Abstract
Background
Psychotic disorders are characterized by aberrant neural connectivity. Alterations in gyrification, the pattern and degree of cortical folding, may be related to the early development of connectivity. Past gyrification studies have relatively small sample sizes, yield mixed results for schizophrenia (SZ), and are scant for psychotic bipolar (BP) and schizoaffective (SZA) disorders and for relatives of these conditions. Here we examine gyrification in psychotic disorder patients and their first-degree relatives as a possible endophenotype.
Methods
Regional Local Gyrification Index (LGI) values, as measured by FreeSurfer software, were compared between 243 controls, 388 psychotic disorder probands, and 300 of their first-degree relatives. For patients, LGI values were examined grouped across psychotic diagnoses and then separately for SZ, SZA, and BP. Familiality (heritability) values and correlations with clinical measures were also calculated for regional LGI values.
Results
Probands exhibited significant hypogyria compared to controls in three brain regions and relatives with axis II cluster A disorders showed nearly significant hypogyria in these same regions. LGI values in these locations were significantly heritable and uncorrelated with any clinical measure. Observations of significant
Conclusions
Psychotic disorders appear to be characterized by significant regionally localized hypogyria, particularly in cingulate cortex. This abnormality may be a structural endophenotype marking risk for psychotic illness and it may help elucidate etiological underpinnings of psychotic disorders.
Keywords: Cortical folding, gyrification, psychosis, schizophrenia, bipolar, schizoaffective
Introduction
The underlying genetic architecture of psychotic disorders has proven difficult to establish, partly because of the disorders' complex nature, clinical heterogeneity, and imprecise diagnostic boundaries (1). Endophenotype strategies have been increasingly employed in efforts to identify liability-conferring genes and clarify disease etiology (2). Endophenotypes, or intermediate phenotypes, are measurable biological traits that are “inteiflediate” between genotype and clinical syndrome. Because endophenotypes are presumed relatively proximal to the neurobiological action of genes, they may provide footholds in the study of the genetic underpinnings of disease (3; 4). Gottesman and Gould (5) and others (3; 6; 7) proposed criteria for useful endophenotypes, including illness association, heritability, state independence, and greater presentation in unaffected family members than in the general population.
Abnormal gyrification, the degree and pattern of folding ofbrain cortex, has been proposed as a schizophrenia endophenotype candidate (8). Schizophrenia is characterized by aberrant connectivity (9-11) and gyrification may be related to the early development of neural connectivity (12-15). It has been suggested that cortical connectivity development in the second trimester generates fiber tension, which draws densely connected regions together, forming bulging gyri, whereas more sparsely connected regions drift apart and are separated by inward sulci (16). The case for abnormal gyrification being an endophenotype for schizophrenia is supported by the presumed neurodevelopmental nature of the disorder, demonstrated heritability of gyrification (17) and observations of atypical cortical folding in both schizophrenia probands (16; 18-28) and, to a lesser degree, unaffected relatives (29-31).
However, gyrification findings in schizophrenia patients are notably discordant, as studies alternately report hypogyria, hypergyria, and negative findings (Table 1). Evidence is also inconclusive in studies of other psychotic disorders, with gyrification research on psychotic bipolar disorder producing both positive (32-34) and negative findings (35; 36) while research on schizoaffective disorder remains scant. These diverse findings may be due to a variety of factors, including relatively small sample sizes and the heterogeneity of tools used to measure gyrification. In past research, gyrification has most commonly been quantified using the Gyrification Index (GI), a measure in two-dimensional space that may be dependent on imaging parameters such as slice thickness and orientation (20).
Table 1.
Review of gyrification research in schizophrenia after studies reviewed by White and Hilgetag (16)
| Authors (Year) | Patients (n) | Controls (n) | Patient population | Mean Age (SD) | Method for gyrification | Significant results (Patients compared to controls) |
|---|---|---|---|---|---|---|
| Janssen et al. (2009) (37) | 49 | 34 | First episode, early onset | 15.8 (1.5) | LGI | N.S. |
| Schultz et al. (2010) (19) | 54 | 54 | First episode | 29.1 (9.4) | GI | ↑ right parahippocampal-lingual cortex |
| Palaniyappan et al. (2011) (20) | 57 | 42 | Adults | 26.1 (7.5) | LGI | ↓ left middle frontal, inferior frontal; bilateral superior frontal, frontopolar ↑ bilateral frontomarginal |
| Haukvik et al. (2012) (38) | 54 | 54 | Adults | 41.9 (8.0) | LGI | N.S. |
| Palaniyappan & Liddle (2012) (22; 23; 39) | 57 | 41 | Adults | 26.1 (7.5) | LGI | ↓ left insula, caudal superior/middle frontal, parieto-occipital sulcus, temporal, precuneus; bilateral superior temporal/inferior parietal junction, supramarginal |
| Ronan et al. (2012) (40) | 17 | 15 | Adolescents | 16.1 (1.1) | LGI | N.S. |
| 46 | 44 | Adults | 33.2 (9.0) | ↓ bilateral hemispheres | ||
| 13 | 13 | Adults | 24.8 (4.7) | N.S. | ||
| Bartholomeusz et al. (2013) (26) | 96 | 73 | First episode | 21.3 (3.3) | LGI | N.S. |
| Palaniyappan & Liddle (2013) (24) | 39 | 34 | Adults | 34.0 (2.9) | LGI | ↓ right caudal middle frontal, inferior parietal/superior temporal, lingual. ↓ left insula, precuneus/posterior cingulate,superior and middle frontal, supramarginal. |
| Palaniyappan et al. (2013) (25) | 18 | 19 | Adolescents | 16.1 (1.2) | LGI | ↓ left insula/inferior frontal, right superior temporal at 2 years follow up ↑ Broca's area, adjacent left insula at 2 years follow up. |
| Schultz et al. (2013) (27) | 72 | 72 | Adults | 28.6 (8.9) | Mean Curvature | ↑ bilaterally V1, V2, V5/MT+ |
| Tepest et al. (2013) (28) | 21 | 21 | First episode | 27.1 (5.0) | GI | ↑ frontal and parietal |
It also remains unknown whether abnormal gyrification qualifies more broadly as an endophenotype marking psychosis liability. To our knowledge, no previous study has examined gyrification in psychotic disorders treating schizophrenia, schizoaffective disorder, and psychotic bipolar disorder patients in the same sample. However, psychotic disorders exhibit similar characteristics including cross-cutting symptom profiles (41), overlapping diagnoses within family lineages (42-45), and common susceptibility genes (46-48). This high degree of similarity underscores the importance of evaluating candidate endophenotypes across psychotic diagnoses. It also highlights the nosological uncertainty surrounding psychotic disorders (49) and raises questions about the relationship between their etiologies.
The Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) is a multisite consortium designed to characterize potential endophenotypes across the psychosis spectrum. Here we report gyrification findings from the B-SNIP consortium calculated from three-dimensional surface reconstructions using the Local Gyrification Index (LGI). In so doing, we first evaluated the candidacy of gyrification as a psychotic disorder endophenotype. To address this question, we examined familiality ofLGI measures and also examined LGI measures in first degree relatives as an overall group, as well as relatives defined by the presence of a psychopathology liability trait, i.e. axis II cluster A personality disorders (50; 51).
Second, we examined whether patterns of gyrification differ between psychosis diagnoses to evaluate specificity of this biomarker for symptom-based categories, such as schizophrenia, bipolar, and schizoaffective disorders. Precautions were taken to avoid possible medication confounds, as lithium and antipsychotic usage have been found to have structural effects (52-61).
Methods and Materials
We compared MRI-derived regional local gyrification index data between healthy controls, probands with schizophrenia (SZ), schizoaffective disorder (SZA), or psychotic bipolar disorder (BP), and their first-degree relatives. Data were derived from B-SNIP, which represents a 6-site (Wayne State University, Harvard University, Maryland Psychiatric Research Center, University of Chicago / University of Illinois at Chicago, University ofTexas Southwestern, and the Institute ofLiving / Yale University) to uncover intermediate phenotypes of psychotic disorders.
Study Participants
The study included 257 healthy control participants, 441 probands with a psychotic disorder (177 SZm 106 SZA, and 158 BP) and 309 of their first-degree relatives from the B-SNIP database on whom 3.0 Tesla MRI data, clinical measures, and demographic information were available.
All participants met the following inclusion criteria: (1) ages 15-65; (2) sufficient proficiency in English to understand task instructions; (3) no known history of neurologic disorders including head injury; (4) no history of substance abuse within the last month or substance dependence within the last 6 months; and (5) negative urine toxicology screen on day of testing. Control subjects met the following additional criteria: (1) no personal or family history (first degree) of psychotic or bipolar disorders; (2) no personal history of recurrent mood disorder; (3) no lifetime history of substance dependence; (4) no history of any significant cluster A axis II personality features defined by meeting full or within one criteria of a Cluster A diagnosis using the Structured Interview for DSM-IV-TR Personality (SID-P) (62). Institutional review boards at each site approved the study and all sites used identical diagnostic, clinical, and recruitment techniques (63).
All participants underwent a diagnostic interview using the Structured Clinical Interview for DSM-IV-TR (SCID-IV) (64) and were categorized by diagnosis. Relatives without psychosis and controls were also administered the SID-P. Diagnoses were made at each site by a consensus process led by a senior clinician that included reviews of results from the clinical interviews, psychiatric and medical histories, and medical records when available. Symptom ratings were completed with probands by a trained rater blind to MRI data using the Positive and Negative Symptom Scale (PANSS) (65), the MontgomeryAsberg Depression Rating Scale (MADRS) (66), and the Young Mania Rating Scale (YMRS) (67).
Clinical and structural data were available in 1014 included participants (253 controls, 179 SZ, 100 SZA, 150 BP, and 332 relatives). 83 participants (10 controls, 22 SZ, 10 SZA, 9 BP, and 32 relatives) were excluded due to motion and scanner artifacts. A chi-squared test showed that proportion of images with artifacts differed significantly between groups. 931 subjects were included in the final analysis. Mean age, race distribution, and sex distribution across diagnostic groups are presented in table 2.
Table 2.
Demographics of included participants
| Controls | SZ | SZA | BP | Relatives | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 243 | 157 | 90 | 141 | 300 | ||||||||||
| Mean age (sd)a | 37.5 (12.3) | 34.3 (12.2) | 35.7 (12.2) | 36.6 (13.0) | 39.8 (16.1) | ||||||||||
| Race Distributionb,c | AA | CA | OT | AA | CA | OT | AA | CA | OT | AA | CA | OT | AA | CA | OT |
| 66 | 155 | 22 | 67 | 77 | 13 | 38 | 46 | 6 | 32 | 102 | 7 | 86 | 199 | 15 | |
| 27% | 64% | 9% | 43% | 49% | 8% | 42% | 51% | 7% | 23% | 72% | 5% | 29% | 66% | 5% | |
| Gender Distributionb | F | M | F | M | F | M | F | M | F | M | |||||
| 129 | 114 | 56 | 101 | 50 | 40 | 97 | 44 | 212 | 88 | ||||||
| 53% | 47% | 36% | 64% | 56% | 44% | 69% | 31% | 71% | 29% | ||||||
| Mean Family Hollingshead Score (sd)a | 39 (15) | 42 (16) | 46 (17) | 38 (16) | 42 (16) | ||||||||||
| Mean total PANSS score (sd)a | NA | 66 (17) | 69 (16) | 54 (14) | NA | ||||||||||
| Mean Intracranial Volume (sd)a | 1450 cc (198) | 1486 cc (198) | 1389 cc (186) | 1435 cc (169) | 1435 cc (178) | ||||||||||
| Mean Current Choloropromazine Equivalent Dosage (sd)a | 0 mg/day (0) | 349 mg/day (406) | 378 mg/day (491) | 236 mg/day (456) | 3 mg/day (28) | ||||||||||
| Current Lithium Usage Distributionb,d | Li | No Li | Li | No Li | Li | No Li | Li | No Li | Li | No Li | |||||
| 0 | 243 | 42 | 99 | 8 | 82 | 13 | 144 | 3 | 297 | ||||||
| 0% | 100% | 30% | 70% | 9% | 81% | 8% | 92% | 1% | 99% | ||||||
Significantly different between groups by one way ANOVA
Significantly different between groups by chi-squared test
AA – African American; CA – Caucasian; OT – Other
Li – Presently using lithium; No Li – Not presently using lithium
MRI-structural imaging
Subjects were scanned in 6 sites: Boston (3.0 T, GE Signa); Detroit (3.0 T, Siemens Allegra); Baltimore (3.0 T, Siemens Trio tim); Hartford (3.0 T, Siemens Allegra); Dallas (3.0 T, Philips); and Chicago (3.0 T, GE Signa). High-resolution isotropic T1-weighted MPRAGE scans (TR=6.7 msec, TE=3.1 msec, 8° flip angle, 256×240 matrix size, total scan duration=10:52.6 minutes, 170 sagittal slices, 1mm slice thickness, 1×1×1.2 mm3 voxel resolution) were obtained following the Alzheimer's Disease Neuroimaging Initiative (ADNI) protocol (http://www.loni.ucla.edu/ADNI).
All images underwent rigorous data quality control. First, images were converted to NIFTI format and checked for scanner artifacts by trained raters. When images passed this pre-check, they were run through a first-level auto-reconstruction (auto-recon1) in FreeSurfer (68). After auto-recon 1, the skull stripped brains were checked for remaining dura or sinus that could interfere with accurate segmentation. When non-brain tissue was found, images were edited manually by trained raters. All raters had inter-rater reliabilities (intra-class r) above 95%. When deemed sufficiently clean for segmentation by an independent rater, images were run through auto-recon 2 & 3 , i n which gray matter surface area, thickness, and volume measures were extracted.
Average LGI values were calculated in32 anatomically defined cortical parcellations in each hemisphere (69); combined they cover the entire cortex. As described and validated by Schaer et al. (70), LGI was measured by iteratively quantifying GI in spherical 3D regions of interest. Multiple overlapping spherical regions of interest (~65 cc each) were defined on the convex hull of the brain and paired with the corresponding cortical surface defined during FreeSurfer's normal processing. The LGI measure is the ratio of convex hull surface area to buried cortex surface area.
Statistical analyses
To identify regions showing differences in LGI between groups, we used a hierarchical approach which minimized risk ofType I error using a process with two steps: 1) a selection step and 2) a selective analysis. In the selection step, contrasts were run bilaterally on the six large functionally distinct regions of the brain (frontal, temporal, parietal, occipital, sensorimotor, and cingulate cortex). The mean LGI value of each of these large regions (“supra-regions”) was calculated by taking the average LGI value across the given region's component sub-regions, weighting by the sub-regions' surface areas. When a large region exhibited a trending difference (p<0.1), it was retained for selective analysis. In this selective analysis, for each large region passing the selection step, the initial contrast was run on its component sub-regions, Benjamini-Hochberg adjusting for the number of subregions. To avoid the problem of “double-dipping” into multiple comparison corrections, a selection step were first performed on a randomly chosen XA of the sample whereas the sub-region analysis was performed on the remaining ¾ of the sample. The two steps were thereby run on independent samples to ensure noncircular analysis (71). Outliers were handled by winsorising all values greater than three standard deviations from group means.
The effect of lithium was evaluated by employing this hierarwnjafanalysis to compare LGI values of probands currently using as well as not using lithium. Antipsychotic effect was evaluated by correlating chlorpromazine (CPZ) equivalent dosage and regional LGI values, Benjamini-Hochberg adjusting for the number of regions. Because significantil thium effects were found, lithium usage was included as a categorical covariate in all analyses. No significant correlations were found between current CPZ equivalent dosage and LGI values, and so CPZ equivalent dosage was not included as a covariate.
All probands, all non-psychotic relatives, and all relatives with axis II cluster A disorders were compared to controls using this hierarchical analysis. SZ, SZA, and BP were then also separately compared to controls and SZ was compared to BP.
A post-hoc analysis was conducted in which a composite LGI score was calculated by taking LGI value over brain regions where all probands showed significant differences compared to controls. The composite scores of all no-psychotic relatives and all relatives with axis II cluster A disorders were then compared both to controls and probands using pairwise contrasts.
In regions where all probands differed from controls, probands’ LGI values were correlated with the riscores on clinical scales, Hochberg adjusting for the total number of correlations.
Familiality was quantified using a maximum likelihood method in Sequential Oligogenic Linkage Analysis Routines (SOLAR) version 6.2 (72). Significance of heritability was determined using a maximum likelihood ratio test comparing a model explaining phenotypic variation by family membership to a model assuming no variation is explained by family membership.
Sex, race, scanner site, handedness, duration of illness, current lithium usagei current chloropromazine equivalent usage, age, intracranial volume (ICV), current cognitive ability (measured by Wide Range Achievement Test IV (WRAT4), a measure of premorbid intelligence (73)), and socioeconomic status (measured by Hollingshead index (74)) were tested as potential covariates for analyses. Measures were in cluded as covariates when they both were significantly associated with regional LGI values (by ANOVAs for categorical variables and Pearson's correlations for continuous variables, Benjamini-Hochberg adjusting for the total number of cortical regions) and also differed significantly between controls, probands, and relatives (by chi-squared tests for categorical variables and ANOVAs for continuous variables). According to this process, sex, race, scannerisite, lithiumiusage, age, andi ICV qualified as covariates.
Results
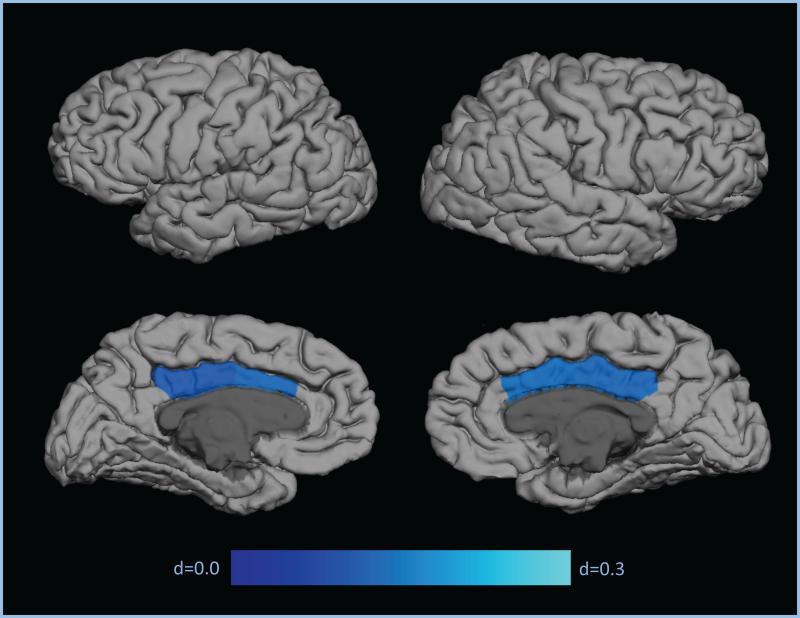
Significant associations with LGI values were observed with sex in 35 regions (p<0.05), with race in 44 regions (p<0.05), with scanner site in 64 regiofc(p<0.01), with lithium usage in the right caudal anterior cingulate and right posterior cingulate (p<0.01), with age in 64 regions (p<0.001), and with ICV in 23 regions (p<0.05). No regions showed interactions between these variables and diagnosis on the LGI imeasures. No significant associations were found for handedness, chloropromazine equivalent usage, cognitiveiability, and socioeconomicistatus. For all of theimeasures significantly associated with regional LGI values, significant differences were found between controls, SZ, SZA, BP, and relatives (p<0.001), and soisex, race, scanner site, lithium usage, age, and ICV were used as covar atesiiniall statistical analyses. Mean LGI values varied significantly between probands and controls in the right poster or cingulate, right caudalianter or cingulate, and left caudal anterior cingulate brain regions (p<0.05; d=0.17-0.19; Figure 1, Table 3). In these three regions of observed significant difference, probands exhibited hypogyria i.e., smaller LGI values. Probands also showed trending hypogyr a comparedito controls in the leftposterior cingulate (p<0.06; d=0.15; Figure 1; Table 3).
Figure 1.
Effect sizes for regional contrasts demonstrating patient hypogyria
Table 3.
Descriptive and comparative statistics for Local Gyrification Index (LGI) in regions of significant and trending proband hypogyria
| Large Region | Region | Mean LGI (SE)a | Effect sizes for contrasts to controls Cohen's d | |||||
|---|---|---|---|---|---|---|---|---|
| Controls (HC) n=243 | All Relatives (Rel) n=300 | Axis IIA Relatives (AxIIA) n=33 | Probands (Prob) n=388 | |||||
| HC-Rel | HC-AxIIA | HC-Prob | ||||||
| Cingulate | Left Caudal Anterior Cingulate | 1.73 (0.01) | 1.72 (0.01) | 1.70 (0.02) | 1.71 (0.01) | 0.087 | 0.221 | 0.175** |
| Left Posterior Cingulate | 1.77 (0.01) | 1.76 (0.01) | 1.76 (0.02) | 1.74 (0.01) | 0.017 | 0.074 | 0.146$ | |
| Right Caudal Anterior Cingulate | 1.78 (0.01) | 1.78 (0.01) | 1.77 (0.02) | 1.76 (0.01) | 0.022 | 0.155 | 0.187** | |
| Right Posterior Cingulate | 1.81 (0.01) | 1.82 (0.01) | 1.80 (0.02) | 1.79 (0.01) | −0.010 | 0.146 | 0.179* | |
| Composite | 1.78 (0.01) | 1.78 (0.01) | 1.76 (0.02) | 1.76 (0.01) | 0.020 | 0.247$ | 0.130* | |
Values adjusted for age, sex, site, race, lithium usage, and intracranial volume
p < 0.06
p<0.05
p<0.01
*** p<0.001 (All p-values reflect Benjamini-Hochberg adjustment)
No significant correlations were found in probands between any clinical measure (PANSS positive, PANSS negative, MADRS, or YMRS) and LGI values in the four regions of significant or trending observed probandihypogyria.
Noisignificant differences with controls were found in LGI values of all relatives or relatives with axis II cluster A disorders. However, in all four regions of significant or trending observed proband hypogyria, mean LGI values were non-significantly smaller in axis II cluster A relatives compared with controls (p>0.12, d=0.07-0.22; Table 3). Over these three regions of observed proband hypogyria, relatives with axis II cluster A disorders exhibited nearly significant reductions in composite LGI score compared with controls (p<0.051; d=0.25; Table 3).
Familiality estimates for LGI were modest but significant in 43 of 68 brain regions. Familiality estimates were significant in all four regions of observed significant or trending proband hypogyria (p<0.05), with h 2R values ranging from 0.26 in the right posterior cingulate to 0.45 in the left posterior cingulate (Table 4).
Table 4.
Heritability values (h2R) for regions of observed significant or trending proband hypogyria
| Region | h2R (SE)a | p |
|---|---|---|
| Left Caudal Anterior Cingulate | 0.33 (0.13) | 0.006** |
| Left Posterior Cingulate | 0.45 (0.13) | 0.0004*** |
| Right Caudal Anterior Cingulate | 0.31 (0.14) | 0.02* |
| Right Posterior Cingulate | 0.26 (0.15) | 0.04* |
Heritability values calculated with a maximum likelihood method in Sequential Oligogenic Linkage Analysis Routines (SOLAR) version 6.2 (68).
p<0.05
p<0.01
p<0.001 (All p-values reflect Benjamini-Hochberg adjustment)
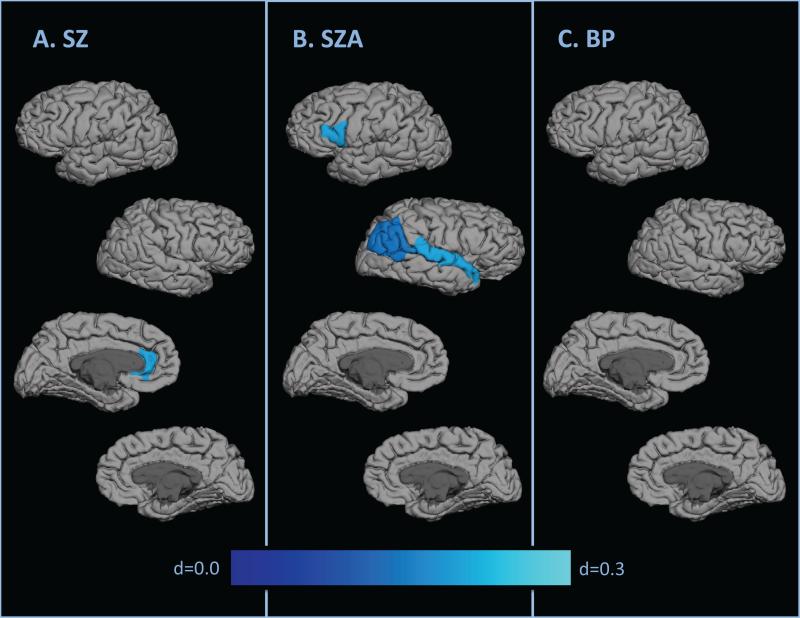
Compared to controls, significant hypogyria was found in the left rostral anterior cingulate for SZ (p<0.01, d=0.26; Figure 2a, Table 5), left parsopercularis, right inferior parietal, right banks of the superior temporal sulcus, and right superior temporal for SZA (p<0.05, d=0.19-0.27; Figure 2b, Table 5), and no regions for BP (Figure 2c). No significant results were found in the direct SZ-BP comparisons.
Figure 2.
Effect sizes for regional contrasts demonstrating hypogyria by diagnosis
Table 5.
Descriptive and comparative statistics for LGI in all regions where diagnosis groups exhibited significant hypogyria
| Large Region | Region | Mean LGI (SE)a | Effect sizes for contrasts to controls Cohen's d | |||||
|---|---|---|---|---|---|---|---|---|
| Controls | Schizophrenia (SZ) | Schizo-affective (SZA) | Psychotic Bipolar (BP) | HC-SZ | HC-SZA | HC-BP | ||
| Frontal | Left Pars Opercularis | 3.38 (0.02) | 3.33 (0.02) | 3.27 (0.02) | 3.40 (0.02) | N.S.b | 0.269* | N.S.b |
| Parietal | Right Inferior Parietal | 2.67 (0.01) | 2.64 (0.02) | 2.62 (0.02) | 2.68 (0.02) | N.S.b | 0.195* | N.S.b |
| Temporal | Right Banks STS | 3.23 (0.01) | 3.20 (0.02) | 3.16 (0.02) | 3.24 (0.02) | N.S.b | 0.226* | N.S.b |
| Right Superior Temporal | 2.31 (0.01) | 2.30 (0.02) | 2.25 (0.02) | 2.34 (0.02) | N.S.b | 0.262* | N.S.b | |
| Cingulate | Left Rostral Anterior Cingulate | 1.83 (0.01) | 1.79 (0.01) | 1.82 (0.01) | 1.83 (0.01) | 0.265** | N.S.b | N.S.b |
Values adjusted for sex, age, site, and race
Large region contrast did not justify regional contrast
N.S. – Not Significant
Discussion
In this study, we found that patients with DSM-IV psychotic disorders exhibited significant hypogyria compared with controls in the right pars opercularis, right transverse temporal gyrus, bilateral posterior cingulate, and bilateral caudal anterior cingulate. Statistically trending hypogyria compared with controls was also found in patients' right superior frontal gyrus, right inferior parietal lobe, and left rostral anterior cingulate. The observed patient-control differences were all in the direction of lower patient gyrification. The consistency of this finding of hypogyria is particularly notable amid the mixed findings of hypogyria and hypergyria in previous literature in schizophrenia (Table 1) and in high risk populations (29-31; 75-77). However, this study's findings appear especially robust given its large sample size, which was more than three times the size of the next largest comparable study of which we are aware. These findings are further bolstered by the rigorous use of multiple comparison corrections and the exclusion of potentially confounded data points, such as patients using lithium.
The regions of observed patient hypogyria are among the most recently evolved cortical regions in heteromodal association cortex, which have previously been reported to exhibit developmental abnormalities in schizophrenia (78-80). Patient hypogyria was particularly localized bilaterally in the cingulate, suggesting that psychotic disorders may be characterized by abnormal cingulate connectivity. This observation is consistentwith in vivo imaging (81) and post-mortem data (82) showing reduced gyral complexity in this brain region. They are also corroborated by a broader body ofliterature implicating structural, function, and neurochemical evidence of cingulate alterations in psychotic disorders (83-95). This cingulate dysfunction has been postulated to disrupt the modulation of prefronto-temporal integration in schizophrenia (84). The observed temporal regions ofhypogyria also are consistent with prior observations of similarly localized surface area, symmetry, and folding abnormalities in schizophrenia (96-99).
We investigated patient hypogyria as a candidate endophenotype for psychotic disorder considering the criteria suggested by Gottesman and Gould (5). Our observations lend some support to this possibility. First, abnormal gyrification was observed to be associated with psychotic disorder as patients exhibited significant reductions of gyrification compared to controls in several cortical regions. Second, gyrification was found to be heritable as familiality estimates were significant, albeit modest, for all six regions of patient hypogyria. These findings match prior demonstration of gyrification heritability using the two-dimensional GI measure (17). Third, the lack of significant correlations between patients' gyrification in regions of abnormality and both their positive and their negative symptoms points to gyrification being primarily state-independent. Fourth, unaffected family members with axis II cluster A disorders exhibited a significant reduction in gyrification compared to controls in composite LGI over regions of patient hypogyria. Non-psychotic relatives with axis II clusterAdisorders, are characterized by traits such as schizotypy that may reflect the genetic liability to schizophrenia (50; 51). The small sample size of the axis II cluster A subset may explain the lack of significance in individual regions since the effect sizes of were comparable to those in the patient-control comparisons (Table 3). In the right pars opercularis, the axis II cluster A relatives exhibited markedly reduced gyrification even compared with patients. The similarity in patients' and this relative subset's patterns ofhypogyria suggests possible continuity between axis I and axis II disorders (100-104). Future studies better powered to investigate the gyrification of individuals with axis II cluster A disorders may help inform this line of research.
The second goal of our study was to evaluate how gyrification abnormalities differ across psychotic diagnoses. We found that each psychotic diagnosis exhibited a somewhat non-overlapping profile of gyrification. Schizoaffective disorder patients were observed to have the most widespread deficits compared to controls. Schizophrenia patients demonstrated significant hypogyria compared to controls bilaterally in the cingulate. Psychotic bipolar disorder patients had only one region of significant difference compared to controls. Although the direct BP-SZ comparison yielded no significant results, these findings of disparate gyrification profiles relative to controls may lend some support for the divides between psychotic diagnoses such as schizophrenia, schizoaffective disorder, and psychotic bipolar disorder. Particularly, the more widespread hypogyria in schizoaffective disorder and schizophrenia than in bipolar disorder suggests that hypogyria may accompany non-affective psychosis more than primary affective psychosis. Our results call into question the construct of the schizoaffective disorder diagnosis. Surprisingly, rather than appearing as a disorder intermediate to schizophrenia and bipolar disorder, schizoaffective disorder appeared to exhibit a pronounced profile of hypogyria. It may be that schizophrenia and affective disorder related genetic factors that may be enriched in this population could interact to increase the likelihood of altered gyrification.
Elucidation of brain structural measures such as LGI may also help further understand the etiopathology of psychotic disorders. Schizophrenia and related psychotic disorders are now widely held to have neurodevelopmental origins, and genes involved in neurodevelopmental processes that could impact on gyrification are being increasingly implicated. For example, genes involved in neuronal adhesion and axonal elongation, such as cadherins and neuregulin have been implicated in schizophrenia and bipolar disorders. Interestingly, a recent candidate gene study showed an association in schizophrenia between polymorphisms of protocadherin 12 (PCDH12), a cell adhesion molecule involved in axonal guidance and synaptic specificity, and cortical folding (105). These leads need confirmation in larger genome-wide association studies.
Despite this study's strengths in its novelty, size, using of a whole brain-based three dimensional approach, and methodological rigor, certain limitations may constrain the generalizability of these findings. The inclusion criteria for probands, including the need for the presence of a family member willing and able to participate and cooperation with the demands of participating in a rigorous research study, may limit the sample representativeness. The somewhat higher number of exclusions of proband scans with artifacts may also limit sample representativeness. Although current lithium usage was included as a covariate and there were no effects of current antipsychotics on LGI, the possible effect of medication confounds cannot be entirely ruled out, as cumulative usage of lithium and antipsychotics was not recorded in this study. Also, the cross-sectional nature of this study precludes the possibility of investigating disease trajectories, which may be studied by instead employing longitudinal data.
Overall, our findings notably indicate that psychotic disorders are characterized by hypogyria, particularly localized in the cingulate. They also suggest that hypogyria may be a structural endophenotype marking clinical and familial risk for psychotic illness across schizophrenia, schizoaffective, and bipolar diagnostic categories. Hypogyria may thus provide an intermediate link in the pathway between psychotic disorders' genetic underpinnings and their clinical syndromes. Given this etiological foothold, next steps should include determining the genes associated with patient hypogyria. Hypogyria and its underlying genes may serve as potential means towards drawing more biologically rooted diagnostic boundaries between psychotic disorders, producing more accurate diagnoses, and identifying possible targets for treatment and intervention.
Supplementary Material
Acknowledgements
This work was supported in part by NIMH grants MH078113, MH077945, MH077852, MH077851, MH077862, MH072767, and MH083888. The authors thank Dr. Shaun Eack, Dr. Gunvant Thaker, Dr. Melvin McKinnis, and Dr. Nash Boutros for their collaboration in the design and implementation of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Dr. Keshavan has received research support from Sunovion and GlaxoSmithKline.
Dr. Tamminga has the following disclosures to make: Intracellular Therapies (ITI, Inc.)—Advisory Board, drug development; PureTech Ventures—Ad Hoc Consultant; Eli Lilly Pharmaceuticals—Ad Hoc Consultant; Sunovion—Ad Hoc Consultant; Astellas—Ad Hoc Consultant; Cypress Bioscience—Ad Hoc Consultant; Merck—Ad Hoc Consultant; International Congress on Schizophrenia Research—Organizer, unpaid volunteer; National Alliance on Mental Illness—Council Member, unpaid volunteer; American Psychiatric Association—Deputy Editor.
Dr. Pearlson has served on an advisory panel for Bristol-Myers Squibb. Dr. Sweeney has been on advisory boards for Bristol-Myers Squibb, Eli Lilly, Pfizer, Roche, and Takeda and has received grant support from Janssen.
The other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Thaker GK. Endophenotypic Studies in Schizophrenia: Promise and Challenges. Schizophrenia Bull. 2006;33:1–2. doi: 10.1093/schbul/sbl062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen AJ, Griss ME, Folley BS, Hawkins KA, Pearlson GD. Endophenotypes in schizophrenia: A selective review. Schizophr Res. 2009;109 doi: 10.1016/j.schres.2009.01.016. NIH Public Access 24-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preston GA, Weinberger DR. Dialogues Clin Neurosci. Vol. 7. Les Laboratoires Servier; 2005. Intermediate phenotypes in schizophrenia: a selective review. p. 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiser M, van Os J, Davidson M. Time for a shift in focus in schizophrenia: from narrow phenotypes to broad endophenotypes. BritJofPsychiat. 2005;187:203–205. doi: 10.1192/bjp.187.3.203. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman II, Gould TD. The Endophenotype Concept in Psychiatry: Etymology and Strategic Intentions. AmJPsychiat. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 6.Bearden CE, Freimer NB. Endophenotypes for psychiatric disorders: ready for primetime? Trends Gen. 2006;22:306–313. doi: 10.1016/j.tig.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Kendler KS, Neale MC. Mol Psychiatr. Vol. 15. Nature Publishing Group; 2010. Endophenotype: a conceptual analysis. pp. 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White TrGottesman I. Brain Connectivity and Gyrification as Endophenotypes for Schizophrenia: Weight ofthe Evidence. Curr Top Med Chem. Vol. 12. Bentham Science Publishers; 2012. pp. 2393–2403. [DOI] [PubMed] [Google Scholar]
- 9.Friston KF, Frith CD. Schizophrenia: A Disconnection Syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 10.Galinowski A. Linking Mind and Brain in the Study of Mental Illnesses: A Project for a Scientific Psychopathology. Science. 1997;275:1586–1593. doi: 10.1126/science.275.5306.1586. [DOI] [PubMed] [Google Scholar]
- 11.Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. NeuroImage. 2012;62:2296–2314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- 12.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 13.Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- 14.Hilgetag CC, Barbas H. AnatEmbryol. Vol. 210. Springer-Verlag; 2005. Developmental mechanics ofthe primate cerebral cortex. pp. 411–417. [DOI] [PubMed] [Google Scholar]
- 15.White T, Su S, Schmidt M, Kao C-Y, Sapiro G. The development of gyrification in childhood and adolescence. Brain Cognition. 2010;72:36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White T, Hilgetag CC. Gyrification and neural connectivity in schizophrenia. DevPsychopathol. 2011;23:339–352. doi: 10.1017/S0954579410000842. [DOI] [PubMed] [Google Scholar]
- 17.Rogers J, Kochunov P, Zilles K, Shelledy W, Lancaster J, Thompson P, et al. On the genetic architecture of cortical folding and brain volume in primates. NeuroImage. 2010;53:1103–1108. doi: 10.1016/j.neuroimage.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penttilä J, Paillére-Martinot M-L, Martinot J-L, Mangin J-F, Burke L, Corrigall R, et al. Global and temporal cortical folding in patients with early-onset schizophrenia. J Am Acad Child Psy. 2008;47:1125–1132. doi: 10.1097/CHI.0b013e3181825aa7. [DOI] [PubMed] [Google Scholar]
- 19.Schultz CC, Koch K, Wagner G, Roebel M, Nenadic I, Gaser C, et al. Increased parahippocampal and lingual gyrification in first-episode schizophrenia. Schizophr Res. 2010;123:137–144. doi: 10.1016/j.schres.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. Folding of the prefrontal cortex in schizophrenia: regional differences in gyrification. Biol Psychiat. 2011;69:974–979. doi: 10.1016/j.biopsych.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Palaniyappan L, Liddle PF. Aberrant cortical gyrification in schizophrenia: a surface-based morphometry study. J Psychiatr Neurosci. 2012;37:399–406. doi: 10.1503/jpn.110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palaniyappan L, Liddle PF. Differential effects of surface area, gyrification and cortical thickness on voxel based morphometric deficits in schizophrenia. NeuroImage. 2012;60:693–699. doi: 10.1016/j.neuroimage.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 23.Palaniyappan L, Liddle PF. Dissociable morphometric differences ofthe inferior parietal lobule in schizophrenia. EurArch Psy Clin N. 2012;262:579–587. doi: 10.1007/s00406-012-0314-y. [DOI] [PubMed] [Google Scholar]
- 24.Palaniyappan L, Liddle PF. Diagnostic Discontinuity in Psychosis: A Combined Study of Cortical Gyrification and Functional Connectivity. Schizophrenia Bull. 2013 doi: 10.1093/schbul/sbt050. doi: 10.1093/schbul/sbt050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palaniyappan L, Crow TJ, Hough M, Voets NL, Liddle PF, James S, et al. Gyrification of Broca's region is anomalously lateralized at onset of schizophrenia in adolescence and regresses at 2 year follow-up. Schizophr Res. 2013;147:39–45. doi: 10.1016/j.schres.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Bartholomeusz CF, Whittle SL, Montague A, Ansell B, McGorry PD, Velakoulis D, et al. Sulcogyral patterns and morphological abnormalities ofthe orbitofrontal cortex in psychosis. Prog Neuro-Psychopha. 2013;44C:168–177. doi: 10.1016/j.pnpbp.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Schultz CC, Wagner G, Koch K, Gaser C, Roebel M, Schachtzabel C, et al. The visual cortex in schizophrenia: alterations of gyrification rather than cortical thickness--a combined cortical shape analysis. Brain Struct Funct. 2013;218:51–58. doi: 10.1007/s00429-011-0374-1. [DOI] [PubMed] [Google Scholar]
- 28.Tepest R, Schwarzbach CJ, Krug B, Klosterkotter J, Ruhrmann S, Vogeley K. Morphometry of structural disconnectivity indicators in subjects at risk and in age-matched patients with schizophrenia. Eur Arch Psy Clin N. 2013;263:15–24. doi: 10.1007/s00406-012-0343-6. [DOI] [PubMed] [Google Scholar]
- 29.Jou RJ, Hardan AY, Keshavan MS. Reduced cortical folding in individuals at high risk for schizophrenia: a pilot study. Schizophr Res. 2005;75:309–313. doi: 10.1016/j.schres.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Falkai P, Honer WG, Kamer T, Dustert S, Vogeley K, Schneider-Axmann T, et al. Disturbed frontal gyrification within families affected with schizophrenia. J Psychiatr Res. 2007;41:805–813. doi: 10.1016/j.jpsychires.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 31.Dauvermann MR, Mukherjee P, Moorhead WT, Stanfield AC, Fusar-Poli P, Lawrie SM, Whalley HC. Relationship between gyrification and functional connectivity of the prefrontal cortex in subjects at high genetic risk of schizophrenia. CurrPharm Des. 2012;18:434–442. doi: 10.2174/138161212799316235. [DOI] [PubMed] [Google Scholar]
- 32.Penttila J, Cachia A, Martinot J-L, Ringuenet D, Wessa M, Houenou J, et al. Cortical folding difference between patients with early-onset and patients with intermediate-onsetbipolar disorder. Bipolar Disord. 2009;11:361–370. doi: 10.1111/j.1399-5618.2009.00683.x. [DOI] [PubMed] [Google Scholar]
- 33.Penttila J, Paillere-Martinot M-L, Martinot J-L, Ringuenet D, Wessa M, Houenou J, et al. J Psychiatr Neurosci. Vol. 34. Canadian Medical Association; 2009. Cortical folding in patients with bipolar disorder or unipolar depression. p. 127. [PMC free article] [PubMed] [Google Scholar]
- 34.McIntosh AM, Moorhead TWJ, McKirdy J, Hall J, Sussmann JED, Stanfield AC, et al. Prefrontal gyral folding and its cognitive correlates in bipolar disorder and schizophrenia. Acta Psychiat Scand. 2009;119:192–198. doi: 10.1111/j.1600-0447.2008.01286.x. [DOI] [PubMed] [Google Scholar]
- 35.Liao Y-L, Sun Y-N, Hsieh J-C, Su T-P, Guo W-Y, Wu Y-T. Cortical complexity analysis of patients with bipolar disorder using three-dimensional gyrification index. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:3933–3936. doi: 10.1109/IEMBS.2008.4650070. [DOI] [PubMed] [Google Scholar]
- 36.Mirakhur A, Moorhead TWJ, Stanfield AC, McKirdy J, Sussmann JED, Hall J, et al. Changes in gyrification over 4 years in bipolar disorder and their association with the brain-derived neurotrophic factor valine(66) methionine variant. Biol Psychiat. 2009;66:293–297. doi: 10.1016/j.biopsych.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Janssen J, Reig S, Aleman Y, Schnack H, Udias JM, Parellada M, et al. Gyral and sulcal cortical thinning in adolescents with first episode early-onset psychosis. Biol Psychiat. 2009;66:1047–1054. doi: 10.1016/j.biopsych.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Haukvik UK, Schaer M, Nesvag R, McNeil T, Hartberg CB, Jonsson EG, et al. Cortical folding in Broca's area relates to obstetric complications in schizophrenia patients and healthy controls. Psychol Med. 2012;42:1329–1337. doi: 10.1017/S0033291711002315. [DOI] [PubMed] [Google Scholar]
- 39.Palaniyappan L, Liddle P. Aberrant cortical gyrification in schizophrenia: a surface-based morphometry study. J Psychiatr Neurosci. 2012;37:399–406. doi: 10.1503/jpn.110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ronan L, Voets NL, Hough M, Mackay C, Roberts N, Suckling J, et al. Consistency and interpretation of changes in millimeter-scale cortical intrinsic curvature across three independent datasets in schizophrenia. NeuroImage. 2012;63:611–621. doi: 10.1016/j.neuroimage.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosen C, Marvin R, Reilly JL, Deleon O, Harris MSH, Keedy SK, et al. Phenomenology of first-episode psychosis in schizophrenia, bipolar disorder, and unipolar depression: a comparative analysis. Clin Schizophr Relat Psychoses. 2012;6:145–151. [PubMed] [Google Scholar]
- 42.Rice J. Arch Gen Psych. Vol. 44. American Medical Association; 1987. The Familial Transmission of Bipolar Illness. pp. 441–447. [DOI] [PubMed] [Google Scholar]
- 43.Kendler KS, Karkowski LM, Walsh D. The structure of psychosis: latent class analysis of probands from the Roscommon Family Study. Arch Gen Psych. 1998;55:492–499. doi: 10.1001/archpsyc.55.6.492. [DOI] [PubMed] [Google Scholar]
- 44.Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laursen TM, Agerbo E, Pedersen CB. Bipolar disorder, schizoaffective disorder, and schizophrenia overlap: a new comorbidity index. J Clin Psychiat. 2009;70:1432–1438. doi: 10.4088/JCP.08m04807. [DOI] [PubMed] [Google Scholar]
- 46.Badner JA, Gershon ES. Meta-analysis ofwhole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatr. 2002;7:405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- 47.Craddock N. Genes for Schizophrenia and Bipolar Disorder? Implications for Psychiatric Nosology. Schizophrenia Bull. 2005;32:9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, et al. Nature. Vol. 460. Nature Publishing Group; 2009. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. pp. 748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keshavan MS, Clementz BA, Pearlson GD. Reimagining psychoses: An agnostic approach to diagnosis. Schizophr Res. 2013;146:10–16. doi: 10.1016/j.schres.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 50.Stefanis NC, Trikalinos TA, Avramopoulos D. Impact schizophrenia candidate genes on schizotypy and cognitive endophenotypes at the population level. Biol Psychiat. 2007;62:784–792. doi: 10.1016/j.biopsych.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 51.Tarbox SI, Pogue-Geile MF. A multivariate perspefciveon schizotypy and familial association with schizophrenia: a review. Clin PsycholRev. 2011;31:1169–1182. doi: 10.1016/j.cpr.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore GJ, Bebchuk JM, Wilds IB, Chen G, Menji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- 53.Sassi RB, Nicoletti M, Brambilla P, Mallinger AG, Bellivier F, Kupfer DJ, et al. Increased gray matter volume in lithium-treated bipolar disorder patients. Neurosci Lett. 2002;329:243–245. doi: 10.1016/s0304-3940(02)00615-8. [DOI] [PubMed] [Google Scholar]
- 54.Scherk H, Falkai P. Effects of antipsychotics on brain structure. Curr Opin Psychiatr. 2006;19:145–150. doi: 10.1097/01.yco.0000214339.06507.d8. [DOI] [PubMed] [Google Scholar]
- 55.Yucel K, Taylor VH, McKinnon MC, MacDonald K, Alda M, Young LT, MacQueen GM. Bilateral Hippocampal Volume Increase in Patients with Bipolar Disorder and Short-term Lithium Treatment. Neuropsychopharmacol. 2007;33:361–367. doi: 10.1038/sj.npp.1301405. [DOI] [PubMed] [Google Scholar]
- 56.Foland LC, Altshuler LL, Sugar CA, Lee AD, Leow AD, Townsend J, et al. Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuro Report. 2008;19:221–224. doi: 10.1097/WNR.0b013e3282f48108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore GJ, Gortese BM, Glitz DA, Zajac-Benitez C, Quiroz JA, Uhde TW, et al. A Longitudinal Study of the Effects of Lithium Treatment on Prefrontal and Subgenual Prefrontal Gray Matter Volume in Treatment-Responsive Bipolar Disorder Patients. J Clin Psychiat. 2009;70:699–705. doi: 10.4088/JCP.07m03745. [DOI] [PubMed] [Google Scholar]
- 58.Lyoo IK, Dager SR, Kim JE, Yoon SJ, Friedman SD, Dunner DL, Renshaw PF. Lithium-Induced Gray Matter Volume Increase As a Neural Correlate of Treatment Response in Bipolar Disorder: A Longitudinal Brain Imaging Study. Neuropsychopharmacol. 2010;35:1743–1750. doi: 10.1038/npp.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moncrieff J, Leo J. Psychol Med. Vol. 40. Cambridge University Press; 2010. A systematic review of the effects of antipsychotic drugs on brain volume. pp. 1409–1422. [DOI] [PubMed] [Google Scholar]
- 60.Ho B-C, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Arch Gen Psych. Vol. 68. American Medical Association; 2011. Long-term Antipsychotic Treatment and Brain Volumes: A Longitudinal Study of First-Episode Schizophrenia. pp. 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewis DA. Arch Gen Psych. Vol. 68. American Medical Association; 2011. Antipsychotic Medications and Brain Volume: Do We Have Cause for Concern? pp. 126–127. [DOI] [PubMed] [Google Scholar]
- 62.Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality: SIDP-IV. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- 63.Skudlarski P, Schretlen DJ, Thaker GK, Stevens MC, Keshavan MS, Sweeney JA, et al. Diffusion Tensor Imaging White Matter Endophenotypes in Patients With Schizophrenia or Psychotic Bipolar Disorder and Their Relatives. Am J Psychiat. 2013;170:886–898. doi: 10.1176/appi.ajp.2013.12111448. [DOI] [PubMed] [Google Scholar]
- 64.First MB, Gibbon M. User'sguidefor the structured clinical interviewfor DSM-IVaxis I disorders: SCID-1 clinician version. American Psychiatric Association; Washington, DC: 1997. [Google Scholar]
- 65.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophrenia Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 66.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Brit J of Psychiat. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 67.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. BritJofPsychiat. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 68.Fischl B. FreeSurfer. NeuroImage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 70.Schaer M, Cuadra MB, Tamarit L. A surface-based approach to quantify local cortical gyrification. IEEE TMed Imaging. 2008;27:161–170. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- 71.Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature neuroscience. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in generalpedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilkinson GS, Robertson GJ. Wide RangeAchievement Test 4 Professional Manual. Psychological Assessment Resources; Lutz, FL: 2006. [Google Scholar]
- 74.Hollingshead A de B. Four Factor Index ofSocial Status. 1975 Retrieved from http//elsinore.cis.yale.edu/sociology/yjs/yjs_fall_2011.pdf#page=21.
- 75.Harris JM, Yates S, Miller P, Best JJK, Johnstone EC, Lawrie SM. Gyrification in first-episode schizophrenia: a morphometric study. Biol Psychiat. 2004;55:141–147. doi: 10.1016/s0006-3223(03)00789-3. [DOI] [PubMed] [Google Scholar]
- 76.Harris JM, Whalley H, Yates S, Miller P, Johnstone EC, Lawrie SM. Abnormal cortical folding in high-risk individuals: a predictor of the development of schizophrenia? Biol Psychiat. 2004;56:182–189. doi: 10.1016/j.biopsych.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 77.Harris JM, Moorhead TWJ, Miller P, McIntosh AM, Bonnici HM, Owens DGC, et al. Increased Prefrontal Gyrification in a Large High-Risk Cohort Characterizes Those Who Develop Schizophrenia and Reflects Abnormal Prefrontal Development. BiolPsychiat. 2007;62:722–729. doi: 10.1016/j.biopsych.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 78.Ross CA, Pearlson GD. Schizophrenia, the heteromodal association neocortex and development: potential for a neurogenetic approach. Trends Neurosci. 1996;19:171–176. doi: 10.1016/s0166-2236(96)10022-9. [DOI] [PubMed] [Google Scholar]
- 79.Pearlson GD, Petty RG, Ross CA, Tien AY. Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacol. 1996;14:1–17. doi: 10.1016/S0893-133X(96)80054-6. [DOI] [PubMed] [Google Scholar]
- 80.Cannon TD, Thompson PM, van Erp TGM, Toga AW, Poutanen V-P, Huttunen M, et al. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. P NatlAcadSci USA. 2002;99:3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yotter RA, Nenadic I, Ziegler G, Thompson PM, Gaser C. Local cortical surface complexity maps from spherical harmonic reconstructions. NeuroImage. 2011;56:961–973. doi: 10.1016/j.neuroimage.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 82.Wheeler DG, Harper CG. Localised reductions in gyrification in the posterior cingulate: Schizophrenia and controls. Prog Neuro-Psychopha. 2007;31:319–327. doi: 10.1016/j.pnpbp.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 83.Benes FM. Neurobiological investigations in cingulate cortex of schizophrenic brain. Schizophrenia Bull. 1993;19:537–549. doi: 10.1093/schbul/19.3.537. [DOI] [PubMed] [Google Scholar]
- 84.Fletcher P. Abnormal Cingulate Modulation of Fronto-Temporal Connectivity in Schizophrenia. NeuroImage. 1999;9:337–342. doi: 10.1006/nimg.1998.0411. [DOI] [PubMed] [Google Scholar]
- 85.Bouras C, Kovari E, Hof PR, Riederer BM, Giannakopoulos P. Acta Neuropathol. Vol. 102. Springer-Verlag; 2001. Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. pp. 373–379. [DOI] [PubMed] [Google Scholar]
- 86.Kubicki M, Westin C-F, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiat. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yucel M, Wood SJ, Fornito A, Riffkin J, Velakoulis D, Pantelis C. J Psychiatr Neurosci. Vol. 28. Canadian Medical Association; 2003. Anterior cingulate dysfunction: Implications for psychiatric disorders? p. 350. [PMC free article] [PubMed] [Google Scholar]
- 88.Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation ofthe initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006:1073–1074. 25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 89.Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiat. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 90.Yücel M, Brewer WJ, Harrison BJ, Fornito A, O'Keefe GJ, Olver J, et al. Anterior cingulate activation in antipsychotic-naive first-episode schizophrenia. ActantychiatScand. 2007;115:155–158. doi: 10.1111/j.1600-0447.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- 91.Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, et al. Meta-Analysis of Gray Matter Anomalies in Schizophrenia: Application of Anatomic Likelihood Estimation and Network Analysis. Biol Psychiat. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fornito A, Yucel M, Wood SJ, Bechdolf A, Carter S, Adamson C, et al. Anterior cingulate cortex abnormalities associated with a first psychotic episode in bipolar disorder. BritJ Psychiat. 2009;194:A19–A19. doi: 10.1192/bjp.bp.107.049205. [DOI] [PubMed] [Google Scholar]
- 93.Fornito A, Yucel M, Wood SJ, Adamson C, Velakoulis D, Saling MM, et al. Surface-based morphometry of the anterior cingulate cortex in first episode schizophrenia. Hum Brain Mapp. 2008;29:478–489. doi: 10.1002/hbm.20412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fornito A, Yucel M, Dean B, wood SJ, Pantelis C. Anatomical Abnormalities of the Anterior Cingulate Cortex in Schizophrenia: Bridging the Gap Between Neuroimaging and Neuropathology. Schizophrenia Bull. 2009;35:973–993. doi: 10.1093/schbul/sbn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zalesky A, Fornito A, Seal ML, Cocchi L, Westin C-F, Bullmore ET, et al. Disrupted Axonal Fiber Connectivity in Schizophrenia. Biol Psychiat. 2011;69:80–89. doi: 10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am JPsychiat. 1990;147:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- 97.Petty RG, Barta PE, Pearlson GD. Reversal of asymmetry of the planum temporale in schizophrenia. Am J Psychiat. 1995;152:715–721. doi: 10.1176/ajp.152.5.715. [DOI] [PubMed] [Google Scholar]
- 98.Pearlson GD, Barta PE, Powers RE, Menon RR. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiat. 1997;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- 99.Barta PE, Pearlson GD, Brill LB, Royall R, McGilchrist IK, Pulver AE, et al. Planum temporale asymmetry reversal in schizophrenia: replication and relationship to gray matter abnormalities. Am J Psychiat. 1997;154:661–667. doi: 10.1176/ajp.154.5.661. [DOI] [PubMed] [Google Scholar]
- 100.Krueger RF, Tackett JL. Personality and psychopathology: working toward the bigger picture. J Pers Disord. 2003;17:109–128. doi: 10.1521/pedi.17.2.109.23986. [DOI] [PubMed] [Google Scholar]
- 101.Ruocco AC. Reevaluating the distinction between Axis I and Axis II disorders: the case of borderline personality disorder. J Clin Psychol. 2005;61:1509–1523. doi: 10.1002/jclp.20205. [DOI] [PubMed] [Google Scholar]
- 102.Flanagan E, Blashfield R. Do clinicians see Axis I and Axis II as different kinds of disorders? Compr Psychiat. 2006;47:496–502. doi: 10.1016/j.comppsych.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 103.Livesley WJ, Jang KL. The Behavioral Genetics ofPersonality Disorder. Annu Rev Clin Psychol. 2008;4:247–274. doi: 10.1146/annurev.clinpsy.4.022007.141203. [DOI] [PubMed] [Google Scholar]
- 104.Røysamb E, Kendler KS, Tambs K, Orstavik RE, Neale MC, Aggen SH, et al. The joint structure of DSM-IV Axis I and Axis II disorders. J Abnorm Psychol. 2011;120:198–209. doi: 10.1037/a0021660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gregorio SP, Sallet PC, Do K-A, Lin E, Gattaz WF, Dias-Neto E. Polymorphisms in genes involved in neurodevelopment may be associated with altered brairymSplftlogy in schizophrenia: Preliminary evidence. Psychiat Res. 2009;165:1–9. doi: 10.1016/j.psychres.2007.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.