Abstract
DNA methylation has been implicated in the etiopathology of various complex disorders. DNA methyltransferases are involved in maintaining and establishing new methylation patterns. The aim of the present study was to investigate the inherent genetic variations within DNA methyltransferase genes in predisposing to susceptibility to schizophrenia. We screened for polymorphisms in DNA methyltransferases, DNMT1, DNMT3A, DNMT3B and DNMT3L in 330 schizophrenia patients and 302 healthy controls for association with Schizophrenia in south Indian population. These polymorphisms were also tested for subgroup analysis with patient's gender, age of onset and family history. DNMT1 rs2114724 (genotype P = .004, allele P = 0.022) and rs2228611 (genotype P = 0.004, allele P = 0.022) were found to be significantly associated at genotypic and allelic level with Schizophrenia in South Indian population. DNMT3B rs2424932 genotype (P = 0.023) and allele (P = 0.0063) increased the risk of developing schizophrenia in males but not in females. DNMT3B rs1569686 (genotype P = 0.027, allele P = 0.033) was found to be associated with early onset of schizophrenia and also with family history and early onset (genotype P = 0.009). DNMT3L rs2070565 (genotype P = 0.007, allele P = 0.0026) confers an increased risk of developing schizophrenia at an early age in individuals with family history. In-silico prediction indicated functional relevance of these SNPs in regulating the gene. These observations might be crucial in addressing and understanding the genetic control of methylation level differences from ethnic viewpoint. Functional significance of genotype variations within the DNMTs indeed suggest that the genetic nature of methyltransferases should be considered while addressing epigenetic events mediated by methylation in Schizophrenia.
Introduction
Schizophrenia is one of the most debilitating mental disorders affecting 1% of the world population. Being a complex disorder with heterogeneous nature of symptom presentations the etiology of schizophrenia is still an enigma. The disorder has a well established heritability, while studies done in identifying the genetic susceptibility factors were invariantly inconsistent. Genome wide association studies and Linkage analyses have tried to identify susceptibility loci but, no common genetic variant confers in itself more than twice the risk in susceptibility for schizophrenia in general population [1]. Apart from genetic factors, several environmental factors have been implicated in the etiology of schizophrenia which includes migration [2], urbanicity during upbringing [3], prenatal famine [4], season of birth [5], Paternal age [6], in utero exposure to influenza epidemics [7], cannabis use [8] etc. Several studies have been done worldwide to elucidate the role of Gene X Environment interaction in vulnerability to schizophrenia [9]. DNA sequence variations, epigenetic regulations and environmental cues act stochastically to contribute in the etiopathogenecity of schizophrenia [10]. Recent advances in the field of epigenetics have increased understanding of this interaction by identifying molecular mechanisms that mediate environmental influences on gene expression and activity. Epigenetic mis - regulations in response to a variety of environmental factors have been suggested as a mechanism to explain the increasing risk of schizophrenia in adulthood [11]. Epigenetic mechanisms represent a form of cellular memory that contributes to either short- or long-term changes in neuronal function in response to a variety of behavioral experiences, environmental factors, and pharmacological stimuli [12].
Epigenetic changes are mediated by chemical modification of DNA such as DNA methylation or by protein modifications such as histone acetylation and methylation. DNA methylation is indeed the most studied and probably the best understood type of epigenetic modification. DNA methylation is a covalent modification occurring at the cytosine residues of DNA with lasting heritable effects. DNA methylation usually at the CpG sites near to the regulatory regions of genes and regulate the transcription of these genes. DNA methylation at the gene promoters can affect gene transcription by altering the accessibility of RNA polymerase and transcription factors [13]. Methylation of DNA typically leads to transcriptional repression. The process of DNA methylation is regulated by several external and internal factors which include age, diet, folate levels, methionine turn over and the molecular level maintenance machinery. DNA Methyltransferases are a family of enzymes that mediate the process of DNA methylation as a significant component of molecular level maintenance machinery. DNMTs catalyses the transfer of methyl group from S-Adenosyl methionine to the cytosine residue of DNA. There are mainly two classes of DNA methyltransferases; maintenance methyltransferases (DNMT1) and de novo methyltransferases (DNMT3A & 3B). DNMT1 has a preference for hemi methylated DNA and helps in maintaining the methylation pattern through generations. DNMT3A and 3B induces de novo methylation to establish tissue specific DNA methylation pattern during development and in response to environmental factors. DNMT3L lacks methyltransferase activity but orchestrates the process of methylation by the de novo methyltransferases. It also interacts with other DNA binding proteins in chromatin remodeling complex. Genetic variants which influence the DNA methylation need to be considered given the importance of this in regulation of this epigenetic process in response to the environmental cues. The aim of the present study was to identify the role of genetic variants in DNA MethylTransferases such as maintenance methyltransferase gene (DNMT1) and genes for de novo methyltransferases (DNMT3A & 3B) and DNMT3L in predisposing to schizophrenia. The role of these variants would further be investigated and evaluated in terms of age of onset, gender and family history.
Materials and Methods
Subject selection
Patients in this study were recruited from various Mental Health Centres across Kerala, a South Indian state. The study group consisted of 330 patients and 302 controls. All the patients and controls belong to Malayalam speaking ethnic communities reflecting their genetic stratification. Linguistic ethnic background of Malayalam speaking ethnic communities from Kerala also indicates that the population was genetically and epigenetically stratified by considering the food, environment and regional parameters. Patients diagnosed with schizophrenia were recruited by psychiatrists after evaluation for symptoms using ICD10/DSM IV criteria and were rated using BPRS-E for symptom severity. Demographic details regarding age, ethnicity, family history, diet etc was collected through personal interviews during sample collection. Age of onset was determined as the time when the first schizophrenia symptoms occurred based on interview with patient and family informants or from medical records. Age of onset ≤18 years was considered as early onset and was compared for association against the normal and late onset patients. 10 ml peripheral blood sample was collected in EDTA vials, for DNA isolation, from the study subjects. Genomic DNA was isolated from peripheral lymphocytes using conventional Phenol Chloroform method. All the participants gave informed, written consent in a standard consent form to participate in the study after being provided with, and receiving a full explanation of study protocols and objectives. All potential participants who declined to participate or otherwise did not participate were eligible for treatment (if applicable) and were not disadvantaged in any other way by not participating in the study. Whenever the patient had a compromised capability to consent next of kin or legally authorised representative had consented on the behalf of participants. The present study was approved by the Institutional Ethics Committee for Biomedical Subject of Rajiv Gandhi Center for Biotechnology, as per the guidelines of Indian Council for Medical Research and was also approved by Directorate of Health Services, Govt. of Kerala. Age, Sex and ethnicity matched controls were recruited from the normal population.
SNP selection and Genotyping
SNPs were selected based on functional significance, minor allele frequency and their tagging status. Eight SNPs from DNMT1, four from DNMT3A, three from DNMT3B and four from DNMT3L were selected. The details of the selected SNPs are given in Table 1 . Genotyping was done using fluorescence-based competitive allele-specific PCR, KASPar (KBiosciences LGC, Herts, UK) as per the manufacturer's protocol and confirmed by sequencing in representative samples. The genotype calling based on the respective allele specific fluorescence was done by allelic discrimination utility of the SDS 7500 v2.0.5 software (Applied Biosystems, Foster City, CA, USA) at an ambient temperature of 25°C and genotype clusters were plotted.
Table 1. Details of Seleted SNPs.
| Gene | SNP ID | Alleles | Genotyping Method | Possible Functional Effects |
| DNMT1 | rs10418707 | G/A | KASPar | Intronic enhancer |
| rs2114724 | C/T | KASPar | Intronic enhancer | |
| rs2228611 | G/A | KASPar | Splicing Regulation | |
| rs10423341 | C/A | KASPar | Intronic enhancer | |
| rs2228612 | A/G | KASPar | Missense (conservative) | |
| rs2162560 | G/A | KASPar | Intronic enhancer | |
| rs759920 | G/A | KASPar | Intronic enhancer | |
| rs16999593 | T/C | KASPar | Missense (non-conservative); Splicing regulation | |
| DNMT3A | rs1550117 | G/A | Sequencing | |
| rs2304429 | G/A | KASPar | Intronic enhancer | |
| rs2289195 | G/A | KASPar | ||
| rs734693 | C/T | KASPar | Intronic enhancer | |
| DNMT3B | rs1569686 | T/G | KASPar | Promoter/regulatory region |
| rs2424913 | C/T | KASPar | ||
| rs2424932 | G/A | KASPar | ||
| DNMT3L | rs8129776 | G/A | KASPar | |
| rs762424 | G/A | KASPar | Intronic enhancer | |
| rs2070565 | C/T | KASPar | Splicing site | |
| rs2838535 | C/T | KASPar |
Statistical Analysis
Genotype and allele frequencies were computed and were checked for deviation from hardy Hardy-Weinberg equilibrium (ihg2.helmholtz-muenchen.de/cgi-bin/hw/hwa1.pl). Case-control genetic comparisons were performed using the chi-square test and allelic and model based odds ratios (OR), and 95% confidence intervals (CI) were calculated by Fisher's exact test (two-tailed). Further stratification of the patients was done to understand the role of DNMT variants with the gender, age of onset and family history. All statistical analyses were performed using the Graph Pad Prism 5.01, (GraphPad software Inc. San Diego, CA, USA). We considered p-value of <0.05 as significant. We carried out Bonferroni's correction to test for multiple comparisons. However due to the exploratory nature of this study, we prefer to present the results with no adjustment for multiple testing as well, so as not to penalize the data with the possibility of missing important findings. Haplotype analysis was performed using unphased 3.1.3 and P values were visualized using a Microsoft excel based graphical tool GrASP v0.82 beta. Linkage Disequilibrium plots for controls and patients were generated using Haploview 4.2. LD plots for different world populations were generated for the selected SNPs (snpinfo.niehs.nih.gov/snpinfo/snptag.htm). Functional prediction of the deleterious effect if any, of the associated SNP with respect to the functional categories such as protein coding, splicing regulation, transcriptional regulation, and post-translation was assessed in silico using F-SNP program (compbio.cs.queensu.ca/F-SNP/), FastSNP (fastsnp.ibms.sinica.edu.tw), SNP Function Prediction (FuncPred) (snpinfo.niehs.nih.gov/snpinfo/snpfunc.htm), SNP Nexus (www.snp-nexus.org/), HaploReg (www.broadinstitute.org/mammals/haploreg).
Results
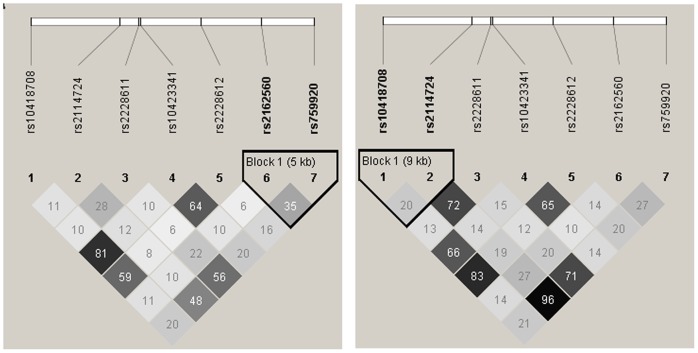
The demographic characteristics of the patients enrolled in this study are shown in Table 2 . There were no significant differences in the mean age and sex distribution between patients and controls. There was no evidence of a deviation from Hardy-Weinberg equilibrium among the controls (P>0.05). Observations from association analyses of SNPs in DNMT1, DNMT3A, DNMT3B, and DNMT3L indicated that DNMT1 rs2114724 and rs2228611 were strongly associated with schizophrenia at allelic and genotypic level ( Table 3 , Table S1). Subgroup analysis of DNMT polymorphisms within the patient based on Gender, Age of onset and Family history is shown in Table 4 . Association analysis of DNMT1, DNMT3A, DNMT3B and DNMT3L polymorphisms are summarised by -Log P value for schizophrenia in a South Indian population ( Figure 1 ). In DNMT1 rs2114724 the allelic (P = 0.022, OR 1.3, CI = 1.04–1.62) and genotypic (P = 0.004) association show an overrepresentation of TT genotype and T allele while in DNMT1 rs2228611 the allelic (P = 0.002 OR 1.43, CI = 1.14–1.8) and genotypic (P = 0.003) association show and overrepresentation of AA genotype and A allele in schizophrenia patients ( Table 3 ). In-Silico analysis of DNMT1 rs2114724 and rs2228611 SNPs, indicate highest functional significance within the DNMT1 gene. DNMT1 rs2114724 indicated enhancer like function with F score of 0.21 while rs2228611 indicated functional relevance for splicing regulation with an F-score of 0.31 ( Table 5 ). While evaluating the allele and genotype frequency differences of the associated DNMT1 SNPs with HapMap populations, we do observe variations within the Indian population i.e between Gujarati Indian (GIH) and South Indian (KER) population. The minor allele of DNMT1 rs2228611 in South Indian and Han Chinese (CHB) populations turns out to be a major allele in Mexican and CEU population (Figure S1) while the minor allele DNMT1 rs2114724 in south Indian population turns out to be a major allele in CHB population (Figure S2). Linkage disequilibrium analysis revealed distinct differences in the LD pattern between patients and controls of DNMT1 due to the associated SNPs ( Figure 2 ).
Table 2. Demographic characteristics of Patients.
| Cases | Total | 330 |
| Females | 196 | |
| Males | 134 | |
| Mean Age, | Females | 36.77±11.65 |
| Males | 34.85±8.93 | |
| Mean Age of onset | Females | 25.3±9.06 |
| Males | 24.03±7.47 | |
| Positive Family History | 41% | |
| Age of onset ≤18 years | 27% |
Table 3. Association analysis of DNMT1 polymorphisms in schizophrenia.
| SNP | Cohort | Genotypes | P value | Allele | P value | Odds Ratio | 95% C.I | |||
| G/G | A/G | A/A | G | A | ||||||
| DNMT1 | PATIENTS | 179(0.58) | 111(0.36) | 18(0.06) | 0.249 | 469(0.76) | 147(0.24) | 0.6388 | 1.068 | 0.8212–1.390 |
| rs10418707 | CONTROLS | 159(0.53) | 126(0.42) | 13(0.04) | 439(0.75) | 147(0.25) | ||||
| C/C | C/T | T/T | C | T | ||||||
| DNMT1 | PATIENTS | 97(0.30) | 147(0.45) | 81(0.25) | 0.004 | 347(0.53) | 311(0.47) | 0.0223 | 1.3 | 1.04–1.62 |
| rs2114724 | CONTROLS | 98(0.33) | 160(0.53) | 43(0.14) | 356(0.59) | 246(0.41) | ||||
| G/G | A/G | A/A | G | A | ||||||
| DNMT1 | PATIENTS | 97(0.30) | 155(0.48) | 68(0.21) | 0.003 | 349(0.55) | 291(0.45) | 0.0019 | 1.43 | 1.14–1.80 |
| rs2228611 | CONTROLS | 113(0.38) | 152(0.51) | 34(0.11) | 378(0.63) | 220(0.37) | ||||
| C/C | A/C | A/A | C | A | ||||||
| DNMT1 | PATIENTS | 185(0.59) | 112(0.36) | 17(0.05) | 0.512 | 482(0.78) | 134(0.22) | 0.3623 | 0.88 | 0.67–1.16 |
| rs10423341 | CONTROLS | 163(0.56) | 115(0.40) | 12(0.04) | 441(0.76) | 139(0.24) | ||||
| A/A | A/G | G/G | A | G | ||||||
| DNMT1 | PATIENTS | 182(0.59) | 112(0.36) | 14(0.05) | 0.33 | 476(0.77) | 140(0.23) | 0.1505 | 0.82 | 0.63–1.07 |
| rs2228612 | CONTROLS | 153(0.54) | 111(0.39) | 19(0.07) | 417(0.74) | 149(0.26) | ||||
| G/G | A/G | A/A | G | A | ||||||
| DNMT1 | PATIENTS | 156(0.49) | 130(0.41) | 33(0.10) | 0.905 | 442(0.69) | 196(0.31) | 0.6659 | 0.95 | 0.74–1.21 |
| rs2162560 | CONTROLS | 139(0.47) | 124(0.42) | 32(0.11) | 402(0.68) | 188(0.32) | ||||
| G/G | A/G | A/A | G | A | ||||||
| DNMT1 | PATIENTS | 103(0.32) | 153(0.47) | 68(0.21) | 0.096 | 359(0.55) | 289(0.45) | 0.2069 | 1.16 | 0.92–1.45 |
| rs759920 | CONTROLS | 96(0.32) | 157(0.53) | 43(0.15) | 349(0.59) | 243(0.41) |
Table 4. Association analysis based on gender, Age of onset and Family history.
| Demographic variables | SNP ID | Gene | Associated genotype/Allele | Genotype P Value | Allele P Value |
| Male Patients | DNMT3B | GG/G | 0.023 | 0.0063 | |
| Age of Onset below 18 | rs1569686 | DNMT3B | TT/T | 0.027 | 0.033 |
| Family history + Age of Onset below 18 | rs1569686 | DNMT3B | TT/T | 0.009 | 0.07 |
| Family history + Age of Onset below 18 | rs2070565 | DNMT3L | CC/C | 0.007 | 0.0026 |
Figure 1. Association analysis of DNMT1, DNMT3A, DNMT3B and DNMT3L polymorphisms represented in -Log P value with schizophrenia in a South Indian population.
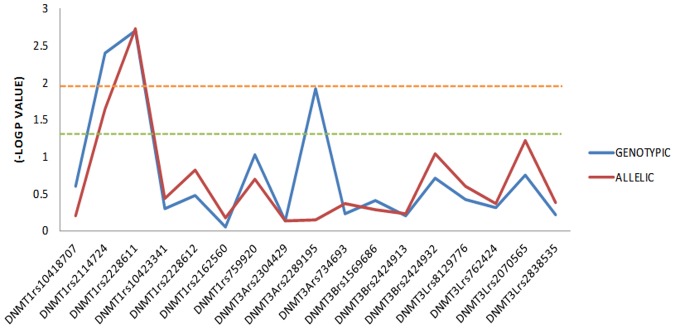
Table 5. In-Silico functional analysis of associated SNP of DNMTs in schizophrenia.
| SNP | F Score | Functional role | Binding Affinity scores | Source | ||
| rs2114724 | 0.208 | Regulatory motifs altered | Motif | T | C | Haploreg |
| ETF | −0.4 | 11.3 | ||||
| rs2228611 | 0.314 | Regulatory motifs altered | Motif | A | G | Haploreg |
| Bach1 | 6.9 | −5 | ||||
| Bach2 | 11.8 | −0.1 | ||||
| ROα1_3 | 12.3 | 0.4 | ||||
| Splice Regulation | Exon splicing enhancer | A | G | ESEfind | ||
| SRp55 | 3.08 | - | ||||
| SF2ASF1 | - | 4.03 | ||||
| rs1569686 | - | Regulatory motifs altered | Motif | G | T | Haploreg |
| GR_disc5 | 12.1 | 3.1 | ||||
| Gfil_1 | 0.8 | 11.6 | ||||
| Gfil_2 | 9.1 | 11.6 | ||||
| Gfilb | 9.2 | 11.5 | ||||
| LBP-1_2 | −0.4 | 11.6 | ||||
| NF-kappaB_disc3 | 14.2 | 10 | ||||
| rs2424932 | - | miRNA Binding | miRNA | A | G | SNP FuncPred |
| hsa-miR-920 | 140 | - | ||||
| hsa-miR-4802-5p | 152 | - | mirSNP | |||
| rs2070565 | 1.00 | Regulatory motifs altered | Motif | T | C | Haploreg |
| ATF4 | 2.2 | 13.7 | ||||
| Maf_known1 | 13.1 | 3.8 | ||||
| NF-E2_disc1 | 10.7 | 10.6 | ||||
| Nrf-2_2 | 16.2 | 6.5 | ||||
| nrf-2_3 | 13.9 | 11.3 |
Figure 2. Linkage Disequilibrium plot showing R″ value in patients (A) and controls (B).
Among de novo methyltransferases the DNMT3A rs2289195 was found to be associated at genotypic level (P = 0.013) with schizophrenia. None of the selected SNPs in DNMT3B, and DNMT3L were found to be associated with schizophrenia. We further analysed the association of DNMT polymorphisms within the patient group by classifying the patients into different subgroups based on Gender, Age of onset and Family history ( Table 4 ). DNMT3B rs2424932 GG genotype (P = 0.023) and G allele (P = 0.0063) was found to be associated with the Schizophrenia in male patients when compared against male controls. Similar association was not observed in females. In the subgroup analysis with age of onset and family history we found that DNMT3B rs1569686 was associated with genotypic (P = 0.027) and allelic (P = 0.033) level with an overrepresentation of TT genotype and T allele in patients with early onset of Schizophrenia. Patients with positive family history of psychiatric illness were further subgrouped into early onset and late onset. Interestingly here too DNMT3B rs1569686 was also found to be associated significantly at genotypic level (P = 0.009) with family history and early age of onset. rs1569686 (−579 G/T) is a promoter SNP inducing changes in Transcription factor binding affinity (mbs.cbrc.jp/research/db/ TFSEARCH.html). Further, the subgroup analysis of positive family history and early onset and normal or late onset indicated that DNMT3L rs2070565 was significantly associated at genotypic (P = 0.007) and allelic (P = 0.0026) level with over representation of CC genotype and C allele in early onset subgroup ( Table 4 ). In-silico analysis indicated that DNMT3L rs2070565 is a splice site variant with an F-score of 1. rs2070565 T/C is an intronic SNP in DNMT3L which result in alteration of splice regulatory motifs ( Table 5 ).
Discussion
In the present study we report that the polymorphisms in the DNMT1, rs2114724 and rs2228611 are significantly associated with schizophrenia in Malayalam speaking south Indian population. Subsequently, while stratifying the Schizophrenia patient group based on the demographic variables, we report a strong association with de novo DNMTs. Among the de novo DNMTs in the present study, DNMT3B rs2424932 was strongly associated with gender and DNMT3B rs1569686 early age of onset while DNMT3L rs2070565 was found to be associated with family history and early age of onset. DNMT1 is known to be responsible in maintaining the intrinsic methylation machinery through generations while de novo DNMTs induces de novo methylation to establish tissue specific DNA methylation pattern during development and in response to environmental factors. These observations suggest that while addressing the alterations in methylation events contributing to schizophrenia, it is important to evaluate the underlying genetic variants in methyltransferases to understand the reasons for differential methylation.
Till date most of the studies relating to schizophrenia has been restricted to either expression based analysis or methylation analysis at candidate gene or global methylation level changes. Hypomethylation of leukocyte DNA in male subjects with schizophrenia was reported in Japanese population [14]. Interestingly, in another study on Israel population no differences in global methylation status was observed inspite of an increase in homocysteine levels in schizophrenia patients [15]. Gene specific methylation and subsequent alteration in their expression level has been studied with many candidate genes in different populations. Methylation in RELN, SOX10, MB-COMT, 5HTR, DRD2 etc. has been studied extensively. The hypermethylation of the CpG islands flanking a CRE and SP1 binding site observed at upstream to Reelin promoter in the post-mortem brains of schizophrenic patients [16]–[17]. MB-COMT promoter DNA has been reported to be hypomethylated in schizophrenia and bipolar disorder patients, compared with the controls [18]. However, these findings could not be confirmed when replicated in other studies [19]–[21]. Increased DNA methylation in the promoter region of the 5HTR1A gene has also been reported in SCZ and BPD [22]. However, no significant difference in the overall DNA methylation of MAOA promoter was reported in schizophrenia patients [23]. In cortical Brodmann's area 10 (BA10) and peripheral blood lymphocytes of Schizophrenia patients, DNMT1 and to a lesser extent DNMT3a, mRNAs were reported to be upregulated [24].The increased expression of S-adenosyl methionine and DNA methyltransferase-1 has been postulated to contribute to promoter cytosine 5-methylation and to downregulation of the expression of mRNAs encoding for reelin and GAD67 in cortical GABAergic neurons of schizophrenia and bipolar disorder patients [25]. Abberant gene expression of brain-derived neurotrophic factor (BDNF) has been implicated in onset of several mental illnesses subsequent to early-life adversity. Maltreatment during infancy leads to increased methylation in BDNF promoter and a subsequent decrease in its expression. [26] Interestingly, all these studies refer to alterations in methylation levels as an influence by the extrinsic factors such as environment, food intake etc. As a result, the success of these observations on methylation levels or expression levels of candidate genes has been limited to individual studies which could not be replicated in many other studies as number of extrinsic and intrinsic factors could modulate the outcome. None of these studies have investigated the role of intrinsic mechanisms in maintaining the methylation machinery to recognize the inherent genetic factors that are crucial to influence the methylation levels in disease causation. The present study on the role of polymorphisms in DNMTs could help in understanding the discrepancies of inconsistencies on differential methylation in previous studies.
In the present study the DNMT1 rs2114724 TT genotype and T allele and DNMT1 rs2228611 AA genotype and A allele were observed to be significantly associated with schizophrenia. These observations are interesting as DNMT1 rs2114724 is an intronic SNP with mutant allele T rendering an intronic enhancer effect. In Korean population, this DNMT1 rs2114724 has been reported to be associated with HBV clearance through intronic enhancer effect [27]. LINE-1 methylation which is a surrogate marker for global methylation was reported to be higher in men with atleast one mutant allele compared to wild type genotype [28]. Moreover the positive correlation of LINE-1 methylation with S-Adenosyl methionine which was seen in individuals with wildtype genotype was lost in men with heterozygous and mutant genotypes [28]. Similarly, DNMT1 rs2228611 which is a synonymous SNP found in exon 17 and was found to be associated with schizophrenia in our study, has a possible splice regulatory function with G/A resulting in loss of three Exonic splicing enhancer binding motif (see Supplementary data). AG and AA genotype of DNMT1 rs2228611 SNP has been reported to be associated with strong inverse relation of LINE-1 methylation with Cadmium exposure in Argentinean women [29] suggesting a possible role in regulating the methylation level in response to environmental cues. Functional significance of genotype variations within the DNMTs indeed suggest that the genetic nature of methyltransferases should be considered while addressing the issue of methylation in Schizophrenia.
The intrinsic mechanisms such as age, gender, ethnicity and genotype and extrinsic mechanisms such as food and environment, if not understood in relation to each other, can also drastically influence inferences on epigenomic alterations. Epigenomic changes are strongly influenced by age, gender, ethnicity and environment. None of the earlier studies on schizophrenia have discussed on these variables. In the present study there were no significant differences in the mean age and sex distribution between patients and controls, indicating that the variables such as age and gender did not contribute to epigenomic alteration. Subject selection based on Malayalam speaking ethnicity also indicated that the populations were genetically stratified. In an earlier study we have reported that Malayalam speaking ethnic communities are genetically distinct from the north Indian communities [30]. While comparing the associated allele and genotype distributions of DNMT1 with the HapMap population we do observe distinct variations within the Indian population i.e Gujarati Indian and South Indian population. Even while comparing with the global populations we observe that the minor allele of DNMT1 rs2228611 in South Indian and CHB populations turns out to be a major allele in Mexican and CEU population while the minor allele DNMT1 rs2114724 in south Indian population turns out to be a major allele in CHB population. In India it is also possible to generalize the epigenetic parameters based on linguistic background of the ethnic population as all linguistic background have distinct food habits which is different from each linguistic ethnic population. In the present study the since the sampling was done from within the Malayalam speaking ethnic community from Kerala which also indicates that the samples were epigenetically stratified as they have similar life style based on their food habits. These observations indicate that genetic selection based on ethnicity might have differential impact on genetic association and epigenomic studies significantly.
Stratifying the patients based on their genotypes and demographic variables we observe a strong association of DNMT3B rs2424932 GG genotype and G allele with schizophrenia in male patients. Interestingly, the DNMT3B rs2424932 A allele was recently reported to be associated with suicidal tendency in people with psychiatric illness along with increase in global DNA methylation level in the suicide attempters compared to non attempters [31]. This SNP is found in 3′UTR and is present in miRNA binding site. Insilico analyses revealed that presence of A allele adds an additional regulation to the gene through binding of hsa_mir-920. In our study, we report an association of DNMT3B rs1569686 TT genotype and T allele with early onset of schizophrenia. In-silico analysis revealed that this DNMT3B rs1569686 (−579 G/T) is a promoter SNP inducing changes in Transcription factor binding affinity (mbs.cbrc.jp/research/db/ TFSEARCH.html). Earlier reports do indicate that null mutations of the mammalian DNA methyltransferases (DNMTs) can be lethal either at prenatal or and postnatal stage [32]–[33]. The exact functional role of the DNMT3B rs1569686 (−579 G/T) polymorphism is still not yet completely elucidated. The DNMT3B has nearly 40 known splice variants expressed in a tissue- and disease-specific manner, but very little is known about the role of these splice variants in modulating DNMT3B function. The polymorphic regions −149C>T, −283T>C and −579G>T within the DNMT3B gene has two transcriptional start sites, which exist in different exons (exon 1A and 1B) and the expression is regulated by different promoters. One promoter is nested within a CpG-rich area, whereas the other promoter is found in CpG poor. The DNMT3B - rs6058870 (283T>C, −283 bp from exon 1A transcription start site) and rs1569686 (−579G>T, −579 bp from exon 1B transcription site) polymorphisms are located in the CpG-rich and CpG-poor promoters, respectively. Some authors have suggested rs1569686 (−579G>T) might directly impair promoter activity while majority observe that it might induce its effect through a tag SNP of functional haplotypes with −149C>T (rs2424913) and 283T>C (rs6058870), which have been functionally associated with promoter activity and gene expression levels. It is difficult to synthesize a construct of a haplotype containing −149C>T, −283T>C and −579G>T polymorphisms by PCR since the −579G>T polymorphism is located at 17171 bp from the −283T>C polymorphism. This makes haplotype specific functional observation difficult. A recent study has demonstrated the potential of combining functional genomics and population genetics approaches for understanding gene regulation (Spivakov et al. 2012). Therefore, while evaluating the LD patterns of SNPs and SNPs proximal to the studied SNPs, among different HapMap population we observe distinct differences between various ethnicities. LD patterns of GIH population show distinct differences with African and Mongoloid populations. DNMT3L rs2070565 CC genotype and C allele was found to be significantly higher in patients with family history of mental illness and who developed the disease at an earlier age compared to those who developed at normal or older age. This suggests the role of DNMT3L rs2070565, in predisposing individuals with a positive family history with increased risk of developing Schizophrenia at an early age. This could be mediated through an alteration in splice regulatory motifs.
To date this is the first exhaustive study implicating the role of DNMT1 with Schizophrenia, DNMT3B with male gender and early onset and DNMT3L rs2070565 with early onset linked to positive family history patients. Implicating the role of DNMT1 polymorphisms that are crucial in maintenance of an already established DNA methylation patterns at replication and de novo DNMT3A, 3B and 3L that are crucial in establishing new methylation patterns is indeed a step forward towards understanding methylation level differences in Schizophrenia. These observations might be crucial in addressing and understanding the genetic control of methylation level differences from ethnic viewpoint. It would be interesting to identify the implication of these observations in global methylation, genome wide methylation and schizophrenia candidate gene specific methylation.
Supporting Information
Allele (A) and genotype (B) frequency of DNMT1 rs2228611 in HapMap population in comparison to South Indian population (KER).
(TIF)
Allele (A) and genotype (B) frequency of DNMT1 rs2114724 in HapMap population in comparison to South Indian population (KER).
(TIF)
Association analysis of DNMT3A, DNMT3B and DNMT3L polymorphisms in schizophrenia.
(DOC)
Funding Statement
KRS was supported by Council for Scientific and Industrial Research (CSIR) New Delhi through a research fellowship. MB was supported through intramural project support from Rajiv Gandhi Center for Biotechnology (RGCB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sullivan PF (2005) The genetics of schizophrenia, PLoS Med 2: , e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selten JP, Cantor-Graae E, Kahn RS (2007) Migration and schizophrenia. Curr Opin Psychiatry 20: , 111–115. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen C, Mortensen P (2001) Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch Gen Psychiatry 58: , 1039–1046. [DOI] [PubMed] [Google Scholar]
- 4. Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, et al. (1996) Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 53: 25–31. [DOI] [PubMed] [Google Scholar]
- 5.Davies G, Welham J, Chant D, Torrey E, McGrath J (2003) A systematic review and meta-analysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophr Bull 29: , 587–593. [DOI] [PubMed] [Google Scholar]
- 6.Sipos A, Rasmussen F, Harrison G, Tynelius P, Lewis G, et al. (2004) Paternal age and schizophrenia: a population based cohort study. BMJ 329: , 1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takei N, Mortensen PB, Klaening U, Murray RM, Sham PC, et al. (1996) Relationship between in utero exposure to influenza epidemics and risk of schizophrenia in Denmark. Biol Psychiatry 40: , 817–824. [DOI] [PubMed] [Google Scholar]
- 8.Weiser M, Noy S (2005) Interpreting the association between cannabis use and increased risk for schizophrenia. Dialogues Clin Neurosci. 7: , 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuang M (2000) Schizophrenia: genes and environment, Biol Psychiatry 47: , 210–220. [DOI] [PubMed] [Google Scholar]
- 10.Petronis A (2004) The origin of schizophrenia: genetic thesis, epigenetic antithesis, and resolving synthesis. Biol Psychiatry 55: , 965–970. [DOI] [PubMed] [Google Scholar]
- 11.Gabriel O, Arturas P (2008) Environmental studies of Schizophrenia through the prism of epigenetics. Schizophr Bull. 34, 1122–1129. [DOI] [PMC free article] [PubMed]
- 12.Ronald SD, Samuel SN (2007) Epigenetic marking and neuronal plasticity. Biol Psychiatry 62: , 1–3. [DOI] [PubMed] [Google Scholar]
- 13.Jaenisch R, Bird A (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic andenvironmental signals. Nat. Genet. 33 (suppl), 245–254. [DOI] [PubMed] [Google Scholar]
- 14. Shimabukuro M, Sasaki T, Imamura A, Tsujita T, Fuke C, et al. (2007) Global hypomethylation of peripheral leukocyte DNA in male patients with schizophrenia: a potential link between epigenetics and schizophrenia. J Psychiatr Res. 41: 1042–1046. [DOI] [PubMed] [Google Scholar]
- 15.Bromberg A, Levine J, Nemetz B, Belmaker RH, Agam G (2008) No association between global leukocyte DNA methylation and homocysteine levels in schizophrenia patients. Schizophr Res 101: , 50–57. [DOI] [PubMed] [Google Scholar]
- 16.Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, et al. (2005) Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci U S A 102: , 9341–9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, et al. (2005) Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Amer J Med Genet B: Neuropsychiatr Genet 134: , 60–66. [DOI] [PubMed] [Google Scholar]
- 18.Abdolmaleky HM, Cheng KH, Faraone SV, Wilcox M, Glatt SJ, et al. (2006) Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum Mol Genet 15: , 3132–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, et al. (2008) Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Amer J Hum Genet 82: , 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dempster EL, Mill J, Craig IW, Collier DA (2006) The quantification of COMT mRNA in post mortem cerebellum tissue: diagnosis, genotype, methylation and expression. BMC Med Genet 7: , 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tochigi M, Iwamoto K, Bundo M, Sasaki T, Kato N, et al. (2008) Gene expression profiling of major depression and suicide in the prefrontal cortex of postmortem brains. Neurosci Res 60: , 184–191. [DOI] [PubMed] [Google Scholar]
- 22.Carrard A, Salzmann A, Malafosse A, Karege F (2011) Increased DNA methylation status of the serotonin receptor 5HTR1A gene promoter in schizophrenia and bipolar disorder. J Affect Disord 132: , 450–453. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Zhang J, Zhang L, Shen Y, Xu Q (2012) Effects of MAOA promoter methylation on susceptibility to paranoid schizophrenia, Hum Genet 131: , 1081–1087. [DOI] [PubMed] [Google Scholar]
- 24.Zhubi A, Veldic M, Puri N, Kadriu B, Caruncho H, et al. (2009) An upregulation of DNA-methyltransferase 1 and 3a expressed in telencephalic GABAergic neurons of schizophrenia patients is also detected in peripheral blood lymphocytes. Schizophr Res 111: , 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guidotti A, Ruzicka W, Grayson DR, Veldic M, Pinna G, et al. (2007) S-adenosyl methionine and DNA methyltransferase-1 mRNA overexpression in psychosis. Neuroreport 18: , 57–60. [DOI] [PubMed] [Google Scholar]
- 26.Roth TL, Lubin FD, Funk AJ, Sweatt JD (2009) Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry 65: , 760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji-Yong C, Joon SB, Tae JP, Kim JY, Byung LP, et al. (2009) Putative association of DNA methyltransferase 1 (DNMT1) polymorphisms with clearance of HBV infection. BMB Rep 42: , 834–839. [DOI] [PubMed] [Google Scholar]
- 28. Inoue-Choi M, Nelson HH, Robien K, Arning E, Bottiglieri T, et al. (2013) Plasma S-adenosylmethionine levels, genetic polymorphisms of DNMT1 and DNMT3A, and LINE-1 DNA methylation among Chinese in Singapore, BMC Cancer. 13: 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammad Bakhtiar H, Marie V, Gabriela C, Karin B (2012) Low-level environmental cadmium exposure is associated with DNA hypomethylation in Argentinean women, Environ Health Perspect. 120: , 879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomas R, Nair S, Banerjee M (2006) A crypto-Dravidian origin for the nontribal communities of South India based on human leukocyte antigen class I diversity. Tissue Antigens 68: 225–234. [DOI] [PubMed] [Google Scholar]
- 31.Murphy TM, Mullins N, Ryan M, Foster T, Kelly C, et al. (2013) Genetic variation in DNMT3B and increased global DNA methylation is associated with suicide attempts in psychiatric patients. Genes, Brain Behav 12: , 125–132. [DOI] [PubMed] [Google Scholar]
- 32.Li E, Bestor TH, Jaenisch R (1992) Targeted mutation of the DNA methyltransferase gene results in embryonic lethality, Cell 69: , 915–926. [DOI] [PubMed] [Google Scholar]
- 33.Okano M, Bell DW, Haber DA, Li E (1999) DNA methyltransferases DNMT3a and DNMT3b are essential for de novo methylation and mammalian development, Cell 99: , 247–257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Allele (A) and genotype (B) frequency of DNMT1 rs2228611 in HapMap population in comparison to South Indian population (KER).
(TIF)
Allele (A) and genotype (B) frequency of DNMT1 rs2114724 in HapMap population in comparison to South Indian population (KER).
(TIF)
Association analysis of DNMT3A, DNMT3B and DNMT3L polymorphisms in schizophrenia.
(DOC)