Summary
Purpose
Post-traumatic epilepsy (PTE) occurs in a proportion of traumatic brain injury (TBI) cases, significantly compounding the disability, risk of injury, and death for sufferers. To date, predictive biomarkers for PTE have not been identified. This study used the lateral fluid percussion injury (LFPI) rat model of TBI to investigate whether structural, functional, and behavioral changes post-TBI relate to the later development of PTE.
Methods
Adult male Wistar rats underwent LFPI or sham-injury. Serial MR and PET imaging, and behavioral analyses were performed over six months post-injury. Rats were then implanted with recording electrodes and monitored for two consecutive weeks using video-EEG to assess for PTE. Of the LFPI rats, 52% (n=12) displayed spontaneous recurring seizures and/or epileptic discharges on the video-EEG recordings.
Key findings
MRI volumetric and signal analysis of changes in cortex, hippocampus, thalamus, and amygdala, 18F-FDG PET analysis of metabolic function, and behavioral analysis of cognitive and emotional changes, at one week, one month, three months, and six months post-LFPI, all failed to identify significant differences on univariate analysis between the epileptic and non-epileptic groups. However, hippocampal surface shape analysis using high dimensional mapping-large deformation identified significant changes in the ipsilateral hippocampus at one week post-injury relative to baseline that differed between rats that would go onto become epileptic versus those who did not. Furthermore, a multivariate logistic regression model that incorporated the one week, one month, and three month 18F-FDG PET parameters from the ipsilateral hippocampus was able to correctly predict the epileptic outcome in all of the LFPI cases. As such, these subtle changes in the ipsilateral hippocampus at acute phases after LFPI may be related to PTE and require further examination.
Significance
These findings suggest PTE may be independent of major structural, functional, and behavioral changes induced by TBI, and suggest more subtle abnormalities are likely involved. However, there are limitations associated with studying acquired epilepsies in animal models that must be considered when interpreting these results, in particular the failure to detect differences between the groups may be related to the limitations of properly identifying/separating the epileptic and non-epileptic animals into the correct group.
Keywords: Post-traumatic epilepsy, Lateral fluid percussion injury, MRI, PET, Epileptogenesis
Introduction
Traumatic brain injuries (TBI) are induced by biomechanical forces to the brain, and represent a global health concern and socioeconomic problem (Maas et al., 2008). Amongst the chronic disabilities associated with TBI, post-traumatic epilepsy (PTE) occurs in approximately 25% of TBI patients (Asikainen et al., 1999; Garza & Lowenstein, 2006), and accounts for up to 20% of symptomatic epilepsy cases (Annegers et al., 1998; Garza & Lowenstein, 2006). Although TBI can induce numerous neurobiological changes in the brain that could potentially result in PTE (D’Ambrosio & Perucca, 2004a; Kharatishvili & Pitkänen, 2010; Li et al., 2011), the causal mechanisms of PTE have yet to be identified (Garza & Lowenstein, 2006; Kharatishvili & Pitkänen, 2010). Consequently, there are currently no reliable biomarkers for PTE (Dichter, 2009; Dash et al., 2010; Mishra et al., 2011; Pitkänen et al., 2011), or effective pharmaceutical treatments known to prevent its onset (Beghi, 2003; Temkin et al., 2009; Loane & Faden, 2010). Although anticonvulsants might suppress seizures at acute stages after TBI, they do not prevent or suppress the chronic spontaneous seizures that reflect the culmination of the epileptogenic process (Beghi, 2003; Kharatishvili & Pitkänen, 2010). There is a clear need to better understand the changes evoked by TBI that may contribute to PTE (Garza & Lowenstein, 2006; Temkin, 2009). Given the limitations involved in addressing this issue in the clinical population, the use of an animal model to study PTE may be beneficial (Pitkänen et al., 2011).
The lateral fluid percussion injury (LFPI) is one of the most commonly used experimental models of TBI, and is capable of inducing physiological, pathological, and behavioral changes in rodents consistent with those occurring in the clinical condition (Thompson et al., 2005; Jones et al., 2008a; Liu et al., 2010). For example, previous works from our laboratory have found progressive structural, functional, and behavioral changes in rats given LFPI (Jones et al., 2008a; Liu et al., 2010). LFPI has also been shown to be a valid model of PTE, as the proportion of rats given LFPI that develop PTE is similar to the frequency reported in TBI patients, and LFPI induces a number of cerebral changes thought to be associated with PTE (Thompson et al., 2005; Kharatishvili & Pitkänen, 2010; Pitkänen et al., 2011). Past LFPI studies have reported initial evidence that imaging techniques, such as MRI, may be able to detect TBI-induced changes that predict the onset of PTE (Kharatishvili & Pitkänen, 2010; Pitkänen et al., 2011). However, these studies are limited to a small number of imaging modalities (Pitkänen et al., 2011), and may have confounding factors (Kharatishvili et al., 2007). In light of these issues and the need to better understand TBI-induced changes that may underlie or predict PTE, the current study investigated whether structural or functional abnormalities, as assessed by serial in vivo T2-weighted MRI and 18F-FDG PET imaging over six months post-injury, were associated with the occurrence of PTE in rats that had suffered a severe LFPI. As neurological symptoms, such as emotional disturbances, are known to occur in epilepsy patients and might be associated with PTE (Beyenburg et al., 2005), behavioral analyses were also completed.
Materials and Methods
Subjects
Subjects were 70 male Wistar rats obtained from an inbred colony at the Royal Melbourne Hospital Biological Research Facility. Rats were 8–12 weeks of age at the time of LFPI, were housed individually under a 12 h light/dark cycle, and given access to food and water ad libitum for the duration of the experiment. All experimental procedures were approved by the Melbourne Health Animal Ethics Committees (AEC#0705687). We have previously published data obtained from these rats (Jones et al., 2008a; Liu et al., 2010).
Experimental groups
Rats were assigned to receive either a sham-injury (n=25) or a LFPI (n=45). Of the LFPI rats, 11 (24%) died immediately post-LFPI, one died during electrode surgery, and 10 others were excluded from the current study due to compromised video-EEG recordings. Thus, a total of 23 rats given LFPI were included in this study. Based on video-EEG analysis for measures of PTE (as described below), the LFPI rats were further divided into epileptic (n=12) and non-epileptic (n=11) groups. Here we compared only the epileptic and non-epileptic groups. For results comparing sham-injury and LFPI groups see Jones et al., 2008a and Liu et al., 2010.
Lateral fluid percussion injury (LFPI)
LFPI and sham-injury procedures were based on a standard protocol as previously described and used by our group (McIntosh et al., 1989; Thompson et al., 2005; Jones et al., 2008a). Briefly, under anesthesia a 5mm craniotomy, positioned 4mm lateral and 4mm posterior to bregma, was performed to create a circular window exposing the intact dura mater of the brain. A modified female Luer-Lock cap was secured over the craniotomy window by dental acrylic. The rat was then removed from anesthesia and attached to the fluid percussion device via the modified female Luer-Lock cap. Once the rat responded to a toe pinch, a severe-intensity (3.2–3.5 atmospheres) fluid pulse of silicone oil generated by the fluid percussion device was with pure oxygen post-injury if required. Upon resumption of spontaneous breathing, and return to pre-LFPI levels of heart rate and oxygenation status, the dental acrylic caps were removed and the wound sutured closed. Sham-injury rats underwent the same procedures as LFPI rats, with the exception that the fluid pulse was not given.
Acute neuromotor assessment
As previously described, acute neurological injury was assessed in all rats on the day prior to injury, and on every day for three days after injury, using a composite neuromotor score (McIntosh et al., 1989; Jones et al., 2008a). Briefly, neuromotor assessment included ability to traverse a flat wooden beam, rotarod, forelimb flexion when the rat is elevated by its tail, reflex to a loud startle, and the light escape task. Each task was judged on an ordinal score of 0 (pass) or 1 (fail), except the forelimb flexion, which has an ordinal graded severity (maximum deficit score of 2/limb).
Neuroimaging
Acquisition
T2-weighted MRI scans were performed at baseline, and both MRI and 18F-FDG PET scans were performed at 1 week, 1, 3 and 6 months post-LFPI. The details of MRI and 18F-FDG PET scanning procedures and protocols used in this study have been previously described (Liu et al., 2010). Briefly, T2-weighted small-animal MRI data were acquired using a 4.7-T 47/30 Advance small-animal spectrometer with Paravision 3.0 (Bruker Biospec), with a shield-gradient set appropriate for rats (Bouilleret et al., 2009). PET images were acquired using a Mosaic Animal PET scanner (Philips), and completed 1–3 days before the corresponding MRI scans. Rats were injected intraperitoneally (i.p.) with 37–74 MBq (1–2 mCi) of 18F-FDG at 30 min before scanning. Rats were then anesthetized with 2% isoflurane (1:1 oxygen:air) and placed onto an acrylic platform for a 30 min acquisition. Corrections were applied for dead-time loss, decay, and activity injected (standardized uptake value [kBq/mL]).
MRI volumetric analysis
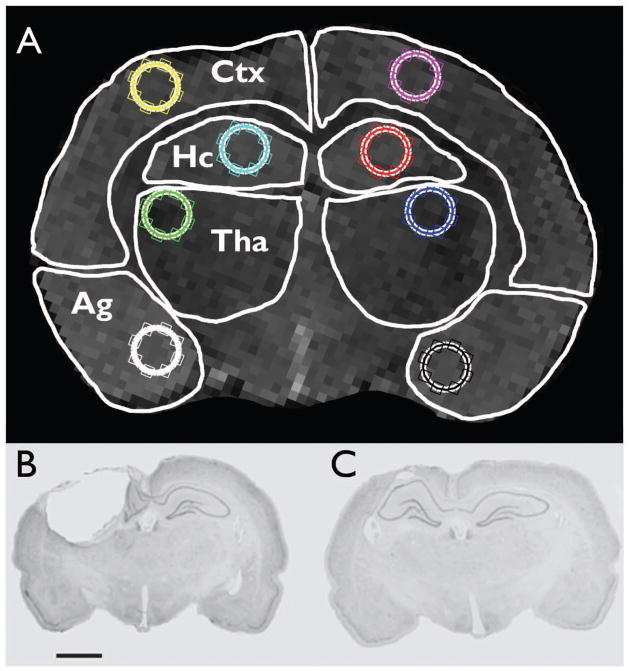
Two rats from the epileptic group were excluded from all imaging analysis due to incomplete scans. All imaging analysis procedures followed those previously described (Liu et al., 2010). Briefly, T2-weighted MRI volumes of selected brain regions were quantified with manually drawn regions of interest (ROIs) using Analyze (Analyze; Mayo Foundation). Ten ROIs, including the cortex, hippocampus, thalamus, amygdala, and lateral ventricles from both hemispheres, were drawn as previously described (Bouilleret et al., 2009; see Figure 1). ROIs were drawn on consecutive axial MRI slices by an investigator blinded to experimental conditions. Only slices containing hippocampus were analyzed. Before ROIs were drawn, the MR image set was co-registered to a template MR image with the ROIs predefined. The ROIs were then redrawn for the target MR image on the basis of this template.
Figure 1.
(A) Regions of interest (ROI) used in imaging analyses. White outlines illustrate cortex (Ctx), hippocampus (Hc), thalamus (Tha), and amygdala (Ag) ROIs used for MRI and PET analyses. The circles within each ROI were used for intensity analysis. (B and C) Representative coronal thionin-stained histological images of LFPI (B) and sham-injury lesions (C; scale bar = 2mm; see Jones et al., 2008a for additional details).
MRI intensity analysis
As previously described (Onyszchuk et al., 2009), analysis was performed with Matlab to measure mean intensities in ROIs (cortex, hippocampus, thalamus, and amygdala from both hemispheres) drawn on coronal MRI slices by an investigator blinded to experimental conditions (see Figure 1). Only slices containing hippocampus were analyzed. ROI intensities were normalized to muscle tissue intensity.
Large-deformation high-dimensional mapping of hippocampal morphometry
Large-deformation high-dimensional mapping (HDM-LD) of hippocampal morphometry was also completed as previously described (Hogan et al., 2004; Liu et al., 2010). HDM-LD utilizes computer-assisted shape recognition to identify patterns within MRI data (Bouilleret et al., 2009; Hogan et al., 2009). Computational anatomic techniques produce 3-dimensional surface representations of the hippocampus with resolution at a subvoxel level, enabling visualization of details of hippocampal surface anatomy (Gardner & Hogan, 2005). Changes in morphology can be detected even when total volume changes are not significantly different (Csernansky et al., 1998; Posener et al., 2003; Hogan et al., 2004; Hogan et al., 2008). Here we generated coordinates for hippocampal surface transformation for each image using a common template. Hippocampal surfaces were first corrected for global size differences by registering the corresponding brain volume to an average rat atlas and obtaining global scaling estimates in the three spatial directions (Snyder, 1996). After scaling, the surfaces were registered to a common hippocampal surface template. Average hippocampal deformations for the baseline and 1 week scans for both groups were then computed. Vertex-level differences within and between groups were entered as a dependent variable in ANOVA and post-hoc unpaired t-tests, corrected for multiple comparisons with a cluster-constrained empiric noise estimation model.
PET analysis
For PET ROI analysis, each 18F-FDG PET image was co-registered to its corresponding MRI scan, on which the 10 ROIs were defined. The mean activity was measured for all 10 ROIs, and mean value of activity for the ROIs was calculated. For PET statistical parametric mapping (SPM) analysis of PET data we used SPM (SPM5; Welcome Department of Cognitive Neuroscience). For PET preprocessing, the PET sub-volumes encompassing the brain were extracted and resampled to a 0.11718 mm3 voxel size into the three-dimensional space of a reference MR image using trilinear interpolation (Analyze; Mayo Foundation). We chose one sham-control PET scan with a high degree of symmetry, alignment, and freedom from artifacts as a reference PET image. We then created a mean image for use as the PET template by realigning six other individual PET scans using a rigid transformation and trilinear interpolation to the reference PET image (Casteels et al., 2006). To account for brain size variations over time, all individual PET images were spatially normalized to the PET template using the affine spatial normalization option in SPM and a trilinear interpolation. The voxel size of the normalized and resampled PET images was 0.1 mm3. Flexible factorial analysis with three independent factors was applied: treatment (sham or LFPI), time (1 week and 1, 3, or 6 months), and subject (rat number), with an interaction between factors one and two to perform the analysis. The PET images were proportionally normalized using the cerebellum mean value derived from the manual analysis. Contrasts assessing differences between epileptic and non-epileptic at each time point were derived and tested at a p < 0.01.
Behavioral Assessment
As previously described (Jones et al., 2008a; Liu et al., 2010), all rats underwent well-validated assessments of anxiety-like behavior (elevated-plus maze and open-field test; Prut & Belzung, 2003; Carobrez & Bertoglio, 2005), learning and memory (water maze; Jones et al., 2009), and depression-like behavior (sucrose preference test and forced swim test; Porsolt et al., 1977; Willner et al., 1987). All of the tests, except the forced swim test, were performed at 1, 3, and 6 months post-injury. As it is moderately stressful to the animal, forced swim testing only occurred at the 6-month post-injury time point (Jones et al., 2008a).
Assessment for the development of epilepsy
Electrode Implantation
Upon completion of the behavioral and imaging studies, all rats were implanted with six extradural screw-electrodes. The surgical procedure of electrode implantation has been previously described in detail (Stroud et al., 2005; Jones et al., 2008b). Briefly, rats were anaesthetized with isoflurane in equal parts medical air and oxygen. Electrodes were custom-made by soldering gold ‘female’ connector sockets (Ginder Scientific, Canada) onto stainless steel Teflon-coated wire (SDR Clinical Technology) with a 1.4 × 3mm stainless steel screw (Mr. Specs, Australia) attached at the opposite end. Each screw was placed into the skull via a separate burr hole, and the electrodes were inserted into a nine-pin ABS plug (GS09PLG-220, Ginder Scientific, Canada). The entire assembly was secured with dental acrylic and the skin suture around the headpiece.
Video-EEG monitoring
Following electrode implantation and a one-week recovery period, brain electrical activity was monitored for two weeks using continuous video-EEG recording in order to assess for PTE. As previously described (Stroud et al., 2005; Jones et al., 2008b), EEG cables were connected to the electrode headpiece of each rat, and to a computer running Compumedics™ video/EEG acquisition system (32 channel series, Compumedics Limited, Australia). For each rat, four of the six implanted electrodes were used for EEG recording, plus one electrode each for ground and reference. Using this configuration, each rat used 4 channels, allowing for a total of 8 rats to be recorded at once on a 32-channel system. To monitor behavior during seizures, rats were continuously recorded by an overhead video camera (3–8mm, Pentax, USA). The camera was equipped with an infrared light and a wide-angle lens, which allowed for day and night video recording of up to eight rats simultaneously (Van Raay et al., 2009).
Video-EEG analysis
Two separate experienced reviewers who were blinded to the experimental groups analyzed all EEG recordings. Determination of seizure expression followed protocols described in our previous work (Stroud et al., 2005; Jones et al., 2008b). Electroencephalographic seizure activity was defined as high-amplitude, rhythmic discharges that represented clearly a new pattern of tracing. This included repetitive spikes, spike-and-wave discharges and slow waves. The event must have lasted at least 5s and showed an evolution in the dominant frequency. If epileptic events occurred within an interval of less than 5s without return to baseline, it was defined as belonging to the same seizure event (Stroud et al., 2005; Jones et al., 2008b). If an electrographic event was observed, the behavioral severity was determined from the corresponding video recording and scored for seizure class according to the Racine scale (Racine, 1972). For the event to be classified as a post-traumatic seizure both reviewers had to identify the electroencephalographic seizure activity and classify it as such.
Statistical Analyses
The MRI and PET data for each ROI were analyzed separately using a 2-way ANOVA for repeated measures, with epilepsy status and time after LFPI as the independent variables. Neuromotor and behavioral tests were analyzed using 2-way ANOVA, with epilepsy status and time after LFPI as the independent variables. Multivariate logistic regression analyses were conducted to determine whether a combination of the parameters affected by LFPI, or the dynamics of changes within these parameters over time, might predict epileptic outcome. Analyses were performed using Statistica or SPSS software. Statistical significance was set at p < 0.05 and all data were expressed as mean ± SEM.
Results
Occurrence of post-traumatic epilepsy (PTE)
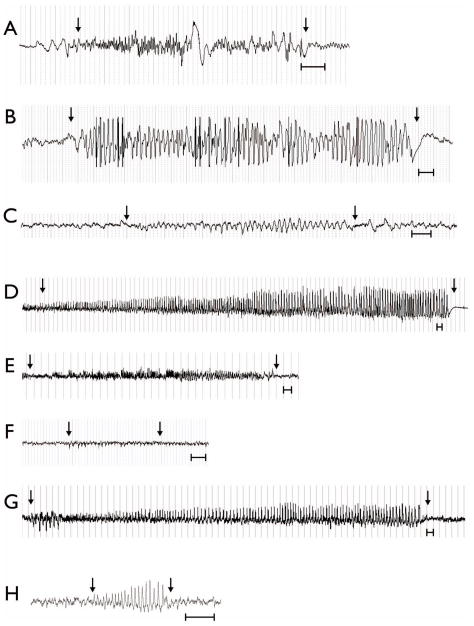
There was a 98% inter-rater reliability between reviewers, with both reviewers identifying 44/45 seizures. Spontaneous recurrent seizures were observed in 30% of rats (n=7), with these rats experiencing an average of 6.3 seizures (median = 4; range = 2–17) over the two-week video-EEG recording period (see Figure 2). The mean duration of the seizures was 52.9 s (median = 14.6 s; range = 6 – 676 s). Epileptic discharges were observed in an additional 22% of rats (n=5), with these rats experiencing an average number of 11.5 discharges (median = 10.5; range = 2 – 20) over the two-week video-EEG recording period (see Figure 2). Unless stated otherwise, these 12 rats (52%) composed the epilepsy group for all remaining analyses. The remaining 48% of rats (n=11) given LFPI were not observed to experience spontaneous recurrent seizures or epileptic discharges and composed the non-epileptic LFPI group. The sham-injury rats displayed no evidence of spontaneous recurrent seizures or epileptic discharges and were therefore not included in any other analyses. For details regarding comparisons between the sham-injury and LFPI groups see Jones et al., 2008a and Liu et al., 2010.
Figure 2.
Representative examples of EEG traces of electroencephalographic seizure activity from each of the 7 rats that had recurrent spontaneous seizures recorded on video-EEG monitoring (A–G), and an example of an epileptic discharge recorded on an additional 5 rats (H), six months after LFPI. Arrows indicate beginning and end of seizure activity. Scale bars = 1 s.
Large-deformation high-dimensional mapping of hippocampal morphometry
HDM-LD analysis revealed that both of the LFPI groups (epileptic and non-epileptic) displayed a significant deformation of the ipsilateral hippocampus at one week post-injury relative to baseline measures (see Figure 3). However, these effects differed between the epileptic and non-epileptic groups. Specifically, the epileptic group displayed an increase in lateral regions of the hippocampus, whereas the non-epileptic rats displayed a decrease in the medial-ventral regions.
Figure 3.
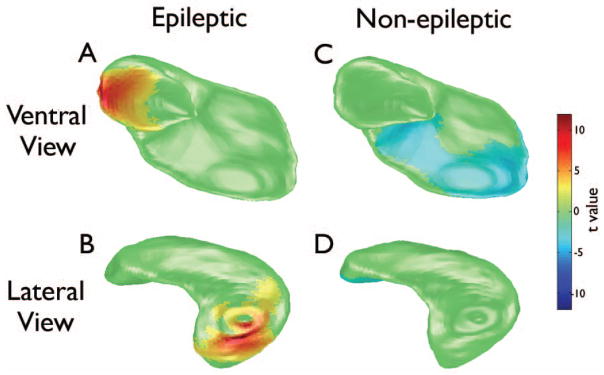
Large-deformation high-dimensional mapping (HDM-LD) of the ipsilateral hippocampus illustrating changes in epileptic (A and B) and non-epileptic rats (C and D) one week after LFPI compared to baseline. Epileptic rats displayed significantly thicker surface in the lateral region of the hippocampus at one-week post-LFPI (red-yellow) compared to baseline (unpaired two-tailed t test, p < .05, corrected for multiple comparisons). Non-epileptic rats displayed significantly thinner surface in the medial-ventral region at one-week post-LFPI (blue) compared to baseline.
T2-weighted MRI and PET
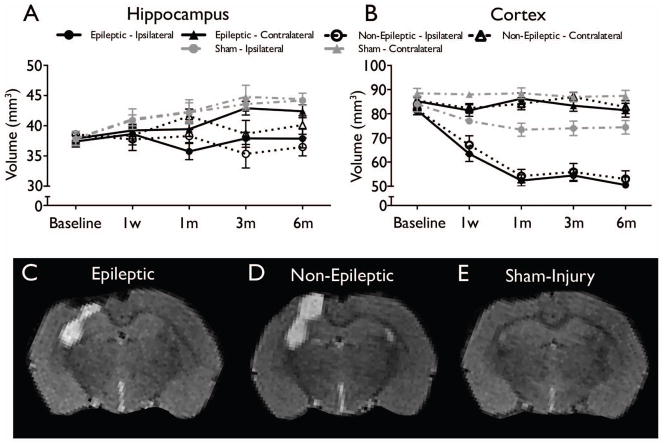
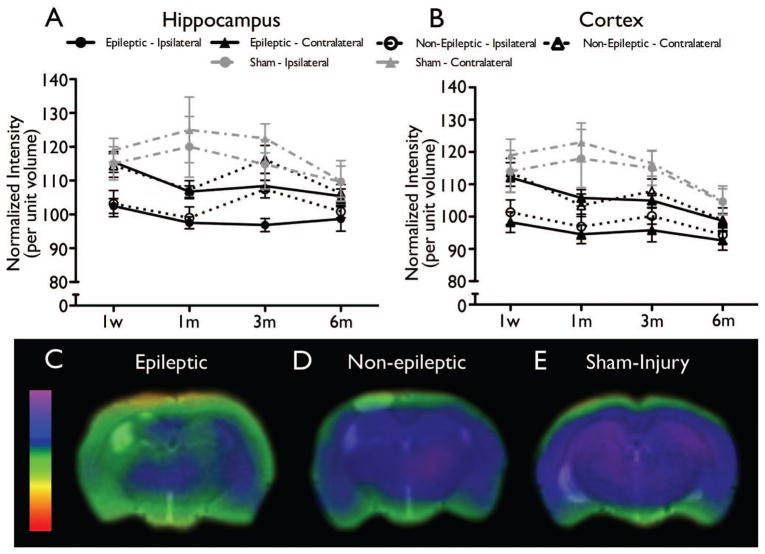
There were no significant differences found between epileptic and non-epileptic rats in T2-weighted MRI (all ps > .05, see Figure 4) or PET measures (all ps > .05, see Figure 5).
Figure 4.
MRI-based analysis of structural damage after LFPI found no significant differences between epileptic and non-epileptic rats. Time course of volume changes from baseline to six months in hippocampus (A) and cortex (B) are shown (mean ± SEM). (C–E) Representative T2-weighted images at six months post-injury demonstrate brain damage in both the LFPI groups (epileptic - C and non-epileptic - D) relative to sham-injury (E; see Liu et al., 2010 for additional details), but no significant differences between the epileptic and non-epileptic groups.
Figure 5.
PET-based ROI analysis of functional changes after LFPI found no significant differences between epileptic and non-epileptic rats. However, a multivariate logistic regression model that incorporated the one week, one month, and three month PET parameters from the ipsilateral hippocampus was able to predict the epileptic outcome in all of the LFPI cases. Time course of metabolic changes on PET scans in the hippocampus (A) and the cortex (B) are shown (mean ± SEM). Representative PET images at three months post-injury demonstrate a decrease in metabolism in both the LFPI groups (epileptic - C and non-epileptic - D) relative to sham-injury (E; see Liu et al., 2010 for additional details), with worsened hypometabolism observed in the ipsilateral hippocampus of the epileptic group.
Neuromotor and behavioral assessment
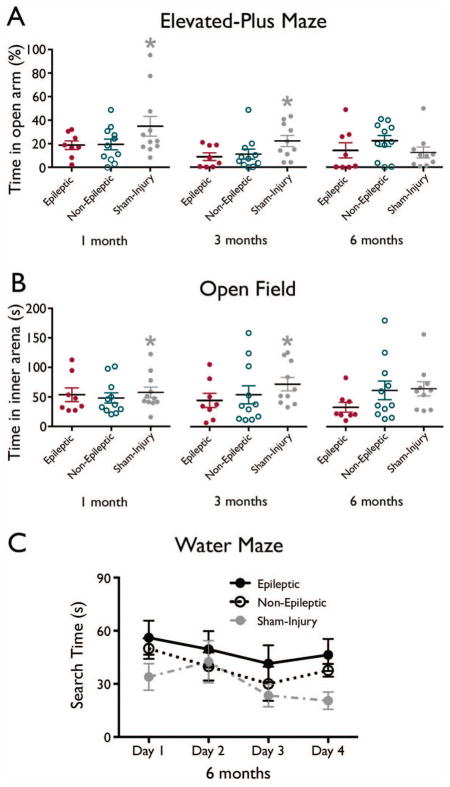
There were no significant differences in acute neuromotor scores (all ps > .05, data not shown), indicating that both groups experienced similar levels of injury severity. Behavioral measures also found no differences between epileptic and non-epileptic LFPI rats (all ps > .05, see Figure 6).
Figure 6.
Behavioral outcomes (mean ± SEM) in the elevated-plus maze (A), open field, and (C) water maze. There were no significant differences between epileptic and non-epileptic groups. However, rats given LFPI are hyperanxious compared to sham-injured rats, * significantly greater than LFPI rats, p < .05, see Jones et al., 2008a for additional details regarding differences between sham and LFPI rats.
Multivariate logistic regression model
Multivariate logistic regression analyses determined that none of the neuromotor, behavioural, or MRI parameters were significant predictors of epileptic outcome (all ps > .05). However, a multivariate logistic regression model that incorporated the 18F-FDG PET parameters from the ipsilateral hippocampus at one week, one month, and three months post-injury blocked over time was able to significantly predict the epileptic outcome in 100% of the LFPI cases (χ2(3) = 19.121, p < .001; see Figure 5)
Analyses excluding rats with epileptic discharges
To ensure that the sensitivity of the analyses were not limited by the inclusion of rats experiencing epileptic discharges in the absence of spontaneous seizures, additional statistical analyses that excluded these rats were also conducted. Consistent with the previous analyses, there were no significant differences between the epilepsy and non-epilepsy groups on any of the measures (all ps > .05). Furthermore, the multivariate logistic regression model incorporating the serial ipsilateral hippocampus PET parameters was again able to significantly predict the epileptic outcome in 100% of the cases (χ2(3) =17.845, p < .001), while the neuromotor, behavioural, and MRI parameters where unable to significantly predict epileptic outcome (all ps > .05).
Discussion
Here we report that a proportion of rats given LFPI display evidence of PTE at six months post-injury. Rats were divided into epileptic and non-epileptic groups in order to investigate whether structural, functional, and behavioral changes were predictive of PTE. HDM-LD analysis based on T2-weighted MRI revealed that the epileptic and non-epileptic rats displayed minor but significant differences with regards to changes in the ipsilateral hippocampus at one week post-injury relative to baseline, with epileptic rats showing an increased thickness in the lateral region and non-epileptic rats showing thinning in the medial-ventral region. Additionally, a multivariate logistic regression model including the serial 18F-FDG PET parameters from the ipsilateral hippocampus was able to significantly predict the epileptic outcome in all of the LFPI cases. With the exception of these findings, the detailed serial MRI, PET, and behavioral analyses for six months post-injury found no other differences between epileptic and non-epileptic rats.
The Validity and Limitations of LFPI as an animal model of PTE
Previous studies have reported inconsistent findings regarding the use of LFPI as an animal model of PTE. While D’Ambrosio and colleagues (2004b, 2005) report PTE in 92–100% of rats in chronic stages after severe LFPI, Kharatishvili and colleagues (2006) report incidence rates of 43–50%. The variability in these findings is likely a result of key methodological differences (i.e. age and injury parameters), as well as the differences in the definition of what is a seizure between these studies. The determination of what is a seizure in rodent acquired epilepsy models is a highly controversial area, with no generally accepted criteria (Dudek and Bertram 2010, D’Ambrosio and Miller 2010). In this study we have used a rigorous definition that had been used in previous publications on experimental post-traumatic epilepsy in the FPI model by Kharatishvili and colleagues (Kharatishvili et al., 2006; Kharatishvili et al., 2007) and subsequently applied in our previous work (Bouilleret et al., 2009; Bouilleret et al., 2011). The determination was done by two independent experienced reviewers who were blinded to the experimental groups, with 44/45 of the seizures identified by both of our blinded reviewers and no seizures were identified in sham animals. Therefore, it is unlikely that the identified events are normal oscillations or electrical noise or genetically determined absence seizures. As our methods and definition of PTE closely align with those of Kharatishvili and colleagues (2006, 2007), it is reassuring that our finding that 30–52% of rats given severe LFPI displayed either spontaneous seizures and/or epileptic discharges are similar to those reported by this group (Kharatishvili et al., 2006). This is also consistent with rates of PTE in patients following TBI (Englander et al., 2003; Frey, 2003; Christensen et al., 2009), and supports the use of LFPI in rats to model and study PTE. However, it is important to acknowledge that some workers in the field would question the relevance of brief seizures, even when lasting longer than 5 seconds, recorded in rodents post experimental TBI to human post traumatic epilepsy (Dudek and Bertram 2010). The median duration of seizures recorded in this study was 14.6 seconds, with a range of 6 – 676 seconds.
There are also limitations with the current study related to the LFPI – PTE model that must be considered when interpreting the present results. This was done because previous studies had demonstrated that the majority of animals given LFPI who develop PTE do so by six months (Kharatishvili et al., 2006), two weeks of continuous video-EEG recording was the most detailed analysis at six months post-LFPI previously reported (Kharatishvili et al., 2006), and compatibility issues associated with the EEG-recording electrodes and MRI scans meant that the EEG electrodes had to be implanted following completion of the serial MRI acquisitions. However, we acknowledge that post-LFPI epileptic animals can experience prolonged seizure-free intervals, and initial seizures may take over six months to occur (Kharatishvili et al., 2006). Therefore, we cannot exclude the possibility that our ‘non-epileptic’ group included epileptic animals who experienced seizures outside of the monitoring period, or may have gone on to develop seizures had the study continued to a later time point. This may have contributed to the lack of differences between epileptic and non-epileptic animals on many of the measures, and, as such, the remaining discussion should be interpreted in light of this concern. Furthermore, it is important for future studies investigating PTE to consider these limitations and incorporate the most detailed seizure analysis that is feasible.
Neuroimaging and behavioral predictors of PTE
Here, using advanced analysis of MRI and PET parameters, we were able to identify subtle changes that may be predictive of PTE after LFPI in the rat. Specifically, MRI-based HDM-LD hippocampal morphometry analysis identified significant surface changes in the ipsilateral hippocampus that differed between epileptic and non-epileptic rats, and a multivariate logistic regression model that incorporated the serial PET parameters from the ipsilateral hippocampus at one week, one month, and three months post-injury was able to significantly predict the epileptic outcome in each of the cases. Although these subtle findings reached significance we interpret them cautiously given that no other measures in the study comparing the epileptic and non-epileptic groups identified differences. Nonetheless, the strength of HDM-LD and multivariate logistic regression analyses is their ability to detect subtle findings (Csernansky et al., 1998; Hogan et al., 2009). Thus, the differences reported here may indicate hippocampal abnormalities that occur at various time points post-TBI that are involved in PTE. Indeed, numerous other LFPI studies have implicated hippocampal abnormalities with PTE (Pitkänen et al., 2009), and some suggest that hippocampal changes identified by MRI-based analyses could serve as a surrogate marker for PTE (Kharatishvili et al., 2007). Furthermore, other studies have found that 18F-FDG PET hypometabolism in the hippocampus, similar to that observed in the epileptic rats here, may be associated with epileptogenesis (Jupp et al., 2012). However, whether the findings reported here are truly related to the epileptogenic process will require larger and more detailed studies, perhaps focusing histopathological and biochemical examinations on the regions that showed significant HDM-LD changes, as well as more temporally sensitive PET imaging and EEG analysis.
Despite the use of serial T2-weighted MRI and 18 F-FDG PET imaging to assess structural and functional changes for six months post-LFPI, we found no other significant differences between epileptic and non-epileptic rats. The lack of major structural changes associated with PTE reported here are consistent with previous reports that cortical damage assessed with serial T2-weighted MRI was not related to seizure susceptibility in rats given LFPI (Kharatishvili et al., 2007). Taken together with the lack of functional differences detected by the serial 18F-FDG PET, the ROI techniques used here were unable to detect any major structural and metabolic changes post-TBI predictive of PTE, limiting their use as predictive tools and suggests that PTE occurs independent of the major structural and functional changes induced by TBI. However, while the ROI techniques used here may lack the capability (i.e. spatial resolution) necessary to solely detect more subtle abnormalities involved in PTE, other neuroimaging modalities, such as diffusion or functional MRI, may be more useful (Kharatishvili et al., 2007).
The behavioral measures also failed to detect significant changes related to epileptic status, indicating that these are not reliable indicators of PTE. However, it is important to note that while no differences were found between epileptic and non-epileptic rats, as a whole the rats given LFPI did display significant imaging and behavioral abnormalities relative to sham-injured rats (see Jones et al., 2008a; Liu et al., 2010; Figures 4–6). Therefore, although behavioral and imaging abnormalities would be predicted to occur in epilepsy (Golarai et al., 2001; Jones et al., 2008b; Pitkänen et al., 2011), similar changes induced by TBI may have confounded the sensitivity of our measures. Furthermore, the behavioral testing may have occurred at time-points not sensitive to changes related to PTE, and the behavioral tasks were not comprehensive to the entire spectrum of behaviors potentially associated with PTE. For example, the water maze task used in this study was specific to spatial learning and memory and failed to identify significant group differences, whereas other cognitive tasks (Saucier et al., 2008), or variations of the water maze (Shultz et al., 2009), could be used to assess whether other learning and memory abnormalities may serve as PTE biomarkers. Thus, future studies that employ more detailed techniques may be able to detect slight differences between epileptic and non-epileptic rats post-TBI that were not observed here.
In conclusion, here we examined rats given LFPI using serial MRI, PET, and behavioral assessments for six months post-injury. Based on video-EEG monitoring, rats were identified as either epileptic or non-epileptic, and comparisons were made to examine structural, functional, and behavioral changes related to PTE. A subtle difference was identified between epileptic and non-epileptic rats in hippocampal morphometry, and a multivariate logistic regression model that included the serial PET parameters from the ipsilateral hippocampus was able to accurately predict epileptic outcome. While these findings provide cautious optimism and might direct future studies, unfortunately no other readily identifiable differences between the epileptic and non-epileptic rats were detected. Taken together, these findings indicate that PTE may not be related to major structural, functional, and behavioral changes, and suggest other more subtle changes or mechanisms may be involved.
Acknowledgments
We wish to acknowledge the staff of the Howard Florey Institute Small Animal MRI Facility, and the Peter MacCallum Cancer Centre. Funding for this project was provided by the Victorian Government Transport Accident Commission (DP0023). N.J. and T.O. acknowledge the financial support of the NHMRC of Australia.
Footnotes
None of the authors have any conflict of interest to disclose.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338:20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- Asikainen I, Kaste M, Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia. 1999;40:584–589. doi: 10.1111/j.1528-1157.1999.tb05560.x. [DOI] [PubMed] [Google Scholar]
- Beghi E. Overview of studies to prevent posttraumatic epilepsy. Epilepsia. 2003;44:21–26. doi: 10.1046/j.1528-1157.44.s10.1.x. [DOI] [PubMed] [Google Scholar]
- Beyenburg S, Mitchell AJ, Schmidt D, Elger CE, Reuber M. Anxiety in patients with epilepsy: systemic review and suggestions for clinical management. Epilepsy Behav. 2005;7:161–171. doi: 10.1016/j.yebeh.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Hogan RE, Velakoulis D, Salzberg MR, Wang L, Egan GF, O’Brien TJ, Jones NC. Morphometric abnormalities and hyperanxiety in genetically epileptic rats: a model of psychiatric comorbidity? Neuroimage. 2009;45:267–274. doi: 10.1016/j.neuroimage.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Cardamone L, Liu YR, Koe AS, Fang K, Williams JP, Myers DE, O’Brien TJ, Jones NC. Confounding neurodegenerative effects of manganese for in-vivo MR imaging in rat models of brain insults. Journal of Magnetic Resonance Imaging. 2011;34:774–84. doi: 10.1002/jmri.22669. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Casteels C, Vermaelen P, Nuyts J, Van Der Linden A, Baekelandt V, Mortelmans L, Bormans G, Van Laere K. Construction and evaluation of multitracer small-animal PET probabilistic atlases for voxel-based functional mapping of the rat brain. J Nucl Med. 2006;47:1858–1866. [PubMed] [Google Scholar]
- Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet. 2009;373:1105–1110. doi: 10.1016/S0140-6736(09)60214-2. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, Grenander U, Miller MI. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci USA. 1998;95:11406–11411. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Perucca E. Epilepsy after head injury. Curr Opin Neurol. 2004a;17:731–735. doi: 10.1097/00019052-200412000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio RD, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004b;127(2):304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio RD, Fender JS, Fairbanks JP, Simon EA, Born DE, Doyle DL, Miller JW. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 2005;132:2805–2821. doi: 10.1093/brain/awh337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio RD, Miller JW. What is an epileptic seizure? Unifying definitions in clinical practice and animal research to develop novel treatments. Epil Curr. 2010;10:61–66. doi: 10.1111/j.1535-7511.2010.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Zha J, Hergenroede G, Moore AN. Biomarkers for the diagnosis, prognosis, and evaluation of treatment efficacy for traumatic brain injury. Neurotherapeutics. 2010;7:100–114. doi: 10.1016/j.nurt.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter MA. Posttraumatic epilepsy: the challenge of translating discoveries in the laboratory to pathways to a cure. Epilepsia. 2009;50:41–45. doi: 10.1111/j.1528-1167.2008.02009.x. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Bertram EH. Counterpoint to “What is an epileptic seizure?” by D’Ambrosio and Miller. Epil Curr. 2010;10:91–94. doi: 10.1111/j.1535-7511.2010.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander J, Bushnik T, Duong TT, Cifu DX, Zafonte R, Wright J, Hughes R, Bergman W. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch Phys Med Rehabil. 2003;84:365–373. doi: 10.1053/apmr.2003.50022. [DOI] [PubMed] [Google Scholar]
- Frey L. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia. 2003;44:11–17. doi: 10.1046/j.1528-1157.44.s10.4.x. [DOI] [PubMed] [Google Scholar]
- Gardner R, Hogan RE. Three-dimensional deformation-based hippocampal surface anatomy, projected on MRI images. Clin Anat. 2005;18:481–487. doi: 10.1002/ca.20183. [DOI] [PubMed] [Google Scholar]
- Garza N, Lowenstein DH. Posttraumatic epilepsy: a major problem in desperate need of major advances. Epilepsy Curr. 2006;6:1–5. doi: 10.1111/j.1535-7511.2005.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan RE, Wang L, Bertrand ME, Willmore LJ, Bucholz RD, Nassif AS, Csernansky JG. MRI-based high-dimensional hippocampal mapping in mesial temporal lobe epilepsy. Brain. 2004;127:1731–1740. doi: 10.1093/brain/awh197. [DOI] [PubMed] [Google Scholar]
- Hogan RE, Carne RP, Kilpatrick CJ, Cook MJ, Patel A, King L, O’Brien TJ. Hippocampal deformation mapping in MRI negative PET positive temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2008;79:636–640. doi: 10.1136/jnnp.2007.123406. [DOI] [PubMed] [Google Scholar]
- Hogan RE, Bouilleret V, Liu YR, Wang L, Williams JP, Jupp B, Myers D, O’Brien TJ. MRI-based large deformation high dimensional mapping of the hippocampus in rats: development and validation of the technique. J Magn Reson Imaging. 2009;29:1027–1034. doi: 10.1002/jmri.21766. [DOI] [PubMed] [Google Scholar]
- Jones NC, Cardamone L, Williams JP, Salzberg MR, Myers D, O’Brien TJ. Experimental traumatic brain injury induces a pervasive hyperanxious phenotype in rats. J Neurotrauma. 2008a;25:1367–1374. doi: 10.1089/neu.2008.0641. [DOI] [PubMed] [Google Scholar]
- Jones NC, Salzberg MR, Kumar G, Couper A, Morris MJ, O’Brien TJ. Elevated anxiety and depressive-like behavior in a rat model of genetic generalized epilepsy suggesting common causation. Exp Neurology. 2008b;209:254–260. doi: 10.1016/j.expneurol.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Jones NC, Kumar G, O’Brien TJ, Morris MJ, Rees MR, Salzberg MR. Anxiolytic effects of rapid amygdala kindling, and the influence of early life experience in rats. Behav Brain Res. 2009;203:81–87. doi: 10.1016/j.bbr.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Jupp B, Williams J, Binns D, Hicks RJ, Cardamone L, Jones N, Rees S, O’Brien TJ. Hypometabolism precedes limbic atrophy and spontaneous recurrent seizures in a rat model of TLE. Epilepsia. 2012;53:1233–1244. doi: 10.1111/j.1528-1167.2012.03525.x. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Nissinen JP, McIntosh TK, Pitkänen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140:658–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Immonen R, Gröhn O, Pitkänen A. Quantitative diffusion MRI of the hippocampus as a surrogate marker for posttraumatic epileptogenesis. Brain. 2007;130:3155–3168. doi: 10.1093/brain/awm268. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Pitkänen A. Posttraumatic epilepsy. Curr Opin Neurol. 2010;23:183–188. doi: 10.1097/WCO.0b013e32833749e4. [DOI] [PubMed] [Google Scholar]
- Li H, McDonald W, Parada I, Faria L, Graber K, Takahashi DK, Yunyong M, Prince D. Targets for preventing epilepsy following cortical injury. Neurosci Lett. 2011;497:172–176. doi: 10.1016/j.neulet.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YR, Cardamone L, Hogan RE, Gregoire M-C, Williams JP, Hicks RJ, Binns D, Koe A, Jones NC, Myers DE, O’Brien TJ, Bouilleret V. Progressive metabolic and structural cerebral perturbations after traumatic brain injury: an in vivo imaging study in the rat. J Nucl Med. 2010;51:1788–1795. doi: 10.2967/jnumed.110.078626. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas AIR, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Mishra AM, Bai H, Gribizis A, Blumenfeld H. Neuroimaging biomarkers of epileptogenesis. Neurosci Lett. 2011;497:194–204. doi: 10.1016/j.neulet.2011.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyszchuk G, Levine SM, Brooks WM, Berman NEJ. Post-acute pathological changes in the thalamus and internal capsule in aged mice following controlled cortical impact injury: a magnetic resonance imaging, iron histochemical, and glial immunohistochemical study. Neurosci Lett. 2009;452:204–208. doi: 10.1016/j.neulet.2009.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Immonen RJ, Grohn OHJ, Kharatishvili I. From traumatic brain injury to posttraumatic epilepsy: what animal models tell us about the process and treatment options. Epilepsia. 2009;50:21–29. doi: 10.1111/j.1528-1167.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Bolkvadze T, Immonen R. Anti-epileptogenesis in rodent post-traumatic epilepsy models. Neurosci Lett. 2011;497:163–171. doi: 10.1016/j.neulet.2011.02.033. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Posener JA, Wang L, Price JL, et al. High-dimensional mapping of the hippocampus in depression. Am J Psychiatry. 2003;160:83–89. doi: 10.1176/appi.ajp.160.1.83. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Saucier DM, Shultz SR, Keller AJ, Cook CM, Binsted G. Sex differences in object location memory and spatial navigation. Anim Cogn. 2008;11:129–137. doi: 10.1007/s10071-007-0096-1. [DOI] [PubMed] [Google Scholar]
- Shultz SR, MacFabe DF, Martin S, Jackson J, Taylor R, Boon F, Ossenkopp K-P, Cain DP. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long-Evans rat: further development of a rodent model of autism. Behav Brain Res. 2009;200:33–41. doi: 10.1016/j.bbr.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Snyder AZ. Difference image versus ratio image error function forms in PET-PET realignment. In: Bailey D, Jones T, editors. Quantification of Brain Function using PET. San Diego: Academic Press; 1996. pp. 131–137. [Google Scholar]
- Stroud LM, O’Brien TJ, Jupp B, Wallengren C, Morris MJ. Neuropeptide Y suppresses absence seizures in a genetic rat model. Brain Res. 2005;1033:151–156. doi: 10.1016/j.brainres.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50:10–13. doi: 10.1111/j.1528-1167.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- Van Raay L, Morris MJ, Reed R, O’Brien TJ, Dedeurwaerdere S. A novel system allowing long-term simultaneous video-electroencephalography recording, drug infusion and blood sampling in rats. J Neurosci Methods. 2009;179:184–190. doi: 10.1016/j.jneumeth.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]