Abstract
Background
Inverse associations between micronutrient intake and cardiovascular outcomes have been previously shown, but did not focus on diabetic patients.
Objective
To systematically review the role of micronutrients in the development/presence of cardiovascular outcomes in patients with diabetes.
Methods
We searched Medline, Embase, and Scopus (January/1949-March/2012) for observational studies that evaluated micronutrients and cardiovascular outcomes in patients with diabetes, and then selected and extracted the data (two independent reviewers).
Results
From the 15 658 studies identified, five were included, comprising three case-control and two cohorts, with a follow-up of 7-15 years. A meta-analysis was not performed due to the different antioxidant micronutrients (types and measurement methods) and outcomes evaluated. The micronutrients assessed were vitamin C intake in diet and/ or supplementation, chromium and selenium in toenail samples, and α-tocopherol and zinc in serum levels. Intake of > 300 mg of vitamin C through supplementation was associated with increased risk of cardiovascular disease, coronary artery disease (CAD), and stroke (RR 1.69-2.37). High levels of α-tocopherol in serum were associated with 30% lower CAD risk in another study (HR 0.71; 95%CI 0.53-0.94). Among minerals (zinc, selenium, and chromium), an inverse association between zinc and CAD was observed; levels lower than 14.1 μmol/L were associated with an increased risk for CAD (RR 1.70; 95%CI 1.21-2.38).
Conclusion
The information available on this issue is scarce. Further prospective studies are needed to elucidate the role of these nutrients in the cardiovascular risk of patients with diabetes.
Keywords: Micronutrients, Antioxidants, Risk Factors, Cardiovascular Diseases, Diabetes Mellitus
Introduction
Biological research supports a key role for oxidative stress in atherogenesis; free radical-mediated damage induces oxidative changes in low-density cholesterol particles that initiate and promote atherosclerotic changes. This process could be reversed or prevented by the use of antioxidants1. Observational studies have shown inverse associations between antioxidant intake and cardiovascular events2, but randomized clinical trials have not shown any benefit of antioxidants in cardiovascular events3. However, these studies did not focus on patients with diabetes mellitus (DM), a population with high cardiovascular disease risk.
Coronary and cerebrovascular diseases, which are caused primarily by atherosclerosis, are the major cause of morbidity and mortality in patients with DM4, and occur more frequently and more severely in these patients5. Hyperglycemia in DM is characterized by a high oxidative stress state6, which is closely related to the genesis of chronic complications of diabetes, including cardiovascular diseases7. Particular attention has been given to the applicability of antioxidant therapy (endogenous enzymes and dietary substances) in the prevention and management of diabetic complications, especially atherosclerotic cardiovascular disease8.
Lifestyle changes and a healthy diet are included in the prevention and/or treatment of atherosclerosis in patients with or without DM. High intake of fruits, vegetables, whole grains, and oil seeds and low intake of sodium are usually recommended9. Epidemiological studies have shown that certain foods with antioxidant properties are associated with a reduction in inflammatory markers and low-density cholesterol oxidation8, and consequently, improved endothelial function10.
The aim of this study was to systematically review the role of vitamins (vitamins A, C, and E) and minerals (zinc, selenium, chromium, manganese, and copper) with antioxidants properties in the presence or development of clinical cardiovascular outcomes in patients with DM.
Methods
Literature search
The search was performed to select observational studies that evaluated the role of antioxidant micronutrient intake (vitamins and minerals) in the presence or development of cardiovascular events in patients with DM. The databases used in the search were Medline from Pubmed, Embase, and Scopus for the period from January 1949 to March 2012. The search strategy included terms referring to antioxidant micronutrients: "micronutrients," "antioxidant micronutrient," "trace elements," "biometals," "antioxidants," "vitamins," "antioxidant vitamins," "vitamin C," 'ascorbic acid," 'vitamin E," "tocopherols," "alfa-tocopherol," "β-carotene," "vitamin A," "pro-vitamin A," "minerals," "antioxidant minerals," "diet," "diet therapy," "zinc," "copper," "manganese," "chromium," "selenium", to patients (type 1 or type 2 DM): "Diabetes Mellitus, Type 1," "Diabetes Mellitus, Insulin-Dependent," "Diabetes Mellitus, Juvenile-Onset," "Diabetes Mellitus, Sudden-Onset," "Diabetes Mellitus, Type I," "IDDM," "Diabetes Mellitus, Brittle," "Diabetes Mellitus, Ketosis-Prone," Autoimmune Diabetes, "Diabetes Mellitus, Type 2," "Diabetes Mellitus, Ketosis-Resistant," "Diabetes Mellitus, Non-Insulin-Dependent," "Diabetes Mellitus, Slow-Onset," Stable Diabetes Mellitus, "Diabetes Mellitus, Type II," NIDDM, "Diabetes Mellitus, Adult-Onset," "Diabetes Mellitus, Noninsulin Dependent," "Maturity-Onset Diabetes Mellitus," and type of study (observational), using a previously validated list of terms available at: http://www.sign.ac.uk/methodology/filters.html#obs.
The search strategy described above was used to identify studies on Pubmed. Similar terms were searched for in other databases. There was no restriction of the language used in the publications. The article references included in this review were consulted to identify other potentially eligible studies.
Inclusion and exclusion criteria
We included observational studies (case-control studies and cohorts irrespective of their prospective or retrospective nature) that evaluated the role of antioxidant micronutrient intake (from diet and/or supplements) in the presence or development of major cardiovascular events such as myocardial infarction or revascularization, stroke, sudden death, and death from cardiovascular causes in patients with type 1 or type 2 DM.
In selecting the studies, the antioxidant micronutrients looked at were vitamin A (beta-carotene), vitamin C (ascorbic acid), vitamin E (tocopherol), zinc, selenium, chromium, manganese, and copper. The outcomes considered were major cardiovascular events (cardiovascular death, stroke, myocardial infarction, and myocardial revascularization) and their individual components (fatal and non-fatal myocardial infarction, fatal and non-fatal stroke, sudden death or myocardial revascularization).
Study selection and data extraction
Two reviewers (R.A.S. and F.M.S.) independently reviewed the titles and abstracts of each article identified in the literature search. In this first stage all articles that clearly did not meet the inclusion criteria were rejected. The selected articles were analyzed by reading the full text, and the eligible articles were then identified. Disagreements between reviewers at this stage of article analysis were resolved by discussion. The concordance, estimated by Kappa coefficient, was good (Kappa = 0.79).
Data extraction from each study included in this review was conducted independently by two reviewers (R.A.S. and F.M.S.) using a standardized instrument. The data extracted were publication identification, study design, sample size, follow-up duration (in cohort studies), and participants' general characteristics (type of DM, age, gender, body mass index, diabetes treatment, hypertension, and smoking). The data on diet characteristics and micronutrient antioxidants evaluated (quantity, measurement unit, and assessment method) were also extracted. The data extracted concerning cardiovascular outcomes were event type, case numbers, and the estimated risk as presented in the manuscript [relative risk (RR), odds ratio (OR), or hazard ratio (HR)]. We extracted the risk estimate data that considered the largest number of covariates in the analyses.
Quality assessment of studies
The methodological quality of each study included in this review was assessed independently by two reviewers (R.A.S. and F.M.S.) from a questionnaire developed by the authors. The questionnaire was based on four instruments for quality assessment of observational studies designed by the Scottish Intercollegiate Guidelines Network and Critical Appraisal Skills Programme, as proposed in the Cochrane Handbook11. The questionnaire included issues related to the study aim (clarity and specificity), the inclusion and exclusion criteria used to select the participants, sample size of groups, number of patients lost from each group, assessment form of exposure status to the factor studied, and outcomes (if standardized assessment was made by blinded investigators as to the participant exposure status).
Results
Literature search
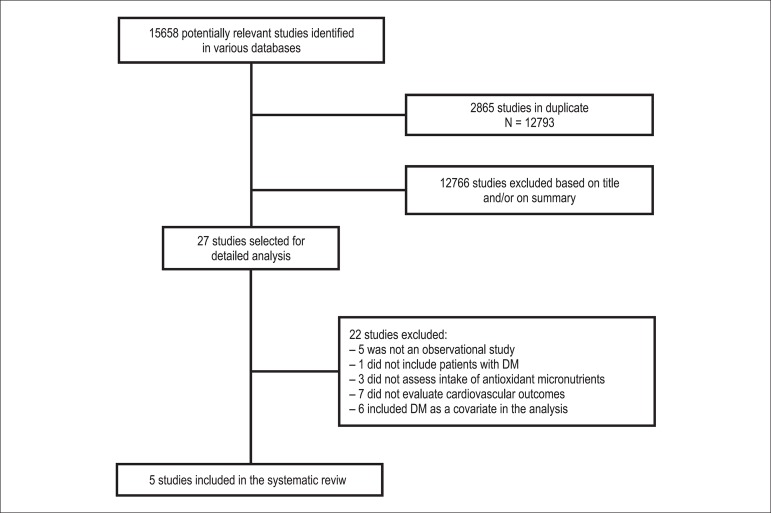
From the 15 658 articles identified, 2865 were excluded because they were duplicated among the databases searched. After analysis of titles and abstracts, 12 766 articles were excluded because they did not meet the inclusion criteria and 27 articles were selected for reading the full text. After evaluating the full texts, 22 articles were excluded because of the following criteria: five studies were not observational, one study involved patients with pre-diabetes and without diabetes, three studies did not assess antioxidant micronutrient effect, seven studies did not evaluate cardiovascular outcomes, and six studies included DM as a covariate in estimating cardiovascular risk and sub-analyses did not include only patients with DM. New articles were not identified from the reference lists of studies consulted. Therefore, five studies were included in this review12-16. The study selection flow diagram is shown in Figure 1.
Figure 1.
The study selection flow diagram.
General characteristics of the studies
The main characteristics of the five studies included are described in Table 1. Three of them presented a case-control design12,14,15 and two were cohort studies13,16 with a follow-up ranging from 716 to 15 years13. One study was conducted in patients with type 1 DM15, one study included patients with type 2 DM16, and in one manuscript the authors reported that the majority of the participants had type 2 DM13. The other two studies did not specify the type of diabetes12,14. Sample size ranged from 12115 to 1923 participants13. The age of the patients ranged from 34 to 75 years. Two studies included both men and women15,16, two studies were performed only in men12,14, and one study was conducted only in women13. Two studies described the treatment of DM; in one of them most of the participants were using oral antidiabetic agents16; whereas in the other study, approximately 70% of patients were using insulin and/or oral antidiabetic agents13. Only two studies reported the number of hypertensive participants, current smokers, and the waist-to-hip ratio values13,15.
Table 1.
Main features of studies
| Author, year | Design (follow-up) | n | DM | DM duration | Age (years) | Gender | BMI (kg/m2) | Micronutrient |
|---|---|---|---|---|---|---|---|---|
| Rajpathak et al12(2004) | case-control | 886 | not reported | not reported | 40-75 | 100% men | not reported | chromium |
| Lee et al13 (2004) | cohort (15 years) | 1923 | not reported | 10.3 years | 62.2 | 100% women | 30.1 | vitamin C |
| Rajpathak et al14 (2005) | case-control | 886 | not reported | not reported | 40-75 | 100% men | not reported | selenium |
| Costacou et al15 (2006) | case-control | i2i | type 1 | 26.7 years | 34.6 | 47.9% women | 24.2 | a-tocopherol |
| Soinio et al16 (2007) | cohort (7 years) | i059 | type 2 | not reported | 45-64 | 45.1% women | 27.9 | zinc |
DM: Diabetes Mellitus; BMI: body mass index.
Different antioxidant micronutrients were evaluated in the studies and different methods were used to measure them. Vitamin C provided in dietary intake and/or supplementation was assessed by food frequency questionnaire13, chromium and selenium were quantified in samples12,14, and α-tocopherol and zinc were measured in serum15,16. The usual diet composition was not described in any study, only a partial dietary description of saturated fatty acids, vitamin E, and beta-carotene was reported in one study13.
Cardiovascular outcomes were differently evaluated among the studies: two studies evaluated the presence of cardiovascular disease12,14, two reported the presence of coronary artery disease15,16 and one other study reported mortality by cardiovascular disease, coronary heart disease, and stroke13. Because of these differences we could not perform a meta-analysis of the data extracted. Therefore, the main results of each study included in this review are shown in Table 2 and discussed.
Table 2.
Main results of the studies Included In the review
| Author (year) | Micronutrient (measure unit) | Statistical analysis criteria | Outcome (number of cases /total number) | RR/OR/HR (CI 95%) | Variables considered for adjustment in multivariate analysis | |
|---|---|---|---|---|---|---|
| Rajpathak et al. (2004)12 | toenail chrome (μg/g) | upper quartile (>2.08) vs. other quartiles | CVD (198/886) | OR = 0.68 (0.42-1.10) | Age, BMI, alcohol, smoking, family history of AMI, physical activity, hypercholesterolemia, hypertension, dietary fats, fiber, glycemic load, folate and selenium levels, and mercury in toenail. | |
| Lee et al. (2004)13 | vitamin C (mg/day) | diet and supplementation | upper quintile (>667) vs. other quintiles | CVD (281/1923) | RR = 1.84 (1.12-3.01) | Age, energy, WHR, BMI, physical activity, smoking, alcoholism, education, marital status, HRT, treatment and duration of DM, dietary fats, vitamin E, β-carotene and folate. |
| CAD (175/1923) | RR = 1.91 (1.05-3.48) | |||||
| Stroke (57/1923) | RR = 2.57 (0.86-7.66) | |||||
| only diet | upper quintile (>251) vs. other quintiles | CVD (281/1923) | RR = 1.11 (0.66-1.87) | Age, energy, WHR, BMI, physical activity, smoking, alcoholism, education, marital status, HRT, treatment and DM duration, dietary fats, vitamin E, p-carotene, folate,and vitamin C supplements. | ||
| CAD (175/1923) | RR = 1.08 (0.57-2.06) | |||||
| Stroke (57/1923) | RR = 1.89 (0.60-6.03) | |||||
| only supplementation | upper quartile (>300) vs. other quartiles | CVD (281/1923) | RR = 1.69 (1.09-2.44) | Age, energy, WHR, BMI, physical activity, smoking, alcoholism, education, marital status, HRT treatment and DM duration, and vitamin C. | ||
| CAD (175/1923) | RR = 2.07 (1.27-3.38) | |||||
| Stroke (57/1923) | RR = 2.37 (1.01-5.57) | |||||
| Rajpathak et al. (2005)14 | toenail selenium (μg/g) | upper quartile (>1.20) vs. other quartiles | CVD (198/886) | OR = 1.47 (0.92-2.35) | Age, BMI, alcohol, smoking, family history of MI, physical activity, hypercholesterolemia, hypertension, dietary fats, fiber, glycemic load, folate and chromium, and mercury levels in toenail. | |
| Costacou et al. (2006)15 | serum α-tocopherol (μg/ml) | high levels (>10.45) vs. low levels | CAD (54/121) | HR = 0.71 (0.53-0.94) | Adjustment model is not specified. | |
| Soinio et al. (2007)16 | serum zinc (μmol/L) | lower quartile (<14.1) vs. other quartiles | Fatal CAD (156/1059) | RR = 1.70 (1.21-2.38) | Age, sex, DM duration, total cholesterol, HDL-c, triglycerides, HbAlc, GFR, hypertension, smoking, BMI, residence place, and DM treatment. | |
| Fatal CAD or non-fatal AMI (254/1059) | RR = 1.37 (1.03-1.82) | |||||
CAD: coronary artery disease; CVD: cardiovascular disease; DM: Diabetes Mellitus; AMI: acute myocardial infarction; OR: odds ratio; HR: hazard ratio; RR: relative risk; CI: confidence interval; WHR: waist-to-hip ratio; BMI: body mass index; HRT hormone replacement therapy; HDL-c: HDL-cholesterol; HbA1c: glycated hemoglobin; GFR: glomerular filtration rate.
Main findings of the studies
Antioxidant vitamins and cardiovascular outcomes
The role of antioxidant vitamins in cardiovascular disease development was evaluated in two studies13,15.
The relationship between vitamin C intake (assessed by a food frequency questionnaire validated in a subsample of the study population) and cardiovascular outcomes in postmenopausal women with DM was evaluated in a prospective cohort study followed for 15 years13. Cardiovascular outcomes (cardiovascular disease mortality, coronary heart disease and stroke) were defined based on the International Classification of Diseases: the codes potentially related to the diagnoses of interest were selected according to the description in the records of local deaths (Iowa, USA). Vitamin C intake of more than 667 mg/day (diet and/or by supplementation) approximately doubled the risk of mortality from cardiovascular disease and coronary artery disease in patients with diabetes. When dietary and supplemental vitamin C were analyzed separately, only supplemental vitamin C showed a positive association with mortality endpoints: the use of at least 300 mg/day of vitamin C supplements was associated with higher risk of cardiovascular disease mortality (RR 1.69; 95%CI 1.09-2.44), coronary artery disease (RR 2.07; 95%CI 1.27-3.38) and stroke (RR 2.3; 95%CI 1.01-5.57) than using smaller quantities of supplementation.
The effect of α-tocopherol, γ-tocopherol, and retinol on the incidence of coronary artery disease in patients with type 1 DM was evaluated in a study of 54 cases and 67 controls derived from a cohort study conducted in Pittsburgh, USA15. Cases were defined by the participants who first developed coronary artery disease, as determined by one of the following criteria: physician-diagnosed angina, myocardial infarction confirmed by Q-waves on electrocardiogram, hospital records (Minnesota code 1.1 or 1.2), angiographic stenosis ≥50%, coronary artery bypass surgery, angioplasty, or ischemic electrocardiographic changes during the follow-up period. Serum levels of α-tocopherol ≥10.45 µg/ml were inversely associated with coronary artery disease (HR 0.71; 95%CI 0.53-0.94). However, when multivitamin supplement users were compared to nonusers, the protective effect of this micronutrient was observed only among supplement users (HR 0.22; 95%CI 0.10-0.49). It is noteworthy that the authors did not specifically report the type of supplement used by the study participants.
Antioxidant minerals and cardiovascular risk
The possible association between zinc, chromium and selenium and presence or development of cardiovascular events in patients with DM were evaluated by three studies12,14,16.
A cohort study with 7 years of follow-up investigated serum zinc levels as a predictor of coronary artery disease in 1050 patients with type 2 DM from Finland16. The outcomes evaluated were mortality from coronary artery disease based on medical records and death certificates, and myocardial infarction incidence according to the World Health Organization criteria (chest pain, enzyme changes and electrocardiogram). Patients with ≤ 14.1 µmol/L of serum zinc at baseline were at higher risk of death from coronary artery disease (RR 1.7; 95%CI 1.21-2.38) and fatal and non-fatal myocardial infarction (RR 1.37; 95%CI 1.03-1.82) than patients with serum levels ≥14.1 µmol/L.
In a case-control study (derived from the Health Professionals Follow-up Study), toenail levels of chromium12 or selenium14 were determined in 198 male patients with DM and prior cardiovascular disease, as well as 688 male patients with DM and without cardiovascular disease. Cardiovascular disease was considered present when subjects presented fatal or non-fatal myocardial infarction as defined by the World Health Organization criteria, coronary artery bypass grafting, angioplasty or stroke. In a multivariate analysis adjusted for the other potential confounding factors, there was no association between chromium12 or selenium14 levels and cardiovascular outcomes.
Quality evaluation
Quality evaluation is shown in Table 3. None of the studies included meets all the criteria previously established to evaluate methodological quality. However, all five studies were for the purpose of answering a clear and focused question and four of them assessed the exposure status and outcomes in a standardized and valid method. The information regarding the outcomes was collected from population-based registries in the two cohort studies selected13,16. In the three case-control studies12,14,15, outcomes were measured in a valid and standardized way. Potential confounding factors were considered in analysis of data from four studies12-14,16. Only one study showed no clear results, because the authors did not describe which covariates were used for multivariate regression adjustment15. Among the three case-control studies, none of them described whether the follow-up losses were similar between the groups12,14,15. In the two cohort studies13,16, follow up duration was considered appropriate and the selection of participants was controlled for potential confounding factors.
Table 3.
Methodological quality of studies included in this review
| Cohort studies | Case-control studies | ||||
|---|---|---|---|---|---|
| Lee et al. (2004)13 | Soinio et al. (2007)16 | Rajpathak et al. (2004)12 | Rajpathak et al. (2005)14 | Costacou et al. (2006)15 | |
| Items related to all observational studies | |||||
| Issue clear, focused, and appropriate | Yes | Yes | Yes | Yes | Yes |
| Exposure status assessed by valid and standardized way | Yes | Yes | Yes | Yes | No |
| Outcomes assessed by valid and standardized way | Yes | Yes | Yes | Yes | Yes |
| Outcomes evaluated by investigators blinded to the exposure | Not described | Not described | Yes | Yes | Not described |
| Potential confounding factors considered in the analysis of data | Yes | Yes | Yes | Yes | Not described |
| Results clearly presented and discussed | Yes | Yes | Yes | Yes | No |
| Items related to cohort studies | |||||
| Sufficient follow-up duration | Yes | Yes | Not applicable | Not applicable | Not applicable |
| Selection of participants controlled for potential confounders | Yes | Yes | Not applicable | Not applicable | Not applicable |
| Items related to case-control studies | |||||
| Sample size similar between cases and controls | Not applicable | Not applicable | No | No | Yes |
| Data collected similarly for cases and controls | Not applicable | Not applicable | Yes | Yes | Yes |
| Exclusion criteria applied similarly for cases and controls | Not applicable | Not applicable | Not described | Not described | Not described |
| Clearly defined cases | Not applicable | Not applicable | Yes | Yes | Yes |
| Controls clearly defined | Not applicable | Not applicable | Yes | Yes | Yes |
| Follow-up losses similar between cases and controls | Not applicable | Not applicable | Not described | Not described | Not described |
Discussion
The purpose of this systematic review was to evaluate the role of antioxidant micronutrients in the presence or development of cardiovascular events in patients with DM. However, the high clinical heterogeneity among the studies obtained hindered the performance of a meta-analysis. Moreover, information on this issue is scarce and of low quality. Vitamin C, vitamin E (α-tocopherol), zinc, selenium, and chromium were micronutrients with antioxidant properties evaluated by the case-control and cohort studies included in this review. The outcomes analyzed were myocardial infarction, stroke, myocardial revascularization, sudden death, and death from cardiovascular causes.
The use of more than 300 mg/day of vitamin C by supplementation was associated with increased cardiovascular risk13. Interestingly, this is not what is reported for subjects without diabetes2,17. A systematic review of 15 cohort studies with 374,488 subjects without DM showed an inverse association between higher intake of vitamin C (diet and supplement) and risk of coronary artery disease (RR 0.84; 95%CI 0.73-0.95)2, but the results were not confirmed with the use of supplemental vitamin C only in the same study2. In clinical trials with long follow-up periods analyzed in other reviews, vitamin C supplement use had no significant effect on the risk of myocardial infarction and stroke in subjects without diabetes17. The inconsistency of these findings may be partially explained by the presence of diabetes and the recommended daily intake of the vitamin. Vitamin C can act as a pro-oxidant interacting with free iron18 and among patients with DM an iron metabolism disorder seems to occur, with an increase in free iron stores19. Alternatively, vitamin C could have promoted protein glycation20 and stimulated lipid peroxidation21, with a possibly deleterious effect on the cardiovascular system as higher doses were administered. The daily vitamin C supplementation amount used was higher than the recommended daily intake for adults (90 mg/day for men and 75 mg/day for women), but lower than the maximum tolerable level (2000 mg/day)22.
Reduced serum levels of α-tocopherol were inversely associated with the incidence of coronary artery disease15, according to prospective observational studies in individuals without DM and/or without previous cardiovascular disease23,24. The α-tocopherol form of vitamin E is the most biologically active and could be considered a good biomarker of the consumption of this vitamin25. However, the beneficial effect observed in the study included in the current review occurred among users of antioxidant supplements, without specifying the supplement type and quantity15. Moreover, increased mortality from all causes3 in subjects without DM was demonstrated with 10-5000 IU/day of vitamin E supplementation in randomized clinical trials. A possible explanation of the adverse effects described is that vitamin E can inhibit platelet function26.
High serum zinc was shown to be protective against the development of cardiovascular disease16, a result that is in accordance with other studies in patients without DM27,28. Patients with type 2 DM presented lower values of serum zinc (9.23 µmol/L vs. 12.46 µmol/L, p < 0.001) compared with patients without DM, suggesting a lower antioxidant capacity in diabetes29. Possibly, the importance of maintaining high serum zinc values is due to their role in an endogenous antioxidant system30 and/or because zinc plays a clear role in the synthesis, storage and secretion of insulin31.
Chromium12 and selenium14, which were measured in the toenail, were not associated with cardiovascular outcomes in patients with DM in the studies included in this review. This result is different from what was observed in subjects without DM in a case-control study performed in eight European countries and Israel (EURAMIC study)32. In the EURAMIC study, chromium levels in nails was inversely associated with the occurrence of myocardial infarction (OR 0.59; 95%CI 0.37-0.95)32. Moreover, chromium33 and zinc31 are beneficial in regulating insulin action and energy metabolism. Better glycemic control could be reflected in lower cardiovascular outcomes34. In a recent systematic review with meta-analysis, chromium supplementation (1.28 to 1000 mcg/day) decreased the glycated hemoglobin values in 381 patients with DM by 0.6% (95%CI -0.9 to -0.2)35. However, cardiovascular outcomes were not evaluated in that study.
The study which evaluated selenium included in the current review was not in accordance with a recent meta-analysis of 25 observational studies36 that demonstrated a reduction of 24% (95%CI 7-38) in the risk of coronary artery disease with an increase of 50% in selenium levels (assessed by different methods). Selenium is another essential mineral involved in antioxidant defense, since it is part of glutathione peroxidase, a selenoprotein. In this context, low serum selenium has been linked to increased risk of cardiovascular disease in subjects without DM37. The effects of selenium supplementation (200 µg/day) in the prevention of cardiovascular events were not confirmed in a randomized clinical trial36 or in a prospective study having a follow-up of 7.6 years38, probably due to its narrow therapeutic range. Selenium deficiency in humans appears to be just one factor in a complex set of nutritional variables that may predispose or protect against cardiovascular disease37. One of the limitations common to studies with selenium and chromium included in this review involves the measurement method adopted. Although the levels of these minerals in the toenail may reflect the long-term intake of the mineral25, samples contamination could be a source of error12,14.
Our systematic review has several limitations: 1. the low quality of the original studies; 2. no study included meets all items previously established to evaluate methodological quality and potential confounding factors were considered in the analysis of data just from four studies12-14,16; 3. no sensibility analysis was carried out due to clinical heterogeneity of studies included; 4. the case-control studies did not allow us to establish a cause-consequence between micronutrients intake and cardiovascular outcomes. Also, the results for supplementation of vitamin C derived from a single cohort study and need to be considered with caution.In conclusion and according to available evidence, information about antioxidant micronutrient intake and cardiovascular risk in individuals with DM is too scarce to determine which micronutrient antioxidants might be related to cardiovascular outcomes in the DM population. Moreover, the antioxidant property of micronutrients appears to be only one factor in a complex set of nutritional variables that may predispose or protect against cardiovascular disease. Further studies should be performed to explore the relationship between antioxidant micronutrient intake and the development of cardiovascular disease in patients with DM, preferably randomized controlled trials. The description of the results of this review will aid researchers interested in investigating the topic to develop their hypotheses.
Footnotes
Author contributions
Conception and design of the research, Acquisition of data and Critical revision of the manuscript for intellectual content: Sarmento RA, Silva FM, Sbruzzi G, Schaan BD, Almeida JC; Analysis and interpretation of the data: Sarmento RA, Silva FM, Schaan BD, Almeida JC; Statistical analysis: Sarmento RA, Silva FM, Sbruzzi G, Schaan BD; Writing of the manuscript: Sarmento RA.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This article is part of Roberta Aguiar Sarmento postgraduation final project at Instituto de Cardiologia - Fundação Universitária de Cardiologia do RS.
References
- 1.Diaz MN, Frei B, Vita JA, Keaney JF Jr. Antioxidants and atherosclerotic heart disease. N Engl J Med. 1997;337(6):408–416. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- 2.Ye Z, Song H. Antioxidant vitamins intake and the risk of coronary heart disease: meta-analysis of cohort studies. Eur J CardiovascPrevRehabil. 2008;15(1):26–34. doi: 10.1097/HJR.0b013e3282f11f95. [DOI] [PubMed] [Google Scholar]
- 3.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics2009 update: a report fromthe American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. Erratum in: Circulation. 2009;119(3):e182. Circulation. 2010;122(1):e11. Circulation. 2011;124(16):e424. [DOI] [PubMed] [Google Scholar]
- 5.Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862. Erratum in: N Engl J Med. 2011 Mar 31;364(13):1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 7.Penckofer S, Schwertz D, Florczak K. Oxidative stress and cardiovascular disease in type 2 diabetes: the role of antioxidants and pro-oxidants. J CardiovascNurs. 2002;16(2):68–85. doi: 10.1097/00005082-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Martini LA, Catania AS, Ferreira SR. Role of vitamins and minerals in prevention and management of type 2 diabetes mellitus. Nutr Rev. 2010;68(6):341–354. doi: 10.1111/j.1753-4887.2010.00296.x. [DOI] [PubMed] [Google Scholar]
- 9.Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, et al. American Diabetes Association Nutrition recommendations and interventions for diabetes:a position statement of the American Diabetes Association. Care. 2008;31(Suppl 1):S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 10.Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventionsversion 5.1.0. The Cochrane Collaboration; [updated March 2011]. 2011. [Cited in 2012 Dec 10]. Availablefrom: http://www.cochrane-handbook.org. [Google Scholar]
- 12.Rajpathak S, Rimm EB, Li T, Morris JS, Stampfer MJ, Willett WC, et al. Lower toenail chromium in men with diabetes and cardiovascular disease compared with healthy men. Diabetes Care. 2004;27(9):2211–2216. doi: 10.2337/diacare.27.9.2211. [DOI] [PubMed] [Google Scholar]
- 13.Lee DH, Folsom AR, Harnack L, Halliwell B, Jacobs DR., Jr Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes. Am J ClinNutr. 2004;80(5):1194–1200. doi: 10.1093/ajcn/80.5.1194. [DOI] [PubMed] [Google Scholar]
- 14.Rajpathak S, Rimm E, Morris S, Hu F. Toenail selenium and cardiovascular disease in men with diabetes. J Am CollNutr. 2005;24(4):250–256. doi: 10.1080/07315724.2005.10719472. [DOI] [PubMed] [Google Scholar]
- 15.Costacou T, Zgibor JC, Evans RW, Tyurina YY, Kagan VE, Orchard TJ. Antioxidants and coronary artery disease among individuals with type 1 diabetes: Findings from the Pittsburgh Epidemiology of Diabetes Complications Study. J Diabetes Complications. 2006;20(6):387–394. doi: 10.1016/j.jdiacomp.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Soinio M, Marniemi J, Laakso M, Pyorala K, Lehto S, Ronnemaa T. Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care. 2007;30(3):523–528. doi: 10.2337/dc06-1682. [DOI] [PubMed] [Google Scholar]
- 17.Asplund K. Antioxidant vitamins in the prevention of cardiovascular disease: a systematic review. J Intern Med. 2002;251(5):372–392. doi: 10.1046/j.1365-2796.2002.00973.x. [DOI] [PubMed] [Google Scholar]
- 18.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. CurrMed Chem. 2005;12(10):1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Real JM, López-Bermejo A, Ricart W. Cross-talkbetween iron metabolism and diabetes. Diabetes. 2002;51(8):2348–2354. doi: 10.2337/diabetes.51.8.2348. [DOI] [PubMed] [Google Scholar]
- 20.Lee KW, Mossine V, Ortwerth BJ. The relative ability of glucose and ascorbate to glycate and crosslink lens proteins in vitro. off. Exp Eye Res. 1998;67(1):95–104. doi: 10.1006/exer.1998.0500. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292(5524):2083–2086. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- 22.US National Academy of Sciences . Dietary reference intakes for vitamin C, vitamin E, selenium and carotenoids. Washington, DC: NationalAcademy Press; 2000. [PubMed] [Google Scholar]
- 23.Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willet WC. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993;328(20):1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- 24.Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willet WC. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med. 1993;328(20):1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- 25.Mayne ST. Antioxidant nutrients and chronic disease: use of biomarkers of exposure and oxidative stress status in epidemiologic research. J Nutr. 2003;133(3):933S–940S. doi: 10.1093/jn/133.3.933S. [DOI] [PubMed] [Google Scholar]
- 26.Steiner M. Influence of vitamin E on platelet function in humans. J Am CollNutr. 1991;10(5):466–473. doi: 10.1080/07315724.1991.10718173. [DOI] [PubMed] [Google Scholar]
- 27.Reunanen A, Knekt P, Marniemi J, Mäki J, Maatela J, Aromaa A. Serum calcium, magnesium, copper and zinc and risk of cardiovascular death. Eur J ClinNutr. 1996;50(7):431–437. [PubMed] [Google Scholar]
- 28.Lee DH, Folsom AR, Jacobs DR., Jr Iron, zinc, and alcohol consumption and mortality from cardiovascular diseases: the Iowa Women's Health Study. Am J ClinNutr. 2005;81(4):787–791. doi: 10.1093/ajcn/81.4.787. [DOI] [PubMed] [Google Scholar]
- 29.Anetor JI, Senjobi A, Ajose OA, Agbedana EO. Decreased serum magnesium and zinc levels: atherogenic implications in type-2 diabetes mellitusin Nigerians. NutrHealth. 2002;16(4):291–300. doi: 10.1177/026010600201600403. [DOI] [PubMed] [Google Scholar]
- 30.Prasad AS. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. ExpGerontol. 2008;43(5):370–377. doi: 10.1016/j.exger.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Chausmer AB. Zinc, insulin and diabetes. J Am CollNutr. 1998;17(2):109–115. doi: 10.1080/07315724.1998.10718735. [DOI] [PubMed] [Google Scholar]
- 32.Guallar E, Jiménez FJ, van't Veer P, Bode P, Riemersma RA, Gómez-Aracena J, et al. Low toenail chromium concentration and increased risk of nonfatal myocardial infarction. Am J Epidemiol. 2005;162(2):157–164. doi: 10.1093/aje/kwi180. [DOI] [PubMed] [Google Scholar]
- 33.Lai MH. Antioxidant effects and insulin resistance improvement of chromium combined with vitamin C and e supplementation for type 2 diabetes mellitus. J ClinBiochemNutr. 2008;43(3):191–198. doi: 10.3164/jcbn.2008064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 35.Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30(8):2154–2163. doi: 10.2337/dc06-0996. [DOI] [PubMed] [Google Scholar]
- 36.Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. Selenium and coronary heart disease: a meta-analysis. Am J ClinNutr. 2006;84(4):762–773. doi: 10.1093/ajcn/84.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alissa EM, Bahijri SM, Ferns GA. The controversy surrounding selenium and cardiovascular disease: a review of the evidence. Med SciMonit. 2003;9(1):RA9–R18. [PubMed] [Google Scholar]
- 38.Stranges S, Marshall JR, Trevisan M, Natarajan R, Donahue1 RP, Combs GF, et al. Effects of selenium supplementation on cardiovascular disease incidence and mortality: secondary analyses in a randomized clinical trial. Am J Epidemiol. 2006;163(8):694–699. doi: 10.1093/aje/kwj097. [DOI] [PubMed] [Google Scholar]