Abstract
We report the Laser Induced Forward Transfer (LIFT) of antibodies from a liquid donor film onto paper receivers for application as point-of-care diagnostic sensors. To minimise the loss of functionality of the active biomolecules during transfer, a dynamic release layer was employed to shield the biomaterial from direct exposure to the pulsed laser source. Cellulose paper was chosen as the ideal receiver because of its inherent bio-compatibility, liquid transport properties, wide availability and low cost, all of which make it an efficient and suitable platform for point-of-care diagnostic sensors. Both enzyme-tagged and untagged IgG antibodies were LIFT-printed and their functionality was confirmed via a colorimetric enzyme-linked immunosorbent assay. Localisation of the printed antibodies was exhibited, which can allow the creation of complex 2-d patterns such as QR codes or letters for use in a final working device. Finally, a calibration curve was determined that related the intensity of the colour obtained to the concentration of active antibodies to enable quantitative assessment of the device performance. The motivation for this work was to implement a laser-based procedure for manufacturing low-cost, point-of-care diagnostic devices on paper.
INTRODUCTION
The development and applications of point-of-care diagnostic devices have been growing significantly over the last few years for use away from the medical and laboratory infrastructure which is present in a hospital or doctor's surgery.1, 2 These tests should require very small sample volumes (<1 ml) with little or no preparation, and can provide the result much faster than routine lab tests. Additionally, such tests should be implemented and interpreted without any special training or equipment, and can be administered in the home environment, while they are most importantly low-cost to buy and transport. The transfer and immobilisation of biomolecules on the device during its fabrication has a critical role and is usually achieved by spotting, injection, or photolithography.3, 4 The ultimate goal of this study, however, is the development of paper-based diagnostic devices, using a laser-based printing procedure called Laser Induced Forward Transfer (LIFT) as an alternative for the deposition of biomolecules, which enables printing of reagent volumes as low as a few nanolitres.
LIFT is a laser-based direct-write additive technique used for depositing materials from a thin donor film onto a receiver, as shown schematically in Figure 1. The donor film is pre-deposited on a carrier that is transparent to the incident laser light, and photons from the laser source provide the driving force that transfers a small volume of the donor material onto the accepting receiver. The LIFT technique was first proposed by Bohandy et al.5 for deposition of Cu films using an ArF excimer laser, but since then it has been employed for printing a wide range of materials, including metals, oxides, polymers, ceramics, organics as well as biomolecules, for applications as diverse as embedded electronic circuits, integrated photonic devices, and tissue engineering.6, 7, 8, 9, 10 For printing of biological donor materials, the transfer of cells,11, 12, 13, 14, 15, 16 fungi,17 proteins,18, 19, 20, 21 bacteria,22 and DNA23, 24, 25, 26 has already been reported in the literature, where one of the most fundamental requirements is to demonstrate the printing of viable and functional samples, although previous work was not targeted towards point-of-care paper-based diagnostics. This laser-based approach to printing of biomolecules has several inherent advantages; operation in a standard ambient environment allows printing of a wide range of materials with the further possibility of transferring multi-layered 3D structures composed of different materials. In addition, a further flexibility presents itself in the form of precise control of the size and shape of the printed pixels which is possible by controlling the parameters of the incident laser light such as wavelength, pulse duration, energy density, and spatial beam profile.6, 27 Pixelated test patterns in the form of barcodes and QR codes represent an immediate advantage over isolated spot-printing when the outcome of the test results should remain secure or be otherwise encoded until interpreted by trained staff. We have chosen for this work a modified LIFT procedure, referred to as Dynamic Release Layer (DRL-LIFT),28 which employs the use of a sacrificial layer that absorbs the energy of the incident laser pulses and therefore protects the biomolecules from radiation. Materials commonly used as DRLs include gold,29 silver,17 titanium,22, 25 or more cost-effective polymers, such as triazene.12, 28, 30, 31
Figure 1.
Schematic of the LIFT setup for liquid donor transfer.
The use of paper as a platform to build low-cost sensing devices was proposed by Whitesides et al. in 2007, and since then its use for developing such sensing devices is well-documented.1, 32 Paper is readily available in a range of different grades, thicknesses and forms, and has a wide variety of properties such as wettability, porosity, and capability to wick liquid solutions.32 Additional features that make paper very attractive for point-of-care sensors are that liquid flow can occur without the need for external pumps, and the flow rate can also be adjusted by suitable pre-treatment processes.33 Finally, it is easily and inexpensively stored and transported, is biocompatible and can be incinerated for easy and safe disposal after use. With all these intrinsic benefits as well as fulfilling the low-cost requirement, we have chosen paper as the receiver medium onto which the sensor would be fabricated. The present work demonstrates the use of one of the most routinely employed papers—cellulose-based filter paper—as the receiver for our LIFT-printing studies.
In this paper, we present a DRL-LIFT-printing approach that uses an Au DRL to transfer a liquid donor that contains either enzyme-tagged or untagged antibodies. The functionality of the antibodies post-transfer was validated by an indirect colorimetric Enzyme Linked Immunosorbent Assay (ELISA),34 thereby demonstrating a LIFT-printed paper-based ELISA. ELISA is a commonly used method for biochemical analysis that provides high sensitivity and selectivity through enzymatic signal amplification. Paper-based ELISA tests35 allow for quick and reliable diagnostics with minimal reagent volumes and in conjunction with the patterning ability of LIFT should make possible the fabrication of personalised biosensors, and this concept is further described in a later section. Our results demonstrate the first steps towards our final goal of laser-printed, low-cost, point-of-care paper-based diagnostic sensors.
EXPERIMENTAL METHODS
Materials and methods
The untagged antibodies used in the present experiments were purified mouse IgG2a (BD Biosciences, UK, 557353). The enzyme-conjugated antibodies were goat anti-mouse IgG (H + L) (Life Technologies, UK, G21040) tagged with horseradish peroxidase (HRP), an enzyme which has the ability to catalyze the conversion of the chromogenic substrate, 3,3′,5,5′-Tetramethylbenzidine (TMB) (Sigma-Aldrich, T0440), which when oxidised yields a characteristic colour change (to blue) that is easily detectable visually by the human eye or a camera. The blocking solution was >98% lyophilised bovine serum albumin (BSA) (Sigma-Aldrich, A3059) in a 2% concentration of Phosphate Buffered Saline (PBS) (PAA Laboratories, UK, H15-001). The glycerol used for the donor film was >99% pure (Fisher Scientific, G/0600/08) and Tween20 (Sigma-Aldrich, P1379) was used as a surfactant in the “washing” and “blocking” stages in a 0.05% concentration in PBS.
Experimental setup
The schematic in Fig. 1 shows the setup used for these trial LIFT-printing experiments. The laser used was a KrF excimer operating at 248 nm with a repetition rate of 1 Hz, pulse duration of ∼10 ns, and delivering a maximum energy of ∼400 mJ per pulse. This laser was chosen due to its immediate availability and to demonstrate the technique as a proof-of-principle, while other less expensive lasers can be used. An aperture was used to select a smaller central area of the beam, which had an acceptable spatial uniformity, and this was subsequently imaged via a lens with a focal length of 30 cm onto the interface of the fused silica carrier and the donor providing a laser spot area of ∼700 μm × 700 μm, large enough to produce a transfer-printed pixel that could be observed without the aid of an imaging device. The energy density required for printing should exceed the LIFT threshold, which was experimentally determined to be ∼200 mJ/cm2. Two standard manual xyz-axes translational stages were used for positioning the donor and the receiver, which were separately controllable, allowing for LIFT-printing from different areas of the donor onto selected areas of the receiver. The vertical axis of both translation stages was adjusted such that the plane of the donor was set at the image plane of the aperture and the receiver was set at a separation of ∼100 μm from the donor surface.
Preparation of the donor
A 1 mm thick fused silica carrier, pre-cleaned using a standard solvent cleaning procedure, was then coated with a 25 nm Au film using e-beam evaporation, and this acted as the DRL. In order to improve the hydrophilicity, and hence adhesion, the Au DRL film was treated with an O2 plasma for 20 s. A liquid donor film, consisting of a glycerol–PBS solution (80% v/v) was then formed on the carrier by containing it in a square well using tape (Fig. 2) or by using a wire coater (Gardco, US). In the first case, the addition of the donor solution near one edge of the predefined well was followed by spreading across the well by a glass slide to remove the excess liquid donor and thereby form a film. Alternatively, about 200 μl of the solution was pipetted on the carrier and spread with the wire coater whilst removing any excess liquid, leading to the formation of a ∼65 μm thick film. The uniformity and reproducibility of the film's thickness using the wire coater method proved to be better, and hence was the selected approach. The antibodies, either the secondary (enzyme-tagged anti-mouse IgG) or the primary (untagged mouse IgG), were incorporated in this film in concentrations ranging from ng/ml to μg/ml, prior to the forming of the film on the carrier.
Figure 2.
Image showing a glycerol donor film on a fused silica carrier after LIFT-printing of specific pixels. The donor film is transparent and spread across the whole area of the carrier, while being confined by the surrounding tape-window.
The receiver was Whatman grade 1 filter paper. Nitrocellulose paper, which exhibits much higher hydrophobicity and results in localisation and immobilisation of antibodies and other biomolecules, is an alternative material in many point-of-care sensors.32 Our results show that similar attributes are observed for LIFT-printing on simple cellulose paper, where antibodies also exhibited high localisation within the LIFT-printed areas. Indeed it was observed that the glycerol was absorbed in the cellulose paper faster and with greater efficiency than in the nitrocellulose paper, for which the droplets were not completely absorbed within the volume of the paper, but are adsorbed on its surface with excess liquid evaporating over time as the paper is left to dry under ambient lab conditions. Since we could implement the ELISA on a cellulose paper, the more expensive nitrocellulose was not trialled as a receiver in our experiments.
RESULTS AND DISCUSSION
Two sets of LIFT-printing experiments are presented here. The first included the direct LIFT-printing of enzyme-tagged antibodies on paper. In order to show the functionality of the antibody-HRP enzyme conjugate post printing, it is necessary that a change in colour (from white in the absence of HRP to blue in the presence of HRP) is observed at the LIFT-printed positions on paper as a consequence of the presence of the enzyme that induces the colour change on interaction with the chromogenic substrate. This would thus confirm the retention of the biological and chemical properties of the printed antibodies after LIFT-printing. The second set of experiments involved LIFT-printing of untagged antibodies on paper and the subsequent detection of these antibodies via an indirect ELISA test, which provides proof of their immunological functionality and stability following the LIFT-printing process.
Different concentrations, ranging from 50 ng/ml to 500 ng/ml, of the HRP-conjugated antibodies were incorporated into the donor films and pixels were LIFT-printed onto cellulose paper. The volume of each deposited pixel is approximately 35 nl, as calculated by the volume of the donor film that is removed from the carrier. The LIFT-printed pixels were left to dry for ∼10 min, and the chromogenic substrate TMB was added in order to induce a colour change, that would confirm that the antibodies retain their functionality post-LIFT-printing. These initial tests were designed to demonstrate the feasibility of LIFT-printing antibodies and to determine the lowest concentration that can provide a sufficiently intense colour change that is detectable with the human eye or a simple mobile phone camera, the only two options considered to be convenient and beneficial for point-of-care delivery. Spectrophotometry or other more sophisticated and instrument-intensive detection schemes were therefore not used for the functionality testing procedures as our future aim is to develop a device that operates outside normal medical facilities such as hospitals and furthermore can be read by untrained non-medical personnel/individuals. It was observed that a concentration of the HRP-conjugated antibodies, lower than 50 ng/ml in PBS represented the threshold at which detection could still be achieved. Concentrations of antibodies lower than this did not induce a colour change intense enough to be observable.
The colour intensities of the LIFT-printed pixels were quantified by recording the digital images with a simple 1.3 megapixel USB camera under ambient laboratory lighting conditions, followed by analysis of the images using a customised MATLAB program. This program uses a 256-bit colour scale where black corresponds to a colour intensity of 255 and white to a colour intensity of zero. Each image is also represented by three values which correspond to red, green, and blue colour components in the image. As seen in Fig. 3, the colour intensities of the LIFT-printed pixels of HRP-conjugated IgG were measured and plotted to create a calibration curve for known concentrations. The same concentrations of the HRP-conjugated antibodies were pipetted on the paper receiver, and their images were captured and analysed to create the calibration curve of the pipetted IgG and identify any loss of antibodies during the LIFT process. The background colour of the paper receiver was deducted from the colour intensity of the LIFT-printed pixels, to improve the signal-to-noise ratio. For a commercial application, however, a standard colour-map printed adjacent to the test can be used to implement the subtract-and-compare measurement. Results indicated that the colour intensity of the LIFT-printed antibodies was reduced by between 2%–4% (when normalised to the full scale of 0–100) compared to the colour intensity of the antibodies which have just been pipetted. This decrease of the colour intensity can likely be attributed to the denaturing of antibodies during transfer due to the heating induced by the laser irradiation. Similar decrease in the functionality of the LIFT-printed biomaterial has been reported in literature, however, these reports describe a reduction in the viability of LIFT-printed cells, and this was found to be dependent on the laser fluence.15
Figure 3.
Plot of the colour intensity of different concentrations of LIFT-printed HRP-conjugated IgG and of pipetted HRP-conjugated IgG along with their linear interpolation curves.
LIFT-printing in patterns and barcodes
One of the important properties of direct-write laser processing is that it allows for precise control of the size and shape of both individual printed pixels, as well as the resultant multiple-pixel patterns that can be printed, which can be varied on an individual basis for specific tests on targeted recipients. Figs. 4a, 4b show the results for a simplistic LIFT-printed trial barcode, which further establishes the flexibility inherent to such a laser-based direct write procedure. The bar code patterns were revealed by addition of the chromogenic substrate TMB onto the paper after LIFT-printing of the enzyme-tagged antibody to form the desired pattern. Printing the biomolecules in a shape of a real full-size barcode or QR code would allow additional information to be added to the test, such as patient-specific details, extra controls such as test duplication, or encoding of the result where a positive diagnosis might otherwise produce unintentional patient anxiety (such as in cancer disease monitoring). In practice, for actual implementation of such tests, barcodes can also identify a specific patient, so that by scanning and sending the test results with a mobile phone camera to the intended recipient, the healthcare professionals can rapidly be alerted of the patient's condition. Thus, in the case of a virus infection, information about their geographical location (via the GPS feature inherent to modern smartphone devices) would enable rapid vectorial mapping of the spread of the virus. For simple tests where secrecy or patient confidentiality was not an issue, word patterns36 such as “yes/no” would facilitate immediate read-out by the patient or person taking the test. Figs. 4c, 4d show such LIFT-printed “yes/no” patterns that have been produced by addition of the chromogenic substrate TMB onto the paper after LIFT-printing the enzyme-tagged secondary antibody to form the desired word patterns.
Figure 4.
LIFT-printed pixels of HRP-conjugated anti-mouse IgG in the form of a QR code, (a) before the detection with the chromogenic substrate TMB and after developing with TMB in the form of (b) a 2-d trial barcode QR code, shown as a black/white pattern in the inset, (c) “yes” and (d) “no.”
LIFT-printed paper-based enzyme linked immunosorbent assay
The second set of experiments involved the LIFT-printing of untagged antibodies from a glycerol donor film onto cellulose paper receivers, and the subsequent validation of their chemical and biological functionality through an ELISA. An ELISA is a form of analytic biochemical assay that uses enzymes to detect the presence of an antigen, such as a virus or an antibody, and is mainly used as a diagnostic tool in medicine and plant pathology, as well as a quality control in food and environmental industries. Depending on the type of ELISA (indirect, sandwich or competitive) that is being implemented, LIFT-printing of at least one type of antibody specific to the antigen is required. Typically, an ELISA is conducted in a polystyrene multi-well plate, which in this report was replaced by plain cellulose paper. Eventually, the target antigen will be in the biological sample to be tested, and the bioassay components will be LIFT-printed. However, in the development phase, the target, a mouse IgG, is first bound or immobilised on the paper surface via the LIFT-printing step. A non-reacting protein (BSA) is then added onto the paper to block any areas of the paper that the target IgG has not been immobilised on, and thereby preventing non-specific binding during the subsequent step which involves the addition of the secondary enzyme-tagged antibody (HRP-conjugated anti-mouse IgG). This HRP-tagged antibody attaches specifically to the target and on addition of the corresponding chromogenic substrate, TMB, the LIFT-printed pixels turn blue because of oxidation of the substrate by the enzyme.
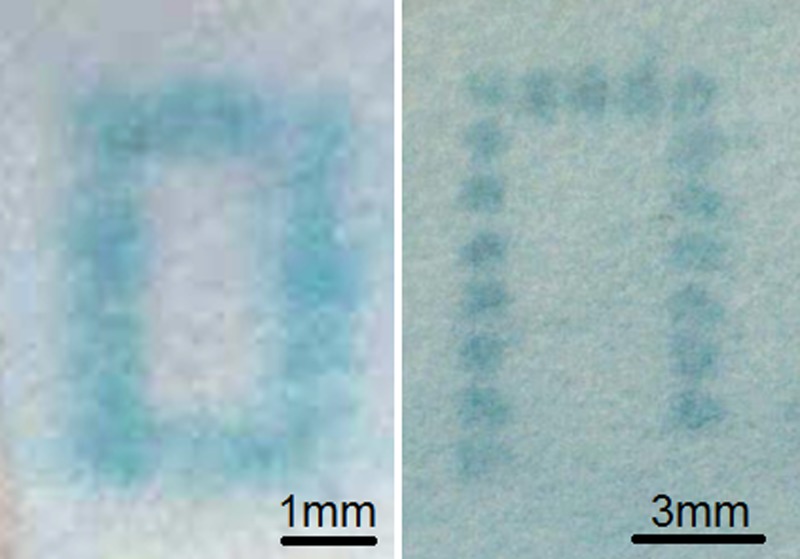
The primary untagged antibodies, in concentrations ranging from 0.1 μg/ml to 5 μg/ml, have been LIFT-printed on the specific areas on the paper and given sufficient time to dry (∼10 min). Subsequently, the paper was blocked with BSA for 1 h at room temperature, to prevent non-specific binding of the antibodies on the paper during the following steps of the ELISA test. Although, there are other blocking solutions, BSA was chosen as it is widely available, are comparatively inexpensive, and commonly used in ELISA tests. We have trialled different blocking concentrations of BSA (1%, 2%, and 5%), and the concentration used was the one found to be optimal in keeping the background to a minimum. The following step involves the tagging of the LIFT-printed antibodies with the enzyme-tagged antibodies (HRP-conjugated anti-mouse IgG). Different concentrations of the secondary tagged antibodies were used to determine the optimum result, defined as the generation of the highest colour intensity with the minimum background signal. A concentration of 50 ng/ml was selected as the optimal value. The paper was immersed in this conjugate solution followed by a washing step to remove any unbound antibodies and other reagents from the paper surface. Washing was repeated three times for five minutes each using a washing solution. The final step involved the addition of the chromogenic substrate TMB which, when combined with the HRP enzymes, produced blue-coloured pixels, highly localised at the LIFT-printed positions as seen in Fig. 5. It is shown that even an individual pixel with a volume of the order of nanolitres can provide a sufficient result, thus minimising the total cost of the test.
Figure 5.
Optical microscopy images of LIFT-printed mouse IgG following the ELISA test, forming continuous lines, and individual pixel arrays.
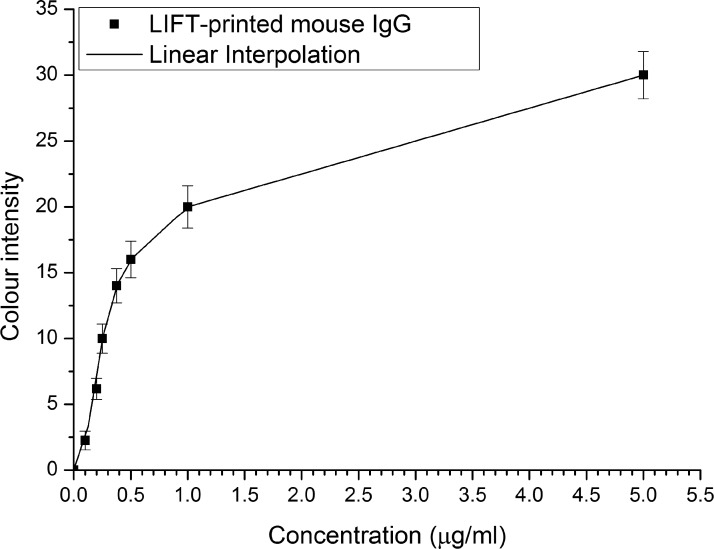
The calibration curve for the paper-ELISA using this particular set of antibodies was obtained as described previously, by capturing images of pixels LIFT-printed with known concentrations of antibodies and subsequently measuring the resulting colour intensities (Fig. 6). The calibration curve would thus allow the determination of unknown concentrations of the target antibodies in a test-sample. It is clear that the blue colour is highly visible after the ELISA has been performed. For a future complete system, however, it is desirable to include a calibration test based on a standard pre-printed blue-coloured control pattern. Current work is designed to establish the optimum and simplest protocol that could be implemented by the individual under test.
Figure 6.
Calibration curve of the mean colour intensity following the ELISA versus the concentration of LIFT-printed antibodies in each pixel.
CONCLUSIONS
Transfer of untagged and enzyme-linked antibodies on paper receivers was performed using Laser Induced Forward Transfer with ns laser pulses and the feasibility of LIFT-printing biomolecules on paper for the development of a paper-based diagnostic sensor was demonstrated. The functionality and immunological reactivity of the LIFT-printed antibodies was confirmed by developing and demonstrating an ELISA protocol and establishing the standard calibration curve for the LIFT-printed pixels. Additionally, it was shown that the localisation of the LIFT-printed pixels and immobilisation of the antibodies, which is a pre-requisite for paper-based diagnostic devices, was maintained throughout the wet-bench process which further justifies our use of the spatial patterning ability of LIFT. This immobilisation of the antibodies through the LIFT-printing process on the paper surface, also allowed the use of plain cellulose paper as opposed to the more expensive nitrocellulose membranes. This will further reduce the final cost and more importantly allow the fabrication of devices that do not need to be confined by hydrophobic barriers in fluidic channels, but can instead work in free-flow conditions. This work demonstrates that LIFT is a technique capable of transferring antibodies onto a paper receiver accurately, reproducibly and with minimal loss of biochemical functionality, and that LIFT represents a promising technology for manufacturing immunologic paper-based point-of-care diagnostic sensors.
The future work of the authors will involve the LIFT-printing of all the necessary reagents on different sites on the paper and the use of lasers to fabricate fluidic channels. Thus, the blocking and washing steps will be automated, making the device easier for an untrained individual to use.
ACKNOWLEDGMENTS
The authors acknowledge the funding received via the EPSRC Grant Nos. EP/J008052/1 and EP/K023454/1. The authors also acknowledge the funding received via the Knowledge Mobilisation Fellowship for Dr. Collin Sones from the Institute for Life Sciences and the Faculty of Health Sciences of the University of Southampton. Saul N. Faust receives funding from the Southampton UK National Institute of Health Research (NIHR) Wellcome Trust Clinical Research Facility and NIHR Respiratory Biomedical Research Unit. Spiros Garbis acknowledges support from the Wessex Medical Trust.
References
- Li X., Ballerini D. R., and Shen W., “ A perspective on paper-based microfluidics: Current status and future trends,” Biomicrofluidics 6, 011301 (2012). 10.1063/1.3687398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A. W., Phillips S. T., and Whitesides G. M., “ Diagnostics for the developing world microfluidic paper-based analytical devices,” Anal. Chem. 82, 3–10 (2010). 10.1021/ac9013989 [DOI] [PubMed] [Google Scholar]
- Barbulovic-Nad I., Lucente M., Sun Y., Zhang M., Wheeler A. R., and Bussmann M., “ Bio-microarray fabrication techniques–A review,” Crit. Rev. Biotechnol. 26, 237–259 (2006). 10.1080/07388550600978358 [DOI] [PubMed] [Google Scholar]
- Blawas A. S. and Reichert W. M., “ Protein patterning,” Biomaterials 19, 595–609 (1998). 10.1016/S0142-9612(97)00218-4 [DOI] [PubMed] [Google Scholar]
- Bohandy J., Kim B. F., and Adrian F. J., “ Metal deposition from a supported metal film using an excimer laser,” J. Appl. Phys. 60, 1538–1539 (1986). 10.1063/1.337287 [DOI] [Google Scholar]
- Arnold C. B., Serra P., and Pique A., “ Laser direct-write techniques for printing of complex materials,” MRS Bull. 32, 23–31 (2007). 10.1557/mrs2007.11 [DOI] [Google Scholar]
- Palla-Papavlu A., Dinca V., Luculescu C., Shaw-Stewart J., Nagel M., Lippert T., and Dinescu M., “ Laser induced forward transfer of soft materials,” J. Opt. 12, 124014 (2010). 10.1088/2040-8978/12/12/124014 [DOI] [Google Scholar]
- Sones C. L., Kaur K. S., Ganguly P., Banks D. P., Ying Y. J., Eason R. W., and Mailis S., “ Laser-induced-forward-transfer: A rapid prototyping tool for fabrication of photonic devices,” Appl. Phys. A 101, 333–338 (2010). 10.1007/s00339-010-5827-5 [DOI] [Google Scholar]
- Stratakis E., Ranella A., Farsari M., and Fotakis C., “ Laser-based micro/nanoengineering for biological applications,” Prog. Quantum Electron. 33, 127–163 (2009). 10.1016/j.pquantelec.2009.06.001 [DOI] [Google Scholar]
- Koch L., Deiwick A., Schlie S., Michael S., Gruene M., Coger V., Zychlinski D., Schambach A., Reimers K., Vogt P. M., and Chichkov B., “ Skin tissue generation by laser cell printing,” Biotechnol. Bioeng. 109, 1855–1863 (2012). 10.1002/bit.24455 [DOI] [PubMed] [Google Scholar]
- Schiele N. R., Corr D. T., Huang Y., Raof N. A., Xie Y., and Chrisey D. B., “ Laser-based direct-write techniques for cell printing,” Biofabrication 2, 032001 (2010). 10.1088/1758-5082/2/3/032001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraiswamy A., Narayan R. J., Lippert T., Urech L., Wokaun A., Nagel M., Hopp B., Dinescu M., Modi R., Auyeung R. C. Y., and Chrisey D. B., “ Excimer laser forward transfer of mammalian cells using a novel triazene absorbing layer,” Appl. Surf. Sci. 252, 4743–4747 (2006). 10.1016/j.apsusc.2005.07.166 [DOI] [Google Scholar]
- Barron J. A., Krizman D. B., and Ringeisen B. R., “ Laser printing of single cells: Statistical analysis, cell viability, and stress,” Ann. Biomed. Eng. 33, 121–130 (2005). 10.1007/s10439-005-8971-x [DOI] [PubMed] [Google Scholar]
- l. Hopp B., Smausz T., Szabó G., Kolozsvári L., Kafetzopoulos D., Fotakis C., and Nógrádi A., “ Femtosecond laser printing of living cells using absorbing film-assisted laser-induced forward transfer,” Opt. Eng. 51, 014302 (2012). 10.1117/1.OE.51.1.014302 [DOI] [Google Scholar]
- Lin Y., Huang Y., and Chrisey D. B., “ Metallic foil-assisted laser cell printing,” J. Biomech. Eng. 133, 025001 (2011). 10.1115/1.4003132 [DOI] [PubMed] [Google Scholar]
- Ringeisen B. R., Kim H., Barron J. A., Krizman D. B., Chrisey D. B., Jackman S., Auyeung R. Y., and Spargo B. J., “ Laser printing of pluripotent embryonal carcinoma cells,” Tissue Eng. 10, 483–491 (2004). 10.1089/107632704323061843 [DOI] [PubMed] [Google Scholar]
- Hopp B., Smausz T., Antal Z., Kresz N., Bor Z., and Chrisey D., “ Absorbing film assisted laser induced forward transfer of fungi (Trichoderma conidia),” J. Appl. Phys. 96, 3478 (2004). 10.1063/1.1782275 [DOI] [Google Scholar]
- Barron J. A., Young H. D., Dlott D. D., Darfler M. M., Krizman D. B., and Ringeisen B. R., “ Printing of protein microarrays via a capillary-free fluid jetting mechanism,” Proteomics 5, 4138–4144 (2005). 10.1002/pmic.200401294 [DOI] [PubMed] [Google Scholar]
- Boutopoulos C., Andreakou P., Kafetzopoulos D., Chatzandroulis S., and Zergioti I., “ Direct laser printing of biotin microarrays on low temperature oxide on Si substrates,” Phys. Status Solidi A 205, 2505–2508 (2008). 10.1002/pssa.200780206 [DOI] [Google Scholar]
- Dinca V., Ranella A., Farsari M., Kafetzopoulos D., Dinescu M., Popescu A., and Fotakis C., “ Quantification of the activity of biomolecules in microarrays obtained by direct laser transfer,” Biomed. Microdevices 10, 719–725 (2008). 10.1007/s10544-008-9183-6 [DOI] [PubMed] [Google Scholar]
- Zergioti I., Karaiskou A., Papazoglou D. G., Fotakis C., Kapsetaki M., and Kafetzopoulos D., “ Femtosecond laser microprinting of biomaterials,” Appl. Phys. Lett. 86, 163902 (2005). 10.1063/1.1906325 [DOI] [Google Scholar]
- Barron J. A., Rosen R., Jones-Meehan J., Spargo B. J., Belkin S., and Ringeisen B. R., “ Biological laser printing of genetically modified Escherichia coli for biosensor applications,” Biosens. Bioelectron. 20, 246–252 (2004). 10.1016/j.bios.2004.01.011 [DOI] [PubMed] [Google Scholar]
- Colina M., Serra P., Fernandez-Pradas J. M., Sevilla L., and Morenza J. L., “ DNA deposition through laser induced forward transfer,” Biosens. Bioelectron. 20, 1638–1642 (2005). 10.1016/j.bios.2004.08.047 [DOI] [PubMed] [Google Scholar]
- Chatzipetrou M., Tsekenis G., Tsouti V., Chatzandroulis S., Thanos D., and Zergioti I., “ Direct laser printing of oligonucleotides for the fabrication of a label-free biosensor,” Procedia Eng. 25, 851–855 (2011). 10.1016/j.proeng.2011.12.209 [DOI] [Google Scholar]
- Fernandez-Pradas J., Colina M., Serra P., Dominguez J., and Morenza J. L., “ Laser-induced forward transfer of biomolecules,” Thin Solid Films 453–454, 27–30 (2004). 10.1016/j.tsf.2003.11.154 [DOI] [Google Scholar]
- Karaiskou A., Zergioti I., Fotakis C., Kapsetaki M., and Kafetzopoulos D., “ Microfabrication of biomaterials by the sub-ps laser-induced forward transfer process,” Appl. Surf. Sci. 208–209, 245–249 (2003). 10.1016/S0169-4332(02)01396-X [DOI] [Google Scholar]
- Arnold C. B. and Piqué A., “ Laser direct-write processing,” MRS Bull. 32, 9–15 (2007). 10.1557/mrs2007.9 [DOI] [Google Scholar]
- Banks D. P., Kaur K., Gazia R., Fardel R., Nagel M., Lippert T., and Eason R. W., “ Triazene photopolymer dynamic release layer-assisted femtosecond laser-induced forward transfer with an active carrier substrate,” EPL 83, 38003 (2008). 10.1209/0295-5075/83/38003 [DOI] [Google Scholar]
- Barron J. A., Wu P., Ladouceur H. D., and Ringeisen B. R., “ Biological laser printing: A novel technique for creating heterogeneous 3-dimensional cell patterns,” Biomed. Microdevices 6, 139–147 (2004). 10.1023/B:BMMD.0000031751.67267.9f [DOI] [PubMed] [Google Scholar]
- Kattamis N. T., Purnick P. E., Weiss R., and Arnold C. B., “ Thick film laser induced forward transfer for deposition of thermally and mechanically sensitive materials,” Appl. Phys. Lett. 91, 171120 (2007). 10.1063/1.2799877 [DOI] [Google Scholar]
- Kaur K. S., Fardel R., May-Smith T. C., Nagel M., Banks D. P., Grivas C., Lippert T., and Eason R. W., “ Shadowgraphic studies of triazene assisted laser-induced forward transfer of ceramic thin films,” J. Appl. Phys. 105, 113119 (2009). 10.1063/1.3132822 [DOI] [Google Scholar]
- Yetisen A. K., Akram M. S., and Lowe C. R., “ Paper-based microfluidic point-of-care diagnostic devices,” Lab Chip 13, 2210–2251 (2013). 10.1039/c3lc50169h [DOI] [PubMed] [Google Scholar]
- Nery E. W. and Kubota L. T., “ Sensing approaches on paper-based devices: a review,” Anal. Bioanal. Chem. 405, 7573–7595 (2013). 10.1007/s00216-013-6911-4 [DOI] [PubMed] [Google Scholar]
- Ngo T. T. and Lenhoff H. M., “ Enzymes as versatile labels and signal amplifiers for monitoring immunochemical reactions,” Mol. Cell. Biochem. 44, 3–12 (1982). 10.1007/BF00573840 [DOI] [PubMed] [Google Scholar]
- Cheng C. M., Martinez A. W., Gong J., Mace C. R., Phillips S. T., Carrilho E., Mirica K. A., and Whitesides G. M., “ Paper-based ELISA,” Angew. Chem. 49, 4771–4774 (2010). 10.1002/anie.201001005 [DOI] [PubMed] [Google Scholar]
- Li M., Tian J., Al-Tamimi M., and Shen W., “ Paper-based blood typing device that reports patient's blood type ‘in writing’,” Angew. Chem. 51, 5497–5501 (2012). 10.1002/anie.201201822 [DOI] [PubMed] [Google Scholar]