Abstract
G-protein-coupled receptors (GPCRs) are the most abundant receptors in the heart and therefore are common targets for cardiovascular therapeutics. The activated GPCRs transduce their signals via heterotrimeric G-proteins. The four major families of G-proteins identified so far are specified through their α-subunit: Gαi, Gαs, Gαq and G12/13. Gαi-proteins have been reported to protect hearts from ischemia reperfusion injury. However, determining the individual impact of Gαi2 or Gαi3 on myocardial ischemia injury has not been clarified yet. Here, we first investigated expression of Gαi2 and Gαi3 on transcriptional level by quantitative PCR and on protein level by immunoblot analysis as well as by immunofluorescence in cardiac tissues of wild-type, Gαi2-, and Gαi3-deficient mice. Gαi2 was expressed at higher levels than Gαi3 in murine hearts, and irrespective of the isoform being knocked out we observed an up regulation of the remaining Gαi-protein. Myocardial ischemia promptly regulated cardiac mRNA and with a slight delay protein levels of both Gαi2 and Gαi3, indicating important roles for both Gαi isoforms. Furthermore, ischemia reperfusion injury in Gαi2- and Gαi3-deficient mice exhibited opposite outcomes. Whereas the absence of Gαi2 significantly increased the infarct size in the heart, the absence of Gαi3 or the concomitant upregulation of Gαi2 dramatically reduced cardiac infarction. In conclusion, we demonstrate for the first time that the genetic ablation of Gαi proteins has protective or deleterious effects on cardiac ischemia reperfusion injury depending on the isoform being absent.
Introduction
Cardiovascular disease (CVD) in its various forms is a major cause of morbidity and mortality worldwide. Annually more than 17 million people die from CVD which represent approximately 29% of all deaths. Among those, 7.2 million die due to heart attack resulting from coronary heart disease (WHO 2012). Once considered a disease seen predominantly in industrial nations, nowadays myocardial infarction becomes more common also in developing countries [1]. This underlines the urgent need for strategies to protect the heart from ischemic injury.
In recent years a variety of cardio-protective drugs are used in clinical practice, such as β-adrenergic blockers or adenosine which all signal via G-protein-coupled receptors (GPCRs) [2]. Experimentally, compounds such as adenosine, opioids and bradykinin activating Gαi-coupled receptors have been shown to attenuate myocardial reperfusion injury [3].
Gi-proteins belong to the family of heterotrimeric G-proteins consisting of α, β, and γ subunits of which Gα defines the nature of the G-protein. Upon ligand binding to the GPCR, the receptor catalyzes guanine nucleotide exchange in Gα which then leads to dissociation of Gβγ from the Gα subunit. It allows both entities to interact with downstream effectors, thereby initiating intracellular signaling necessary to elicit the biological response of the cell. Aside from Gi three other families of heterotrimeric G-proteins are known, namely Gs, Gq, and G12/13. The Gi-family includes three closely-related Gα members, Gαi1-3, each encoded by a single gene. The Gαi1-3-isoforms share 85–95% of amino acid sequence identity and are characterized by their sensitivity towards pertussis toxin (PTX) [4], [5]. Gαi1, Gαi2, and Gαi3 display overlapping expression patterns with Gαi2 and Gαi3 abundantly expressed in the cardiovascular system [6], [7]. Current research assumes that Gαi2 and the quantitatively minor Gαi3 isoform exhibit redundant physiological roles which may explain that single Gαi2-deficient mice show only a relatively mild, and single Gαi3-deficient mice no visible phenotype [8]–[10]. In line with the hypothesis that in vivo deletion of a single Gαi-isoform can functionally be at least partially compensated by remaining Gαi-isoforms, Gαi2/Gαi3-double-deficient mice die in utero at early embryonic stages [11]. However, recent studies in mice lacking Gαi2 or Gαi3 disclose distinct biological key roles of these two Gαi-isoforms [12]. In particular, defects of autophagic liver proteolysis, development of axial skeleton, and planar cell polarity in cochlear hair cells are solely caused by Gαi3-deficiency [11], [13], [14]. Contrariwise, defects in skeletal muscle growth, thrombus formation and of various immune functions of leukocytes are detectable only in Gαi2-deficient mice [15]–[18].
Gαi2 has been suggested to play a significant role in ischemia reperfusion injury of the heart while a possible involvement of Gαi3 has been neglected so far [19], [20]. This study was undertaken to analyze isoform-specific consequences of Gαi-deficiency on cardiac ischemic reperfusion injury in mice. Employing a well established and characterized murine in vivo model of heart ischemia and reperfusion in Gαi-deficient mice [21] we show that Gαi2-deficiency leads to massive myocardial ischemia reperfusion injury whereas Gαi3-deficiency is highly protective in this scenario.
Materials and Methods
Ethics statement
Animal experiments were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (FELASA). The protocol was approved by the committee on the Ethics of Animal Experiments of local authority “Regierungspräsidium Tübingen” (permit number: TO4/10). All surgery was performed under anaesthesia as described in the respective method parts and all efforts were made to minimize suffering.
Gαi-deficient mouse strains
The generation and basal phenotypic characterization of Gαi2-deficient and Gαi3-deficient mice as well as their backcrossing on a C57BL/6 background were described elsewhere [10], [11], [16], [17]. As controls wild-type C57BL/6 mice (WT) or littermates were used as indicated. Gαi2-deficient mice were maintained in individually ventilated cages (IVCs) and Gαi3-deficient mice under specific pathogen-free conditions (SPF) according to national guidelines for animal care at the animal facility of the University of Tübingen. The mice used were of either sex as we see no differences. All mice were between 8 to 12 weeks old except animals for expression analysis which were up to 14 weeks of age.
Murine model of myocardial ischemia
Murine model of myocardial ischemic reperfusion injury was performed as described previously [21]. Briefly, after receiving anesthesia (Pentobarbital, 80 mg/kg, i.p.), mice were placed on a temperature-controlled heating table. All animals were intubated, ventilated, and left parasternal thoracotomy was performed to lay open the left coronary artery. Deep anesthesia was controlled regularly to avoid suffering of mice. Coronary artery occlusion was achieved by using the previously described hanging weight system. After reperfusion a double staining technique using triphenyl-tetrazolium chloride (TTC) to mark vital and necrotic tissue and Evans Blue staining to negatively mark the AAR was used [22]. The extent of infarct sizes were determined by calculating the percentage of infarction compared to the area at risk (AAR) from 4–5 discs per heart [21]. Each group of animal consists of at least 6 mice. Planimetric determination of infarct size and AAR was performed using the ImageJ Software version 1.44p.
RT-PCR for transcriptional analysis
Tissue or whole blood cells were homogenized; RNA was isolated, and transcribed into cDNA. Transcriptional expression levels were measured using real-time reverse transcription polymerase chain reaction (iCycler CFX 96; Bio-Rad Laboratories, Munich, Germany) and normalized to two house-keeping genes, namely β-actin and GAPDH. To detect β-actin, GAPDH, Gαi2 and Gαi3 mRNA levels, following primers were used: GAPDH sense 5′-cga gaa tgg gaa gct tgt cat c-3′; GAPDH antisense 5′-cgg cct cac ccc att tg-3′; β-actin sense 5′-ctc tcc ctc acg cca tcc tg-3′; β-actin antisense 5′-tca cgc acg att tcc ctc tca g-3′; Gαi2 sense 5′-gcc aac aag tac gac ggc a-3′; Gαi2 antisense 5′-gta tct ctc acg ctt ctt gtg ct-3′; Gαi3 sense 5′-atg aac cga atg cat gaa agc a-3′; Gαi3 antisense 5′-ttt ggt gtc agt ggc aca ggt a-3′.
Immunoblot detection of Gαi proteins
After homogenization of tissue, samples were resuspended in RIPA buffer, and protein concentrations were measured by standard BCA method following the manufacturers' instructions (Thermoscientific, Illinois, USA). Protein amounts are indicated in the figure legends. Proteins were loaded on either urea-supplemented or 10% SDS polyacrylamide gels and blotted onto nitrocellulose membranes as described. The antibodies detecting Gαi proteins, i.e. anti-Gαcom, anti-Gαi2, and anti-Gαi3 were previously described [17], [23]. Loading conditions were controlled by GAPDH. A horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody served for immunodetection (Santa Cruz Biotechnology, Inc, USA, Santa Cruz). Immunoreactive bands were visualized by using an ECL detection system (GE Healthcare, Braunschweig, Germany). The levels of Gαi2 and Gαi3 in different organs from WTs; in heart tissue of WT, Gαi2 -/-, and Gαi3 -/- mice; and in heart tissue at different time points at and after ischemic events were quantified by densitometric analysis using Image J 1.44p after normalizing to GAPDH level.
Immunofluorescence staining of myocardial tissue
Untreated hearts of WT, Gαi2 -/-, and Gαi3 -/- mice and ischemic hearts (60 min) of mice after different reperfusion condition treatments (0, 60 and 120 min reperfusion) were excised and immediately frozen in Tissue-Tek® (Sakura Finetek, Netherland, Leiden). 0.5 µm thick cryostat sections were mounted on slides, fixed in 4% formaldehyde for 30 min, permeabilized with 0.1% Triton® X-100 (AppliChem, Germany, Darmstadt) for 10 min and blocked with 5% BSA in PBS for 45 min. For immunodetection of G-proteins in heart tissue, the previously described Gαi antibodies, i.e. anti-Gαi2 and anti-Gαi3, were used. DAPI (Invitrogen, USA, Oregon) was applied for nuclei detection. Gαi2 and Gαi3 signals were visualized with an Alexa 488-conjugated mouse anti-rabbit IgG (Invitrogen, USA, Oregon). The fluorescence imaging was performed with an Axiophot Zeiss microscope (Zeiss, Jena) using a digital camera with AxioVision 4.8 software.
Data analysis
Statistics were performed using one-way ANOVA with Bonferroni post test to determine group differences or unpaired student t test where appropriate. A value of P<0.05 was considered to be statistically significant.
Results
In order to get insights into the individual role of the two major Gαi-proteins of the cardiovascular system, i.e. Gαi2 and Gαi3, in the development of myocardial ischemia reperfusion injury (MIRI) we studied Gαi2 -/- and Gαi3 -/- mice in comparison to wild type controls in an acute murine model of heart ischemia and reperfusion [21].
Expression of Gαi2 and Gαi3 in murine organs
First, we compared expression levels of murine Gαi2 and Gαi3 in the heart and various other organs (Figure 1a). In the heart, both Gαi-isoforms were detected on transcriptional and protein level (Figure 1a–c). Although low transcript levels were evident as compared to all other organs tested, significant protein expression was detectable in immunoblot analysis. Notably, as described for many organs and tissues [6], [11], [17] Gαi2 is also the predominant isoform in the heart, although we found significant levels of Gαi3 (Figure 1c).
Figure 1. Expression of the two Gαi-isoforms.
RT-PCR analysis of various mouse organs. Transcriptional levels of Gαi2 (a) and Gαi3 (b) were normalized to β-actin and GAPDH. c. Immunoblot analysis of heart lysates from wildtype male mice with a Gαcommon antibody following urea supplemented SDS-PAGE and western blotting.
Up regulation of Gαi-proteins during myocardial IR
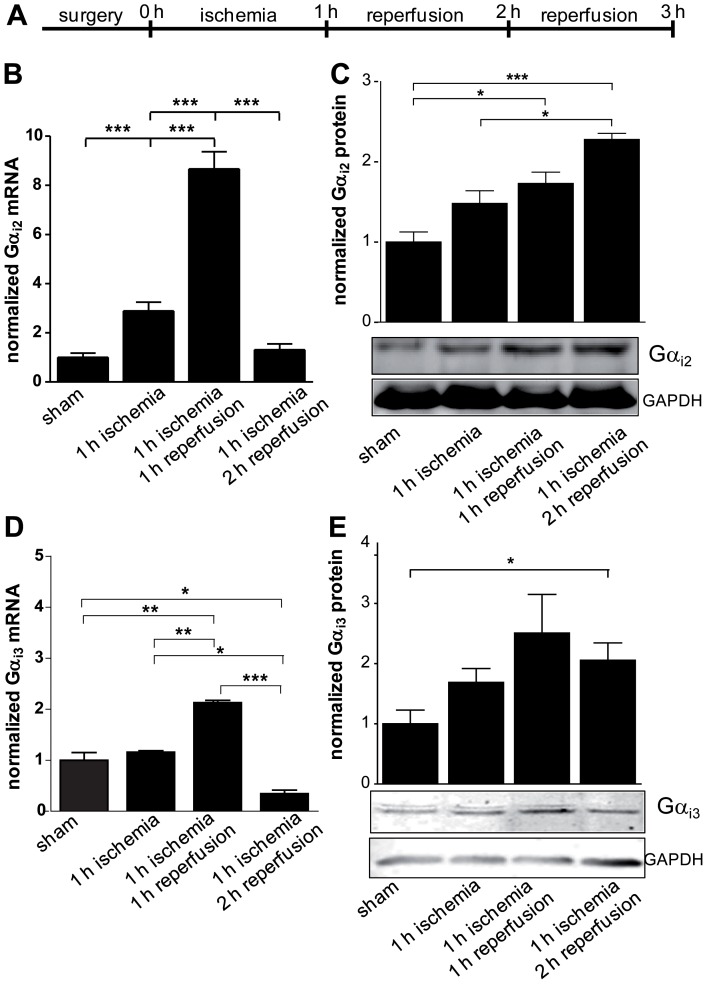
In accordance with its predominant expression, Gαi2 has been reported to play an important role during ischemic injury [19]. Moreover, a recent study showed that enhancement of Gαi2 signaling through loss of its negative regulation by RGS proteins protects the heart from ischemic injury [20]. Therefore, we wondered whether the different phases of myocardial ischemia reperfusion regulate expression levels of Gαi2 since transcript levels were obtained 72 hrs after ischemia [19]. To address this question, the mice were exposed to a one hour period of ischemia followed by two hours of reperfusion (Figure 2a). At defined time points mice were sacrificed and their hearts analyzed. The cardiac tissue from the area at risk (AAR) was excised and transcript and protein expression levels of Gαi2 (Figure 2b and c) and Gαi3 (Figure 2d and e) were measured and compared to samples from sham-operated controls. The Gαi2-specific mRNA was up regulated more than threefold after one hour of ischemia while there was no significant change in the protein level. In the following early phase of reperfusion Gαi2 transcripts peaked with an eightfold increase with subsequent more than twofold increase in the protein level during late phase of reperfusion (Figure 2b and c). Similarly, Gαi3 mRNA was also regulated during ischemia and reperfusion time in a similar manner but less intense (Figure 2d). Interestingly, protein levels increased statistically significant more than twofold during reperfusion phase (Figure 2e). To exclude that this enhanced expression is due to a massive influx of PMNs which highly express Gαi2 and Gαi3 the AAR sections were stained with anti-Gαi- and subsequently with anti-CD15-antibodies (Figure S1). In fact, while leukocytes infiltrated into the heart tissue during the reperfusion phase a clearly enhanced Gαi2- and Gαi3-specific staining of cardiac tissue was evident. Taken together, the results may indicate that cardiac Gαi2 and Gαi3 play similar roles during ischemia reperfusion injury in the heart.
Figure 2. Increased expression of Gαi2 and Gαi3 during IR-injury.
a. Schedule of the IR model with one hour ischemia followed by one or two hour reperfusion. b. Surgeries were performed as indicated with C57BL/6 male mice (WT). After the procedures AAR was excised and transcript levels of Gαi2 were determined by quantitative PCR. c. Protein expression of Gαi2 was analyzed by immunoblotting using a Gαi2-specific antibody. Protein amounts were normalized to GAPDH. Protein amounts loaded were 60 µg. Shown are representative images. d. Transcript levels of Gαi3 after surgeries as described in (a) were determined by quantitative PCR. e. Protein expression of Gαi3 was analyzed by immunoblotting using Gαi3-specific antibodies. Protein amounts were normalized to GAPDH. Protein amounts loaded 60 µg. Shown are representative images. Data are shown as mean ± SEM (n = 3); statistic was calculated with one-way ANOVA, with Bonferroni post test; *P≤0.05; **P≤0.01; ***P≤0.001 as indicated.
Gαi2-deficiency aggravates whereas Gαi3-deficiency ameliorates IR-injury
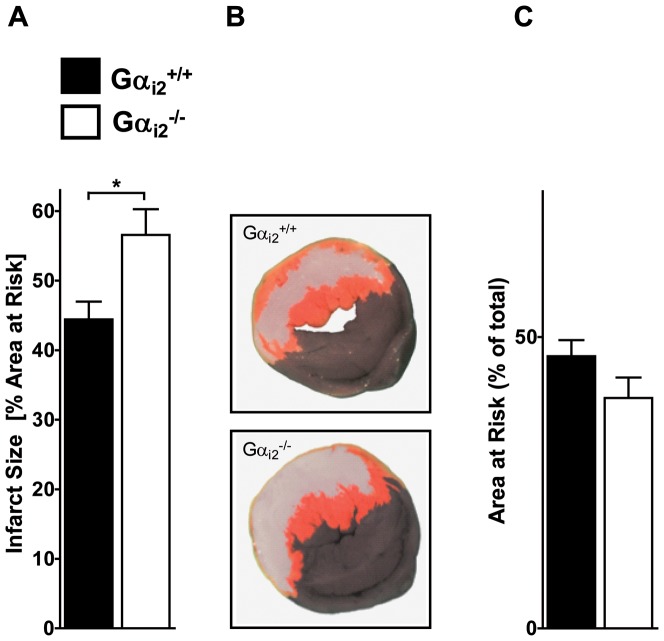
To challenge the concept of redundancy of the two Gαi isoforms in IR-injury, we performed regional myocardial ischemia reperfusion in Gαi2- (Figure 3) and Gαi3-deficient mice (Figure 4). In this model the infarct size is determined by comparing the area of infarction within the area at risk (AAR) [21]. Vital and necrotic tissues within the AAR were identified by double staining with TTC and Evans Blue, respectively. The degree of myocardial destruction was calculated as percentage of infarcted myocardium to AAR. In this experimental setting WT mice show expected infarct sizes of 44.4±2.6%, whereas Gαi2 -/- mice displayed significantly increased infarct areas of 56.6±3.7% (Figure 3a). To illustrate the infarct size in a more visual fashion, one representative heart disc of each WT and Gαi2 -/- mice is depicted (Figure 3b). Concurrently, the area at risk was not significantly different between groups (Figure 3c). This supports and extends the previously described protective role of Gαi2 in ischemia reperfusion [19], [20].
Figure 3. Gαi2-deficiency aggravates IR-injury.
a. Gαi2 +/+ (n = 6) and Gαi2 -/- (n = 7) mice were exposed to one hour ischemia followed by two hours reperfusion. Hearts were stained with Evans Blue to determine the AAR and TTC to mark vital tissue (red) and necrotic tissue (white). Subsequently, infarct size was calculated as percentage of AAR (for details see Method's section). b. Representative heart slice of Gαi2 +/+ and Gαi2 -/- mice are shown. These heart discs have an AAR of 50% (Gαi2 +/+) and 50% (Gαi2 -/-). The infarcted area was 41% (Gαi2 +/+) and 58% (Gαi2 -/-). c. Quantification of AAR as a percentage of the total heart disc (p = 0.14). Data in (a) and (c) are shown as mean ± SEM; statistic was calculated with t-test; *P≤0.05 as indicated.
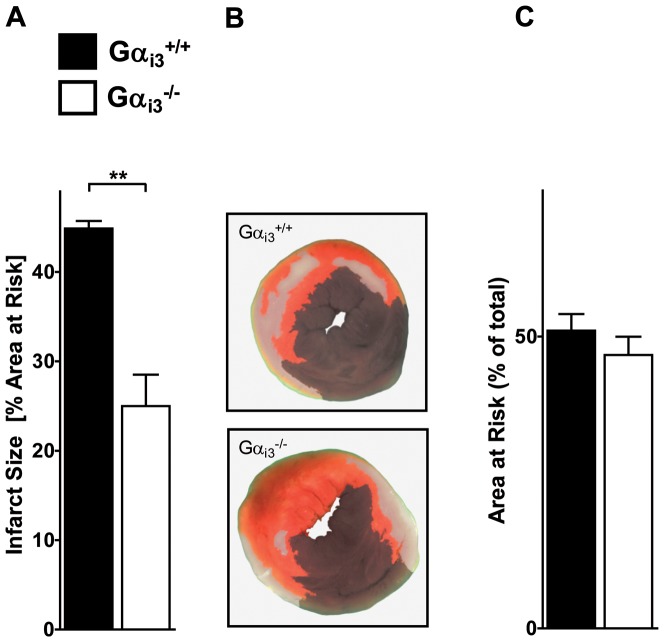
Figure 4. Gαi3-deficiency ameliorates IR-injury.
a. Gαi3 +/+ (n = 7) and Gαi3 -/- (n = 6) mice were exposed to one hour ischemia followed by two hours reperfusion. Hearts were stained with Evans Blue to determine the AAR and TTC to mark vital tissue (red) and necrotic tissue (white). Subsequently, infarct size was calculated as percentage of AAR (for details see Method's section). b. Representative heart slice of Gαi3 +/+ and Gαi3 -/- mice are shown. These heart discs have an AAR of 42% (Gαi3 +/+) and 57% (Gαi3 -/-). The infarcted area was 43% (Gαi3 +/+) and 28% (Gαi3 -/-). c. Quantification of AAR as a percentage of the total heart disc (p = 0.35). Data in (a) and (c) are shown as mean ± SEM; statistic was calculated with t-test; **P≤0.01 as indicated.
Surprisingly, in Gαi3 -/- mice the extent of damage after myocardial IR-injury was dramatically decreased. Gαi3 -/- mice exhibited strong reduction in infarcted areas (25.0±3.5%) compared to controls (44.9±0.8%; Figure 4a) while there was no significant change in AAR (Figure 4c). Again, one representative heart disc of both groups is pictured (Figure 4b). These data reveal an up to now unknown protective mechanism in mice against IR injury in the absence of Gαi3. Therefore, in contrast to current thinking, our data suggest that Gαi2 and Gαi3 play opposite instead of redundant roles in IR injury.
Increased expression of Gαi2 in Gαi3-deficient mice and vice versa
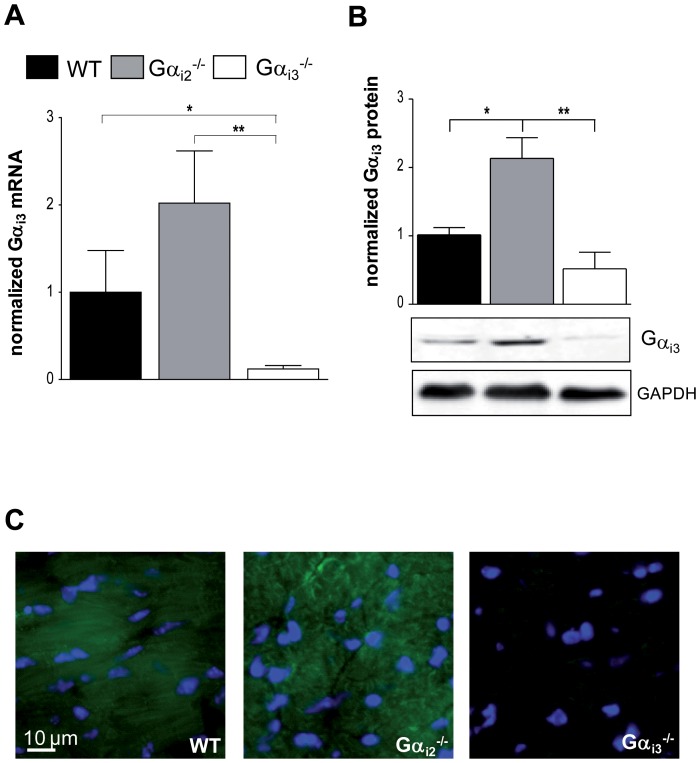
Previously, we detected an up regulation of the remaining isoform in different murine Gαi-deficient tissues and cells for either Gαi2 or Gαi3 [11], [17]. This is thought to represent an important mechanism contributing to functional redundancy and prompted us to ask whether the hearts of the knockout-mice lack compensatory up regulation of the remaining Gαi-isoform. In a previous attempt we have analyzed hearts from knock out-mice using high resolution SDS-PAGE in combination with Gαcommon antibodies [6]. However; only partial resolution of Gαi3 from Gαi2 limited the value of the densitometric analysis. In particular, the upregulation of Gαi3 in the absence of Gαi2 might have been overestimated. To approach this question in a more rigorous way, we measured mRNA and protein levels of Gαi2 in the cardiac tissue from Gαi3 -/- mice and vice versa (Figure 5 and 6). For assessment normalized transcript levels and immunoblot intensity of target proteins were compared to the housekeeping protein GAPDH. Isoform-specific antibodies were used for individual detection of Gαi2 or Gαi3 [11], [17]. Both approaches showed significantly higher expression levels of Gαi2 in Gαi3 -/- mice (Figure 5a and b) and vice versa Gαi3 in Gαi2 -/- mice (Figure 6a and b). In Gαi2 -/- mice which lack the predominant Gαi isoform, Gαi3 was up regulated more than twofold in cardiac tissue, and in Gαi3 -/- mice Gαi2 expression levels were increased by 50%. To strengthen our finding on up regulated protein expression in either knockout mouse model, we performed immunofluorescence staining of murine heart tissue sections (Figure 5c and 6c). Validation of the primary and secondary antibodies is shown in Figure S2. In accordance with the RT-PCR and Western blot results in either case signals of the remaining Gαi-isoform were highly increased in heart tissue of the knock-out counterpart.
Figure 5. Expression of Gαi2 in heart tissue of Gαi3-deficient mice.
a. mRNA levels of Gαi2 in hearts of wildtype (WT), Gαi2-deficient (Gαi2 -/-) and Gαi3-deficient male mice (Gαi3 -/-) (n = 4). b. Representative immunoblots of heart from WT, Gαi2 -/- and Gαi3 -/- male mice detected with a Gαi2-specific antibody. GAPDH was used to normalize the amount of protein. Protein amounts loaded 60 µg. The graph depicts the densitometric analysis (n = 3). c. Immunohistochemical staining of Gαi2 in heart tissue of WT, Gαi2 -/- and Gαi3 -/- mice. Representative pictures are shown. Scale bar: 10 µm. Data are shown as mean ± SEM; statistic was calculated with one-way ANOVA, with Bonferroni post test; *P≤0.05; **P≤0.01; ***P≤0.001 as indicated.
Figure 6. Expression of Gαi3 in heart tissue of Gαi2-deficient mice.
a. mRNA levels of Gαi3 in hearts of wildtype (WT), Gαi2-deficient (Gαi2 -/-) and Gαi3-deficient male mice (Gαi3 -/-) (n = 3). b. Representative immunoblots of heart from WT, Gαi2 -/- and Gαi3 -/- male mice detected with a Gαi3-specific antibody. GAPDH was used to normalize the amount of protein. Protein amounts loaded 60 µg. The graph depicts the densitometric analysis (n = 3). c. Immunohistochemical staining of Gαi3 in heart tissue of WT, Gαi2 -/- and Gαi3 -/- mice. Representative pictures are shown. Scale bar: 10 µm. Data are shown as mean ± SEM; statistic was calculated with one-way ANOVA, with Bonferroni post test; *P≤0.05; **P≤0.01; ***P≤0.001 as indicated.
Therefore, we conclude that the compensatory up regulation of the remaining Gαi isoform did not mask the phenotype seen in the knock-out models.
Discussion
The role of Gi-protein-dependent receptor signaling in the cardiovascular system is still a matter of intense investigations. A variety of therapeutics acting on Gi-PCRs is currently in use for regulation of heart function and protection. Therefore, cardiovascular Gi-PCRs are the most targeted receptors in the pharmacological treatment of cardiac diseases [2], [24]. In principle all these receptors can couple to two Gαi-isoforms, i.e. Gαi2 and Gαi3. However, current thinking implies only Gαi2 as the isoform responsible for eliciting biological effects whereas a role for Gαi3 is neglected in this scenario. Hence, the aim of our study was to focus on a role for Gαi3 in cardiac ischemia.
Here, we show for the first time that the absence of Gαi2 or Gαi3 have opposite effects on the severity of myocardial IR injury in knockout mice. In particular, Gαi2-deficiency led to enhanced myocardial infarct size whereas the absence of Gαi3 was highly protective. Whereas the first observation confirms and extends previous studies [19], [20], the latter finding was unexpected. The increased infarct size visible in Gαi2-deficient mice underlines a protective role of Gαi2 signaling which was reported in previous studies making use of different experimental approaches. For instance, in vivo administration of PTX being considered a functional pan-Gi-inhibitor in combination with an infarct model demonstrated a cardio-protective effect of these G-proteins in rat hearts [25]. We performed similar experiments using our acute mouse model of 60 min. of regional myocardial ischemia followed by 120 min. reperfusion in vivo (Figure S3 and Methods S1). Interestingly, infarct sizes of the PTX-treated animals were even more pronounced as compared to those seen in Gαi2-deficient mice, i.e. 67.0±4.8% vs. 56.6±3.7%, respectively, whereas the values for the controls in either group were almost the same (42.3±2.2% vs. 44.4±2.6%). The latter data argue for a reliable procedure as indicated by similar values in both control groups. PTX modifies Gαi-proteins by ADP-ribosylation of a cystein residue in the extreme C-terminus of sensitive Gαi-proteins. In the afore-mentioned study in rats [25] the degree of PTX-induced in vivo ADP-ribosylation of cardiac Gαi-proteins was assessed by employing a radioactive in vitro approach. Interestingly, this analysis revealed that only a small subpopulation of Gi-proteins in the myocardial membrane was PTX-modified. This is a phenomenon we also see in our studies (data not shown). Since PTX modifies Gαi-proteins with different efficiency, it cannot be excluded that PTX acted in a rather isoform selective way [26]. Moreover, different cells and tissues may exhibit variable sensitivity and kinetics towards PTX. Therefore it remains unclear which Gαi-isoforms in which tissues and organs have contributed to the observed cardio-protective effect. Another study also targeted the interaction of GPCRs with cardiac Gi-proteins in a more specific approach [19]. Mice were created with a transgene expressing an inhibitory carboxyl-terminal 63 amino acid peptide of Gαi2 in cardiac tissue acting in a dominant negative fashion. These mice, when subjected to ischemia/reperfusion induced heart injury, demonstrated an exacerbated ischemic injury as compared to controls. Although the effects of the inhibitory Gαi2-minigene on Gi-dependent signaling pathways were significant, the contribution of the Gαi2- and Gαi3-specific pathways to the observed cardio-protective effect was not investigated. In a recent paper a complementary genetic approach to study the effect of Gαi2-signaling on cardiac ischemia in vitro was described [20]. Knock-in mice were examined in which the endogenous Gαi2 gene was replaced with an RGS-insensitive G184S Gαi2 mutant that was unable to interact with RGS proteins. This resulted in an enhancement of Gαi2 signaling by reversal of its negative regulation by RGS proteins thereby protecting the heart from ischemic injury. Although this study was in accordance with the concept of Gαi2-dependent protection of the heart, it ignored a possible role of Gαi3. Moreover, these mice showed a dramatic and complex phenotype affecting the heart and several other organs which may produce secondary effects on heart function and resulting in premature death [27].
Similar concerns have been raised about the Gαi2 knockout model that we have used in our current study. Initially, these mice have been reported to display a histopathological phenotype resembling ulcerative colitis and adenocarcinoma of the colon [10]. However, when these mice were housed under pathogen-free conditions no obvious signs of intestinal inflammation were visible during the course of the study and they did not show the previously reported lethality phenotype [17]. This allowed us to specifically study the roles of the two Gαi-isoforms in cardiac ischemia injury in vivo.
Surprisingly, mice lacking Gαi3 showed a significantly reduced infarct size following IR injury. It was intriguing that the deletion of one Gαi isoform results in the up regulation of the remaining ones. In fact, we detected an up regulation in heart tissue; a phenomenon we have observed previously in all tissues and cells we analyzed so far [6], [11], [17]. As a consequence, the particular knock-out model exhibits two important features, i.e. the deletion of the target Gαi-isoform and the enhanced expression of the remaining ones. Therefore the deleterious or protective effect might not only be the result of the loss of one isoform but also the enhanced signaling of the remaining ones. For example, the increased infarct size seen in Gαi2-deficient mice could either be due to missing Gαi2 or over-expressed Gαi3. Conversely, the reduced infarct size in Gαi3-deficient mice could either be due to the over-expressed Gαi2 or absent Gαi3. In that respect, it will be interesting to re-evaluate the previous studies discussed above [19], [20]. The main conclusions from these studies were to attach a predominant role of Gαi2 in ischemia reperfusion injury. However, these studies ignored that an altered Gαi2 signaling could affect Gαi3 expression – as observed here and in previous studies – and signaling. This is of special importance since Gαi3 has been shown to play crucial roles in both, its GDP-bound and GTP-bound form [28], [29]. The current view is that Gαi2 and Gαi3 have largely overlapping roles. Some of our recent data contradict such a claim, showing that the absence of the minor Gαi3 isoform cannot be compensated by the remaining Gαi2 isoform [11], [13], [14]. G-protein signaling pathways come in at least two different shapes: a canonical and a non-canonical pathway which may mechanistically establish non-redundant distinct functions [29]. Future works have to concentrate on solving this question.
The current study displays intriguing and highly significant differences between the two Gαi-isoforms albeit it employed a relatively small number of animals. One obvious limitation is the fact that global knockout animals, which lack the respective Gαi-isoform in every tissue or organ, were studied. For future directions of research, in particular additional tools are required to decipher the specific functions of the two Gαi isoforms in cardiac and non-cardiac cells, e.g. cardiomyocytes, endothelial or immune cells. Ideally, experimental approaches may include detailed analyses of tissue-specific mouse models where the Gαi gene of interest is deleted in a constitutive or inducible manner. This allows elucidating the individual contribution of the Gαi-isoforms to the ischemic reperfusion injury in the heart. Furthermore with this approach an up regulation of the remaining isoform may be prevented. Whereas an appropriate Gαi2-model is available [13] the corresponding Gαi3-mouse model has not been created so far.
In conclusion, we provide strong evidence that both the deficiency for Gαi2 and for Gαi3 has profound and opposite effects on IR injury in mice. This may open the rationale to develop biased GiPCR drugs which may allow a different regulation of Gαi2 and Gαi3 by the same receptor.
Supporting Information
PMN infiltration in heart tissue during IR-injury. Surgeries in WT mice were performed as indicated. To stain infiltrated neutrophils, a source for the level changes in Gαi protein expression, immunohistochemistry with an anti-CD15 antibody was performed. Additionally, tissue was stained with a. Gαi2- and b. Gαi3-specific antibodies. Representative images are shown. Scale bar = 10 µm.
(TIF)
Control staining to test antibody specificity. To rule out unspecific binding of the used antibodies in heart tissue control staining were performed as follow. a. Staining of WT tissue with IgG antibody. b. Heart tissue from Gαi2 -/- mice was stained with anti-Gαi2 antibody. c. Heart tissue from Gαi3 -/- mice was stained with anti-Gαi3 antibody. Representative images are shown. Scale bar = 10 µm.
(TIF)
PTX treatment aggravates IR injury. a. WT mice were either injected i.p. with vehicle (n = 6) or Pertussis toxin (PTX)(see Methods S1) (n = 6) and 48 hours later exposed to one hour ischemia and one hour reperfusion. Hearts were counterstained with Evans Blue to determine the AAR and TTC to mark vital tissue (red) and necrotic tissue (white). Subsequently, infarct size was calculated as percentage of AAR. b. Representative heart slice of WT mice treated with NaCl or PTX are shown. These heart discs have an infarcted area of 46% (WT+NaCl) and 69% (WT+PTX). Data in (a) are shown as mean ± SEM; statistic was calculated with t-test; ***P≤0.001 as indicated.
(TIF)
Pertussis Toxin treatment.
(DOCX)
Acknowledgments
We are grateful to Michaela Hoch-Gutbrod and Alice Mager for excellent technical assistance and members of the Nürnberg lab for helpful discussions and critical reading.
Funding Statement
This work was supported by grants of the Deutsche Forschungsgemeinschaft (SFB 612 to B.N. and MO2252/1 to D.K.), the Open Access Publishing Fund of Tuebingen University and the Intramural Research Program of the NIH (project Z01-ES-101643 to L.B.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N Engl J Med 357: 1121–1135 doi:10.1056/NEJMra071667 [DOI] [PubMed] [Google Scholar]
- 2. DeWire SM, Violin JD (2011) Biased ligands for better cardiovascular drugs: dissecting G-protein-coupled receptor pharmacology. Circulation research 109: 205–216. [DOI] [PubMed] [Google Scholar]
- 3. Eisen A, Fisman EZ, Rubenfire M, Freimark D, McKechnie R, et al. (2004) Ischemic preconditioning: nearly two decades of research. A comprehensive review. Atherosclerosis 172: 201–210 doi:10.1016/S0021-9150(03)00238-7; S0021915003002387 [pii]. [DOI] [PubMed] [Google Scholar]
- 4. Murayama T, Ui M (1983) Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. The Journal of biological chemistry 258: 3319–3326. [PubMed] [Google Scholar]
- 5. Simon MI, Strathmann MP, Gautam N (1991) Diversity of G proteins in signal transduction. Science 252: 802–808. [DOI] [PubMed] [Google Scholar]
- 6.Dizayee S, Kaestner S, Kuck F, Hein P, Klein C, et al. (2013) Correction: Galpha- and Galpha-Specific Regulation of Voltage-Dependent L-Type Calcium Channels in Cardiomyocytes. PLoS One 8 . doi:10.1371/annotation/7be097c0-9075-41ae-91e9-d2209de952cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hippe HJ, Ludde M, Schnoes K, Novakovic A, Lutz S, et al. (2013) Competition for Gbetagamma dimers mediates a specific cross-talk between stimulatory and inhibitory G protein alpha subunits of the adenylyl cyclase in cardiomyocytes. Naunyn-Schmiedeberg's archives of pharmacology 386: 459–469. [DOI] [PubMed] [Google Scholar]
- 8. Albarran-Juarez J, Gilsbach R, Piekorz RP, Pexa K, Beetz N, et al. (2009) Modulation of alpha2-adrenoceptor functions by heterotrimeric Galphai protein isoforms. The Journal of pharmacology and experimental therapeutics 331: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagata K, Ye C, Jain M, Milstone DS, Liao R, et al. (2000) Galpha(i2) but not Galpha(i3) is required for muscarinic inhibition of contractility and calcium currents in adult cardiomyocytes. Circulation research 87: 903–909. [DOI] [PubMed] [Google Scholar]
- 10. Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, et al. (1995) Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nature genetics 10: 143–150. [DOI] [PubMed] [Google Scholar]
- 11. Gohla A, Klement K, Piekorz RP, Pexa K, vom Dahl S, et al. (2007) An obligatory requirement for the heterotrimeric G protein Gi3 in the antiautophagic action of insulin in the liver. Proceedings of the National Academy of Sciences of the United States of America 104: 3003–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jin YZ, Thompson BD, Zhou ZY, Fu Y, Birnbaumer L, et al. (2008) Reciprocal function of Galphai2 and Galphai3 in graft-versus-host disease. European journal of immunology 38: 1988–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plummer NW, Spicher K, Malphurs J, Akiyama H, Abramowitz J, et al. (2012) Development of the mammalian axial skeleton requires signaling through the Galpha(i) subfamily of heterotrimeric G proteins. Proceedings of the National Academy of Sciences of the United States of America 109: 21366–21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezan J, Lasvaux L, Gezer A, Novakovic A, May-Simera H, et al. (2013) Primary cilium migration depends on G-protein signalling control of subapical cytoskeleton. Nature cell biology 15: : 1107–1115. ncb2819 [pii]; doi:10.1038/ncb2819 [DOI] [PubMed] [Google Scholar]
- 15. Jantzen HM, Milstone DS, Gousset L, Conley PB, Mortensen RM (2001) Impaired activation of murine platelets lacking G alpha(i2). The Journal of clinical investigation 108: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wiege K, Le DD, Syed SN, Ali SR, Novakovic A, et al. (2012) Defective macrophage migration in Galphai2- but not Galphai3-deficient mice. Journal of immunology 189: 980–987. [DOI] [PubMed] [Google Scholar]
- 17. Wiege K, Ali SR, Gewecke B, Novakovic A, Konrad FM, et al. (2013) Galphai2 is the essential Galphai protein in immune complex-induced lung disease. Journal of immunology 190: 324–333. [DOI] [PubMed] [Google Scholar]
- 18.Minetti GC, Feige JN, Bombard F, Heier A, Morvan F, et al. (2014) Galphai2 signaling is required for skeletal muscle growth, regeneration, and satellite cell proliferation and differentiation. Molecular and cellular biology 34: : 619–630. MCB.00957-13 [pii]; doi:10.1128/MCB.00957-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeGeorge BR Jr, Gao E, Boucher M, Vinge LE, Martini JS, et al. (2008) Targeted inhibition of cardiomyocyte Gi signaling enhances susceptibility to apoptotic cell death in response to ischemic stress. Circulation 117: 1378–1387. [DOI] [PubMed] [Google Scholar]
- 20. Waterson RE, Thompson CG, Mabe NW, Kaur K, Talbot JN, et al. (2011) Galpha(i2)-mediated protection from ischaemic injury is modulated by endogenous RGS proteins in the mouse heart. Cardiovascular research 91: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eckle T, Grenz A, Kohler D, Redel A, Falk M, et al. (2006) Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. American journal of physiology Heart and circulatory physiology 291: 2533–2540. [DOI] [PubMed] [Google Scholar]
- 22. Fishbein MC, Meerbaum S, Rit J, Lando U, Kanmatsuse K, et al. (1981) Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. American heart journal 101: 593–600. [DOI] [PubMed] [Google Scholar]
- 23. Leopoldt D, Harteneck C, Nurnberg B (1997) G proteins endogenously expressed in Sf 9 cells: interactions with mammalian histamine receptors. Naunyn-Schmiedeberg's archives of pharmacology 356: 216–224. [DOI] [PubMed] [Google Scholar]
- 24.Salazar NC, Chen J, Rockman HA (2007) Cardiac GPCRs: GPCR signaling in healthy and failing hearts. Biochim Biophys Acta 1768: : 1006–1018. S0005-2736(07)00058-2 [pii]; doi:10.1016/j.bbamem.2007.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schultz JE, Hsu AK, Barbieri JT, Li PL, Gross GJ (1998) Pertussis toxin abolishes the cardioprotective effect of ischemic preconditioning in intact rat heart. Am J Physiol 275: H495–H500. [DOI] [PubMed] [Google Scholar]
- 26. Exner T, Jensen ON, Mann M, Kleuss C, Nurnberg B (1999) Posttranslational modification of Galphao1 generates Galphao3, an abundant G protein in brain. Proceedings of the National Academy of Sciences of the United States of America 96: 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang X, Fu Y, Charbeneau RA, Saunders TL, Taylor DK, et al. (2006) Pleiotropic phenotype of a genomic knock-in of an RGS-insensitive G184S Gnai2 allele. Molecular and cellular biology 26: 6870–6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garcia-Marcos M, Ghosh P, Farquhar MG (2009) GIV is a nonreceptor GEF for G alpha i with a unique motif that regulates Akt signaling. Proceedings of the National Academy of Sciences of the United States of America 106: 3178–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamakura S, Nomura M, Hayase J, Iwakiri Y, Nishikimi A, et al. (2013) The Cell Polarity Protein mInsc Regulates Neutrophil Chemotaxis via a Noncanonical G Protein Signaling Pathway. Developmental cell 26: 292–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PMN infiltration in heart tissue during IR-injury. Surgeries in WT mice were performed as indicated. To stain infiltrated neutrophils, a source for the level changes in Gαi protein expression, immunohistochemistry with an anti-CD15 antibody was performed. Additionally, tissue was stained with a. Gαi2- and b. Gαi3-specific antibodies. Representative images are shown. Scale bar = 10 µm.
(TIF)
Control staining to test antibody specificity. To rule out unspecific binding of the used antibodies in heart tissue control staining were performed as follow. a. Staining of WT tissue with IgG antibody. b. Heart tissue from Gαi2 -/- mice was stained with anti-Gαi2 antibody. c. Heart tissue from Gαi3 -/- mice was stained with anti-Gαi3 antibody. Representative images are shown. Scale bar = 10 µm.
(TIF)
PTX treatment aggravates IR injury. a. WT mice were either injected i.p. with vehicle (n = 6) or Pertussis toxin (PTX)(see Methods S1) (n = 6) and 48 hours later exposed to one hour ischemia and one hour reperfusion. Hearts were counterstained with Evans Blue to determine the AAR and TTC to mark vital tissue (red) and necrotic tissue (white). Subsequently, infarct size was calculated as percentage of AAR. b. Representative heart slice of WT mice treated with NaCl or PTX are shown. These heart discs have an infarcted area of 46% (WT+NaCl) and 69% (WT+PTX). Data in (a) are shown as mean ± SEM; statistic was calculated with t-test; ***P≤0.001 as indicated.
(TIF)
Pertussis Toxin treatment.
(DOCX)