mins at 37°C prior adding 6 µg/ml of anti-CD44 monoclonal antibody and further incubated for 24 hrs at 37°C (19). Neutrophil activation was performed for 4 hrs at 37°C in the presence of 1 µM ionomycin, TNFα (50 ng/ml), IL-6 (100ng/ml), IL8e (100ng/ml), IL8m (100ng/ml), f-MLP (100 nM), G-CSF (100ng/ml), GM-CSF (100ng/ml), HKLM (1×108 HKLM/ml), flagellin (1 µg/ml), CL075 (5 µg/ml), or CpG-C (1 µM). After 2 washes with PBS, reactions were stopped by adding SDS-sample buffer and boiling. As controls, neutrophils were immediately lysed and boiled in SDS-sample buffer after purification (that is, time 0’) or incubated for 4 hrs at 37°C before lysis and boiling in SDS-sample buffer.
Perforin-mediated assays
Physiologic (also known as sublytic) concentrations of perforin which are required to deliver granzyme B, but not to kill the target cell (that is, that induce 10–15% cells permeabilization) (24), were individually defined for 293T cells and human neutrophils as described elsewhere (67) in 30 µl reactions containing 1.75 × 105 293T cells or 2.5 × 105 neutrophils (HBSS/10 mM Hepes/2 mM CaCl2). Physiologic concentrations of perforin were 300 ng/ml for 293T cells and 500 ng/ml for neutrophils. Under these conditions, target cells were incubated with or without perforin in the absence or presence of 2.0 µg/ml purified granzyme B for 4 hrs at 37°C. After terminating the reactions, the samples were electrophoresed and analyzed by immunoblotting.
Cytotoxic-cell mediated assays
LAK cells were generated as described elsewhere (68). Purified neutrophils labeled with anti-CD16 (30 mins at 4°C) and LAK cells were incubated alone or co-incubated for 4 hrs at 37°C. After 3 washes with PBS, reactions were stopped by adding SDS-sample buffer and boiling. When required, target cells were preincubated with30 nM CMA (2 hrs at 37°C), 10 µM z-DEVD-FMK or 50 µM z-VAD-FMK (30 mins at 4°C), or target cell killing was performed in the presence of 8 mM EGTA. The cytotoxic assays were performed using serum free AIM-V medium to avoid the presence of complement from serum.
Complement-mediated cell lysis
Purified neutrophils were TNP modified as previously described (69), labeled with anti-DNP antibodies, resuspended at 1.6 × 106 cells/ml in gelatin veronal buffer containing 1 mM CaCl2, and incubated for 1 hr at 37°C in the absence or presence of increasing amounts of autologous serum (serum dilutions at 1:32, 1:16 and 1:8), or complement C7-deficient serum at 1:12.5 dilution (concentration suggested by the manufacturer) with or without increasing amounts of purified human complement C7 (100–1000 ng/ml). After 3 washes with PBS, reactions were stopped by adding SDS-sample buffer and boiling.
Mass spectrometric analysis
Samples from 2 RA SFs (RA-SF1 and RA-SF7) or from neutrophils incubated alone (control) or in the presence of perforin or complement were used for mass spectrometric analysis using FASP protocol with spin ultrafiltration units (Expedeon) as described previously (70). Briefly, the protein lysate was mixed with 8 M urea, then put through the spin ultrafiltration unit with centrifugation at 14000 g at 20°C for 15 min. This step was performed twice. 0.05 M iodoacetamide in 8 M urea was added and incubated for 20 min in the dark. Next, filters were washed twice with of 8M urea followed by two washes with 40 mM NH4HCO3. Finally, Lys-C (Promega) was added to each filter in the protein to enzyme ratio of 50:1. Samples were incubated overnight at 37°C, centrifugated prior to analysis with Easy-nLC 1000 in-line with an Orbitrap Elite equipped with a nano-flex ion source (Thermo Scientific). The data were searched against the Human International Protein Index (IPI) database version 3.80 using the Sorcerer 2-SEQUESTalgorithm (Sage-N Research). Data were further analyzed using Scaffold PTM version 1.1.2. A protein probability >99%, a peptide probability >95% (which corresponds to FDR<1%), and a minimum number of two peptides per protein were applied as filters to generate the protein list. Precursor and product ion tolerances were set at 50 ppm and 0.6 Da correspondingly. Search parameters included Lys C specificity, up to two missed cleavages, variable carbamidomethylation (C, +57 Da), variable deamidation (NQ, +0.984 Da), oxidation (M, +16 Da), and deimination on Cit (R, +0.98 Da). The citrullination sites assignments were evaluated by manual inspection of tandem mass spectra. For the citrullinated peptide, in addition to backbone cleavage products (b and y ions), the neutral loss ion was observed adjacent to the precursor ion. All spectra were manually validated.
Statistical analysis
Linear correlations were analyzed using Pearson's correlation coefficient using a two-tailed α of 0.05. Graphpad Prism 5.0 was used to perform analysis and generate figures. Unsupervised hierarchical clustering were performed using the Cluster and TreeView software programs (71).
Supplementary Material
Acknowledgments
We thank Drs. Chris Cheadle and Andrea Fava for assistance in qPCR and statistical analysis, respectively, and Irina Tchernyshyov for technical assistance in mass spectrometry analysis. We thank the reviewers, particularly reviewer 1, for their detailed and insightful comments, which have greatly improved the paper.
Funding: F.A. was supported by The Dana Foundation Scholars Program in Human Immunology, The Donald B. and Dorothy L. Stabler Foundation, Ira T. Fine Discovery Fund, and NIH grant P30 AR053503. U.J.H was supported by the Johns Hopkins Arthritis Center Discover Fund. P.A.N. was supported by the Cogan Family Fund. J.v.E. was supported by NIH grants 1R21HL112586-01 and HHSN268201000032C. A.R. was supported by NIH Grant R37 DE-12354 and ACR-REF Within our Reach grant.
Footnotes
This manuscript has been accepted for publication in Science Translational Medicine. This version has not undergone final editing. Please refer to the complete version of record at www.sciencetranslationalmedicine.org/. This manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Author contributions: V.R. designed and performed experiments, and analyzed the data. U.J.H., D.M.L. and P.A.N. provided patient samples. J.F-B. and J.v.E. performed mass spectrometry analysis. A.R. and E.D. provided advice and data analysis/interpretation. F.A. planned the study, designed and performed experiments, and analyzed/interpreted the data. All authors contributed to preparation of the manuscript.
Competing interests: The authors declare that they have no competing interests.
References
- 1.Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. BioEssays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- 2.Wegner N, Lundberg K, Kinloch A, Fisher B, Malmstrom V, Feldmann M, Venables PJ. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol. Rev. 2010;233:34–54. doi: 10.1111/j.0105-2896.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- 3.Mortier A, Gouwy M, Van DJ, Proost P. Effect of posttranslational processing on the in vitro and in vivo activity of chemokines. Exp. Cell Res. 2011;317:642–654. doi: 10.1016/j.yexcr.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masson-Bessiere C, Sebbag M, Girbal-Neuhauser E, Nogueira L, Vincent C, Senshu T, Serre G. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J. Immunol. 2001;166:4177–4184. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- 6.Kinloch A, Lundberg K, Wait R, Wegner N, Lim NH, Zendman AJ, Saxne T, Malmstrom V, Venables PJ. Synovial fluid is a site of citrullination of autoantigens in inflammatory arthritis. Arthritis Rheum. 2008;58:2287–2295. doi: 10.1002/art.23618. [DOI] [PubMed] [Google Scholar]
- 7.Chang X, Yamada R, Suzuki A, Sawada T, Yoshino S, Tokuhiro S, Yamamoto K. Localization of peptidylarginine deiminase 4 (PADI4) and citrullinated protein in synovial tissue of rheumatoid arthritis. Rheumatology. (Oxford) 2005;44:40–50. doi: 10.1093/rheumatology/keh414. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo K, Xiang Y, Nakamura H, Masuko K, Yudoh K, Noyori K, Nishioka K, Saito T, Kato T. Identification of novel citrullinated autoantigens of synovium in rheumatoid arthritis using a proteomic approach. Arthritis Res. Ther. 2006;8:R175. doi: 10.1186/ar2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J. Immunol. 2008;180:1895–1902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 10.Darrah E, Giles JT, Ols ML, Bull HG, Andrade F, Rosen A. Erosive Rheumatoid Arthritis Is Associated with Antibodies That Activate PAD4 by Increasing Calcium Sensitivity. Sci. Transl. Med. 2013;5:186ra65. doi: 10.1126/scitranslmed.3005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill HR, Augustine NH, Jaffe HS. Human recombinant interferon gamma enhances neonatal polymorphonuclear leukocyte activation and movement, and increases free intracellular calcium. J Exp. Med. 1991;173:767–770. doi: 10.1084/jem.173.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pozzan T, Lew DP, Wollheim CB, Tsien RY. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983;221:1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- 13.Brandolini L, Bertini R, Bizzarri C, Sergi R, Caselli G, Zhou D, Locati M, Sozzani S. IL-1 beta primes IL-8-activated human neutrophils for elastase release, phospholipase D activity, and calcium flux. J Leukoc. Biol. 1996;59:427–434. doi: 10.1002/jlb.59.3.427. [DOI] [PubMed] [Google Scholar]
- 14.Wong K, Kwan-Yeung L. Sphingosine mobilizes intracellular calcium in human neutrophils. Cell Calcium. 1993;14:493–505. doi: 10.1016/0143-4160(93)90008-t. [DOI] [PubMed] [Google Scholar]
- 15.Norberg B, Bjelle A, Eriksson S. Joint fluid leukocytosis of patients with rheumatoid arthritis evidence for neutrophil and monocyte chemotaxis in vivo. Clin. Rheumatol. 1983;2:237–242. doi: 10.1007/BF02041397. [DOI] [PubMed] [Google Scholar]
- 16.Li HL, Zhu H, Xu CJ, Yuan JY. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 17.Savage JE, Theron AJ, Anderson R. Activation of neutrophil membrane-associated oxidative metabolism by ultraviolet radiation. J. Invest Dermatol. 1993;101:532–536. doi: 10.1111/1523-1747.ep12365905. [DOI] [PubMed] [Google Scholar]
- 18.Dwivedi N, Upadhyay J, Neeli I, Khan S, Pattanaik D, Myers L, Kirou KA, Hellmich B, Knuckley B, Thompson PR, Crow MK, Mikuls TR, Csernok E, Radic M. Felty's syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum. 2012;64:982–992. doi: 10.1002/art.33432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mihalache CC, Yousefi S, Conus S, Villiger PM, Schneider EM, Simon HU. Inflammation-associated autophagy-related programmed necrotic death of human neutrophils characterized by organelle fusion events. J. Immunol. 2011;186:6532–6542. doi: 10.4049/jimmunol.1004055. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkmann V, Goosmann C, Kuhn LI, Zychlinsky A. Automatic quantification of in vitro NET formation. Front Immunol. 2012;3:413. doi: 10.3389/fimmu.2012.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, Malawista SE, de Boisfleury CA, Zhang K, Conly J, Kubes P. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012 doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keefe D, Shi L, Feske S, Massol R, Navarro F, Kirchhausen T, Lieberman J. Perforin Triggers a Plasma Membrane-Repair Response that Facilitates CTL Induction of Apoptosis. Immunity. 2005;23:249–262. doi: 10.1016/j.immuni.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Darrah E, Rosen A, Giles JT, Andrade F. Peptidylarginine deiminase 2, 3 and 4 have distinct specificities against cellular substrates: novel insights into autoantigen selection in rheumatoid arthritis. Ann. Rheum. Dis. 2012;71:92–98. doi: 10.1136/ard.2011.151712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu KT, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT. Caspase-3-generated fragment of gelsolin: Effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- 27.Kataoka T, Shinohara N, Takayama H, Takaku K, Kondo S, Yonehara S, Nagai K. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J. Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- 28.Pridgeon C, Lennon GP, Pazmany L, Thompson RN, Christmas SE, Moots RJ. Natural killer cells in the synovial fluid of rheumatoid arthritis patients exhibit a CD56bright,CD94bright,CD158negative phenotype. Rheumatology. (Oxford) 2003;42:870–878. doi: 10.1093/rheumatology/keg240. [DOI] [PubMed] [Google Scholar]
- 29.Goto M, Zvaifler NJ. Characterization of the natural killer-like lymphocytes in rheumatoid synovial fluid. J Immunol. 1985;134:1483–1486. [PubMed] [Google Scholar]
- 30.Young LH, Joag SV, Lin PY, Luo SF, Zheng LM, Liu CC, Young JD. Expression of cytolytic mediators by synovial fluid lymphocytes in rheumatoid arthritis. Am. J. Pathol. 1992;140:1261–1268. [PMC free article] [PubMed] [Google Scholar]
- 31.Dupuy dA, Monier S, Jorgensen C, Gao Q, Travaglio-Encinoza A, Bologna C, Combe B, Sany J, Reme T. Increased percentage of CD3+, CD57+ lymphocytes in patients with rheumatoid arthritis. Correlation with duration of disease. Arthritis Rheum. 1993;36:608–612. doi: 10.1002/art.1780360506. [DOI] [PubMed] [Google Scholar]
- 32.Warrington KJ, Takemura S, Goronzy JJ, Weyand CM. CD4+,CD28- T Cells in Rheumatoid Arthritis Patients Combine Features of the Innate and Adaptive Immune Systems. Arthritis Rheum. 2001;44:13–20. doi: 10.1002/1529-0131(200101)44:1<13::AID-ANR3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Ren J, Feng Z, Lv Z, Chen X, Li J. Natural killer-22 cells in the synovial fluid of patients with rheumatoid arthritis are an innate source of interleukin 22 and tumor necrosis factor-alpha. J Rheumatol. 2011;38:2112–2118. doi: 10.3899/jrheum.101377. [DOI] [PubMed] [Google Scholar]
- 34.Law RH, Lukoyanova N, Voskoboinik I, Caradoc-Davies TT, Baran K, Dunstone MA, D'Angelo ME, Orlova EV, Coulibaly F, Verschoor S, Browne KA, Ciccone A, Kuiper MJ, Bird PI, Trapani JA, Saibil HR, Whisstock JC. The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature. 2010;468:447–451. doi: 10.1038/nature09518. [DOI] [PubMed] [Google Scholar]
- 35.Luzio JP, Daw RA, Hallett MB, Richardson PJ, Campbell AK. The rapid increase in intracellular free calcium ion concentration induced by complement and its role in cell damage. Biochem. Soc. Trans. 1979;7:1066–1068. doi: 10.1042/bst0071066. [proceedings]. [DOI] [PubMed] [Google Scholar]
- 36.Okroj M, Heinegard D, Holmdahl R, Blom AM. Rheumatoid arthritis and the complement system. Ann. Med. 2007;39:517–530. doi: 10.1080/07853890701477546. [DOI] [PubMed] [Google Scholar]
- 37.Ballanti E, Perricone C, Greco E, Ballanti M, Di MG, Chimenti MS, Perricone R. Complement and autoimmunity. Immunol. Res. 2013 doi: 10.1007/s12026-013-8422-y. [DOI] [PubMed] [Google Scholar]
- 38.Kolb WP, Haxby JA, Arroyave CM, Muller-Eberhard HJ. Molecular analysis of the membrane attack mechanism of complement. J Exp. Med. 1972;135:549–566. doi: 10.1084/jem.135.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vossenaar ER, Despres N, Lapointe E, van der HA, Lora M, Senshu T, van Venrooij WJ, Menard HA. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res. Ther. 2004;6:R142–R150. doi: 10.1186/ar1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinloch A, Tatzer V, Wait R, Peston D, Lundberg K, Donatien P, Moyes D, Taylor PC, Venables PJ. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res. Ther. 2005;7:R1421–R1429. doi: 10.1186/ar1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki A, Yamada R, Ohtake-Yamanaka M, Okazaki Y, Sawada T, Yamamoto K. Anti-citrullinated collagen type I antibody is a target of autoimmunity in rheumatoid arthritis. Biochem. Biophys. Res. Commun. 2005;333:418–426. doi: 10.1016/j.bbrc.2005.05.137. [DOI] [PubMed] [Google Scholar]
- 42.Goeb V, Thomas-L'Otellier M, Daveau R, Charlionet R, Fardellone P, Le LX, Tron F, Gilbert D, Vittecoq O. Candidate autoantigens identified by mass spectrometry in early rheumatoid arthritis are chaperones and citrullinated glycolytic enzymes. Arthritis Res. Ther. 2009;11:R38. doi: 10.1186/ar2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Beers JJ, Schwarte CM, Stammen-Vogelzangs J, Oosterink E, Bozic B, Pruijn GJ. The rheumatoid arthritis synovial fluid citrullinome reveals novel citrullinated epitopes in apolipoprotein E, myeloid nuclear differentiation antigen, and beta-actin. Arthritis Rheum. 2013;65:69–80. doi: 10.1002/art.37720. [DOI] [PubMed] [Google Scholar]
- 44.Harlow L, Rosas IO, Gochuico BR, Mikuls TR, Dellaripa PF, Oddis CV, Ascherman DP. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2013;65:869–879. doi: 10.1002/art.37881. [DOI] [PubMed] [Google Scholar]
- 45.Andrade F, Darrah E, Gucek M, Cole RN, Rosen A, Zhu X. Autocitrullination of human peptidyl arginine deiminase type 4 regulates protein citrullination during cell activation. Arthritis Rheum. 2010;62:1630–1640. doi: 10.1002/art.27439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Bolt M, Guertin MJ, Chen W, Zhang S, Cherrington BD, Slade DJ, Dreyton CJ, Subramanian V, Bicker KL, Thompson PR, Mancini MA, Lis JT, Coonrod SA. Peptidylarginine deiminase 2-catalyzed histone H3 arginine 26 citrullination facilitates estrogen receptor alpha target gene activation. Proc. Natl. Acad. Sci. U. S. A. 2012;109:13331–13336. doi: 10.1073/pnas.1203280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tak PP, Spaeny-Dekking L, Kraan MC, Breedveld FC, Froelich CJ, Hack CE. The levels of soluble granzyme A and B are elevated in plasma and synovial fluid of patients with rheumatoid arthritis (RA) Clin. Exp. Immunol. 1999;116:366–370. doi: 10.1046/j.1365-2249.1999.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffiths GM, Alpert S, Lambert E, McGuire J, Weissman IL. Perforin and granzyme A expression identifying cytolytic lymphocytes in rheumatoid arthritis. Proc. Natl. Acad. Sci. U. S. A. 1992;89:549–553. doi: 10.1073/pnas.89.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraan MC, Haringman JJ, Weedon H, Barg EC, Smith MD, Ahern MJ, Smeets TJ, Breedveld FC, Tak PP. T cells, fibroblast-like synoviocytes, and granzyme B+ cytotoxic cells are associated with joint damage in patients with recent onset rheumatoid arthritis. Ann. Rheum. Dis. 2004;63:483–488. doi: 10.1136/ard.2003.009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldbach-Mansky R, Suson S, Wesley R, Hack CE, El-Gabalawy HS, Tak PP. Raised granzyme B levels are associated with erosions in patients with early rheumatoid factor positive rheumatoid arthritis. Ann. Rheum. Dis. 2005;64:715–721. doi: 10.1136/ard.2003.007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ronday HK, van der Laan WH, Tak PP, de Roos JA, Bank RA, TeKoppele JM, Froelich CJ, Hack CE, Hogendoorn PC, Breedveld FC, Verheijen JH. Human granzyme B mediates cartilage proteoglycan degradation and is expressed at the invasive front of the synovium in rheumatoid arthritis. Rheumatology. (Oxford) 2001;40:55–61. doi: 10.1093/rheumatology/40.1.55. [DOI] [PubMed] [Google Scholar]
- 52.Saito S, Murakoshi K, Kotake S, Kamatani N, Tomatsu T. Granzyme B induces apoptosis of chondrocytes with natural killer cell-like cytotoxicity in rheumatoid arthritis. J. Rheumatol. 2008;35:1932–1943. [PubMed] [Google Scholar]
- 53.Arai K, Yamamura S, Seki S, Hanyu T, Takahashi HE, Abo T. Increase of CD57+ T cells in knee joints and adjacent bone marrow of rheumatoid arthritis (RA) patients: implication for an anti-inflammatory role. Clin. Exp. Immunol. 1998;111:345–352. doi: 10.1046/j.1365-2249.1998.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Starkebaum G, Arend WP, Nardella FA, Gavin SE. Characterization of immune complexes and immunoglobulin G antibodies reactive with neutrophils in the sera of patients with Felty's syndrome. J Lab Clin. Med. 1980;96:238–251. [PubMed] [Google Scholar]
- 55.Starkebaum G, Singer JW, Arend WP. Humoral and cellular immune mechanisms of neutropenia in patients with Felty's syndrome. Clin. Exp. Immunol. 1980;39:307–314. [PMC free article] [PubMed] [Google Scholar]
- 56.Ditzel HJ, Masaki Y, Nielsen H, Farnaes L, Burton DR. Cloning and expression of a novel human antibody-antigen pair associated with Felty's syndrome. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9234–9239. doi: 10.1073/pnas.97.16.9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burks EJ, Loughran TP., Jr Pathogenesis of neutropenia in large granular lymphocyte leukemia and Felty syndrome. Blood Rev. 2006;20:245–266. doi: 10.1016/j.blre.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Starkebaum G. Chronic neutropenia associated with autoimmune disease. Semin. Hematol. 2002;39:121–127. doi: 10.1053/shem.2002.31918. [DOI] [PubMed] [Google Scholar]
- 59.Breedveld FC, Lafeber GJ, de VE, van Krieken JH, Cats A. Immune complexes and the pathogenesis of neutropenia in Felty's syndrome. Ann. Rheum. Dis. 1986;45:696–702. doi: 10.1136/ard.45.8.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu MC, Lai NS, Yu HC, Huang HB, Hsieh SC, Yu CL. Anti-citrullinated protein antibodies bind surface-expressed citrullinated Grp78 on monocyte/macrophages and stimulate tumor necrosis factor alpha production. Arthritis Rheum. 2010;62:1213–1223. doi: 10.1002/art.27386. [DOI] [PubMed] [Google Scholar]
- 61.BOYLE AJ, MOSHER RE, McCANN DS. Some in vivo effects of chelation. I. Rheumatoid arthritis. J Chronic. Dis. 1963;16:325–328. doi: 10.1016/0021-9681(63)90082-1. [DOI] [PubMed] [Google Scholar]
- 62.Leipzig LJ, BOYLE AJ, McCANN DS. Case histories of rheumatoid arthritis treated with sodium or magnesium EDTA. J Chronic. Dis. 1970;22:553–563. doi: 10.1016/0021-9681(70)90032-9. [DOI] [PubMed] [Google Scholar]
- 63.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, Kaplan MJ. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013;5:178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Almyroudis NG, Grimm MJ, Davidson BA, Rohm M, Urban CF, Segal BH. NETosis and NADPH oxidase: at the intersection of host defense, inflammation, and injury. Front Immunol. 2013;4:45. doi: 10.3389/fimmu.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Senshu T, Akiyama K, Kan S, Asaga H, Ishigami A, Manabe M. Detection of deiminated proteins in rat skin: probing with a monospecific antibody after modification of citrulline residues. J. Invest Dermatol. 1995;105:163–169. doi: 10.1111/1523-1747.ep12317070. [DOI] [PubMed] [Google Scholar]
- 66.Andrade F, Casciola-Rosen LA, Rosen A. Generation of novel covalent RNA-protein complexes in cells by ultraviolet B irradiation: implications for autoimmunity. Arthritis Rheum. 2005;52:1160–1170. doi: 10.1002/art.20992. [DOI] [PubMed] [Google Scholar]
- 67.Martinvalet D, Thiery J, Chowdhury D. Granzymes and cell death. Methods Enzymol. 2008;442:213–230. doi: 10.1016/S0076-6879(08)01411-0. [DOI] [PubMed] [Google Scholar]
- 68.Andrade F, Roy S, Nicholson D, Thornberry N, Rosen A, Casciola-Rosen L. Granzyme B directly and efficiently cleaves several downstream caspase substrates: Implications for CTL-induced apoptosis. Immunity. 1998;8:451–460. doi: 10.1016/s1074-7613(00)80550-6. [DOI] [PubMed] [Google Scholar]
- 69.Wonderlich J, Shearer G, Livingstone A, Brooks A. Induction and measurement of cytotoxic T lymphocyte activity. Curr. Protoc. Immunol. 2006;Chapter 3(Unit 3):11. doi: 10.1002/0471142735.im0311s72. [DOI] [PubMed] [Google Scholar]
- 70.Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat. Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 71.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheadle C, Vawter MP, Freed WJ, Becker KG. Analysis of microarray data using Z score transformation. J Mol. Diagn. 2003;5:73–81. doi: 10.1016/S1525-1578(10)60455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.