Abstract
Apart from their role in hemostasis and thrombosis, platelets are involved in many other biological processes such as wound healing and angiogenesis. Percutaneous coronary intervention is a highly thrombogenic procedure inducing platelets and monocytes activation through endothelial trauma and contact activation by intravascular devices. Platelet P2Y12 receptor activation by adenosine diphosphate facilitates non-ADP agonist-mediated platelet aggregation, dense granule secretion, procoagulant activity, and the phosphorylation of several intraplatelet proteins, making it an ideal drug target. However, not all compounds that target the P2Y12 receptor have similar efficacy and safety profiles. Despite targeting the same receptor, the unique pharmacologic properties of each of these P2Y12 receptor-directed compounds can lead to very different clinical effects.
Keywords: Receptor Aggregation, Platelet Aggregation, Percutaneous Coronary Intervention
Introduction
Apart from their role in hemostasis and thrombosis, platelets are involved in many other biological processes such as wound healing and angiogenesis. Percutaneous coronary intervention is a highly thrombogenic procedure inducing platelets and monocytes activation through endothelial trauma and contact activation by intravascular devices. Platelet P2Y12 receptor activation by adenosine diphosphate facilitates non-ADP agonist-mediated platelet aggregation, dense granule secretion, procoagulant activity, and the phosphorylation of several intraplatelet proteins, making it an ideal drug target. However, not all compounds that target the P2Y12 receptor have similar efficacy and safety profiles. Despite targeting the same receptor, the unique pharmacologic properties of each of these P2Y12 receptor-directed compounds can lead to very different clinical effects.
Platelet adhesion, activation and aggregation
Platelet aggregation exerts an important role in ischemic complications in patients submitted to percutaneous coronary intervention (PCI). Both the atherosclerotic plaque instability and factors related to the procedure itself (endothelial trauma) and contact of thrombogenic structures and the blood are responsible for this process exacerbation1. Despite thromboxane A2 and adenosine diphosphate (ADP) act sinergistically in platelet activation, ADP interaction with its receptors, specially P2 receptors, enhances and sustains this activation. For this reason, these receptors have been the main target of current antiplatelet drugs2.
Platelets are non-nucleated fragments of megakaryocytes that circulate in the blood stream and have a flattened disk conformation when not activated. They participate in a number of biological processes, from fighting infectious agents to initiating tissue repair by activating angiogenesis. Thus, their functions go well beyond the participation in the coagulation cascade, and they play a key role in modulating the whole tissue repair process3.
In normal conditions, platelets are not activated by the endothelial surface. The endothelial monolayer acts as an antithrombotic surface, since it does not allow the interaction of platelets and sub endothelial proteins and produces prostacyclin I2 and nitric oxide, which are, both, platelet activation inhibitors. Endothelial cells also express the CD39 enzyme, which converts adenosine triphosphate (ATP) to ADP and ADP to adenosine monophosphate (AMP), avoiding platelet activation by ATP and ADP4.
In the presence of vascular injury or trauma, sub endothelial proteins, such as Von Willebrand factor and collagen, are exposed and platelet adhesion occurs with the objective of promoting tissue healing. The interaction between these proteins and platelets is mediated by a number of receptors at the platelet surface (GPIbα, GPVI α2β1), and platelet activation occurs concomitantly to adhesion. Multiple metabolic pathways are stimulated, leading to an increase in calcium intracellular concentration. This increase activates phospholipase A2 and actin-myosin ATPase, leading to thromboxane A2 formation and platelet conformational change, respectively. When activated, platelets release their granules (containing ADP, ATP, serotonin, calcium, fibrinogen, Von Willebrand factors, cytokines and pro-thrombotic factors), increasing their volume and reactivity. In order to form this aggregate, platelets interact through the binding of fibrinogen and glycoprotein IIb/IIIa receptor5.
Platelet deposition at the vessel wall, leucocyte recruitment and smooth muscle cell migration promote an alteration in the arterial structure, known as vascular remodeling. This remodeling, together with the chronic inflammatory state mediated by platelets, consists in a key step in atherosclerotic plaque formation, intimal hyperplasia and stent restenosis6.
ADP and P2 platelet receptors
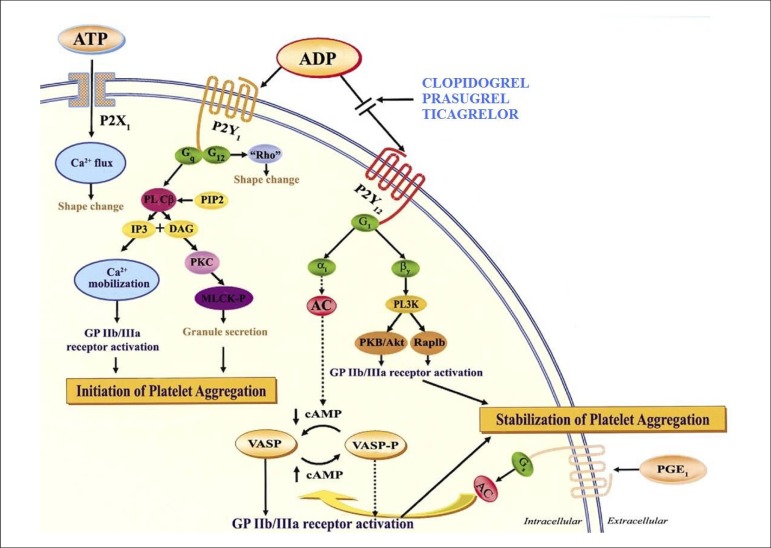
Secreted by red blood cells, endothelial cells and released in platelet granules, ADP is an important mediator of the activation and platelet aggregation amplification. ADP interacts with the platelet surface through receptors of the P2 family, whose two subtypes can be differentiated by the intracellular activation pathway: P2X (binded to ion channels) and P2Y (coupled to G protein). There is currently a new classification based on the agonist type: P2X1, activated by ATP; P2Y1 and P2Y12, activated by ADP (Figure 1)7.
Figure 1.
P2 platelet receptors.
Reprinted from Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F,Macaya C, Bass TA et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am CollCardiol. 2007;49(14):1505-16, with permission of Elsevier.
The P2X1 receptors are responsible for a transient conformational change in platelets, which is associated to the rapid calcium influx. Thus, though not capable of sustaining platelet aggregation, they contribute to collagen-induced activation4.
P2Y1 receptors can be found in multiple tissues, including the heart, blood vessels, smooth muscular cells, nervous tissues, testicles, prostate and ovaries. In response to ADP-mediated activation, calcium is mobilized from platelet storage, leading to conformational change and transient aggregation. This receptor has a key role in the beginning of ADP-induced activation, but, for the effective stabilization of platelet thrombus, the activation of other receptors is required4,5.
P2Y12 receptors, besides being found in platelets, are also present in the microglia, endothelial cells and smooth muscle cells. These receptors have a central role in the amplification of the aggregation induced by all platelet agonists, such as collagen, thrombin, thromboxane A2, adrenaline and serotonin. Despite that, the agonist with the highest affinity, as observed with P2Y1 receptors, is ADP. The intracellular response to its activation is the inhibition of cAMP (cyclic adenosine monophosphate) production, vasodilator-stimulated phosphoprotein (VASP) dephosphorylation and GTPase Rap1B and phosphoinositide 3-kinase (PI3-K) activation. The activation of both P2 receptors is important to ADP-induced aggregation, since the selective inhibition of one receptor leads to an important reduction in platelet aggregation8.
P2Y12 receptor inhibitors
Antiplatelet drugs are essential in the management of patients submitted to PCI. There are three groups of antiaggregation drugs with proven clinical efficacy: cyclooxygenase inhibitors (AAS), P2Y12 receptor inhibitors and glycoprotein IIb/IIIa antagonists9.
The P2Y12 receptor is the main target of oral inhibitory agents, since it is directly involved in the amplification of the platelet reactivity required for thrombus formation. There are three classes of P2Y12 receptors: thienopyridines, ATP analogues and ciclopentil-triazolo pyrimidines (Table 1).
Table 1.
P2Y12 receptor inhibitors
| Drug | Route | Action | Dosing (bolus/maintenance) | Peak effect | Main studies |
|---|---|---|---|---|---|
| Clopidogrel | Oral | Irreversible Hepatic metabolization | 600 mg 75 mg/d | 3 h | CURE-PCI CLARITY-PCI |
| Prasugrel | Oral | Irreversible Hepatic metabolization | 60 mg 10 mg/d | 30 min | TRITON-TIMI 18 |
| Cangrelor | IV | Reversible Direct inhibition | 30μ/Kg/min 4 μ/Kg/min | 1 min | CHAMPION - PLATFORM |
| Ticagrelor | Oral | Reversible Direct inhibition | 180 mg 90 mg 12/12 h | 30 min | PLATO |
CURE-PCI (Effects of pretreatment with Clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention)10; CLARITY-PCI (Effect of Clopidogrel pretreatment before percutaneous coronary intervention in patients with ST elevation myocardial infarction treated with fibrinolytics)11; TRITON-TIMI 18 (Prasugrel versus Clopidogrel in patients with acute coronary syndromes)12; PLATO(Ticagrelor versus Clopidogrel in patients with acute coronary syndromes)13; CHAMPION-PLATFORM (Intravenous platelet blockade with cangrelor during PCI)14.
Thienopyridines
Clopidogrel
The first and the second generation of thienopyridines are represented by ticlopidine and clopidogrel, respectively. Ticlopidine's utilization is limited by a greater incidence of hematologic adverse effects, such as neutropenia and agranulocytosis. Clopidogrel is a pro-drug that must be metabolized in a two-step process by cytochrome P450 (CP450) in the liver to an active metabolite, which will irreversibly bind to the P2Y12 receptors. The majority of the absorbed clopidogrel (85-90%) is hydrolyzed in inactive carboxylic acid and the remaining is rapidly metabolized by CP45015. The 75-mg clopidogrel dose starts acting after two hours, but three to seven days are needed to achieve maximum platelet inhibition. The time for achieving its peak action, however, can be reduced with the utilization of loading doses. With a 300 mg- or 600 mg loading dose, maximum inhibition is achieved in 12 and 3 hours, respectively.
It is worth highlighting that these are mean population values which do not reflect the necessary individual aggregation degree, since a number of pharmacokinetics studies were performed in normal individuals without coronary artery disease (CAD) and no damage to organs responsible for drug excretion or metabolization. Doses higher than 600 mg did not lead to more antiaggregation, since these doses did not lead to an increase in the concentration of the active metabolite4.
Clopidogrel, despite showing efficacy, cannot be considered the ideal antiaggregant. Its main inconveniences are its great individual variability, due to metabolic differences, the irreversible inhibition of the receptors, increasing bleeding risk specially in patients submitted to surgical procedures, and its latency to achieving the peak of action (reducing its benefit to acute coronary syndrome (ASC) patients needing fast platelet activity inhibition16.
Approximately 30% of the patients taking conventional doses of clopidogrel develop resistance or low response to the drug. This percentage represents a clinically vulnerable population with a high risk of major cardiovascular events, including AMI, stent thrombosis and death17.Various factors influence this individual variability, including obesity, diabetes mellitus, ASC, age and mutations in the genes coding P450 cytochrome enzymes.
The patients that are homozygous for mutant alleles of CYP2C19 present a high risk of cardiovascular events, mainly stent thrombosis2.Due to this evidence, the FDA issued an alert recommending that the utilization of other antiaggregation agent or unusual doses of clopidogrel be considered for these patients, individualizing platelet antiaggreagation18.
Individualized therapy is common in clinical cardiology. Various drug classes are dosed according to the clinical or laboratory response of the patient, such as anti-hypertensive and anticoagulant drugs, respectively.
The utilization of laboratory exams that allow a more precise evaluation of the individual variability in antiaggregant response is thus necessary. Currently, two test groups are available for this purpose: genetic and platelet reactivity tests. Since the genotype is constant, its evaluation is not capable of adequately measuring the cumulative influence and the dynamics of the various factors that interfere in platelet reactivity; thus, despite still limited by technical factors, it is more appropriate to evaluate the final phenotype than the genotype.
Platelet aggregation evaluation
The gold standard for platelet function evaluation is light transmission aggregometry. Despite that, the standardization of this method is difficult, demanding approximately four hours for its performance and requiring specific training. Bedside tests, such as VerifyNow®, have been highlighted by their easy utilization, rapid results, and for the lack of sample preparation requirement19.
A number of studies employed the ROC curve for defining the ideal platelet reactivity value relative to the thrombotic risk (cardiovascular mortality, stent thrombosis and non-fatal AMI). The best match between sensitivity and specificity was obtained with 240 P2Y12 reaction units (PRU)15. More recently, the ADAPT-DES study reported that a PRU > 208 and a platelet inhibition percentage equal or inferior to 11% were independently associated to stent thrombosis20.
The clopidogrel dose guided by VerifyNow® was evaluated in the GRAVITAS study. Chronic CAD patients that had been submitted to stent angioplasty and were considered resistant to clopidogrel (PRU > 240) were randomized to a doubled dose (bolus plus maintenance) or standard dose of clopidogrel. Despite the smaller PRU levels seen in the patients taking the higher clopidogrel dose, no difference was observed regarding primary outcome (death, non-fatal AMI and non-fatal stroke)21. In the ARTIC study, the number of events was not reduced by using platelet aggregation guided therapy after drug-eluting stents in the chronic CAD patient population22.
However, the study ADAPT-DES showed that, in patients with CAS, it was possible to evaluate stent thrombosis risk using PRU value, confirming the importance of the clinical presentation type (acute/chronic) to antiaggregation intensity20.
Another method for evaluating platelet aggregation is quantifying VASP (vasodilator-stimulated phosphoprotein) using flux cytometry. VASP is an intracellular protein activated by the binding of agonists to P2Y12 receptors12. In basal conditions, this protein is not phosphorylated and is regulated by the cAMP pathway, which is activated by prostaglandin E1 action and inhibited by ADP via the P2Y12 receptors. VASP phosphorylation is associated to P2Y12 receptor inhibition and the non-phosphorylated form is associated to these receptors' activation. This method, however, demands intense laboratory work and is expensive15.
The difficulty in simulating in vitro hemostasis is the main barrier for the utilization of these tests23. The limitations for the evaluation of the platelet-endothelium and platelet-leukocyte interactions as well as the utilization of thrombotic surfaces as potential aggregation activators cannot be ignored; besides that, the utilization of agonists separately and in fixed concentrations is not similar to the physiological process. Other relevant point is that measuring aggregation in one blood sample is different from quantifying activity in the specific site of the tissue damage. Thus, the agonist may bind to the receptor, but intracellular pathways may exist that contribute to different responses when measuring platelet reactivity.
Prasugrel
Prasugrel, a third-generation thienopyridine drug, is a pro-drug with pharmacokinetic profile similar to clopidogrel which needs one less step to be converted to its active metabolite. Its distinct chemical structure allows a smaller dependency from CP450 for its activation. Its pharmacokinetics is more stable than clopidogrel since it is not influenced by CP450 mutations, resulting in a faster onset of action, and peak aggregation after 30 minutes16.
In the randomized study TRITON-TIMMI 38, prasugrel, when compared to clopidogrel, showed greater efficacy in significantly reducing IAM rates (7.4% vs 9.4%) and stent thrombosis (2.4% vs 1.1%) in ACS patients; however, patients treated with prasugrel presented higher bleeding rates (2.4% vs 1.8%) and the mortality rates were not significantly different. The subgroup analysis did not recommend its use by patients with past history of stroke/transient ischemic attack (TIA), individuals older than 75 years old or weighing less than 60 kg, due to increased bleeding risk. The patients with past history of stroke/TIA presented net damage with the utilization of prasugrel12. The drug was not able to reproduce the benefit seen with clopidogrel in patients with stable CAD or ACS without ST elevation treated conservatively24,25.
Despite prasugrel being considered more potent than clopidogrel, in vivo and in vitro studies showed that their active metabolites have equivalent potencies. Thus, its clinical benefit can be better explained by its better pro-drug conversion into active compounds than clopidogrel. Consequently, most patients treated with this drug tend to have faster inhibition of platelet aggregation16.
Cangrelor
ATP analogues are represented by cangrelor. This drug is a potent reversible inhibitor of P2Y12 receptors that does not need to be metabolized in order to act and is given only intravenously. Since it is an active metabolite, its peak of action is achieved in 2 to 30 minutes when given as a bolus or not, respectively. Its plasma half-life is six minutes, leading to a return to basal platelet aggregation one to two hours after finishing infusion. The studies that compared cangrelor and clopidogrel, however, were not able to show clinical difference between these drugs14,26.
The BRIDGE study compared cangrelor to placebo in patients who discontinued the thienopyridine due to upcoming surgical bypass. Cangrelor was able to maintain antiaggregation until the intervention without, however, increasing bleeding rates during surgery. Cangrelor utilization as a bridge to patients subject to high thrombotic risk that need to be submitted to surgical procedures seems to be promising27.
Ticagrelor
The most recent P2Y12 receptor inhibitor class is the ciclopentil-triazolo pyrimidines, represented by ticagrelor. Unlike the thienopyridines, ticagrelor does not need to be metabolized by the liver, interacts with platelet receptors reversibly and has faster onset and peak of action28. Action onset and peak are similar to clopidogrel in non-responding patients29.
Both efficacy and safety of ticagrelor were evaluated in the PLATO study, in which 18,624 ACS patients were randomized for receiving clopidogrel (75 mg/day, with a loading dose of 300 to 600 mg) or ticagrelor (90 mg twice daily, with a 180 mg loading dose). The primary combined endpoint (mortality due to vascular cause, AMI or stroke) in 12 months was significantly smaller in the ticagrelor group (9.8% vs 11.7%). No significant difference was verified on major bleeding rates when the study's major bleeding rate was used. However, when TIMI's major bleeding criteria were used, the bleeding rate was higher in patients not submitted to myocardial bypass in the ticagrelor group (2.8% vs 2.2%; p = 0.03).The isolated analysis of AMI, vascular mortality and all-cause mortality rates showed a statistically significant rate in patients taking ticagrelor. In this study, the main adverse effects were dyspnea and bradycardia13.
One possible hypothesis for explaining the mortality reduction obtained with ticagrelor is that this drug has other effects besides antiplatelet action. Ticagrelor inhibits adenosine reuptake by red blood cells and is structurally related to adenosine, suggesting that the latter may be one of its metabolites. Adenosine has cytoprotective, anti-inflammatory, antifibrotic and cardioprotective properties, which could explain ticagrelor benefits. Adenosine can also justify the main adverse effects of this drug, such as dyspnea and ventricular pauses. More evidence is required, though, for confirming the direct association of ticagrelor and adenosine30,31.
Due to its short half-life, platelet reactivity returns to basal levels after 3 to 4 missing doses. This is important for patients requiring surgical intervention. However, bad drug adherence quickly exposes the patient to the risk of ischemic events.
Prasugrel vs. Ticagrelor
There are no studies comparing these drugs clinically; thus, care must be taken when extrapolating data from different papers. The study TRITON randomized the great majority of its patients (99%) with knowledge of their coronary anatomy, while PLATO, on the other side, randomized patients at the emergency room. The clopidogrel dose allowed in the two studies was also different. Approximately 50% of the patients in the PLATO study and 33% of the TRITON study patients were taking proton pump inhibitors. A final diagnosis of ACS with ST elevation was made in 37% of the patients in the PLATO study and 26% of the patients in the TRITON study. In PLATO, with the exception of patients submitted to thrombolysis, all therapeutic regimens were evaluated (interventionist, surgical and clinical), while in TRITON patients were randomized after intervention was indicated12,13. Thus, the two studies have different populations and designs, and it is not possible to compare the drugs.
Biondi-Zoccai et al32 evaluated both drugs in an indirect metanalysis and demonstrated the benefit of prasugrel regarding stent thrombosis and ticagrelor regarding major bleeding related to surgical bypass. No difference was observed regarding mortality, AMI or stroke32.
In a pharmacodynamic analysis of 44 ACS patients with high platelet reactivity after taking clopidogrel, Alexopoulos et al33 showed that ticagrelor provided lesser platelet inhibition than prasugrel (32.9 PRU vs. 101.3 PRU; p < 0.001).
Conclusions
Platelet antiaggregation is essential in the management of patients submitted to PCI. The risks of bleeding and thrombotic events must guide antiaggreagation therapy intensity. The more intense the antiaggregation, higher the peri and post-surgery bleeding risks. These risks must always be evaluated, since bleeding complications per se lead to a worse prognosis34. In the group of patients with chronic renal dysfunction, for example, dual platelet antiaggregation therapy greatly increases hemorrhagic events, reducing or even overriding PCI benefit on a medium term basis35. The bleeding risk can be evaluated by clinical prediction scores such as CRUSADE, but these scores do not have good prediction values, limiting proper evaluation of the hemorrhagic risk36.
In ACS patients, there is a high risk of thrombotic complications, an ideal scenario for early and intense platelet anti-aggregation; the same is not true for chronic CAD patients which, for this reason, are not to be exposed to high hemorrhagic risk.
Other option, not yet evaluated in randomized clinical studies, would be the utilization of more potentP2Y12 receptor inhibitors (prasugrel or ticagrelor) in the acute phase of the coronary events, followed by a bridge with clopidogrel, aiming to reduce medium and long term bleeding risk. However, the duration of each therapy or whether this strategy would be really efficacious from a clinical point of view is still unknown and should not be recommended in daily clinical practice.
Antiplatelet selection must thus be carefully evaluated taking into account all adverse events, since its discontinuation imposes and increased risk of ischemic events to patients submitted to PCI. Accessing antiplatelet reactivity may allow anti-aggregation therapy individualization. However, tests for evaluating the response to platelet anti-aggregation drugs are still expensive, lack sensitivity and still require robust evidence showing clinical benefit.
Footnotes
Author contributions
Conception and design of the research: Chan M; Writing of the manuscript: Falcão FJA, Carvalho L; Critical revision of the manuscript for intellectual content: Alves CMR, Carvalho ACC, Caixeta AM.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any post-graduation program.
References
- 1.Chan MY, Weitz JI, Merhi Y, Harrington RA, Becker RC. Catheter thrombosis and percutaneous coronary intervention:fundamental perspectives on blood, artificial surfacesand antithrombotic drugs. J Thromb Thrombolysis. 2009;28(3):366–380. doi: 10.1007/s11239-009-0375-6. [DOI] [PubMed] [Google Scholar]
- 2.Gurbel PA, Tantry US. Do platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents?: platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents. Circulation. 2012;125(10):1276–1287. doi: 10.1161/CIRCULATIONAHA.111.031195. [DOI] [PubMed] [Google Scholar]
- 3.Broos K, Feys HB, De Meyer SF, Vanhoorelbeke K, Deckmyn H. Platelets at work in primary hemostasis. Blood Rev. 2011;25(4):155–167. doi: 10.1016/j.blre.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Oqueli E, Hiscock M, Dick R. Clopidogrel resistance. Heart Lung Circ. 2007;16( Suppl 3):S17–S28. doi: 10.1016/j.hlc.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Abrams CS. Platelet biology. [Access in 2012 Jan 9]. Available from: http://www.uptodate.com/contents/platelet_biology.
- 6.Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105(Suppl 1):S13–S33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 7.Gachet C. Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- 8.Gachet C. The platelet P2 receptors as molecular targets for old and new antiplatelet drugs. Pharmacol Ther. 2005;108(2):180–192. doi: 10.1016/j.pharmthera.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Cattaneo M. The platelet P2Y receptors as targets for new antithrombotic drugs. J Thromb Haemost. 2003;1(6):1133–1135. doi: 10.1046/j.1538-7836.2003.00286.x. [DOI] [PubMed] [Google Scholar]
- 10.Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study(2001) Lancet. 2001;358(9281):527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 11.Sabatine MS, Cannon CP, Gibson CM, López-Sendón JL, Montalescot G, Theroux P, et al. Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics. The PCI-CLARITY study. JAMA. 2005;294(10):1224–1232. doi: 10.1001/jama.294.10.1224. [DOI] [PubMed] [Google Scholar]
- 12.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 13.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, PLATO Investigators. et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt DL, Lincoff AM, Gibson CM, Stone GW, McNulty S, Montalescot G, et al. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med. 2009;361(24):2330–2341. doi: 10.1056/NEJMoa0908629. [DOI] [PubMed] [Google Scholar]
- 15.Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56(12):919–933. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 16.Cattaneo M. New P2Y12 inhibitors. Circulation. 2010;121(1):171–179. doi: 10.1161/CIRCULATIONAHA.109.853069. [DOI] [PubMed] [Google Scholar]
- 17.Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Jukema JW, Huisman MV. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J. 2007;154(2):221–231. doi: 10.1016/j.ahj.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Food and Drug Administration (FDA) Reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug. Silver Spring; 2010. [Access in 2013 Jan 10]. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888.htm. [Google Scholar]
- 19.Harrison P. Platelet function testing. [Access in 2012 Jan 10]. Available from: http://www.uptodate.com/contents/platelet-function-testing]
- 20.Stone GW, compiler. In: Late-breaking clinical trials and first report investigations at TCT 2010 will have significant impact on practice of interventional cardiovascular medicine. http://wwwcrf.org/aboutus/news_and_events/214_late_breathingclinical_trials_and_first_report [Google Scholar]
- 21.Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, et al. Standard- vs. high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized Trial. JAMA. 2011;305(11):1097–1105. doi: 10.1001/jama.2011.290. Erratum in JAMA. 2011;305(21);2174. [DOI] [PubMed] [Google Scholar]
- 22.Collet JP, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, ARCTIC Investigators. et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367(22):2100–2109. doi: 10.1056/NEJMoa1209979. [DOI] [PubMed] [Google Scholar]
- 23.Michelson AD. Platelet function testing in cardiovascular diseases. Circulation. 2004;110(19):e489–e493. doi: 10.1161/01.CIR.0000147228.29325.F9. [DOI] [PubMed] [Google Scholar]
- 24.Trenk D, Stone GW, Gawaz M, Kastrati A, Angiolillo DJ, Müller U, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) Study. J Am Coll Cardiol. 2012;59(24):2159–2164. doi: 10.1016/j.jacc.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Gurbel PA, Erlinge D, Ohman EM, Neely B, Neely M, Goodman SG, et al. Platelet function during extended prasugrel and clopidogrel therapy for patients with ACS treated without revascularization. The TRILOGY ACS platelet function substudy. JAMA. 2012;308(17):1785–1794. doi: 10.1001/jama.2012.17312. [DOI] [PubMed] [Google Scholar]
- 26.Harrington RA, Stone GW, McNulty S, White HD, Lincoff AM, Gibson CM, et al. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361(24):2318–2329. doi: 10.1056/NEJMoa0908628. [DOI] [PubMed] [Google Scholar]
- 27.Angiolillo DJ, Firstenberg MS, Price MJ, Tummala PE, Hutyra M, Welsby IJ, BRIDGE Investigators. et al. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trial. JAMA. 2012;307(3):265–274. doi: 10.1001/jama.2011.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120(25):2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 29.Gurbel PA, Bliden KP, Butler K, Antonino MJ, Wei C, Teng R, et al. Response to Ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation. 2010;121(10):1188–1199. doi: 10.1161/CIRCULATIONAHA.109.919456. [DOI] [PubMed] [Google Scholar]
- 30.Serebruany VL. Adenosine release: apotential explanation for the benefits of ticagrelor in the PLATelet inhibition and clinical outcomes trial? Am Heart J. 2011;161(1):1–4. doi: 10.1016/j.ahj.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 31.Wittfeldt A, Emanuelsson H, Brandrup-Wognsen G, van Giezen JJ, Jonasson J, Nylander S, et al. Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans. J Am Coll Cardiol. 2013;61(7):723–727. doi: 10.1016/j.jacc.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Biondi-Zoccai G, Lotrionte M, Agostoni P, Abbate A, Romagnoli E, Sangiorgi G, et al. Adjusted indirect comparison meta-analysis of prasugrel versus ticagrelor for patients with acute coronary syndromes. Int J Cardiol. 2011;150(3):325–331. doi: 10.1016/j.ijcard.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 33.Alexopoulos D, Galati A, Xanthopoulou I, Mavronasiou E, Kassimis G, Theodoropoulos KC, et al. Ticagrelor versus prasugrel in acute coronary syndrome patients with high on-clopidogrel platelet reactivity following percutaneous coronary intervention: a pharmacodynamic study. J Am Coll Cardiol. 2012;60(3):193–199. doi: 10.1016/j.jacc.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 34.Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, et al. A risk score to predict bleeding in patients with acute coronary syndromes. Am Coll Cardiol. 2010;55(6):2556–2566. doi: 10.1016/j.jacc.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 35.Palmer SC, Di Micco L, Razavian M, Craig JC, Perkovic V, Pellegrini F, et al. Effects of antiplatelet therapy on mortality and cardiovascular and bleeding outcomes in persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012;156(6):445–459. doi: 10.7326/0003-4819-156-6-201203200-00007. [DOI] [PubMed] [Google Scholar]
- 36.Amador P, Santos JF, Gonçalves S, Seixo F, Soares L. Comparison of ischemic and bleeding risk scores in non-ST elevation acute coronary syndromes. Acute Card Care. 2011;13(2):68–75. doi: 10.3109/17482941.2011.567287. [DOI] [PubMed] [Google Scholar]