Summary
Erythropoietin (EPO) hyporesponsiveness is demonstrated by a persistence of anemia despite high dose of recombinant human erythropoietin (rHuEPO) or requirement of large doses to maintain the target hemoglobin concentration. Tolerance to rHuEPO is defined by a diminished erythropoietic response while rHuEPO concentrations are maintained at a high level. We observed a tolerance phenomenon in rats receiving multiple doses of rHuEPO. We further studied the dynamics of erythroid cells in bone marrow, spleen and blood as well as the neocytolysis of reticulocytes after a single intravenous injection of rHuEPO. The results suggest that the tolerance phenomenon observed in the peripheral blood might be due to depletion of the bone marrow precursor cells induced by rHuEPO treatment. This mechanism may be a contributing factor to the EPO hyporesponsiveness. Our findings support the dose reduction of erythropoiesis stimulating agents for patients demonstrating hyporesponsiveness.
Keywords: Erythropoietin, hyporesponsiveness, tolerance, erythroid precursor depletion, dynamics
Introduction
Erythropoiesis is a process of producing red blood cells (RBC) from pluripotent hematopoietic stem cells (HSC) (1). Erythropoietin (EPO) is the main regulator of erythropoiesis. It is a 30.4 kD protein hormone endogenously produced by adult kidney (2). EPO acts through binding to EPO receptor (EPOR) on the early erythroid precursor cells and stimulates their survival, proliferation and differentiation. In erythroid lineage, the earliest committed progenitors are burst-forming units-erythroid (BFU-E), which develop into colony-forming units-erythroid (CFU-E). BFU-E and CFU-E are detected in vitro using colony-forming cell assays (3). CFUs differentiate through several morphologically identifiable precursors till circulating RBC, including proerythroblast, basophilic erythroblast, polychromatophilic erythroblast, orthochromatic erythroblast, and reticulocyte (RET) (1). Erythropoietin is required for the survival of CFU-E and BUF-E, but not erythroblasts (4). The anti-apoptotic effect induced by EPO-EPOR interaction is one of the most recognized mechanisms by which EPO stimulates RBC production (5). Erythroid precursors are produced continuously in bone marrow, but a substantial portion dies before reaching the blood circulation (6). By inhibiting apoptosis, EPO increases the number of cells that will be able to differentiate into circulating RBC (6). This apparently futile cycle of cell production and cell death serves to maintain a supply of cells that can be readily mobilized when needed (7).
EPO also stimulates early release of reticulocytes from bone marrow into blood (8, 9). The effect of EPO on the release of reticulocytes is independent of its effect on erythroid precursor cells and the mechanism remains to be defined. It is generally accepted that EPO influences reticulocyte attachment to the erythroblastic island the bone marrow tissue through regulating the expression of adhesive molecules on cell surface (10). The premature reticulocytes observed in circulation are also known as “stress reticulocytes”. Membrane of circulating stress reticulocytes is rigid and unstable (11, 12). Consequently, they may have shortened survival and are more prone to be removed by spleen (13). Spleen is also considered as an organ contributing to neocytolysis, a process which selectively destructs the youngest circulating RBC (14-16). Neocytolysis is an efficient adaptive mechanism in response to the acute plethora induced by microgravity in spaceflight and descent from high altitude (17-19). EPO continues to be considered as a key regulator for this process and a fast decline of EPO concentration has been suggested to trigger neocytolysis (15, 20).
The recombinant human erythropoietin (rHuEPO) has been used for the treatment of anemia associated with chronic kidney disease (CKD) and anemia induced by chemotherapy in cancer patients. This treatment has been proven effective. However, up to 10% of anemic CKD patients demonstrated a persistence of anemia despite high dose of rHuEPO or requirement of large doses to maintain the target hemoglobin concentration, which has been generally recognized as the EPO hyporesponsiveness (21). The EPO hyporesponsiveness is of clinical importance because it is associated with increased risk of death or cardiovascular events (22-24). The EPO hyporesponsiveness can be due to many factors and have been extensively reviewed in the literature (25-30). The main cause is the iron deficiency. The inflammation associated with chronic renal failure is another factor (29). The management of anemia in patients demonstrating EPO hyporesponsiveness needs to be individualized after the cause is identified. Dose reduction has also been suggested since the increased mortality and cardiovascular events might be due to high concentration of rHuEPO (31).
Our group has observed reduced erythropoiesis in rats during multiple dosing regimens of rHuEPO. This phenomenon, that we called a tolerance effect, was demonstrated by the decline of RET count, RBC count and hemoglobin (HGB) levels despite the fact that rHuEPO concentrations were maintained at a high level (32). A similar phenomenon was also observed in humans, where the reticulocyte count peaked and started to decline at day 10, even though the multiple dosing regimens continued for 4 weeks (33). Since RBC are continuously produced from bone marrow, the tolerance effect observed in the RET response might originate from this tissue. Another possibility is that the RET are hemolyzed and removed through neocytolysis, which has been recently suggested to play a role in EPO hyporesponsiveness (30). In this report, we present results of our studies of the dynamics of erythroid cells in bone marrow, spleen and blood as well as neocytolysis of reticulocytes in response to rHuEPO in rats. Our findings suggest that a diminished degree of erythropoiesis observed in the peripheral blood might be due to the depletion of bone marrow precursor cells induced by erythropoietin. This mechanism may be another contributing factor to the EPO hyporesponsiveness.
Methods
Animals
Male Wistar rats of weight from 300 to 400 g were used for study (Charles River Laboratories, Inc., Raleigh, NC). Rats receiving multiple rHuEPO injections were fed with standard food supplemented with 1% (wt/wt) carbonyl iron (Sigma Chemical, St Louis, MO), whereas control rats were fed with standard diet only. All study protocols were approved by the Institutional Animal Care and Use (IACUC) of the State University of New York, University at Buffalo.
Pharmacokinetic studies
A pharmacokinetic study of single dose rHuEPO was carried out using nine rats. These rats were divided into 3 groups. RHuEPO (EPOGEN 10,000 units/ml; Amgen Inc., Thousand Oaks, CA) was diluted with saline (B. Braun Medical Inc., Irvine, CA) containing 0.25% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) immediately before injection. Drug (100 IU/kg) was administered through the tail vein. After the injection, blood samples were collected in a rotating manner from each group to minimize the total blood volume withdrawn from each animal (34). Each rat was sampled no more than four times. Blood (100-150 μl) was drawn via the tail vein at 5, 30 min and 1, 2, 4, 8, 12, 24, 32, 48, 60 h for determination of rHuEPO concentration. Plasma anticoagulated with EDTA was stored at -20°C until assayed.
For the pharmacokinetic study with multiple IV doses, the experiment procedure has been previously described (32). Briefly, twelve rats were randomized to 4 groups with 3 animals in each group. Animals in 3 groups (n = 9) received 450 IU/kg of rHuEPO thrice-weekly for two weeks. Each rat was sampled 3 times every 48 hours. Blood was drawn via the tail vein at 0, 5, 15, 30 min and 1, 2, 4, 8, 12, 16, 24, 32, 48 hours after the dose. Full pharmacokinetic profiles were measured on days 0, 7 and 11. Serum samples were used for the determination of rHuEPO concentration. Iron status was monitored by measuring plasma concentrations of transferrin and ferritin, during and after cessation of the treatment. The presence of anti-rHuEPO antibodies was examined using an ELISA method.
Pharmacodynamic studies
Pharmacodynamics was evaluated for both single and multiple dosing regimens. Pharamcodynamic markers included RET, mature RBC, RBC counts and HGB concentrations in the peripheral blood. Thirty-three rats were used for the single dose of 100 IU/kg rHuEPO group and twenty-seven rats were used for the control group. Vehicle (saline with 0.25% of bovine serum albumin) was given to rats in the control group. Both drug and vehicle were administered through the tail vein on the same day. For the treatment group, rats (n = 3) were sacrificed by exsanguination under anesthesia on every day until day 7, and subsequently on days 10, 14, 17, and 21. For the control group, rats (n = 3) were sacrificed at predose and on days 1, 3, 5, 7, 10, 14, 17, 21. Spleen and femur bone were extracted and processed immediately for flow cytometry analysis. Blood anticoagulated with EDTA was used for hematological measurements. For multiple dosing regimens, the pharmacodynamic study was performed simultaneously with the pharmacokinetic study. The experimental procedure has been described previously (35). Briefly, the erythropoietic effects of rHuEPO were evaluated every day until day 14, then every 2 days until day 30.
rHuEPO assay
Plasma or serum concentrations of rHuEPO were determined using the Quantikine IVD EPO ELISA (R&D Systems Inc., Minneapolis, MN). The standards are solutions with rHuEPO concentration ranging from 0 to 200 mIU/mL. The lower limit of quantification is 2.5 mIU/mL.
Hematological measurements
Complete blood counts were measured using BC-2800Vet auto hematology analyzer (Mindray Medical International Limited, Shenzhen, China). Hematological parameters in blood were measured immediately after the blood collection. RET counts were enumerated using Retic-COUNT (BD Biosciences, San Jose, CA). Flow cytometry analysis was performed by analyzing forward scatter, side scatter, and green fluorescence (FL1). The RBC population was gated on a scattergram (forward scatter versus side scatter) to exclude debris and platelets. Percent of reticulocytes (%RET) in blood was determined in a FL1 histogram by selecting positive green fluorescence, compared with the unstained control. The absolute RET counts were calculated by multiplying %RET by the RBC counts obtained from the hematology analyzer. Mature RBC counts were obtained by subtracting the absolute RET counts from RBC counts.
Single cell suspension
Femoral bone and spleen were processed immediately after sacrificing the animal. Femoral bone marrow was obtained by flushing a femur with 3 mL cold phosphate-buffered saline and 0.5% BSA (PBS/0.5% BSA). The bone marrow tissue was dispersed mechanically. Spleen was weighted first. Then, tissue was minced with scissors and mechanistically dissociated by gently mashing against a syringe plunger in a petridish containing 5 mL of cold PBS/0.5% BSA. Bone marrow and spleen single cell suspension were prepared by filtering the sample through Bellco tissue sieve.
Total marrow and spleen cellularity
The cell concentration in the single cell suspension was measured using CountBright™ absolute counting beads (Invitrogen, Carlsbad, CA) following the user's instruction manual. Total femur bone marrow and spleen cellularity was calculated by multiplying the volume of single cell suspension by the cell concentration. The total marrow cellularity was calculated based on the ratio of cellularity between total skeleton and femur. This ratio is 14.5, which was calculated from a linear relationship between body weight and this ratio based on values for mice and rabbits obtained from literature (36, 37). The total number of bone marrow cells was calculated as: femur bone marrow cellularity × 14.5.
Immunostaining and flow cytometry
Freshly isolated bone marrow or spleen cells were incubated with rat IgG (200 μg/mL, Jackson ImmunoResearch, West Grove, PA) in PBS/0.5% BSA for 30 min to block Fc receptors. Then, 106 cells were incubated with 0.03 μg of biotin-conjugated anti-rat erythroid cells antibody (BD Biosciences, San Jose, CA) and 0.03 μg of PE-conjugated anti-rat CD71 (BD Biosciences, San Jose, CA) in 100 μl PBS/0.5% BSA with presence of rat IgG for 30 min. After three washes, cells were further incubated with APC-conjugated streptavidin (BD Biosciences, San Jose, CA).
Flow cytometry analysis was carried out on a BD Biosciences FACSCalibur flow cytometer. At least 200,000 cells were collected from each sample. The anti-rat erythroid cell antibody reacts specifically with an antigen found on erythrocytes and erythroid precursors, but not on the other myeloid cells lineage (38). The transferrin CD71 is not specific to erythroid lineage. It is expressed at a high level on early erythroid precursors and its level decreases as erythroid cells mature (39). We also considered the forward scatter (FSC) parameter, which is a function of cell size and has been used to evaluate erythroid maturation (40). The gating scheme is shown in Fig 1A. The erythroid cell population was first identified based on their reactivity to the anti-rat erythroid cell antibody and CD71 level. The erythroid cells were further classified into three subpopulations P1, P2 and P3, based on FSC parameter and CD71 level. Data were analyzed with FCS Express (De Novo Software, Los Angeles, CA).
Fig 1.
Flow cytometry analysis of bone marrow and spleen erythroblasts. (A) Flow cytometry analysis of fresh isolated bone marrow (upper two panels) and spleen (lower two panels) stained with antibodies against rat erythroid cells and CD71. The left panels show all cells. The gated population in the left panel was further analyzed in terms of the forward scatter (FSC) parameter shown in the right panel. Three cell populations P1, P2 and P3 are gated based on their FSC and CD71 level. (B) P1, P2 and P3 cells were sorted and stained with May Grunwald/Giemsa solution.
Cytospins
Cells from each subpopulation (P1, P2 and P3) were sorted. Cell sorting was performed on a BD FACSAria cell sorter. Cytospin preparations of cells from each population were stained with May-Grunwald Giemsa (Electron Microscopy Science, Hatfield, PA).
Statistical analysis
The difference of erythropoietic response between rHuEPO treated and vehicle treated animals was tested using one-way ANOVA with Dunnett's test. All animals given vehicle comprised the control group. The animals given rHuEPO were grouped based on their experimental date. In the Dunnett's test, all treatment groups were compared against the control group. Statistical analysis was performed using SAS (SAS Institute, Cary, NC).
Assessment of RET loss
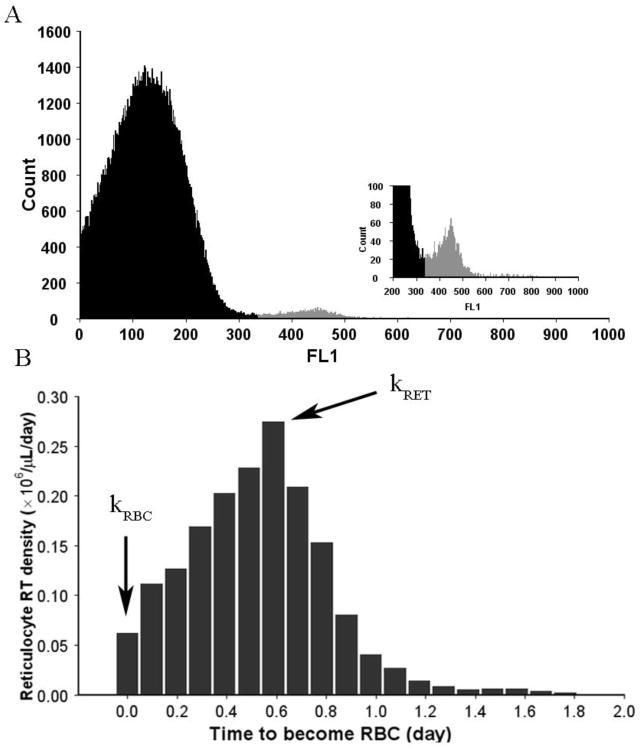
Flow cytometry reticulocyte readings were used to calculate production rates of the circulating RET (kRET) and mature RBC (kRBC) as shown in Fig 2. These parameters are obtained from the reticulocyte age density m(RT) where RT is the time that a reticulocyte needs to become a mature RBC as previously described (41). The reticulocyte production rate kRET is represented by the peak of m(RT) whereas kRBC is equal to the value of the density function at RT = 0 (35). If kloss denotes the rate of reticulocyte loss from the circulation, then the rate of RET change in the circulation is
Fig 2.
Reticulocyte flow cytometry analysis. (A) A distribution of the green fluorescent signal (FL1). The shaded region represents reticulocytes. (B) Reticulocyte age density distribution obtained from the FL1 distribution. The RET age is described by the time that a RET needs to become a mature RBC (RT). The density at RT=0 is the mature RBC production rate (kRBC). The peak RT density approximates the RET production rate (kRET).
Consequently, the accumulated reticulocyte loss at time T since the rHuEPO injection can be calculated as
where RET(T) denotes the RET value at time T. The integrals at the right-hand side of above equation were calculated as areas under kRET(t) and kRBC(t) curves using the trapezoidal rule.
Results
Tolerance and rebound phenomenon of erythropoietic response in rat peripheral blood during rHuEPO multiple dosing regimens
The rHuEPO pharmacokinetic profile during multiple dosing regimens is shown in Fig 3A. The full pharmacokinetic profiles were measured after the 1st, 4th and 6th dose. It can be seen that rHuEPO concentrations were maintained above the endogenous EPO level during the whole dosing period. Panels B-D in Fig 3 depict the RET, RBC and HGB responses to multiple dosing regimens. For RET, there was a progressive increase during the first week. The first peak occurred at day 5. Afterwards, a slow and oscillatory decline was observed before the administration of the last dose on day 11. The RET response was declining even though animals were exposed to the same weekly dosing of rHuEPO, a phenomenon referred to as a tolerance effect. The RET kept decreasing below the baseline and reached a nadir on day 22, followed by a slow return to the baseline on day 28. This temporal behavior of the RET response is named as a rebound phenomenon. The RBC and HGB responses exhibited a similar profile. They both kept increasing and peaked around day 10. The tolerance effect was demonstrated by their declines before the last dose. RBC and HGB further decreased below the baseline on day 20 and did not fully return at the end of the study. Plasma transferrin and ferritin concentrations of treated animal were either at or above the baseline level, indicating that iron was not depleted during rHuEPO multiple dosing regimens. No anti-rHuEPO antibody was found in animals.
Fig 3.
Pharmacokinetics and pharmacodynamics of rHuEPO in multiple dosing regimens. (A) rHuEPO serum concentration vs time profile during multiple dosing regimens of rHuEPO. Solid grey circles represent individual observations. Solid black line represents mean profile with standard deviation (SD) error bars. Arrows represent the dosing events. Dashed line represents endogenous EPO level. Pharmacodynamic measurements include (B) reticulocytes, (C) red blood cells and (D) hemoglobin. Solid circles represent individual observations. Solid lines represent mean data with SD error bars. Black color is used for the treatment group and grey color is used for the control group. Arrows represent the dosing events.
Erythropietic response in rat bone marrow and spleen after a single intravenous injection of rHuEPO
The pharmacokinetic profile of rHuEPO after IV injection is presented in Figure 4A. The peak concentration was about 2000 mIU/mL. The mean rHuEPO plasma concentration declined to 7.0 mIU/mL at 32 h post-injection, which was close to the baseline level for EPO (3.15 mIU/mL). The immunostaining of bone marrow and spleen cells identified three distinct subpopulations, P1, P2 and P3. A morphology check from the cytospin stained with May-Grunwald Giemsa showed that they formed an erythroid developmental sequence. P1 cells represent a mixture of pro-, basophilic, polychromatophilic, and orthochromatic erythroblasts. P2 cells represent orthochromatic erythroblasts. P3 cells represent RET and mature RBC.
Fig 4.
Pharmacokinetic profile and spleen weight changes after a single IV injection of rHuEPO. (A) rHuEPO plasma concentration vs time profile after a single dose of rHuEPO. Solid grey circles represent individual observations. Solid black line represents mean profile with SD error bars. Arrow represents the dosing event. Dashed line represents endogenous EPO level. (B) Spleen weight vs time since rHuEPO administration profile for control and treatment groups. Solid circles represent individual observations. Solid lines represent mean data with SD error bars. Black color is used for the treatment group and grey color is used for the control group. Arrow represents the dosing event. * P < .05.
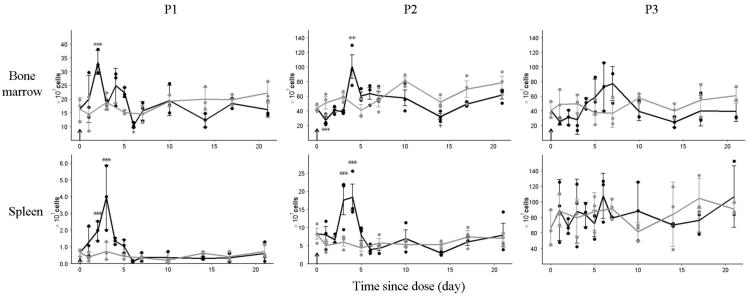
The time courses of cell populations P1, P2 and P3 in bone marrow and spleen for the control and treatment groups are shown in Fig 5. These cell populations in the control group represent their baseline level at steady-state erythropoiesis. Compared with the control group, the bone marrow P1 from the treatment group increased significantly on day 1. It increased 2.2-fold to reach a peak value on day 2, followed by an oscillatory decline. At day 6, it decreased below the baseline. This oscillation continued till the end of the experiment and stayed below the baseline. The bone marrow P2 from treatment group decreased immediately below the baseline level on day 1. It stayed at the lower value until day 3, when it started to rise. The peak of bone marrow P2 cells occurred at day 4, when it was 1.5 times higher than the baseline level. This peak was followed by a decline further below the baseline around day 8. The P2 cells stayed below the baseline level afterward until the end of the study and the nadir occurred at day 14. The response pattern of bone marrow P3 cells to rHuEPO was very similar to that of the P2 cells, but delayed. The total number of P2 cells from the control group was approximately 4 times the P1 cells, indicating that P1 cells went through some divisions when they developed into P2 cells. The total number of P3 cells was close to P2 cells, suggesting that P2 cells were late stage erythroid precursor cells and did not divide. This conclusion is consistent with the differentiation based on their morphology shown in the cytospin staining (Fig 1B).
Fig 5.
Erythropoietic response in bone marrow and spleen after single IV injection of rHuEPO. Panels show time courses of P1, P2 and P3 cell populations in bone marrow and spleen. Solid circles represent individual observations. Solid lines represent mean profile with SD error bars. Grey color denotes the control group and black color denotes the treatment group. Arrow represents the dosing event. * P < .05, ** P < .001, *** P < .001.
Spleen P1 from the treatment group increased immediately on day 2 (Fig 5). It peaked on day 3 and returned to the baseline around day 7. The peak value of P1 cells was about 8 times the baseline value. Spleen P2 cells from the treatment group started to increase on day 2 and peaked around day 4. It returned to baseline around day 7. The peak value was about 4 times the baseline level. Spleen P3 cells did not show any response, which was confirmed by the statistical analysis. Fig 4B shows changes in spleen weight for treatment and control groups. The spleen weight from the treatment group increased and peaked around day 4 followed by a decrease back to the baseline level.
Erythropoietic response in rat peripheral blood after a single intravenous injection of rHuEPO
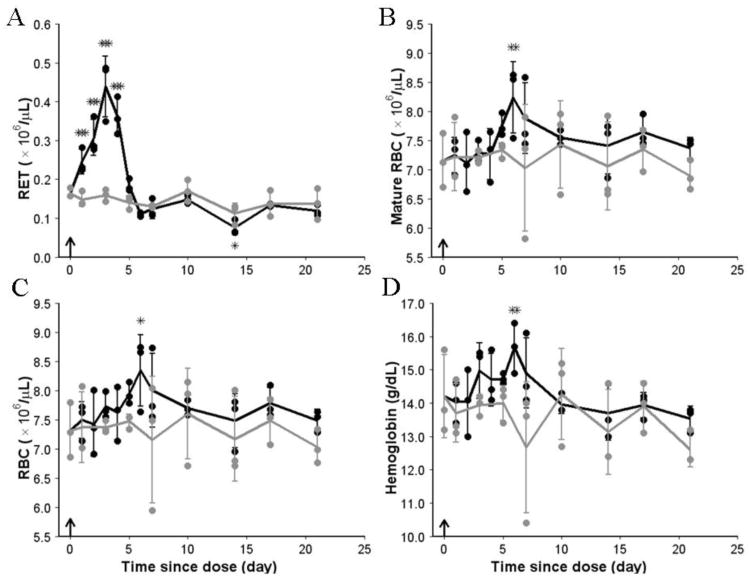
The erythropoietic responses in rat peripheral blood are shown in Fig 6. The hematology parameters from the control group indicated that the baselines for RET, mature RBC, RBC and HGB were fairly stable. They were not subject to change due to the age effect, which was previously observed for younger rats (34). Fig 6A shows that the RET increased immediately after the dose. On day 6, the RET count went down below the baseline level and stabilized at a lower level until the last sampling time. From panels CD in Fig 6, it can be seen that for the treatment group the RBC time course almost parallels the HGB profile. The RBC count and HGB level slowly increased until peaks were achieved approximately on days 5 to 6 at which point RET dropped below the baseline. The increases in RET counts were responsible for the early rise in RBC and HGB because the mature RBC levels were unchanged during that period (Fig 6B). Then, RBC gradually declined as the number of RET available for further maturation into RBC was below their baseline level.
Fig 6.
Pharmacodynamics of rHuEPO in peripheral blood after a single IV injection. (A) RET, (B) mature RBC, (C) RBC, and (D) hemoglobin concentration vs time since dose profiles from control and treatment groups are shown. Solid lines represent mean data with SD error bars. Solid circles represent observations. Black color is used for the treatment group and grey color is used for the control group. Arrow represents the dosing event. * P < .05, ** P < .001, *** P < .001.
Loss of stress reticulocytes
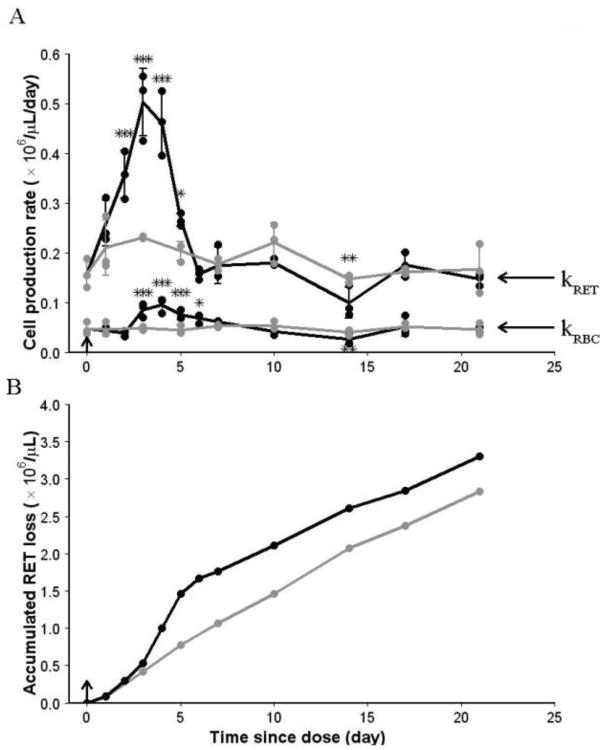
Fig 7A shows time courses of RET and RBC production rates for control and treatment groups. They increase from the pre-dose baseline values to a peak on days 3 to 4 and return to the baseline on day 7. The area below kRET represents the total amount of reticulocytes released to the circulation since the rHuEPO dosing event. These cells combined with RET at the time of rHuEPO administration comprised the circulating RET and the total number of RET eliminated from the circulation, either due to their loss or their maturation to RBC. Since the RET count is known and the accumulated maturation to RBC can be calculated from the area below kRBC, the accumulated RET loss can be obtained. The plots of accumulated RET loss are shown in Fig 7B. The slopes of these curves are reflective of the RET loss rates. The lost reticulocytes consist of cells that were destroyed and stored in peripheral tissues of the reticuloendothelial system. For the control group, the linear profile of the accumulated RET loss indicated that the RET loss rate was constant during the study period. For the treatment group, the accumulated RET loss superimposed with that from the control group until day 3, indicating that the RET loss rate was unchanged at the early stage. The RET loss rate increased between day 3 and day 5, demonstrating an increased loss of RET. After day 5, the accumulated RET loss profile became parallel to the curve from control group, suggesting that the RET loss rate returned to the baseline.
Fig 7.
Loss of reticulocytes. (A) Cell production rate vs time since rHuEPO administration profiles for control and treatment groups. Solid circles represent individual observations. Solid lines represent mean data with SD error bars. kRET and kRBC represent RET and mature RBC production rates, respectively. (B) Accumulated RET loss vs time since rHuEPO administration profiles, which were calculated based on the mean data. Black color is used for the treatment group and grey color is used for the control group. Arrow represents dosing event. * P < .05, ** P < .001, *** P < .001.
Discussion
The aim of this study was to demonstrate and identify underlying mechanisms of the tolerance effect in erythropoietic response during rHuEPO treatment. The tolerance phenomenon is demonstrated in Fig 3, which shows a diminished erythropoietic response in rats while the concentrations of the drug are maintained at a high level. Additionally, a prolonged rebound phase was observed for RET, RBC and HGB after cessation of the treatment. By using flow cytometric immunophenotyping, we identified rat erythroid precursor cells from different developmental stages in bone marrow and spleen. Study of their responses after a single injection of rHuEPO (at 100 IU/kg, which is selected based on its starting dose in humans) showed that the expansion of precursor cells moved as a wave through erythroid developmental stages. This observation is consistent with an early study on erythroid precursors in mice treated with rHuEPO (Bugelski et al, 2008). Comparison of bone marrow precursor cells between treatment and control groups revealed that the precursor pool was partially depleted after rHuEPO injection, resulting in a decreased reticulocyte count in blood.
As shown in the upper panels in Fig 5, the peak in bone marrow P1, P2 and P3 from treatment group occurred sequentially, indicating a wave of erythroid cells maturing through these stages. It can be seen that the bone marrow P3 was initially decreased for the treatment group until day 4. This is consistent with the stimulatory effect of EPO on the early release of premature reticulocytes from bone marrow into blood, which is responsible for the increase of reticulocytes in blood during the first 5 days as shown in Fig 6A. A striking observation is that the peak around day 7 in the bone marrow P3 profile (Fig 5) cannot be found in the time course of circulating RET (Fig 6A). We also did not detect any increase in RET loss after day 5, as shown in Fig 7B. An inevitable explanation is that the increase of RET in the peripheral blood was supposed to be followed by a deep rebound after day 5 due to the earlier depletion of the bone marrow P3 cells and this rebound was compensated by the bone marrow P3 cells from the peak. This observation provides evidence that the precursor pool depletion is responsible for the decrease of RET that occurred from day 5 to day 10 after rHuEPO injection. After day 10, it can be seen that the bone marrow P3 cells were below the baseline for the treatment group, demonstrating a sustained reduction in the RET production from bone marrow.
The spleen erythroid precursor response to rHuEPO was different from that observed in bone marrow. As shown in lower panels in Fig 5, the rebound phenomenon was not present in spleen after the rHuEPO treatment. The spleen P1 population increased after the rHuEPO treatment. However, the peak of spleen P1 population was about 8 times the baseline, while the peak of bone marrow P1cells was about 2 times the baseline. It has been previously demonstrated that rHuEPO stimulates migration of BFU-E and CFU-E from bone marrow to spleen (42). This substantial difference in the response of P1 population between spleen and bone marrow is consistent with this conclusion, suggesting that early precursors from bone marrow migrate to spleen and develop into the spleen P1 cells. The peak of spleen P1 cells occurred one day later than the peak of bone marrow P1 cells, which might reflect the time required to accommodate these cells in spleen. The absence of rebound in the precursor cells in the spleen also implies that these cells did not originate from spleen. At steady-state erythropoiesis, about 95% of the spleen erythroid cells are P3 cells, indicating that most of these cells are RBC from the circulation.
The tolerance effect following the rHuEPO treatment could be produced by a feedback mechanism involving the mature erythroid cells induced apoptosis of immature erythroid cells via activation of the Fas/FasL system (43). This was not observed in our data because the increase of P2 cells in bone marrow did not correspond to a decrease in P1 cells. An observation of such a mechanism might be obstructed by the dynamic behavior of erythroid precursor cells. It is of importance to mention that Fig 5 shows the dynamics of P1, P2 and P3 in terms of the total cell number. The fractions of precursor cells in the whole erythroid population or bone marrow population have also been used to describe the response of precursor cell to rHuEPO (40, 44, 45). This might account for some differences in results between current study and previous studies (40, 45). Our results suggest that the total number of erythroid cells oscillate after the rHuEPO treatment, which leads to the alteration of their relative fractions. Recently, it has been reported that no difference was observed for the femur bone cellularity between the rHuEPO treated and the control mice (46). From Fig 5, it can be seen that the likelihood of detecting such a difference is affected by the sampling time. Nevertheless, it has been demonstrated that high rHuEPO concentrations suppress Fas/FasL pathway (40). Therefore, this mechanism might not be operative in multiple dosing regimens of rHuEPO.
One possible explanation of the RET tolerance in the peripheral blood is neocytolysis (15). A selective destruction of stress RET after they enter blood could be responsible for the rebound phenomena (Fig 6A). To test this hypothesis, we developed a method that allows a quantitative assessment of the RET loss in blood. This method utilizes a proportionality between the fluorescent FL1 signal recorded by flow cytometry and the amount of residual RNA which is a marker of RET age. The detection limit of the residual RNA serves as a check point for RET maturation to RBC. This well-defined FL1 signal is used to measure the RBC production rate kRBC. On the other hand, the peak of the FL1 signal reflects the most frequent younger RET and serves as an approximate measurement of the RET production rate kRET. It must be noted that kRET underestimates the actual rate of RET release to the circulation. The accelerated destruction of RET was only detected from day 3 to day 5 for the treatment group (Fig 7B), which coincided with the occurrence of the peak of RET in blood (Fig 6A). This observation is consistent with the previous finding that the stress RET are more prone to be removed by spleen (13). The accumulation of RET in spleen cannot be observed in the spleen P3 cells due to the presence of large amount of circulating RBC. However, the increased spleen weight from day 3 to day 5 indirectly supports the accumulation of stress RET in spleen as well as the hypothesis that spleen is the organ for neocytolysis (16). The accelerated destruction of RET happened when the rHuEPO concentration was low (Fig 4A), which agrees with a hypothesis that neocytolysis is induced by a decline of EPO concentration (15). After day 5, the RET loss rate returned to the baseline level for the treatment group (Fig 7B), which suggests that the reduced RET counts after day 5 (Fig 6A) observed for the treatment group was not due to neocytolysis.
Erythropoiesis is a dynamic process originated from bone marrow. However, the pharmacodynamics of rHuEPO in bone marrow is less studied because the bone marrow compartment is not easily accessible. The occurrence of anemia has been previously demonstrated after cessation of an intensive EPO treatment in rats (47). This anemia was attributed to the exhaustion of progenitor cells (BFU-E and CFU-E) after the rHuEPO treatment. Our findings further suggest that the exhaustion of precursor cells occurs during the rHuEPO treatment and is responsible for the tolerance and rebound effect in the erythropoietic response in multiple dosing regimens. More importantly, the EPO induced erythroid precursor depletion may contribute to the hyporesponsiveness. It has been observed in clinical trials that the patients with the worst outcomes were those who showed most resistance to the treatment and received the highest dose of erythropoiesis stimulating agent (ESA) (48). Clearly, if the erythroid precursor cells were depleted, an increase of dose would not show much benefit, because of the smaller number of cells to initiate the erythropoiesis. These findings also provide additional justification for reducing ESA dose for anemic patients demonstrating hyporesponsiveness.
Acknowledgments
This work was supported by the National Institutes of Health Grant GM 57980
References
- 1.Koury MJ, Sawyer ST, Brandt SJ. New insights into erythropoiesis. Curr Opin Hematol. 2002;9(2):93–100. doi: 10.1097/00062752-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Elliott S, Pham E, Macdougall IC. Erythropoietins: a common mechanism of action. Exp Hematol. 2008;36(12):1573–1584. doi: 10.1016/j.exphem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15(3):146–155. doi: 10.1016/j.tcb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Koury MJ, Bondurant MC. Maintenance by erythropoietin of viability and maturation of murine erythroid precursor cells. J Cell Physiol. 1988;137(1):65–74. doi: 10.1002/jcp.1041370108. [DOI] [PubMed] [Google Scholar]
- 5.Testa U. Apoptotic mechanisms in the control of erythropoiesis. Leukemia. 2004;18(7):1176–1199. doi: 10.1038/sj.leu.2403383. [DOI] [PubMed] [Google Scholar]
- 6.Wintrobe MM, Greer JP. Wintrobe's clinical hematology. Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 7.Raff MC. Social controls on cell survival and cell death. Nature. 1992;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 8.Hillman RS. Characteristics of marrow production and reticulocyte maturation in normal man in response to anemia. J Clin Invest. 1969;48(3):443–453. doi: 10.1172/JCI106001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Major A, Bauer C, Breymann C, Huch A, Huch R. rh-erythropoietin stimulates immature reticulocyte release in man. Br J Haematol. 1994;87(3):605–608. doi: 10.1111/j.1365-2141.1994.tb08320.x. [DOI] [PubMed] [Google Scholar]
- 10.Rodak BF, Fritsma GA, Doig K. Hematology: clinical principles and applications. 3rd. St Louis, MO: Saunders Elsevier; 2007. [Google Scholar]
- 11.Chasis JA, Prenant M, Leung A, Mohandas N. Membrane assembly and remodeling during reticulocyte maturation. Blood. 1989;74(3):1112–1120. [PubMed] [Google Scholar]
- 12.Waugh RE. Reticulocyte rigidity and passage through endothelial-like pores. Blood. 1991;78(11):3037–3042. [PubMed] [Google Scholar]
- 13.Noble NA, Xu QP, Hoge LL. Reticulocytes II: Reexamination of the in vivo survival of stress reticulocytes. Blood. 1990;75(9):1877–1882. [PubMed] [Google Scholar]
- 14.Alfrey CP, Udden MM, Leach-Huntoon C, Driscoll T, Pickett MH. Control of red blood cell mass in spaceflight. J Appl Physiol. 1996;81(1):98–104. doi: 10.1152/jappl.1996.81.1.98. [DOI] [PubMed] [Google Scholar]
- 15.Alfrey CP, Rice L, Udden MM, Driscoll TB. Neocytolysis: physiological down-regulator of red-cell mass. Lancet. 1997;349(9062):1389–1390. doi: 10.1016/S0140-6736(96)09208-2. [DOI] [PubMed] [Google Scholar]
- 16.Trial J, Rice L, Alfrey CP. Erythropoietin withdrawal alters interactions between young red blood cells, splenic endothelial cells, and macrophages: an in vitro model of neocytolysis. J Investig Med. 2001;49(4):335–345. doi: 10.2310/6650.2001.33899. [DOI] [PubMed] [Google Scholar]
- 17.Rice L, Alfrey CP. Modulation of red cell mass by neocytolysis in space and on Earth. Pflugers Arch. 2000;441(2-3 Suppl):R91–94. doi: 10.1007/s004240000333. [DOI] [PubMed] [Google Scholar]
- 18.Rice L, Ruiz W, Driscoll T, et al. Neocytolysis on descent from altitude: a newly recognized mechanism for the control of red cell mass. Ann Intern Med. 2001;134(8):652–656. doi: 10.7326/0003-4819-134-8-200104170-00010. [DOI] [PubMed] [Google Scholar]
- 19.Risso A, Turello M, Biffoni F, Antonutto G. Red blood cell senescence and neocytolysis in humans after high altitude acclimatization. Blood Cells Mol Dis. 2007;38(2):83–92. doi: 10.1016/j.bcmd.2006.10.161. [DOI] [PubMed] [Google Scholar]
- 20.Trial J, Rice L. Erythropoietin withdrawal leads to the destruction of young red cells at the endothelial-macrophage interface. Curr Pharm Des. 2004;10(2):183–190. doi: 10.2174/1381612043453423. [DOI] [PubMed] [Google Scholar]
- 21.Macdougall IC, Cooper AC. Erythropoietin resistance: the role of inflammation and pro-inflammatory cytokines. Nephrol Dial Transplant. 2002;17(11):39–43. doi: 10.1093/ndt/17.suppl_11.39. [DOI] [PubMed] [Google Scholar]
- 22.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339(9):584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 23.Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008;74(6):791–798. doi: 10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010;363(12):1146–1155. doi: 10.1056/NEJMoa1005109. [DOI] [PubMed] [Google Scholar]
- 25.Elliott J, Mishler D, Agarwal R. Hyporesponsiveness to erythropoietin: causes and management. Adv Chronic Kidney Dis. 2009;16(2):94–100. doi: 10.1053/j.ackd.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Johnson DW, Pollock CA, Macdougall IC. Erythropoiesis-stimulating agent hyporesponsiveness. Nephrology (Carlton) 2007;12(4):321–330. doi: 10.1111/j.1440-1797.2007.00810.x. [DOI] [PubMed] [Google Scholar]
- 27.Kanbay M, Perazella MA, Kasapoglu B, Koroglu M, Covic A. Erythropoiesis stimulatory agent- resistant anemia in dialysis patients: review of causes and management. Blood Purif. 2010;29(1):1–12. doi: 10.1159/000245041. [DOI] [PubMed] [Google Scholar]
- 28.Kwack C, Balakrishnan VS. Managing erythropoietin hyporesponsiveness. Semin Dial. 2006;19(2):146–151. doi: 10.1111/j.1525-139X.2006.00141.x. [DOI] [PubMed] [Google Scholar]
- 29.Macdougall IC, Cooper AC. Hyporesponsiveness to erythropoietic therapy due to chronic inflammation. Eur J Clin Invest. 2005;35(3):32–35. doi: 10.1111/j.1365-2362.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- 30.van der Putten K, Braam B, Jie KE, Gaillard CA. Mechanisms of Disease: erythropoietin resistance in patients with both heart and kidney failure. Nat Clin Pract Nephrol. 2008;4(1):47–57. doi: 10.1038/ncpneph0655. [DOI] [PubMed] [Google Scholar]
- 31.Parfrey PS. Erythropoietin-stimulating agents in chronic kidney disease: a response to hyporesponsiveness. Semin Dial. 2011;24(5):495–497. doi: 10.1111/j.1525-139X.2011.00949.x. [DOI] [PubMed] [Google Scholar]
- 32.Ait-Oudhia S, Scherrmann JM, Krzyzanski W. Time-dependent clearance and hematological pharmacodynamics upon erythropoietin multiple dosing in rats. Biopharm Drug Dispos. 2010;31(5-6):298–315. doi: 10.1002/bdd.712. [DOI] [PubMed] [Google Scholar]
- 33.Cheung WK, Goon BL, Guilfoyle MC, Wacholtz MC. Pharmacokinetics and pharmacodynamics of recombinant human erythropoietin after single and multiple subcutaneous doses to healthy subjects. Clin Pharmacol Ther. 1998;64(4):412–423. doi: 10.1016/S0009-9236(98)90072-8. [DOI] [PubMed] [Google Scholar]
- 34.Woo S, Krzyzanski W, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after intravenous and subcutaneous administration in rats. J Pharmacol Exp Ther. 2006;319(3):1297–1306. doi: 10.1124/jpet.106.111377. [DOI] [PubMed] [Google Scholar]
- 35.Wiczling P, Ait-Oudhia S, Krzyzanski W. Flow cytometric analysis of reticulocyte maturation after erythropoietin administration in rats. Cytometry A. 2009;75(7):584–592. doi: 10.1002/cyto.a.20736. [DOI] [PubMed] [Google Scholar]
- 36.Donohue DM, Reiff RH, Hanson ML, Betson Y, Finch CA. Quantitative measurement of the erythrocytic and granulocytic cells of the marrow and blood. J Clin Invest. 1958;37(11):1571–1576. doi: 10.1172/JCI103750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papayannopoulou T, Finch CA. On the in vivo action of erythropoietin: a quantitative analysis. J Clin Invest. 1972;51(5):1179–1185. doi: 10.1172/JCI106911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hermans MH, Opstelten D. In situ visualization of hemopoietic cell subsets and stromal elements in rat and mouse bone marrow by immunostaining of frozen sections. J Histochem Cytochem. 1991;39(12):1627–1634. doi: 10.1177/39.12.1940317. [DOI] [PubMed] [Google Scholar]
- 39.Lok CN, Ponka P. Identification of an erythroid active element in the transferrin receptor gene. J Biol Chem. 2000;275(31):24185–24190. doi: 10.1074/jbc.M000944200. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Pop R, Sadegh C, Brugnara C, Haase VH, Socolovsky M. Suppression of Fas-FasL coexpression by erythropoietin mediates erythroblast expansion during the erythropoietic stress response in vivo. Blood. 2006;108(1):123–133. doi: 10.1182/blood-2005-11-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiczling P, Krzyzanski W. Method of determination of the reticulocyte age distribution from flow cytometry count by a structured-population model. Cytometry A. 2007;71(7):460–467. doi: 10.1002/cyto.a.20408. [DOI] [PubMed] [Google Scholar]
- 42.Kato M, Kato Y, Sugiyama Y. Mechanism of the upregulation of erythropoietin-induced uptake clearance by the spleen. Am J Physiol. 1999;276(5 Pt 1):E887–895. doi: 10.1152/ajpendo.1999.276.5.E887. [DOI] [PubMed] [Google Scholar]
- 43.De Maria R, Testa U, Luchetti L, et al. Apoptotic role of Fas/Fas ligand system in the regulation of erythropoiesis. Blood. 1999;93(3):796–803. [PubMed] [Google Scholar]
- 44.Bugelski PJ, Nesspor T, Volk A, et al. Pharmacodynamics of recombinant human erythropoietin in murine bone marrow. Pharm Res. 2008;25(2):369–378. doi: 10.1007/s11095-007-9372-7. [DOI] [PubMed] [Google Scholar]
- 45.Socolovsky M, Murrell M, Liu Y, Pop R, Porpiglia E, Levchenko A. Negative autoregulation by FAS mediates robust fetal erythropoiesis. PLoS Biol. 2007;5(10):e252. doi: 10.1371/journal.pbio.0050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singbrant S, Russell MR, Jovic T, et al. Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood. 2011;117(21):5631–5642. doi: 10.1182/blood-2010-11-320564. [DOI] [PubMed] [Google Scholar]
- 47.Piron M, Loo M, Gothot A, Tassin F, Fillet G, Beguin Y. Cessation of intensive treatment with recombinant human erythropoietin is followed by secondary anemia. Blood. 2001;97(2):442–448. doi: 10.1182/blood.v97.2.442. [DOI] [PubMed] [Google Scholar]
- 48.Locatelli F, Del Vecchio L. Erythropoiesis-stimulating agents in renal medicine. Oncologist. 2011;16(3):19–24. doi: 10.1634/theoncologist.2011-S3-19. [DOI] [PubMed] [Google Scholar]