Abstract
Background
Chagas disease affects more than 15 million people worldwide. Although vector-borne transmission has decreased, oral transmission has become important. Recently, our group published the clinical and epidemiological characteristics of the largest outbreak of orally transmitted Chagas disease reported till date. Objective: To describe electrocardiographic changes occurring in the study population during the outbreak caused by ingestion of contaminated guava juice.
Methods
We evaluated 103 positive cases, of which 76 (74%) were aged ≤ 18 years (average age: 9.1 ± 3.1 years) and 27 (26%) were aged > 18 years (average age: 46 ± 11.8 years). All patients underwent clinical evaluations and ECG. If the patients had palpitations or evident alterations of rhythm at baseline, ambulatory ECG monitoring was performed.
Results
A total of 68 cases (66%; 53 children and 15 adults) had ECG abnormalities. Further, 69.7% (53/76) of those aged ≤ 18 years and 56% (15/27) of those aged >18 years showed some ECG alteration (p = ns). ST-T abnormalities were observed in 37.86% cases (39/103) and arrhythmias were evident in 28.16% cases (29/103). ST alterations occurred in 72% of those aged ≤18 years compared with 19% of th ose aged >18 years (p < 0.0001).
Conclusion
This study reports the largest number of cases in the same outbreak of acute Chagas disease caused by oral contamination, with recorded ECGs. ECG changes suggestive of acute myocarditis and arrhythmias were the most frequent abnormalities found.
Keywords: Chagas Disease / complications; Electrocardiography; Chagas Cardiomyopathy; Arrhthmias, Cardiac; Juices; Contamination
Abbreviations
T. cruzi - Trypanosoma cruzi
ECG - Electrocardiogram
NS - Non-significant
AHA - American Heart Association
ACC - American College of cardiology
HRS - Heart Rhythm Society
ST - ST segment
T - T wave
Right BBB - Right bundle branch block
Left BBB - Left bundle branch block
Introduction
Chagas disease is caused by Trypanosomacruzi and transmitted by several types of triatomines1. It is endemic in Latin America, although migration flows have resulted in the spread of the disease in Europe and the United States as well2,3. It has been estimated that there are approximately 15 million diagnosed cases and approximately 109 million people at risk of contracting this illness1. Although these numbers have shown a decrease from 1990 to 20061, in recent years, the description of endemic outbreaks of orally transmitted disease have opened a new area of study and analysis4-8. From being an unknown route of contamination, the oral route has become one of the most active in cases reported in Venezuela, Brazil, and Colombia4-8.
Our group previously published epidemiological and clinical characteristics of the largest outbreak of orally transmitted Chagas disease reported till date9. Here we analyze ECG manifestations of this outbreak, considered unique for occurring in a closed environment (in a school) in a Latin American capital.
Methods
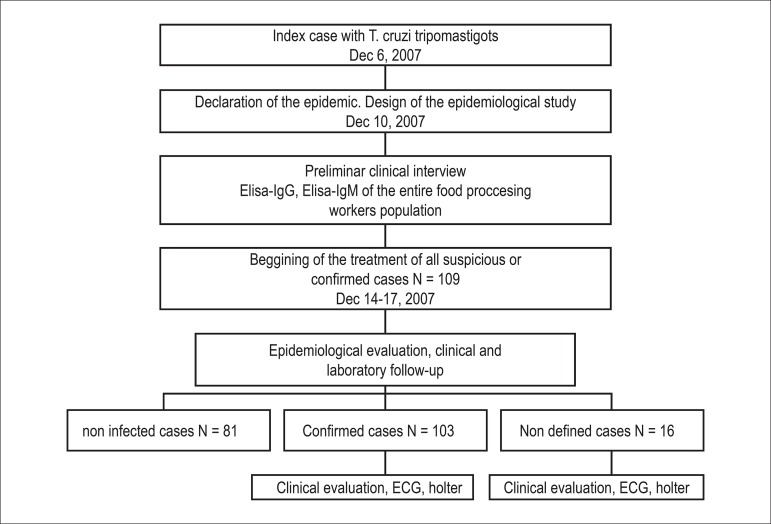
The epidemiology of the outbreak is shown in Figure 19. Of the total positive cases (n = 103), 76 were aged ≤ 18 years (average age: 9.1 ± 3.1 years), whereas 27 were aged >18 years (average age: 46 ± 11.8 years). All positive or undefined patients underwent ECG. Before ECG, the patients were interviewed and physically examined by a cardiologist. The criteria for ECG alterations were based on the AHA / ACCF / HRS recommendations for the standardization and interpretation of ECGs9. ST segment alterations were defined by an elevation ≥1 mm in one or more derivations in which it is not present normally. T wave alterations were defined as a negative T wave in one or more derivations in which it is not present normally. Only confirmed patients (n = 103) were analyzed in this study. If the patient reported palpitations, or if there was any evidence of rhythm disturbance in the baseline ECG (arrhythmias), an ambulatory ECG monitoring (Holter) was performed. Echocardiogram was performed in patients with ECG abnormalities. The results were analyzed differentiating patients younger or older than 18 years using descriptive statistics. Statistical significance was analyzed by comparing proportions.
Figure 1.
Epidemiological description of an acute Chagas disease outbreak in Caracas in 2007.
Results
A total of 68 cases (66%; 53 children and 15 adults) had ECG alterations, whereas 42 (33.9%; 23 children and 12 adults) had normal ECGs. Some major ECG changes were identified in 69.7% patients (53/76) aged <18 years and 55.5% (15/27) of those aged >18 years (p = ns). ECG manifestations are described in Table 1. Because some patients had more than one ECG alteration, the total number of alterations identified is greater than the number of patients.
Table 1.
ECG alterations detected In confirmed cases (n = 103)
| < 18 a n = 53 | > 18 a n = 15 | Total n = 68 | % of the total of cases (n = 103) | |
|---|---|---|---|---|
| ST T changes (ST elevation and T invertion) | 38 | 1 | 39 | 37,86 |
| QT prolongation | 3 | 0 | 3 | 2,91 |
| Microvoltage | 2 | 2 | 4 | 3,88 |
| Right BBB | 2 | 0 | 2 | 1,94 |
| First degree AV block | 2 | 0 | 2 | 1,94 |
| Left BBB | 1 | 2 | 3 | 2,91 |
The most common finding was alteration of the ST segment and T wave that was present in 37.86% cases (39/103). QT prolongation analyzed by the method of Bazett was present in 2.91% cases (3/103).
Blockade of the right branch was present in 1.94% cases (2/103), whereas inhibition of the left branch of the anterior subdivision was present in 2.91% cases (3/103). Looking at age groups, it was clear that those aged ≤ 18 years had a higher incidence of ST abnormalities compared with those aged >18 years (72% vs. 19%) (p < 0.00001). There were no significant differences in other ECG alterations between both groups.
Echocardiograms were performed in 84% cases with ECG alterations (57/68). The echocardiograms of 68% cases were normal, whereas 32% showed mild to moderate pericardial effusion.
Arrhythmias were evident in 32% cases (33/103). The different types of arrhythmias observed are shown in Table 2. Supraventricular arrhythmias occurred in 22.3% cases (23/103), ventricular in 5.8% cases (6/103), and AV block in 2.91% cases (3/103).
Table 2.
Arrhythmias detected in ECG or 24-h Holter monitoring
| < 1B a | > 18 a | Total | % of total number of cases (n = 103) | |
|---|---|---|---|---|
| Ectopic atrial tachycardia | 7 | 2 | 9 | 8,74 |
| Inapropiate sinus tachycardia | 3 | 7 | 10 | 9,71 |
| Supraventricular extrasystols | 1 | 0 | 1 | 0,97 |
| Atrial fibrilation | 2 | 1 | 3 | 2,91 |
| First degree AV block | 2 | 0 | 2 | 1,94 |
| Second degree AV block Weckenbach type | 1 | 0 | 1 | 0,97 |
| Ventricular extrasystols | 1 | 0 | 1 | 0,97 |
| Non sustained ventricular tachycardia | 0 | 1 | 1 | 0,97 |
| Sustained ventricular tachycardia | 1 | 0 | 1 | 0,97 |
Inappropriate sinus tachycardia was the most common arrhythmia occurring in 9.71% cases (10/193), followed by atrial ectopic arrhythmia that was present in 8.74% cases (9/103). Because of the reduced number of cases, statistical analysis in relation to arrhythmias was performed by comparison of proportions between age groups.
Discussion
The presence of blood trypomastigotes in orally inoculated animals who later developed Chagas disease10 was shown as early as 1921; however, it was not until 1991 that 26 acute cases of the disease caused by ingestion of infected cane juice in the state of Paríba in Brazil were described11. Although the description of ECG changes associated with vector-borne Chagas disease has been extensive and detailed12-16, the same cannot be said for cases caused by oral transmission17-19.
The fact that the cases we investigated occurred in a city in a closed environment (school) allowed us to analyze both non-contaminated and contaminated populations and administer rapid medical intervention in the entire population. Thus, this investigation is unique and can be a point of reference. Moreover, it is important to highlight that ours is the only investigation that can compare ECG manifestations in children and adults, because of the number of patients studied. We have compared our results with those reported by other studies of vector-borne and orally transmitted disease.
Table 3 shows a comparison of our results with those reported in different studies of oral infection4,11,17,18. It is worth noting that some reports represent recollection of cases and not an outbreak of the disease itself. In addition, the time between the onset of symptoms and realization of ECG is variable. As shown, abnormal ECG was observed in 50%-100% cases (66% in our study). The most frequent ECG alteration was the change of the T wave and ST segment, ranging between 16.6% and 100% (37.86% in our study). The presence of right branch blockage, characteristic of chronic disease, was present only in 5%-25% cases (1.94% in our study). The presence of atrial fibrillation varies between 0% and 8% (2.9% in our study).
Table 3.
Comparison of ECG changes in acute Chagas disease induced by oral contamination
| Shikanai et al11 | Pinto et al17 | Pinto4 | Bastos21 | Marques et al | |
|---|---|---|---|---|---|
| N | 24 | 188 (multiple outbreaks analysis) | 11 | 13 | 103 |
| Age range | 11-75 | Not described | 17-70 | 9-61 | 5-65 |
| Cases with ECG alterations | 12/24 (50%) | 96/188 (51,1%) | 6/11 (55%) | 12/12 (100%) 1 ECG not performed | 68/103 (66%) |
| ST Tchanges | 4/24 (16,6%) | 40/188 (21,27%) | 4/11 (36%) | 12/12 (100%) | 39/103 (37,86%) |
| Right BBB | 2/24 (8,3%) | 5/188 (5%) | Não | 3/12 (25%) | 2/103 (1,94%) |
| Atrial fibrilation | 0/12 (0%) | 5/96 (2,6%) | Não | 1/12 (8%) | 3/103 (2,9%) |
| Days between symthoms and ECG register | 33-35 | Not described | Not described | 7-37 dias | 32 dias |
In Table 4 we compare our findings on ECG changes in acute Chagas disease transmitted via vector with those reported by Parada et al21 in Venezuela and Shikanai-Yasuda et al11 in Brazil. In our study, we observed a higher incidence of ST-T changes (37.86%) compared with that reported by Parada et al20 (4.4%) and Shikanai-Yasuda et al11 (6.9%). Right branch blockage occurred in 1.94% of our cases, compared with 5.1% in the study of Parada et al20 and 1.1% in the study of Shikanai-Yasuda et al11.
Table 4.
Comparison of ECG changes in acute cases of Chagas disease induced by oral vs. vector-borne contamination
| Parada et al11 | Shikanai-Yasuda et al11 | Marques et al | |
|---|---|---|---|
| N | 58 | 180 | 103 |
| Age range | 17-50 | 1-50 | 5-65 |
| Cases with ECG alterations | 24/58 (41%) | 78/180 (43,4%) | 68/103 (66%) |
| ST T Change | 4/58 (6,9%) | 8/180 (4,4%) | 39/103 (37,86%) |
| Right BBB | 3/58 (5,1%) | 2/180 (1,1%) | 2/103 (1,94%) |
| Atrial fibrilation | 1/58 (1,72%) | Not described | 3/103 (2,9%) |
| Microvoltages | 10/58 (17,2%) | 15/180 (8,3%) | 4/103 (3,88%) |
| Days between symptoms and ECG register | Not described | Not described | 32 dias |
Anselmi et al21 used an experimental model of intraperitoneal inoculation and observed signs of acute myocarditis in 54% of cases (17/31), underscoring the importance of the transmission route and parasite load in clinical manifestations and ECGs.
As we can see, there are differences when comparing vector-borne and oral routes of transmission, as well as among studies of oral transmission. Possible explanations for these differences may be as follows:
1. - Differences in pathogenicity of strains of T. cruzi. Camandaroba et al22 showed differences in pathogenicity between different strains administered to mice orally as well as intraperitoneally. Comparing Peruvian and Colombian strains, they found that Peruvian strains had high infectivity and pathogenicity when administered intraperitoneally, but low infectivity and pathogenicity when administered orally; in contrast, Colombian strains (biodeme type III) had high pathogenicity following oral administration.
2. - Differences in oral pathogenicity. Covarrubias et al23 used a strain of T. cruzi related to an outbreak of orally transmitted disease in Santa Catarina, Brazil, to analyze the expression of surface molecules and infectivity when administered to mice orally. They found that metacyclic trypomastigotes, that in vitro have high concentrations of gp90, a surface molecule that acts as a negative regulator of the invasion of host cells, when administered orally have low levels of themselves, because they are destroyed by gastric acid. [Remark 2] In contrast, gp89, a surface molecule that promotes cellular invasion, is resistant to gastric fluid. Thus, surface molecules that promote cell invasion persist, whereas the inhibitors are destroyed by gastric fluid, which causes the same strain to become much more aggressive when administered orally than when studied in vivo or administered parenterally.
3. - Differences in host-parasite interaction. Although an increase in infectivity has been demonstrated when the parasite is administered orally, because of the destruction of gp9023,24 by gastric juice, there have been no studies that assess how aging-related variations in pH of the stomach could be associated with greater or lesser infectivity according to the capacity to destroy gp90.
4. - A more controlled study group. Our study is the largest investigation of cases occurring in a "closed" environment, in this case a school. Thus, we could study all the people exposed, which does not occur in other investigations.
5. - An earlier stage of the disease. In our study, the time between ingestion of the contaminated guava juice (October 28, 2007), the onset of symptoms, (first patient: November 9, 2007), diagnosis of the index case (December 6, 2007), and ECG recording of the population (December 11-14, 2007) was extremely accurate. This preciseness of records is not common in other studies, and could result in differences when analyzing other moments of the clinical profile.
It is important to mention that ECG alterations were not related to the presence of pericardial effusion. A full description of echocardiographic alterations will be discussed in a different paper. Analysis of ECG evolution in these patients, which would more clearly define the different phases of the disease after oral infection, is the next step.
Conclusion
This is the largest study of clinical cases from a single outbreak of orally transmitted Chagas disease, with recorded ECGs. We found ECG changes suggestive of acute myocarditis and arrhythmias to be the most frequent abnormalities.
Acknowledgments
We are grateful to all patients who agreed to participate in this study.
Footnotes
Author contributions
Conception and design of the research and Analysis and interpretation of the data: Marques J, Mendoza I, Noya B; Acquisition of data: Marques J, Mendoza I, Noya B, Acquatella H; Statistical analysis: Marques J; Writing of the manuscript: Marques J, Mendoza I, Marques-Mejias M; Critical revision of the manuscript for intellectual content: Palácios I.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any post-graduation program.
References
- 1.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375(9723):1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 2.Schmunis GA. Epidemiology of Chagas disease in non-endemic countries: the role of international migration. Mem Inst Oswaldo Cruz. 2007;102(1):75–85. doi: 10.1590/s0074-02762007005000093. Erratum in Mem Inst Oswaldo Cruz. 2007;102(8):2. [DOI] [PubMed] [Google Scholar]
- 3.Guerri-Guttenberg RA, Grana DR, Ambrosio G, Milei J. Chagas cardiomyopathy: Europe is not spared. Eur Heart J. 2008;29(21):2587–2591. doi: 10.1093/eurheartj/ehn424. [DOI] [PubMed] [Google Scholar]
- 4.Pinto AY, Ferreira AG, Valente Vda C, Harada GS, Valente SA. Urban outbreak of acute Chagas disease in Amazon region of Brazil: four years follow-up after treatment with benzidazole. Rev Panam Salud Publica. 2009;25(1):77–83. doi: 10.1590/s1020-49892009000100012. [DOI] [PubMed] [Google Scholar]
- 5.Dias JP, Bastos C, Araujo E, Mascarenhas AV, Martins Netto E, Grassi F, et al. Acute Chagas disease outbreak associated with oral transmission. Rev Soc Bras Med Trop. 2008;41(3):296–300. doi: 10.1590/s0037-86822008000300014. [DOI] [PubMed] [Google Scholar]
- 6.Valente SA, da Costa Valente V, das Neves Pinto AY, de Jesus Barbosa César M, dos Santos MP, Miranda CO, et al. Analysis of an acute Chagas disease outbreak in the Brazilian Amazon: human cases, triatomines, reservoirs mammals, and parasites. Trans R Soc Trop Med Hyg. 2009;103(3):291–297. doi: 10.1016/j.trstmh.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 7.Nicholls RS. Informe de la Consulta Técnica enepidemiología, prevención y manejo de latransmisión de laenfermedad de Chagas como enfermedad transmitida por alimentos (ETA) Rio de Janeiro: Organización Panamericana de laSalud/Organización Mundial de la Salud; 2006. Enfermedad de Chagas como enfermedad transmitida por alimentos: la experiência en Colombia. [Google Scholar]
- 8.Alarcón de Noya B, Díaz-Bello Z, Colmenares C, Ruiz-Guevara R, Mauriello L, Zavala-Jaspe R. Large urban outbreak of orally acquired acute Chagas disease at a school in Caracas, Venezuela. J Infect Dis. 2010;201(9):1308–1315. doi: 10.1086/651608. [DOI] [PubMed] [Google Scholar]
- 9.Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW, et al. American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology. American College of Cardiology Foundation. Heart Rhythm Society AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53(11):976–981. doi: 10.1016/j.jacc.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Nattan-Larrier Infections a Trypanosomes et voies de penetration des virus. Bull Soc Path Exot. 1921;14:537–542. (Fre). [Google Scholar]
- 11.Shikanai-Yasuda MA, Marcondes CB, Guedes LA, Siqueira GS, Barone AA, Dias JC, et al. Possible oral transmission of acute Chagas disease in Brazil. Rev Inst Med Trop São Paulo. 1991;33(5):351–357. doi: 10.1590/s0036-46651991000500003. [DOI] [PubMed] [Google Scholar]
- 12.Espinoza RA, Pericchi LR, Carrasco HA, Escalante A, Martinez O, Gonzalez R. Prognostic indicators of chronic chagasic cardiopathy. Int J Cardiol. 1991;30(2):195–202. doi: 10.1016/0167-5273(91)90095-7. [DOI] [PubMed] [Google Scholar]
- 13.Moleiro F, Mendoza I. Miocardiopatía crónica Chagásica, un estudio epidemiológico utilizando métodos electrofisológicos de exploración clínica. Acta Cient Venez. 1980;31(6):66–72. [PubMed] [Google Scholar]
- 14.Puigbo JJ, Rhode JR, Barrios HG, Suarez JA, Yepez CG. Clinical and epidemiological study of chronic heart involvement in Chagas disease. Bull World Health Organ. 1996;34(5):655–669. [PMC free article] [PubMed] [Google Scholar]
- 15.Acquatella H, Catalioti F, Gomez-Mancebo JR, Davalos V, Villalobos L. Long term control of Chagas disease in Venezuela: effects on serologic findings, electrocardiographic abnormalities and clinical outcome. Circulation. 1987;76(3):556–562. doi: 10.1161/01.cir.76.3.556. [DOI] [PubMed] [Google Scholar]
- 16.Dias E, Laranja FS, Miranda A, Nobrega G. Chagas´disease: a clinical, epidemiologic and pathologic stud. Circulation. 1956;14(6):1035–1060. doi: 10.1161/01.cir.14.6.1035. [DOI] [PubMed] [Google Scholar]
- 17.Pinto AY, Valente SA, Valente Vda C, Ferreira AG, Junior, Coura JR. Acute phase of Chagas disease in the Brazilian Amazon region: study of 233 cases from Pará, Amapá and Maranhão observed between 1988 and 2005. Rev Soc Bras Med Trop. 2008;41(6):602–614. doi: 10.1590/s0037-86822008000600011. [DOI] [PubMed] [Google Scholar]
- 18.Bastos CJ, Aras R, Mota G, Reis F, Dias JP, de Jesus RS, et al. Clinical outcomes of thirteen patients with acute Chagas disease acquired through oral transmission from two urban outbreaks in northeastern Brazil. PLoS Negl Trop Dis. 2010;4(6):e711. doi: 10.1371/journal.pntd.0000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbosa-Ferreira JM, Guerra JA, Santana FS, Filho, Magalhães BM, Coelho LI, Barbosa MG. Cardiac involvement in Acute Chagas' Disease cases in the Amazon region. Arq Bras Cardiol. 2010;94(6):147–149. doi: 10.1590/s0066-782x2010000600023. [DOI] [PubMed] [Google Scholar]
- 20.Parada H, Carrasco H, Añez N, Fuenmayor C, Inglessis I. Cardiac involvement is a constant finding in acute Chagas disease: a clinical, parasitological and histopathological study. Int J Cardiol. 1997;60(1):49–54. doi: 10.1016/s0167-5273(97)02952-5. [DOI] [PubMed] [Google Scholar]
- 21.Anselmi A, Pifano F, Suarez A, Dominguez A, Diaz Vazquez A, Anselmi G. Experimental Schizotrypanum cruzy myocarditis: correlation between histopathologic and electrocardiographic findings in experimental Chagas' heart disease. Am Heart J. 1965;70(5):638–656. doi: 10.1016/0002-8703(65)90393-5. [DOI] [PubMed] [Google Scholar]
- 22.Camandaroba EL, Pinheiro Lima CM, Andrade SG. Oral transmission of Chagas disease: importance of Trypanosoma cruzi biodeme in the intragastric experimental infection. Rev Inst Med Trop S Paulo. 2002;44(2):97–103. doi: 10.1590/s0036-46652002000200008. [DOI] [PubMed] [Google Scholar]
- 23.Covarrubias C, Cortez M, Ferreira D, Yoshida N. Interaction with host factors exacerbates Trypanosoma cruzi cell invasion capacity upon oral infection. Int J Parasitol. 2007;37(14):1609–1616. doi: 10.1016/j.ijpara.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida N. Trypanosoma cruzi infection by oral route: how the interplay between parasite and host components modulates infectivity. Parasitol Int. 2008;57(2):105–109. doi: 10.1016/j.parint.2007.12.008. [DOI] [PubMed] [Google Scholar]