Abstract
Natural products extracts are commonly highly complex mixtures of active compounds and consequently their purification becomes a particularly challenging task. The development of a purification protocol to extract a single active component from the many hundreds that are often present in the mixture is something that can take months or even years to achieve, thus it is important for the natural product chemist to have, at their disposal, a broad range of diverse purification techniques. Counter-current chromatography (CCC) is one such separation technique utilising two immiscible phases, one as the stationary phase (retained in a spinning coil by centrifugal forces) and the second as the mobile phase. The method benefits from a number of advantages when compared with the more traditional liquid–solid separation methods, such as no irreversible adsorption, total recovery of the injected sample, minimal tailing of peaks, low risk of sample denaturation, the ability to accept particulates, and a low solvent consumption. The selection of an appropriate two-phase solvent system is critical to the running of CCC since this is both the mobile and the stationary phase of the system. However, this is also by far the most time consuming aspect of the technique and the one that most inhibits its general take-up. In recent years, numerous natural product purifications have been published using CCC from almost every country across the globe. Many of these papers are devoted to terpenoids—one of the most diverse groups. Naturally occurring terpenoids provide opportunities to discover new drugs but many of them are available at very low levels in nature and a huge number of them still remain unexplored. The collective knowledge on performing successful CCC separations of terpenoids has been gathered and reviewed by the authors, in order to create a comprehensive document that will be of great assistance in performing future purifications.
Keywords: Counter-current chromatography, Terpenoids, Terpenes, Natural products, Separation, Purification
Introduction
Terpenoids, also referred to as terpenes, are one of the largest and the most diverse group of natural products accounting for more than 40,000 individual compounds, with several new compounds being discovered every year.
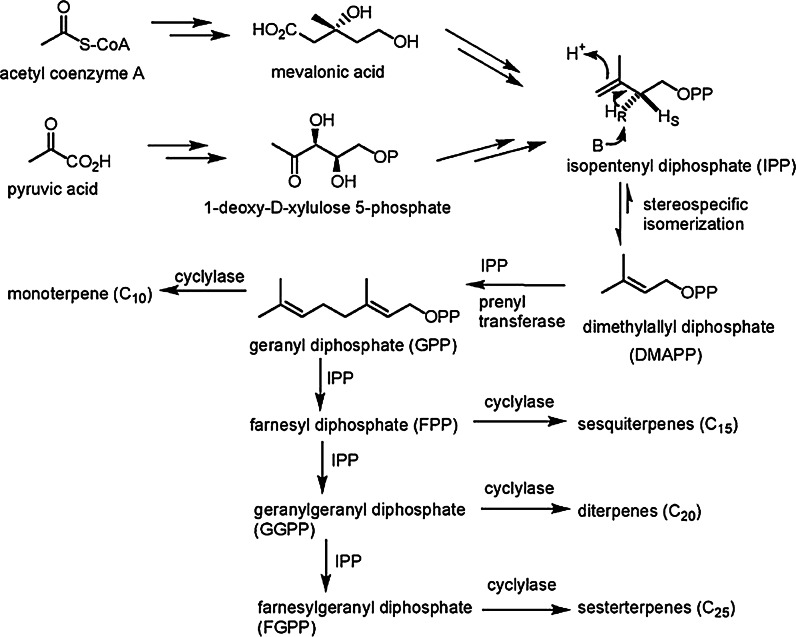
They are synthesized from only two five-carbon isomers: isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). Two biosynthetic routes have been characterized: the classical acetate mevalonate pathway (described in 1967) and the triose phosphate-utilizing non-mevalonate pathway characterized in 2002. Starting from the universal precursors IPP and DMAPP, thousands of enzymes are involved in the biosynthetic pathways for terpenoid chain elongation, cyclization, and functionalization of hydrocarbon chains. The active isoprene unit (IPP) is repetitively added to DMAPP or a prenyl diphosphate in sequential head-to-tail condensations catalyzed by the prenyltrans-ferases. Through consecutive condensations a prenyltransferase can synthesize a variety of products with fixed lengths and stereochemistry (Fig. 1) (Wang et al. 2005; Ajikumar et al. 2008). Based on the number of the building blocks, terpenoids are commonly classified into hemi-, mono-, sesqui-, di-, ses-, tri- and tetraterpenoids (carotenoids) having 1, 2, 3, 4, 5, 6 and 8 isoprenoid residues respectively, and polyterpenes consisting of long chains of many isoprene units (Koch et al. 2008).
Fig. 1.
Biosynthetic pathways of terpenes (Wang et al. 2005)
Terpenoids display a wide range of biological activities. Monoterpenes and sesquiterpenes are the main constituents of essential oils and share responsibility for important properties like antibacterial, antiviral, antioxidant etc. Triterpene saponins—ginsenosides—significantly reduce the production of beta-amyloid which accumulates in the brain of patients with Alzheimer’s disease and play a critical role in pathology by inducing neuronal death. Ginkgolides (cyclic diterpenes of labdane type commonly isolated from Ginkgo biloba) protect neuronal cells from synaptic damage (Yoo and Park 2012). However the antimalarial drug Artemisininn and the anticancer drug paclitaxel (TaxolR) are two renowned terpene-based drugs with established medical applications. Artemisinin, earlier known as Qinghaosu, is a phytoconstituent isolated from Artemisia annua L. and can be described as a compound which possess antimalarial activity. Clinical studies with patients infected with Plasmodium vivax or P. falciparum demonstrated that artemisinin could kill the malarial parasite very quickly at the schizont stage of the parasite’s life cycle (i.e. when it infects the human red blood cell) with no obvious side effects. The molecule has a completely new antimalarial prototype structure with an endoperoxide moiety, which is necessary for activity. Since artemisinin itself has poor bioavailability limiting its effectiveness, several semisynthetic derivatives of artemisinin have been developed (Medhi et al. 2009; Brown 2010). Taxol, a plant diterpenoid widely used as a chemotherapeutic drug against several types of cancer, is known to interact with a specific site of β-tubulin—it binds to microtubules and inhibits their disassembly. Cells treated with taxol are arrested in mitosis and eventually undergo death by apoptosis. This very important activity is strongly depended on its unusual structure. It was shown that the side chain at position C-13 and the taxane ring system are essential for this activity (Xiang et al. 2009).
The purification of natural products is a complex process requiring a comprehensive range of techniques. CCC offers the natural product scientist a different mode of operation to conventional processes. Invented in the mid 1960’s (Ito et al. 1966), to many scientists it is still known as it was back then—a technique that is slow, with separations measuring in hours or days. It was also unreliable as instruments frequently broke down and furthermore had poor capacity with injection amounts measured in tens of milligrams. There was also no opportunity for scale up as the factors required to scale up were poorly understood. However, the technique has been substantially developed since those early days. Advances in engineering and the understanding of the processes involved, particularly in the past 10 years, have created instruments that are fast, robust, permit very high injection loadings and, significantly for the natural products industry, can be rapidly scaled from analytical to pilot level (Sutherland and Fisher 2004). The new generation of coil planet centrifuges operate at higher “g” fields than conventional instruments, enabling higher flow rates to be used so that separation times are measured in minutes rather than hours at the same resolution (Yuan et al. 2008). With a number of important advantages over both solid phase chromatographic techniques and current liquid–liquid extraction techniques, modern high capacity counter-current chromatography is a worthy inclusion in the array of techniques required for natural product purifications.
Theoretical background of CCC
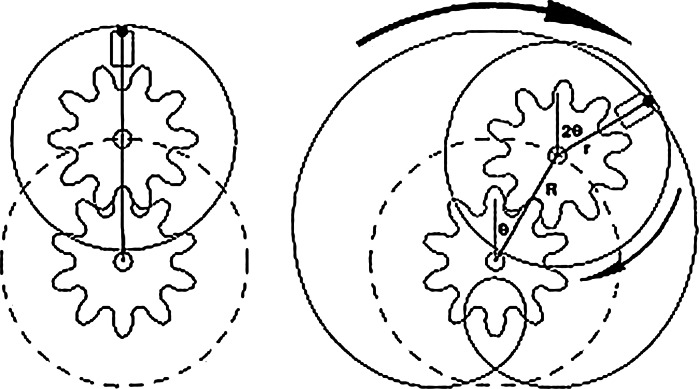
In a counter-current chromatography centrifuge, tubing is wound on a drum which is centrifugally rotated in planetary motion (the holder rotates about its own axis while revolving around the centrifuge axis at the same angular velocity in the same direction) (Fig. 2). A two phase solvent system is introduced into this coil. Although a simple solvent system might consist of hexane and water, a more likely system for a purification would consist of hexane, ethyl acetate, methanol and water or, for biomolecules sensitive to organic solvents, an aqueous two phase system such as aqueous PEG1000 and potassium phosphate salt solution. With the two phase solvent system inside, as the coil travels through its planetary motion cycle, zones of mixing and settling travel along the phases coincident with the low and high accelerations caused by the epicyclic motion of the coil. The mixing zones are coincident with low accelerations and take the form of wave mixing, equivalent to the “swish-swosh” motion that occurs when a tube of liquids is tilted from side to side. The settling zones are coincident with high accelerations and take the form of a smooth interfacial area. There is one mixing and one settling zone per coil loop per revolution. A typical modern analytical CCC instrument may have 40 loops and spin at 2,000 rpm. It will therefore experience 4.8 million partitioning steps per hour (40 × 2,000 × 60). Similarly, a typical preparative instrument may have 30 loops and spin at 1,200 rpm, giving 2.2 million partitioning steps per hour.
Fig. 2.
Motion of the bobbin in the CCC centrifuge (Sutherland et al. 1998)
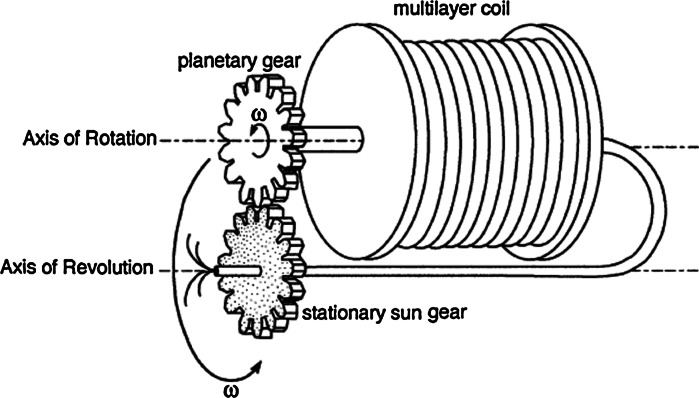
Counter-current chromatography can be achieved not only in the above mentioned hydrodynamic CCC created by two axis of rotation, but also as hydrostatic CCC, typically represented by centrifugal partition chromatography (CPC). This is a single-axis instrument, which has a series of chambers machined circumferentially around a rotor. Rotation of the rotor produces a uniform g-field, which retains the stationary phase in each chamber, while the mobile phase is flowed though in a cascading manner. A CPC instrument requires rotating seals for the mobile phase flow, whereas the hydrodynamic CCC instruments do not. This is because the rotation of the coil about its own axis unwinds the twist produced by its motion around the sun gear, and thus there is no twisting of the flow tubes linking the coil to the pump and the detector (Fig. 3).
Fig. 3.
Type-J planetary motion of a multilayer coil separation column presenting that the column holder rotates about its own axis and revolves around the centrifuge axis at the same angular velocity (ω) in the same direction (Ito 2005)
Solvent selection process
In the past, selection of a suitable two phase solvent system involved a considerable amount of experience and know-how. With aqueous-organic phase systems coming from up to six or more different solvents mixed together, the possibilities were almost limitless. A typical mid-polarity selection table is shown in Table 1 (Garrard 2005). This is a modified version of the table produced by Oka et al. (1991) and runs from moderately polar (System No1: butanol–water) to moderately nonpolar (System No28: heptane–methanol). The italicized systems in the table can be used to rapidly screen the whole table first, allowing the operator to focus into the correct area of interest. Being all multiples of 0.5 ml when 4 ml solvent system is made, these particular systems are quick to make up and test. It is also possible to create the solvent systems on a micro-scale in 96 well plates if the crude sample is in short supply.
Table 1.
Table for selecting a suitable moderately polar two-phase solvent system, graded from polar (No1) to nonpolar (No28)
| No | Heptane | EtOAc | MeOH | Butanol | Water |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 2 | 2 |
| 2 | 0 | 0.4 | 0 | 1.6 | 2 |
| 3 | 0 | 0.8 | 0 | 1.2 | 2 |
| 4 | 0 | 1.2 | 0 | 0.8 | 2 |
| 5 | 0 | 1.6 | 0 | 0.4 | 2 |
| 6 | 0 | 2 | 0 | 0 | 2 |
| 7 | 0.1 | 1.9 | 0.1 | 0 | 1.9 |
| 8 | 0.2 | 1.8 | 0.2 | 0 | 1.8 |
| 9 | 0.29 | 1.71 | 0.29 | 0 | 1.71 |
| 10 | 0.33 | 1.67 | 0.33 | 0 | 1.67 |
| 11 | 0.4 | 1.6 | 0.4 | 0 | 1.6 |
| 12 | 0.5 | 1.5 | 0.5 | 0 | 1.5 |
| 13 | 0.57 | 1.43 | 0.57 | 0 | 1.43 |
| 14 | 0.67 | 1.33 | 0.67 | 0 | 1.33 |
| 15 | 0.8 | 1.2 | 0.8 | 0 | 1.2 |
| 16 | 0.91 | 1.09 | 0.91 | 0 | 1.09 |
| 17 | 1 | 1 | 1 | 0 | 1 |
| 18 | 1.09 | 0.91 | 1.09 | 0 | 0.91 |
| 19 | 1.2 | 0.8 | 1.2 | 0 | 0.8 |
| 20 | 1.33 | 0.67 | 1.33 | 0 | 0.67 |
| 21 | 1.43 | 0.57 | 1.43 | 0 | 0.57 |
| 22 | 1.5 | 0.5 | 1.5 | 0 | 0.5 |
| 23 | 1.6 | 0.4 | 1.6 | 0 | 0.4 |
| 24 | 1.67 | 0.33 | 1.67 | 0 | 0.33 |
| 25 | 1.71 | 0.29 | 1.71 | 0 | 0.29 |
| 26 | 1.8 | 0.2 | 1.8 | 0 | 0.2 |
| 27 | 1.9 | 0.1 | 1.9 | 0 | 0.1 |
| 28 | 2 | 0 | 2 | 0 | 0 |
Quantities (in ml) required to make 4 ml of system using a liquid-handling robot. EtOAc = ethyl acetate, MeOH = methanol. Hexane may be used instead of heptane (Garrard 2005)
In order to achieve an efficient resolution of the target compounds, the K values, which express the solute concentration in the stationary phase divided by that in the mobile phase, should be calculated. The partition coefficient (K) should lie within the approximate range 0.5 < K < 2.0. A smaller K value results in a loss of peak resolution, whilst a larger value produces excessive band broadening.
Solvent systems employed in CCC for terpenoid purification
In order to fully assess the use of two phase systems in CCC of terpenoids, approximately 3,500 scientific papers were studied, published in the last 30 years, that related to all aspects of CCC or CPC. Papers that contained an application example of terpenoids, i.e. a purification performed by CCC or CPC were noted, together with the compounds purified and the solvent system used for the purification. Papers which gave examples of separations reported elsewhere were ignored, as were all symposium abstracts. Only papers which gave specific details of the solvent system and solute were recorded and only natural product secondary metabolites were noted e.g. no synthetic compounds, dyes or chemicals.
In total therefore, 150 solvent systems were listed in Table 2 together with the corresponding solutes that they separated. Some of the solvent systems corresponded to more than one solute, and some of the solutes corresponded to more than one solvent system, but if the same solute and the same solvent system were listed, this was simply a duplicate entry and was therefore removed.
Table 2.
Solvent systems in CCC for terpenoids separations
| Classes of compounds | Purified compounds | References | Type of apparatus/solvent system |
|---|---|---|---|
| Monoterpenoids |
Paeoniflorin Albiflorin |
Huang et al. (2013) | CCC/ethyl acetate–n-butanol–water (3:2.5:5) |
|
Rosiridin Geranyl 1-O-α-l-arabinopyranosyl-(1→6)-β-d-glucopyranoside |
Mudge et al. (2013) | CCC/ethyl acetate–butanol–water (3:2:5) | |
| (S)-3,7-Dimethyl-5-octene-1,7-diol | Knapp et al. (1998) | CCC/chloroform–methanol–water (7:13:8) | |
|
Thymol Carvacrol |
Puertas Mejia et al. (2002) | CCC/n-hexane–tert-butylmethyl ether–acetonitrile (1:0.1:1) | |
| Eugenol | Geng et al. (2007) | CCC/n-hexane–ethyl acetate–methanol–water (1:0.5:1:0.5) | |
|
Chavibetol Methyleugenol |
dos Santos et al. (2009) | CCC/n-hexane–n-butanol–methanol–water (12:4:4:3) | |
| α-Cyperone | Shi et al. (2009) | CCC/n-hexane–ethyl acetate–methanol–water (1:0.2:1.1:0.2) | |
| 1,8-Cineole | Dang et al. (2010) | CPC/petroleum ether–acetonitrile–acetone (4:3:1) | |
| Paeoniflorin | Chen et al. (2004) | CCC/n-butanol–ethyl acetate–water (1:4:5) | |
|
Cuminaldehyde p-Menta-1,4-dien-7-al |
Chen et al. (2011) | CCC/n-hexane–methanol–water (5:4:1) | |
|
Linalol Terpinen-4-ol α-Terpineol |
Skalicka-Woźniak et al. (2013) | CCC/heptane–ethyl acetate–methanol–water (5:2:5:2) | |
|
Anethole Foeniculin |
Skalicka-Woźniak et al. (2013) | CCC/heptane–methanol (1:1) | |
| Sesquiterpenoids |
Parthenolide 11,13-Dihydroparthenolide Anhydroverlotorin 3β-Hydroxycostunolide Costunolide diepoxide 3-Hydroxyparthenolide Artemorin Santamarine Reynosin Artecanin Tanaparthin-β-peroxide |
Fischedick et al. (2012) | CPC/heptane-ethyl acetate–methanol–water (1:1:1:1) |
| Artemisinin | Acton et al. (1986) | CCC/iso-octane-ethyl acetate–methanol–water (7:3:6:4) | |
|
Costunolide Dehydrocostuslactone |
Li et al. (2005) | CCC/light petroleum–methanol–water (5:6.5:3.5) | |
| Lactucopicrin | Wu et al. (2007) | CCC/n-hexane–ethyl acetate–methanol–water (1.5:5:2.75:5) | |
|
11β,13-Dihydrolactucin Lactucin |
Wu et al. (2007) | CCC/ethyl acetate–methanol–water (20:1:20) | |
|
Peroxyferolide Lipiferolide |
Graziose et al. (2011) | CPC/n-hexane–ethyl acetate–methanol–water (2:1:2:1) | |
|
14-(3-Methylpentanoyl)-6-deoxybritannilactone 14-(3-Methylbutanoyl)-6-deoxybritannilactone 14-(2-Methylpropanoyl)-6-deoxybritannilactone 1,3-Epi-granilin 11,13-Dihydro-inuchinenolide B Pulchellin C 6-Deacetylbritanin 4H-Tomentosin Gaillardin Britannin |
Fischedick et al. (2013a) | CPC/heptane–ethyl acetate–methanol–water (4:6:4:6) | |
|
3β-Hydroxy-8β-[4′-hydroxytigloyloxy]-costunolide Eupalinolide A Eupalinolide B |
Yan et al. (2012) | CCC/n-hexane–ethyl acetate–methanol–water (1:4:2:3) | |
|
Xanthathin 4-Epi-xanthanol 4-Epi-isoxanthanol |
Pinel et al. (2007) | CPC/n-hexane–ethyl acetate–methanol–water (1:1:1:1) | |
| β-Caryophyllene | Xie et al. (2008) | CCC/n-hexane–dichloromethane–acetonitrile (10:3:7) | |
| Rupestonic acid | Ma et al. (2005) | CCC/n-hexane–ethyl acetate–methanol–water (6:4:3.5:6.5) with 0.5 % acetic acid in stationary-phase | |
| Rupestonic acid | Yang et al. (2010) | CCC/n-hexane–ethyl acetate–methanol–water (3:5:3:5) | |
|
Germacrone Curdione |
Yan et al. (2005) | CCC/light petroleum ether–ethanol–diethyl ether–water (5:4:0.5:1) | |
|
Atractylon Atractylenolide III |
Zhao and He (2006) | CCC/light petroleum–ethyl acetate–ethanol–water (4:1:4:1) | |
| Nootkatone | Xie et al. (2009) | CCC/n-hexane–methanol–water (5:4:1) | |
|
Caryophyllene oxide β-Farnesene Caryophyllene |
Wei et al. (2012) | CCC/n-hexane–acetonitrile–ethanol (5:4:3) | |
| (S)-Dehydrovomifoliol | Yang et al. (2013) | CCC/n-hexane–ethyl acetate–methanol–water (1:5:1:5) | |
|
Curdione Curcumol Germacrone Curzerene β-Elemene |
Dang et al. (2010) | CPC/light petroleum ether–acetonitrile–acetone (4:3:1) | |
| Patchoulol | Li et al. (2011) | CPC/light petroleum ether–acetonitrile (1:1) | |
|
Tussilagone 14-Acetoxy-7β-(3′-ethyl cis-crotonoyloxy)-lα-(2′-methyl butyryloxy)-notonipetranone 7aβ-(3′-Ethyl cis-crotonoyloxy)-lα-(2′-methyl butyryloxy)-3,14-dehydro-Z-notonipetranone |
Wang et al. (2011a) | CCC/n-hexane–ethyl acetate–methanol–water (1:0.5:1.1:0.3) | |
| Blumenol C | Roscher and Winterhalter (1993) | CCC/chloroform–methanol–water (7:13:8) | |
| Solanesol | Hu et al. (2007) | CCC/n-hexane–methanol (10:7) | |
| Solanesol | Du et al. (2006) | CCC/light petroleum ether‐ethanol‐methanol (200:1:100) | |
| Kudtdiol | Rodrigues et al. (2009) | CCC/ethyl acetate–methanol–water (2:1.75:1) | |
|
8ß-Hydroxyeremophil-3,7(11)-dien-12,8α 15,6α-Diolide and 8β-methoxyeremophil-3,7(11)-dien-12,8α;15,6α-diolide |
Shi et al. (2008) | CCC/light petroleum–ethyl acetate–methanol–water (9:1:8:2) | |
| Diterpenoids | Ptaerobliquol | Agostinho et al. (2013) | CPC/heptane–ethyl acetate–methanol–water) (6:5:6:5) |
|
Coniferin Coniferaldehyde glucoside |
Slacanin et al. (1991) | CPC/chloroform–methanol–water (7:13:8) | |
| Salvinorin A | Shirota et al. (2007) | CPC/n-hexane–dichloromethane–methanol–water (8:8:9:2) | |
|
Andrographolide Neoandrographolide |
Du et al. (2003a) | CCC/n-hexane–ethyl acetate–methanol–water (1:4:2.5:2.5) | |
| Phytol | Xiao et al. (2013) | CCC/n-hexane–acetonitrile–methanol (5:5:3) | |
| Carnosol | Fischer et al. (1991) | CCC/n-hexane–ethyl acetate–methanol–water (70:30:14:8) | |
|
15,16-Epoxy-12-hydroxy-8(17),13(16),14-labdatrien-19-oic acid Imbricatolic acid Isocupressic acid Sandaracopimaric acid Isopimaric acid |
Martin et al. (2006) | CPC/chloroform–methanol–isopropanol–water (5:6:1:4) | |
|
Kaurenoic acids Polyalthic acid |
De Souza et al. (2010) | CCC/n-hexane–acetonitrile–ethyl acetate (1:1:0.4) | |
|
Pseudolaric acid B O-β-d-glucopyranoside Pseudolaric acid C Deacetylpseudolaric acid A Pseudolaric acid A O-β-d-glucopyranoside Pseudolaric acid B Pseudolaric acid B methyl ester Pseudolaric acid A Pseudolaric acid H |
He et al. (2012) | CCC/stepwise gradient: n-hexane–ethyl acetate–methanol–water (1:1:1:1) and (3:2:2:3) and (3.5:1:1:3.5) | |
| Oridonin | Lu et al. (2006) | CCC/n-hexane–ethyl acetate–methanol–water (1:2:1:2) | |
| Oridonin | He et al. (2011) | CCC/n-hexane–ethyl acetate–methanol–water (2.8:5:2.8:5) | |
|
Oridonin Ponicidin |
Lu et al. (2007) | 2D-CCC/n-hexane–ethyl acetate–methanol–water (1:5:1:5) in 1st direction and (3:5:3:5) in 2nd direction | |
|
Isoneotriptophenolide Hypolide Triptonide Triptophenolide Triptonoterpene methyl ether VI |
Peng et al. (2008a) | CCC/n-hexane–ethyl acetate–methanol–water (3:2:3:2) | |
|
Pseudolaric acids A and B And their glucosides (O-β-d-glucopyranosides) |
Han et al. (2009) | CCC/n-hexane–ethyl acetate–methanol–water (5:5:5:5) for purification of aglicones and (1:9:4:6) for glycosides | |
|
6β-Angeloyloxykolavenic acid 6β-Tigloyloxykolavenic acid |
Wu et al. (2008) | CCC/n-hexane–ethanol–water (6:5:1) | |
| Pseudolaric acid B | Han et al. (2008) | CCC/n-hexane–ethyl acetate–methanol–water (5:5:5:5) | |
| lolitrem B | Grancher et al. (2004) | CPC/heptane–ethyl acetate–methanol–water (33:33:24:10) | |
|
Carnosic acid Carnosol Carnosaldehyde Epirosmanol Rosmanol 12-Methoxy-carnosic acid Sageone |
Fischedick et al. (2013b) | CPC/heptane–acetone–water (3:5:2) | |
|
Stevioside Rebaudioside A Rebaudioside C |
Huang et al. (2010) | CCC/n-hexane–n-butanol–water (1.5:3.5:5) | |
|
Ginkgolides A Ginkgolides B Ginkgolides C Bilobalide |
Liu et al. (2013b) | CCC/stepwise gradient: n-hexane–ethyl acetate–methanol–water (4:5:1:5) and (4:5:2:5) | |
|
Cryptotanshinone Tanshinone I Tanshinone IIA |
Gu et al. (2004, 2006) | CCC/stepwise gradient: n-hexane–ethanol–water (10:5.5:4.5) and (10:7:3) | |
|
Cryptotanshinone Tanshinone I 1,2-Dihydrotanshinquinone Tanshinone IIA |
Liang et al. (2013) | CCC/n-hexane–ethyl acetate–methanol–water (5:5:7:3) | |
|
Tanshinone I Tanshinone IIA |
Wu et al. (2010) | CCC/n-hexane–ethyl acetate–ethanol–water (8:2:7:3) | |
|
Cryptotanshinone Tanshinone I Tanshinone IIA |
Tian et al. (2000) | CCC/n-hexane–ethanol–water (4:2:2) | |
|
Dihydrotanshinone Cryptotanshinone Methylenetanshiquinone Tanshinone I Tanshinone II Danshenxinkun B |
Li and Chen (2001b) | CCC/stepwise gradient: n-hexane–ethanol–water (10:5.5:4.5) and (10:7:3) | |
|
Tanshinone I Tanshinone IIA Dihydrotanshinone I Cryptotanshinone |
Tian et al. (2002) | CCC/light petroleum–ethyl acetate–methanol–water (2:3:2.5:1.7) | |
| Przewaquinone A | Han et al. (2003) | CCC/carbon tetrachloride–methanol–water–n‐hexane (3:3:2:1) | |
| Triptolide | Ye et al. (2008b) | CCC/n-hexane–ethyl acetate–methanol–water (4:5:4:5) | |
|
Dihydrotanshinone I 1,2,15,16-Tetrahydrotanshiquinone cryptotanshinone Tanshinone I Tanshinone IIA Neo-przewaquinone A Miltirone |
Sun et al. (2011) | CCC/light petroleum–ethyl acetate–methanol–water (6:4:6.5:3.5) | |
| Triterpenoids | 2β,3β,4β-Trihydroxypregnan-16-one | Rodrigues et al. (2009) | CCC/n-hexane–ethyl acetate–methanol–water (1:2:1.75:1) |
| Squalene | Lu et al. (2003) | CCC/n-hexane–methanol (2:1) | |
|
Pristimerin Netzahualcoyene |
Gutiérrez et al. (2007) | CPC/heptane–ethyl acetate–methanol–water (8:1:6:1) | |
| Guyanin | Severino et al. (2009) | CCC/n-hexane–ethanol–acetonitrile–water (10:8:1:1) | |
|
Barbinervic acid Rotungenic acid 24-Hydroxy ursolic acid Ursolic acid |
Fan and He (2006) | CCC/n-hexane–ethyl acetate–methanol–water (3:6:4:2) | |
| Betulinic acid | Frighetto et al. (2005) | CCC/n-hexane–ethyl acetate–methanol–water (10:5:2.5:1) | |
| Ursolic acid | Frighetto et al. (2008) | CCC/n-hexane–ethyl acetate–methanol–water (10:5:2.5:1) | |
|
Bellericagenin B Bellericaside B Arjunglucoside I 28-Nor-17, 22-seco-2α, 3β, 19, 22, 23-pentahydroxy-Δ 12-Oleanane |
Nasser et al. (2006) | CCC/chloroform–methanol–water (43:37:20) | |
|
2α,3α,19β,23β-Tetrahydroxyurs-12-en-28-oic acid 2α,3α,23β-Trihydroxyurs-12-en-28-oic acid |
Liu et al. (2011) | CCC/n-hexane–ethyl acetate–methanol–water (10:5:3:1) | |
|
Oleanolic acid Ursolic acid |
Du et al. (1995) | CCC/n-hexane–ethyl acetate–methanol–water (3:6:2:1) | |
| Shionone | Wang et al. (2012a) | CCC/n-hexane–methanol (2:1) | |
|
Taraxasterol acetate Lupeol acetate β-Amyrin acetate |
Abbott et al. (1989) | CCC/n-hexane–ethyl acetate–methanol–acetonitrile (5:2:4:5) | |
|
Cycloartenyl ferulate 24-Methylene cycloartanyl ferulate |
Liu et al. (2013a) | CCC/n-hexane–acetonitrile (1:1) | |
| Abrusoside A, B, C, D | Fullas et al. (1990) | CCC/chloroform–methanol–water (7:13:8) | |
| No compound names given | Marston et al. (1988) | CCC/chloroform–methanol–water (7:13:8) | |
|
Inotodiol Trametenolic acid |
Du et al. (2011) | CCC/n-hexane–ethyl acetate–methanol–water (1:0.4:1:0.4) | |
|
Ursolic acid Ursolic acid lactone |
Maurya and Srivastava (2011) | CPC/n-hexane–ethyl acetate–methanol–water (1:2:1.5:1) with 2 % ammonia solution in lower aqueous mobile phase (pH 9.5) | |
|
Asiaticoside Madecassoside |
Diallo et al. (1991) | CCC/chloroform–n-butanol–methanol–water (7:3:6:4) | |
| Taraxeryl acetate | Yang et al. (1995) | CCC/chloroform–methanol–water (2:2:1) | |
|
Triterpenoic acid Acetyl-triterpenoic acid |
Ito et al. (1990) | CCC/n-hexane–ethanol–water (6:5:2) | |
| Celastrol | Wu et al. (2004) | CCC/light petroleum–ethyl acetate–tetrachloromethane–methanol–water (1:1:8:6:1) | |
|
Asiatic acid Madecassic acid Asciaticoside Madecassoside |
Du et al. (2004) | CCC/n‐hexane–n‐butanol–0.05 M NaOH (5:1:6) | |
|
Euscaphic acid Tormentic acid 2α-Acetyl tormentic acid 3β-Acetyl tormentic acid |
Rocha et al. (2007) | CCC/gradient: n-hexane–ethyl acetate–methanol–water (1:2:1.25:2) and (1:2:1.5:2) and (1:2:1.75:2) | |
|
Alisol B Alisol B 23-acetate |
Yoon et al. (2009) | CPC/n-hexane–ethyl acetate–methanol–water (10:2:10:7) | |
|
Ganoderic acids A, B, C6, D, E, F, G Ganoderenic acid D |
Cheng et al. (2012) |
CCC/stepwise gradient: light petroleum ether–ethyl acetate–methanol–water (3:5:3:5) and (4:5:4:5) chloroform–methanol–water (13:7:4) + ammonia (22 mM) in aqueous phase and TFA (11 mM) in organic phase for further purification |
|
|
Dehydrosulphurenic acid 3-Ketodehydrosulphurenic |
Zhang et al. (2013a) | CCC/n-hexane–ethyl acetate–methanol–water (1:1.5:1.2:1) | |
| 24-Methylene cycloartanol | Yao et al. (2007) | CCC/n-hexane–ethyl acetate–acetonitrile (5:1:4) | |
| Lupenone | Yao et al. (2007) | CCC/n-hexane–ethyl acetate–acetonitrile (5:2:5) | |
| Triterpenoid saponins (ginsenosides) | Rc, Rb1 and Re | Wang et al. (2010) | CPC/ethyl acetate–n-butanol–water (1:1:2) |
| Rg3, Rk1, Rg5 and F4 | Ha et al. (2007) | CCC/methylene chloride–methanol–water–isopropanol (6:6:4:1) | |
| Rf, Rd, Re and Rb1 | Qi et al. (2010) | CCC/methylene chloride–methanol–5 mM aqueous ammonium acetate–isopropanol (6:2:4:3) | |
| Re | Engelberth et al. (2010) | CPC/heptane–n-butanol–water (3:4:7) | |
| Rg1, Re, Rf, Rh1, Rb1, Rc, Rb2 and Rd | Shehzad et al. (2011) | CCC/chloroform–methanol–water–isopropanol (4:3:2:1) | |
| Re,Rb1, Rc and Rb2 | Cheng et al. (2011) | CCC/methylene chloride–methanol–water–isopropanol (6:2:4:3, v/v) further purification of Rb1, Rc and Rb2 in n-hexane–n-butanol–0.1 % formic acid (0.7:3:4) | |
| Rb1, Rb2, Rc, Rd, Re, Rg1, Rf and Rh 1 | Shehzad et al. (2012) | CCC/stepwise gradient: n-hexane–ethyl acetate–methanol–water (5:6:1:4) and (4:3:1:2) and (3:3:1:2) | |
| Re and Rg1 | Chen et al. (2012) | CCC/ethyl acetate–n-butanol–water (4:1:6) | |
| Rh1, Rf, Rd, Rg1, Re, Rc, Rb2 and Rb1 | Shehzad et al. (2013) | CCC/methylene chloride–methanol–water–isopropanol (1:1:2:1) | |
| Rg6, Rg5, Rk1,F4, Rg3, Rg2, Rf, Rd, Rg1, Re, Rc, Rb2, Rb1 | Shehzad et al. (2013) | CCC/stepwise gradient: methylene chloride–methanol–water–isopropanol (5:4:1:3) and (2:2:1:2) | |
| Rb1 and Rb2 | Wang et al. (2013) | CPC/ethyl acetate–n–butanol–water (0.8:1.2:2) | |
|
Rg, Rd, Re and Rb Notoginsenoside R |
Cao et al. (2003) | CCC/chloroform–2-butanol–methanol–water (5:1:6:4) | |
|
Rg, Rd, Re and Rb Notoginsenoside R |
Cao et al. (2003) | CCC/ethyl acetate–n–butanol–water (1:1:2) | |
|
Rg, Re and Rb Notoginsenoside R |
Du et al. (2003b) | CCC/n-hexane–n-butanol–water (3:4:7) | |
| Ro | Cheng et al. (2010) | CCC/ethyl acetate–isopropanol–0.1 % formic acid (3:1:5) | |
|
Rg1, Re and Rb1 Notoginsenoside R1 |
Wang et al. (2011b) | CPC/ethyl acetate–n-butanol–water (1:1:2) | |
| Triterpenoid saponins |
Lucyoside Q Lucyoside H |
Du and Gao (2006) | CCC/chloroform‐methanol‐water (13:7:8) |
|
Astragaloside I Astragaloside II |
Han et al. (2007) | CCC/stepwise gradient: ethyl acetate–2-propanol–water (5:1:5) and (50:1:50) | |
|
Lancemaside A Foetidissimoside A Astersaponin Hb |
Shirota et al. (2008) | CPC/n-hexane–n-butanol–methanol–0.1 % aqueous formic acid (3:4:1:6) | |
|
Saikosaponins-A Saikosaponins-C |
Yoon and Kim (2009) | CPC/ethyl acetate–n-butanol–methanol–water (15:1:3:1) | |
|
Platycoside E Deapio-platycoside E |
Han et al. (2009) | CCC/n-hexane–n-butanol–water (1:40:20) | |
|
Platycodin D3 Deapio-platycodin D3 Platycodin D Deapio-platycodin D |
Han et al. (2009) | CCC/n-hexane–n-butanol–water (1:10:5) | |
|
2″-O-Acetylplatycodin D 3″-O-Acetylpolygalacin D 2″-O-Acetylpolygalacin 3″-O-Acetylplatycodin D Polygalacin D |
Ha et al. (2011) | CCC/chloroform–methanol–isopropanol–water (3:2:2:3) | |
| No compound names given | Shi et al. (2007) | CCC/ethyl acetate–n-butanol–ethanol–0.05 % TFA (5:10:2:20) | |
|
Astragaloside IV Astragaloside II Astragaloside I Acetylastragaloside I |
Peng et al. (2008b) | CCC/stepwise gradient: n-hexane–ethyl acetate–ethanol–water (1:0.6:0.6:1) and (1:1:1:1) | |
|
Gypsogenin derivatives Dianoside C |
Yao et al. (2008) | CCC/n-hexane–n-butanol–methanol–0.02 % TFA (1:9:1:9) | |
|
Hederagenin 3-O-[β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-l-arabinopyranosyl]-hederagenin |
Xin et al. (2009) | CCC/n-hexane–ethyl acetate–methanol–water (7:3:5:5) | |
|
23-O-Acetylshengmanol-3O-d-xylopyranoside Cimiracemoside D 25-O-acetylcimigenol-3-O-d-Xylopyranoside Cimigenol |
Cicek et al. (2010) | CCC/n‐hexane–acetone–ethyl acetate–isopropanol–ethanol–water (3.5:1:2:1:0.5:2) | |
| Esculentosides A, B, C, and D | Ma et al. (2010) | CCC/chloroform–methanol–water (4:4:2) | |
| Hederagenin | CCC/heptane–acetone–methanol (5:1:4) | ||
| Hederagenin | He et al. (2002) | CPC/n-hexane–ethyl acetate–methanol–water (7:8:5:3) | |
| Elatoside F | Lee et al. (2009) | CCC/chloroform–methanol–water–isopropanol (4:3:3:1) | |
| Tormentic acid | Wang et al. (2012b) | CCC/n-hexane–ethyl acetate–methanol–water (4:5:4:5) | |
|
Arganine A, C, D Tieghemelin |
Gosse et al. (2002) | CCC/tert-butylmethyl ether–n-butanol–acetonitrile–0.5 % TFA (1:3:3:5) | |
| Triacetyl soyasaponin Ab, Aa, Ab, Ae, Ba, Af, Bb, Be and conjugated groups, αg, βg and γg | Zhao et al. (2012) | CCC/n-butanol–acetic acid–water (5:0.05:5)-acid in n-butanol as stationary phase | |
|
Licorice-saponin A3 Glycyrrhizic acid, 3-O-[β-d-glucuronopyranosyl-(1→2)-β-d-Galactopyranosyl]glycyrrhetic acid |
Xu et al. (2013) |
CCC/ethyl acetate–n-butanol–water (2:3:5) with 10 mM TFA in the upper organic Stationary phase and 10 mM ammonia in the lower aqueous mobile phase |
|
| Glycyrrhizin | Jiang et al. (2004) | CCC/ethyl acetate–methanol–water (5:2:5) | |
|
Goyaglycoside-E Momordicoside L Goyaglycoside-a Momordicoside K |
Du and Yuan (2005) | CCC/tert-butylmethyl ether–n-butanol–methanol–water (1:2:1:5) or (1:3:1:5) | |
| Tetranortriterpenoids |
Azadirachtin A Azadirachtin B Azadirachtin H Desacetylnimbin Desacetylsalannin Nimbin Salannin |
Silva et al. (2007) | CCC/n-hexane–n-butanol–methanol–water (1:0.9:1:0.9) |
|
Methyl angolensate 7-Descetoxy-7-oxogedunin Deacetylgedunin 6α-Acetoxygedunin Gedunin Andirobin |
da Silva et al. (2009) | CCC/stepwise gradient: n-hexane–ethyl acetate–methanol–water (2:1:1.5:1) and (2:1:1.75:1) | |
| Tetraterpenoids (Carotenoids) |
Cochloxanthin Dihydrocochloxanthin |
Diallo and Vanhaelen (1988) | CCC/tetrachloromethane–methanol–water (5:4:1) |
| Zeaxanthin | Chen et al. (2005) | CCC/n-hexane–ethyl acetate–ethanol–water (8:2:7:3) | |
| Crocin | Jiang et al. (2011) | CCC/tert-butylmethyl ether–n-butanol–acetonitrile–water (2:2.5:1:5) | |
| Fucoxanthin | Kim et al. (2011) | CPC/n-hexane–ethyl acetate–ethanol–water (5:5:7:3) | |
| Lutein | Wei et al. (2003) | CCC/n‐heptane–chloroform–acetonitrile (10:3:7) | |
|
Zeaxanthin Lutein |
Aman et al. (2005) | CCC/n-hexane–ethanol–water (6:5:1.3) | |
| Lutein | Tsao and Yang (2006) | CCC/n-hexane–ethanol–water (6:4.5:1.5) | |
| Canthaxanthin | Li et al. (2006) | CCC/n-hexane–ethanol–water (10:9:1) | |
| 9′-Cis-neoxanthin | Baldermann et al. (2007) | CCC/n-hexane–ethanol–water (5:5:4.5) | |
| Lycopene | Baldermann et al. (2008) | CCC/n-hexane–dichloromethane–acetonitrile (30:11:18) with 85 mg/L of 3-tert-butyl-4-hydroxyanisol and 2-tert-butyl-p-cresol | |
|
Crocins 1, 2, 5 Picrocrocin |
Lechtenberg et al. (2008) | CPC/n-hexane–ethyl acetate–ethanol–water (1:3:4:7) | |
| Fucoxanthin | Xiao et al. (2012) | CCC/n-hexane–ethyl acetate–ethanol–water (5:5:6:4) | |
| Lutein | Li et al. (2001) | CCC/n-hexane–ethanol–water (4:3:1) | |
| Lycopene | Wei et al. (2001) | CCC/n-hexane–dichloromethane–acetonitrile (10:3.5:6.5) | |
| Astaxanthin | Li and Chen (2001a) | CCC/n-hexane–ethyl acetate–ethanol–water (5:5:6.5:3) | |
|
Tephrosin Deguelin 6a,12a-Dehydrodeguelin |
Ye et al. (2008a) | CCC/n-hexane–ethyl acetate–methanol–water (1:0.8:1:0.6) |
CCC counter-current chromatography, CPC centrifugal partition chromatography
The solvent systems tables mentioned above are presented here, sorted according to class of terpenoid separated. That table is presented for the benefit of CCC users by suggesting possible suitable solvent systems, which can act as a starting point for further refinement and optimization in the composition of the system.
Applications of high speed and high performance CCC
Being a liquid–liquid chromatography system, CCC can select from an almost infinite range of possible two-phase solvent systems for a purification. Most reported purifications with the technique have understandably concentrated on compounds of intermediate polarity. For example, a review of Chinese herbal medicines purified by CCC found a total of 214 different compounds in 198 published papers with a LogP polarity range from −4 to +12 (Sutherland and Fisher 2009). However, more than 60 % of those compounds fell in the narrow intermediate polarity range of 0–4. Nevertheless, CCC and its sister technique, CPC can be particularly useful for purifications in the extreme polar and non-polar range and some impressive examples have been published. In 1995, Gasper and co-workers managed to purify C60 and C70 fullerenes using the non-polar and non-aqueous solvent system of isooctane, dimethylformamide, 1,2-dichlorobenzene (4:2:1) plus 1 % tert-butylmethyl ether (Gasper et al. 1995). Still at the non-polar end of the spectrum, the carotenoid lycopene was isolated from tomato paste using a non-aqueous phase system of n-hexane, dichloromethane and acetonitrile (10:3.5:6.5) (Wei et al. 2001). This separation was performed in a single step from the crude material with 100 mg of crude extract injected onto a 230 ml capacity centrifuge. The purity of the final product was measured as >98 % by HPLC peak area. Lycopene has a calculated LogP value of approximately 17.6. Beyond the carotenoids, non-polar terpenoids can also be challenging to purify with solid phase chromatography, and again CCC offers an alternative approach. In one example, Liu et al. (2013a) successfully separated cycloartenyl ferulate and 24-methylene cycloartanyl ferulate. Due to the weak polarity, a series of low-polar solvent systems, including: n-hexane–ethyl acetate–ethanol (methanol)–water, n-hexane–ethanol (methanol)–water, n-hexane–methanol and n-hexane–ethyl acetate–n-butanol ethanol (methanol)–water were tested, but all without success. Since the HPLC mobile phase for the analysis of those two target compounds consisted of methanol, acetonitrile and isopropanol, these three solvents were further studied and a mixture of hexane and acetonitrile (1:1) was used for the purification. Another application of non-aqueous solvent systems is the purification of shionone—also a low-polar compound. Wang et al. (2012a) tested several hydrophobic two-phase solvent systems. Among these n-hexane–methanol (2:1) and heptane–dichloromethane–acetonitrile (20:7:13) were suitable for the separation.
Turning to the extreme polar end of the spectrum, CCC has been used with aqueous-organic solvent systems for the purification of peptides. For example, the peptide antibiotic colistin (LogP about −4.7) was isolated from a commercial microbial preparation using the polar two-phase system consisting of n-butanol and 0.04 M aqueous trifluoroacetic acid (1:1) (Ikai et al. 1998). Using the salt-based solvent system consisting of 1-propanol, acetonitrile, saturated ammonium sulphate and water (1:0.5:1.2:1) the highly polar glucosinolate glucoraphanin was purified to a purity >98 % from a crude broccoli extract in a single step (Fisher et al. 2005).
Sometimes modifications are necessary. The separation of three closely related triterpenes: sericic acid, trachelosperogenin E and sericoside was achieved using a three-phase solvent system composed of n-heptane, tert-butylmethyl ether, acetonitrile and water. After mixing all solvents in equal volume, the upper phase was separated and tert-butylmethyl ether was shaken with the remaining two-phase solvent system in order to decrease the polarity. The lower phase was used as a stationary phase. Sericic acid was identified in the fractions collected when the upper phase was used as the mobile phase, while the elution of the middle phase led to the separation of trachelosperogenin E and sericoside (Hamzaoui et al. 2013).
Zhao and Du (2007) proposed a novel non-aqueous two-phase solvent system composed of sunflower oil and ethanol to separate solanesol—a non-cyclic terpene alcohol used as a food additive for preventing cardiac arrest and cancer. Before, this target molecule was purified from tobacco leaves extract with petroleum and methanol, both solvents being unsuitable for the food industry.
Sometimes the addition of a small amount of acid can significantly improve the purification. Shirota et al. (2008) purified the saponins lancemaside A, foetidissimoside A and astersaponin Hb from a hot water extract of Codonopsis lanceolata roots used CPC with mixture of n-hexane–n-butanol–methanol–0.1 % aqueous formic acid (3:4:1:6) as the two-phase solvent system. Because the crude extract contained large amounts of sugars, an aqueous phase was chosen as the stationary phase so that these sugars would be retained in the partition cells, whereas an organic phase was chosen as mobile phase for elution of the target compound. Addition of formic acid to the two-phase solvent system retained the carboxy group in the non-ionised form and allowed the separation of the lancemasides from the large amounts of water-soluble sugars present. The addition of acetic acid to the ethyl acetate–n-butanol–water mixture improved the resolution of soyasaponins, naturally occurring triterpenoid glycosides. A suitable mixture n-butanol, acetic acid, water (5:0.05:5) was taken but acetic acid was added only in n-butanol as the stationary phase (Zhao et al. 2012). During the separation, with the movement of the mobile phase the acetic acid was gradually diluted with water and the polarity of the stationary phase changed as a gradient. It is worth mentioning that the main difficulties with soyasaponin isolation and purification are that the soyasaponins coexist in soybeans with the isoflavone glycosides and they share overlapping polarities. There is also the structural similarity of soyasaponin compounds.
CCC is popularly applied for purification of ginsenosides, an important group of terpenoids due to their biological activity. They generally fall in the moderate to polar category and therefore require similar solvent systems for purification (Qi et al. 2010; Shehzad et al. 2013). A good solubility for saponin-like compounds can be provided by the addition of chloroform or other chlorinated solvents and a gradual polarity change between phases can be achieved by varying the methanol ratio in the system or by adding another alcohol like isopropanol (Qi et al. 2010). Shehzad et al. (2013) proposed an efficient CCC separation method in which a flow-rate gradient technique was coupled with a new solvent gradient dilution strategy for the isolation of ginsenosides from Korean red ginseng (steam-treated P. ginseng). The column was first entirely filled with the upper stationary phase mixture of methylene chloride–methanol–isopropanol–water (5:4:1:3) in a reversed phase system. After 300 min, when five ginsenosides had eluted, the flow rate was increased from 1 to 1.2 ml/min and also the dilution of the lower phase was initiated and was changed to 100 % of lower phase composed of the mixture mentioned above in a ratio (2:2:1:2). Overall, 13 ginsenosides including Rg1, Re, Rf, Rg2, Rb1, Rb2, Rc, Rd, Rg3, Rk1, Rg5, Rg6, and F4 were purified (Shehzad et al. 2013). Qi et al. (2010) for the purification of ginsenosides Rf, Rd, Re and Rb1, applied mixtures of methylene chloride, methanol, water and isopropanol (6:2:4:3).
Emulsification can often be a problem in the CCC purification of ginsenosides. Generally, adding an electrolyte, such as salt or acid, can help to eliminate such emulsification. Salt is not recommended because an additional desalting process will be necessary after the separation. Therefore, formic acid is often chosen to prevent emulsification (Cheng et al. 2010). However, Qi et al. (2010) preferred to avoid an acidic environment, which would lead to the decomposition of ginsenosides. Instead, the inorganic salt ammonium acetate was chosen, because it is volatile and can be precipitated in warm acetone for sample recovery. The addition of this salt resulted in a very slight decrease in K values and thus a shorter separation time. In the end, the proposed solvent system was methylene chloride–methanol–5 mg/mL aqueous ammonium acetate–isopropanol (6:2:4:3).
As the solvent system contained methylene chloride and methanol, both toxic to humans and the environment, Wang et al. (2010) chose low toxic solvents such as ethyl acetate and n-butanol. Since the ginsenosides in P. quinquefolium L. have a comparatively larger polarity with solubility in hydrophilic solvents, both phases require a certain hydrophilicity to get a good separation. Initially, the hexane–n-butanol–water solvent systems were scanned at several volume ratios, but all the systems had a poor retention in the CPC column. Then ethyl acetate was then employed instead of hexane in order to enhance the retention of the stationary phase in the column. Because ginsenosides are easily dissolved in n-butanol, and the viscosity of n-butanol is comparatively large, so in an ethyl acetate–n-butanol–water solvent system, the addition of n-butanol could delay the peak elution time and affect the separation. Overall, the system of ethyl acetate–n-butanol–water (1:1:2) was used for successful separation of three ginsenosides Rc, Rb1, and Re.
Counter-current chromatography has also been used for the separation of minor and structurally similar compounds. Fan and He (2006) using HSCCC with n-hexane–ethyl acetate–methanol–water (3:6:4:2) to separate not only ursolic acid, which is a very small content of the leaves of Diospyros kaki, but also two other pentacyclic triterpenes: barbinervic acid and its epimer rotungenic acid, differing only with the configuration of a hydroxyl group at position C3: one contains an axial and the other an equatorial hydroxyl group. HSCCC was also employed for the separation and purification of minor constituents in Platycodi Radix. Platycosides, the saponins that are the major active constituents, are typically composed of oleanene backbones with two side chains; one a glucose unit attached through an ether linkage at the C-3 position of a triterpene, and the other 28-O-arabinoserhamnose-xylose-apiose linked by an ester bond. They also have different substituents at the C-4 position. Because the content of these compounds is very low, conventional methods are frequently not suitable for the separation. Ha et al. (2011) used a two-phase solvent system consisting of chloroform–methanol–isopropanol–water (3:2:2:3) for purification of minor saponins 2″-O-acetylplatycodin D, 3″-O-acetylpolygalacin D, 2″-O-acetylpolygalacin and a mixture of 3″-O-acetylplatycodin D and polygalacin D, which were further successful purified using prep-HPLC (Ha et al. 2011). Ha and Kim (2009) also used HSCCC for the separation of three pairs of platycosides and their deapiose forms. They used an interesting modification: the column was first filled with a mixture of the two phases, thus reducing the amount of time for hydrodynamic equilibrium to be established. The ratio of two phases was optimised at 70:30 (stationary phase–mobile phase) based on the amount of time required to reach hydrodynamic equilibrium. A series of solvent systems were tested. In the gradient elution mode, the retention of the stationary phase was extremely low. The authors decided to perform the separation in two stages. First, platycoside E, deapio-platycoside E, a mixed fraction containing platyodin D and deapiose form, and a fraction containing platycodin D3 and deapiose form were separated using the n-hexane–butanol–water (1:40:20) in reversed-mode. Then mixed fractions I and II were further purified in the normal elution mode with the above mentioned solvent system in the ratio 1:10:5.
CCC/CPC seems to be one of the few efficient approaches for the separation of xanthanolides, a bicyclic subtype of sesquiterpenic lactones characterized by a 5,7-fused system containing a γ-lactone moiety. Because of some well documented and promising activity purification of these compounds is important. The presence of several chiral centres probably explain the difficulty obtaining enantiomericaly pure xanthanolides by total synthesis, thus purification from natural source seems to be the best solution. Xanthonolides are coextracted with chlorophyll and lipids. The pigment crystallization and the delipidation in order to clean the extract is not selective and cause the partial loss of target compounds. Purification of several xanthanolides (xanthathin, 4-epi-xanthanol and 4-epi-isoxanthanol) was realized in one step, directly from the crude chloroformic extract of the leaves of X. macrocarpum with a mixture of heptane–ethyl acetate–methanol–water (1:1:1:1) (Pinel et al. 2007).
High-speed counter-current chromatography can be applied as a method suitable for fingerprinting. Gu et al. (2006) used it for quality control of TCMs and identification of the active compounds of Salvia miltiorrhiza Bunge, a popular traditional Chinese medicine. In order to purify a series of tanshinones a stepwise elution with solvent systems composed of n-hexane–ethanol–water (10:5.5:4.5) and (10:7:3) was used. The method was compared with more conventional approaches, such as high performance liquid chromatography (HPLC), high performance capillary electrophoresis (HPCE), and thin-layer chromatography scan (TLCS). In the HSCCC separation, 12 components were separated, with good resolution and precision, within 13 h. HSCCC showed better performance in the analysis of tanshinones, which produced a fingerprint which contained more chemical information than that of e.g. TLCS.
Lu et al. (2007) proposed this effective two-dimensional counter-current chromatographic method for the simultaneous isolation and purification of oridonin and ponicidin from a crude extract of Rabdosia rubescens with a pair of two-phase solvent systems composed of n-hexane–ethyl acetate–methanol–water (1:5:1:5 and 3:5:3:5, v/v). A combination of stepwise CCC and pH-zone-refining is also possible. Cheng et al. (2012) reported its successful combination in the separation of the main components from G. lucidum. In the first step, a two-phase solvent system composed of petroleum ether–ethyl acetate–methanol–water (3:5:3:5 and 4:5:4:5) led to the separation of ganoderic acids GE, GC6 and GF with high purity in one run. Also two peaks containing GG, GB, GA and GED, GD, respectively, were collected and their separation was followed by pH-zone-refining CCC. Chloroform–methanol–water (13:7:4) with NH4OH in upper aqueous stationary phase and trifluoroacetic acid as the eluter acid was the most suitable. In another example of two-dimensional CCC, a three-step gradient elution and two-step flow-rate gradient elution was applied to separate 8 diterpene compounds within 80 min in a single run from the alcohol extract of Pseudolarix kaempferi. Pseudolaric acid B O-β-d-glucopyranoside, pseudolaric acid C, deacetylpseudolaric acid A, pseudolaric acid A O-β-d-glucopyranoside, pseudolaric acid B, pseudolaric acid B methyl ester, pseudolaric acid A and pseudolaric acid H were obtained with very high purity (He et al. 2012). The separation was performed in normal phase system. In the first step, the mobile phase, composed of hexane–ethyl acetate–methanol–water (1:1:1:1), was used with a flow rate 0.5 ml/min. While the mobile phase (upper) of (2:3:2:3) and (1:3.5:1:3.5) were used in the second and third step, and the flow rate of mobile phase was set at 1 ml/min. When the separation was performed in preparative conditions, the initial flow rate of mobile phase was 25 ml/min in the first step and then increased to 50 ml/min in the second and third step.
Principle advantages and drawbacks of CCC in the purification of natural products
Counter-current chromatography has a number of key advantages in the purification of natural products. For example, the solvent usage is generally far lower than that of solid phase chromatography systems operating at the same scale (about 25 %) (Graham et al. 2001). Furthermore, since the process is frequently an isocratic one, a simple analysis of solvent composition allows the recycling of the solvents, reducing the usage still further (Garrard et al. 2007). The technique also allows for 100 % recovery of the sample components. In other words, the target compound can always be retrieved since there is no solid phase and therefore no possibility of losses arising from irreversible adsorption onto the solid matrix. This is a significant advantage in every purification process. As a solid-free and therefore relatively gentle chromatographic technique, CCC can be used for the isolation of unstable natural compounds. Baldermann et al. (2007) presented the successful purification of 9′-cis-neoxanthin, the predominant isomer of neoxanthin in green vegetables. Major problems during its isolation include isomerization and oxidation, mainly caused by higher temperatures, light or oxygen exposure. When typical solid stationary phase techniques are applied, the rearrangement products can be detected or complete isomerization can be observed. A solid-free technique is a very practical solution for avoiding the above mentioned complications.
Particulates, such as cell debris, are generally well tolerated in CCC, particularly when performed at large scale where the tubing bore may be up to 10 mm in diameter. This is another major advantage over solid phase chromatography systems. Thus filtering a sample is frequently not necessary, depending on the scale of CCC employed, and even if it is required, it is usually a simple filter paper filtration. With processing times similar to that of other purification methods, scale up is also possible with modern instruments with examples existing running from milligram to kilogram levels (Garrard et al. 2008). The technique can be operated in normal batch injection mode, or as a continuous extraction process for better throughput. A wide range of polarities can be processed due to the range of solvents that may be used (the literature reports examples with a logP range from −4.7 (colistin peptide antibiotic) (Ikai et al. 1998) to +17.6 (lycopene) (Wei et al. 2001). Also, the separation of compounds with vastly different polarities from a single extract is possible. For example, in order to purify a wide range of polarity of triterpene saponis from Panax notoginseng, Zhang et al. (2013b) successfully coupled accelerated solvent extraction (ASE) and high-performance counter-current chromatography (HPCCC). First the upper phase of the solvent system ethyl acetate–n-butanol–water (1:1:2 or 1.2:1:2) or ethyl acetate–n-butanol–methanol–water (3:5:1.5:6) was used as both the ASE solvent and HPCCC stationary phase. The polar saponis were eluted. In order to separate fractions with moderate polarity, the upper phase of system ethyl acetate–n-butanol–methanol–water (6:3:2:6 or 7:3:2:7) was used. Finally, the upper phase of the solvent system of n-hexane–n-butanol–methanol–water (8:2:2:8) or n-hexane–ethyl acetate–n-butanol–methanol–water (0.2:10:0.5:1.5:8) was used as both the ASE solvent and HPCCC stationary phase to elute the low polar compounds. This combination of methods allowed the purification of notoginsenosides R6, R1, Spt1 and ginsenosides Rb1, F4, Rh3, Rg3, Rs3 and Rk1 with a wide range of polarity (Zhang et al. 2013b).
Compared to the early instruments, the quality of modern CCC apparatus is very good. In most cases the coils are tough and the machinery robust. A set of coils would be expected to last the lifetime of the centrifuge and maintenance and running costs are low. Unlike solid phase chromatography, there is no change to component retention over time (no column aging effects) as a freshly-filled coil of solvents is used each run. This makes it easier to consistently satisfy current regulatory requirements when performing purifications under a good laboratory practice (GLP) or good manufacturing practice (GMP) environment.
As has been seen in a number of quoted examples, a large advantage of CCC is that the technique can be operated in a number of different modes, since both the mobile and the stationary phase are liquid. Either normal or reverse phase chromatography can be chosen, depending on which solvent phase is selected to be the mobile one. However, it is even possible to switch from normal phase elution to reverse phase (or vice versa) in the middle of a run. Intermittent counter-current extraction (ICCE) is a continuous process where the operation alternates between normal and reverse phase mode at regular intervals, with the sample continuously introduced in the middle of the coil (Hewitson et al. 2009). In the reference quoted, this technique was successfully used to purify a diterpenoid, triptolide, a high value target compound, from a Chinese herbal plant, from 2 % in the crude extract to over 98 % purity. This was achieved by retaining and enriching the target compound within the CCC coil while washing away all the other components of the crude material. Alternatively, components can be recovered by eluting the liquid stationary phase without any compound losses whilst maintaining resolution (Berthod et al. 2003), a technique known as elution-extrusion. This technique is frequently adopted at the end of standard CCC purification runs simply to ensure that no loss of target compound has occurred within the liquid stationary phase. Another possibility is co-current chromatography (Berthod and Hassoun 2006) where both phases are pumped in the same direction. All of these options use a conventional CCC centrifuge and are thus easy to implement in the laboratory or pilot plant. However, with modifications to the CCC coils, continuous counter-current extraction (CCCE) is possible, where one phase is pumped in the opposite direction to the other and the sample is introduced continuously into the centre of the coil (Ito et al. 2006; Van den Heuvel 2007).
The method also allows a two dimensional procedure to be applied. The purification of very similar terpene lactones from G. biloba L, such as bilobalide, ginkgolides A, B, C, and J, is an example. The partitioning experiment assisted the design of a 2D procedure using a pair of orthogonal solvent systems: chloroform–methanol–water (10:7:3) and hexane–ethyl acetate–methanol–water (4:6:4:6) with addition of 0.5 % DMSO to increase the resolution of ginkgolides A and B. This approach separated these almost equipolar lactones (Qiu et al. 2012).
The main drawbacks of the technique, particularly when compared to preparative HPLC and other solid phase chromatography techniques, include a lower efficiency. When measured in terms of theoretical plates, the efficiency of a “good” CCC apparatus is in the low thousand plate range. This figure cannot be directly compared to HPLC plate counts due to the much higher percentage of stationary phase (around 80 % compared to perhaps 5 % active stationary phase sites in HPLC) and the resolution of CCC can be extremely good. However, a low efficiency results in broad peaks, making the technique far more suited to a preparative application as opposed to an analytical one. Also the narrow polarity range within each run should be emphasised. As mentioned above, a wide range of polarities can be processed by CCC by using different solvent systems. However, a single CCC run operates over a relatively narrow polarity window. Although some examples of gradient elution have been reported (see Table 2), these do not give as wide a polarity range as that achievable with HPLC. The narrow polarity window can be used to advantage when it is desired to pluck a single target compound from a complex mixture. However, it is a disadvantage when a dozen pure compounds are required from the mixture in a single purification run. The CCC apparatus does not inherently lend itself to easy automation and thus its operation can appear labour-intensive. In addition, the instruments have not received the same intensive commercial development that has made modern HPLC and GC equipment so sophisticated. This situation will undoubtedly improve in the future, with advances in CCC machine design. Finally, the solvent system selection is undoubtedly time-consuming. As the two phases are liquid, changes in one phase directly affects the other. Analysis of a range of possible solvent systems can be performed by an automated liquid-handling robot but this still requires a number of hours to complete. This is a current area of research in the field and no doubt large improvements in solvent system selection time can be expected over the next few years.
Conclusions
Although initially dismissed by many chemists and purification scientists as slow, unreliable and temperamental, steady development of the technique of CCC on both the engineering and the application side has transformed it into a technique worthy of inclusion in the natural product scientist’s arsenal. On one side, engineering developments have produced machines that are robust, capable of fast, efficient separations and able to accept high injection loadings. On the other side, developments in the application protocols have produced modes of operation and solvent systems to purify out compounds from the full polarity spectrum. Combined, these have produced a technique that is wonderfully suited to natural product purifications, particularly on a large preparative scale, with advantages over solid phase techniques such as the ability to accept particulates and to always recover all components, and advantages over the old-style liquid–liquid techniques such as high speed, high loading and high resolution. To assist users of CCC and those wishing to experiment with the technique, a comprehensive phase selection table has been generated by distilling the solvent system information presented from CCC terpenoids purifications over the last 30 years.
References
- Abbott T, Peterson R, McAlpine J, et al. Comparing centrifugal countercurrent chromatography, nonaqueous reversed phase HPLC and AG ion exchange HPLC for the separation and characterization of triterpene acetates. J Liq Chromatogr Relat Technol. 1989;12:2281–2301. [Google Scholar]
- Acton N, Klayman DL, Rollman IJ, et al. Isolation of artemisinin (ginghaosu) and its separation from artimisitene using the Ito multilayer coil separator-extractor and isolation of arteannuin B. J Chromatogr. 1986;355:448–450. [Google Scholar]
- Agostinho D, Boudesocque L, Thery-Kone I, et al. A new meroterpenoid isolated from roots of Ptaeroxylon obliquum Radlk. Phytochem Lett. 2013;6:560–566. [Google Scholar]
- Ajikumar PK, Tyo K, Carlsen S, et al. Terpenoids: opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol Pharm. 2008;5:167–190. doi: 10.1021/mp700151b. [DOI] [PubMed] [Google Scholar]
- Aman R, Carle R, Conrad J, et al. Isolation of carotenoids from plant materials and dietary supplements by high-speed counter-current chromatography. J Chromatogr A. 2005;1074:99–105. doi: 10.1016/j.chroma.2005.03.055. [DOI] [PubMed] [Google Scholar]
- Baldermann S, Reinhard A, Köhler N, et al. Application of high-speed counter-current chromatography for the isolation of 9′-cis-neoxanthin from fresh spinach. J Chromatogr A. 2007;1151:183–186. doi: 10.1016/j.chroma.2007.02.064. [DOI] [PubMed] [Google Scholar]
- Baldermann S, Ropeter K, Köhler N, et al. Isolation of all-trans lycopene by high-speed counter-current chromatography using a temperature-controlled solvent system. J Chromatogr A. 2008;192:191–193. doi: 10.1016/j.chroma.2008.03.084. [DOI] [PubMed] [Google Scholar]
- Berthod A, Hassoun M. Using the liquid nature of the stationary phase in countercurrent chromatography: IV. The co-current CCC method. J Chromatogr A. 2006;1116:143–148. doi: 10.1016/j.chroma.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Berthod A, Ruiz-Angel MJ, Carda-Broch S. Elution-extrusion countercurrent chromatography: use of the liquid nature of the stationary phase to extend the hydrophobicity window. Anal Chem. 2003;75:5508–5517. doi: 10.1021/ac030208d. [DOI] [PubMed] [Google Scholar]
- Brown GD. The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao) Molecules. 2010;15:7603–7698. doi: 10.3390/molecules15117603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Tian Y, Zhang TY, Liu QH, et al. Separation of dammarane-saponins from notoginseng, root of Panax notoginseng (Burk.) F.H.Chen by HSCCC coupled with evaporative light scattering detector. J Liq Chromatogr Relat Technol. 2003;26:1579–1591. [Google Scholar]
- Chen F, Lu HT, Jiang Y. Purification of paeoniflorin from Paeonia lactiflora Pall. by high speed counter current chromatography. J Chromatogr A. 2004;1040:205–208. doi: 10.1016/j.chroma.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Chen F, Li HB, Wong R, et al. Isolation and purification of the bioactive carotenoid zeaxanthin from the microalga Microcystis aeruginosa by high-speed counter-current chromatography. J Chromatogr A. 2005;1064:183–186. doi: 10.1016/j.chroma.2004.12.065. [DOI] [PubMed] [Google Scholar]
- Chen Q, Hu X, Li J, et al. Preparative isolation and purification of cuminaldehyde and p-menta-1,4-dien-7-al from the essential oil of Cuminum cyminum L. by high-speed counter-current chromatography. Anal Chim Acta. 2011;689:149–154. doi: 10.1016/j.aca.2011.01.038. [DOI] [PubMed] [Google Scholar]
- Chen F, Luo J, Kong L. Fast isolation of ginsenosides Re and Rg1 from the roots of Panax ginseng by HSCCC-ELSD combined with MCI gel CC guided by HPLC-MS. J Liq Chromatogr Relat Technol. 2012;35:912–923. [Google Scholar]
- Cheng Y, Liang Q, Hu P, et al. Combination of normal-phase medium-pressure liquid chromatography and high-performance counter-current chromatography for preparation of ginsenoside-Ro from Panax ginseng with high recovery and efficiency. Sep Purif Technol. 2010;73:397–402. [Google Scholar]
- Cheng Y, Zhang M, Liang Q, et al. Two-step preparation of ginsenoside-Re, Rb1, Rc and Rb2 from the root of Panax ginseng by high-performance counter-current chromatography. Sep Purif Technol. 2011;77:347–354. [Google Scholar]
- Cheng CR, Li YF, Xu PP. Preparative isolation of triterpenoids from Ganoderma lucidum by counter-current chromatography combined with pH-zone-refining. Food Chem. 2012;130:1010–1016. [Google Scholar]
- Cicek SS, Schwaiger S, Ellmerer EP, et al. Development of a fast and convenient method for the isolation of triterpene saponins from Actaea racemosa by high-speed countercurrent chromatography coupled with evaporative light scattering detection. Planta Med. 2010;76:467–473. doi: 10.1055/s-0029-1186236. [DOI] [PubMed] [Google Scholar]
- da Silva VP, Oliveira RR, Figueiredo MR. Isolation of limonoids from seeds of Carapa guianensis Aublet (Meliaceae) by high-speed countercurrent chromatography. Phytochem Anal. 2009;20:77–81. doi: 10.1002/pca.1100. [DOI] [PubMed] [Google Scholar]
- Dang YY, Li XC, Zhang QW, et al. Preparative isolation and purification of six volatile compounds from essential oil of Curcuma wenyujin using high-performance centrifugal partition chromatography. J Sep Sci. 2010;33:1658–1664. doi: 10.1002/jssc.200900453. [DOI] [PubMed] [Google Scholar]
- De Souza PA, Rangel LP, Oigman SS, et al. Isolation of two bioactive diterpenic acids from Copaifera glycycarpa oleoresin by high-speed counter-current chromatography. Phytochem Anal. 2010;21:539–543. doi: 10.1002/pca.1229. [DOI] [PubMed] [Google Scholar]
- Diallo B, Vanhaelen M. Large scale purification of apocarotenoids from Cocleospermum tinctorium by counter-current chromatography. J Liq Chromatogr. 1988;11:227–231. [Google Scholar]
- Diallo B, Vanhaelen Fastre R, Vanhaelen M. Direct coupling of high speed counter current chromatography to thin layer chromatography. Application to the separation of asiaticoside and madecassoside from Centella asiatica. J Chromatogr. 1991;558:446–450. [Google Scholar]
- dos Santos BCB, da Silva JCT, Guerrero PG, Jr, et al. Isolation of chavibetol from essential oil of Pimenta pseudocaryophyllus leaf by high-speed counter-current chromatography. J Chromatogr A. 2009;1216:4303–4306. doi: 10.1016/j.chroma.2009.01.111. [DOI] [PubMed] [Google Scholar]
- Du Q, Gao S. Preparative separation of saponins from the Luffa cylindeica (L.) Roem. by slow rotary countercurrent chromatography. J Liq Chromatogr Relat Technol. 2006;29:2451–2456. [Google Scholar]
- Du Q, Yuan J. Preparation of triterpene saponins from the fruit of L. by high speed countercurrent chromatography (HSCCC) J Liq Chromatogr Relat Technol. 2005;28:1717–1724. [Google Scholar]
- Du Q, Xiong X, Ito Y. Separation of bioactive quadri-terpenic acids from the fruit of Ligustrum lucidum Ait by high-speed countercurrent chromatography. J Liq Chromatogr. 1995;18:1997–2004. [Google Scholar]
- Du Q, Jerz G, Waibel R, et al. Isolation of dammarane saponins from Panax notoginseng by high speed counter current chromatography. J Chromatogr A. 2003;1008:173–180. doi: 10.1016/s0021-9673(03)00988-9. [DOI] [PubMed] [Google Scholar]
- Du Q, Jerz G, Winterhalter P. Separation of andrographolide and neoandrographolide from the leaves of Andrographis paniculata using high speed counter current chromatography. J Chromatogr A. 2003;984:147–151. doi: 10.1016/s0021-9673(02)01831-9. [DOI] [PubMed] [Google Scholar]
- Du Q, Jerz G, Chen P, et al. Preparation of ursane triterpenoids from Centella asiatica using high speed countercurrent chromatography with step-gradient elution. J Liq Chromatogr Relat Technol. 2004;27:2201–2215. [Google Scholar]
- Du Q, Daijie W, Ito Y. Preparation of solanesol from a tobacco leaf extract using high speed countercurrent chromatography. J Liq Chromatogr Relat Technol. 2006;29:2587–2592. [Google Scholar]
- Du D, Zhu F, Chen X, et al. Rapid isolation and purification of inotodiol and trametenolic acid from Inonotus obliquus by high-speed counter-current chromatography with evaporative light scatting detection. Phytochem Anal. 2011;22:419–423. doi: 10.1002/pca.1297. [DOI] [PubMed] [Google Scholar]
- Engelberth AS, Clausen EC, Carrier DJ. Comparing extraction methods to recover ginseng saponins from American ginseng (Panax quinquefolium), followed by purification using fast centrifugal partition chromatography with HPLC verification. Sep Purif Technol. 2010;72:1–6. [Google Scholar]
- Fan JP, He CH. Single-step preparative separation of barbinervic acid and its epimer (rotungenic acid), along with two other pentacyclic triterpene acids from the leaves of Diospyros kaki using HSCCC. J Liq Chromatogr Relat Technol. 2006;29:815–826. [Google Scholar]
- Fischedick JT, Standiford M, Johnson DA, et al. Activation of antioxidant response element in mouse primary cortical cultures with sesquiterpene lactones isolated from Tanacetum parthenium. Planta Med. 2012;78:1725–1730. doi: 10.1055/s-0032-1315241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischedick JT, Pesic M, Podolski-Renic A, et al. Cytotoxic activity of sesquiterpene lactones from Inula britannica on human cancer cell lines. Phytochem Lett. 2013;6:246–252. [Google Scholar]
- Fischedick JT, Standiford M, Johnson DA, et al. Structure activity relationship of phenolic diterpenes from Salvia officinalis as activators of the nuclear factor E2-related factor 2 pathway. Bioorgan Med Chem. 2013;21:2618–2622. doi: 10.1016/j.bmc.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer N, Weinreich B, Nitz S, et al. Applications of high speed counter current chromatography for the separation and isolation of natural products. J Chromatogr. 1991;538:193–202. [Google Scholar]
- Fisher D, Garrard IJ, van den Heuvel R, et al. Technology transfer and scale up of a potential cancer preventative plant dynamic extraction of glucoraphanin. J Liq Chromatogr Relat Technol. 2005;28:1913–1922. [Google Scholar]
- Frighetto N, Welendorf RM, Pereira da Silva AM, et al. Purification of betulinic acid from Eugenia florida (Myrtaceae) by high-speed counter-current chromatography. Phytochem Anal. 2005;16:411–414. doi: 10.1002/pca.860. [DOI] [PubMed] [Google Scholar]
- Frighetto RTS, Welendorf RM, Nigro EN, et al. Isolation of ursolic acid from apple peels by high speed counter-current chromatography. Food Chem. 2008;106:767–771. [Google Scholar]
- Fullas F, Choi YH, Kinghorn D, et al. Sweet-tasting triterpene glycoside constituents of Abrus fruticulosus. Planta Med. 1990;56:332–333. doi: 10.1055/s-2006-960974. [DOI] [PubMed] [Google Scholar]
- Garrard IJ. Simple approach to the development of a CCC solvent selection protocol suitable for automation. J Liq Chromatogr Relat Technol. 2005;28:1923–1935. [Google Scholar]
- Garrard IJ, Janaway L, Fisher D. Minimising solvent usage in high speed, high loading and high resolution isocratic dynamic extraction. J Liq Chromatogr Relat Technol. 2007;30:151–163. [Google Scholar]
- Garrard IJ, Fisher D, Sutherland IA. Dynamic extraction: a high speed, high capacity purification process that is rapidly scaleable. LC/GC N Am. 2008;26:2–7. [Google Scholar]
- Gasper MP, Berthod A, Talabardon K, et al. An evaluation of the differential partitioning and separation of C60 and C70 fullerenes in a biphasic system using centrifugal partition chromatography. J Liq Chromatogr Relat Technol. 1995;18:1019–1034. [Google Scholar]
- Geng Y, Liu J, Lv R, et al. An efficient method for extraction, separation and purification of eugenol from Eugenia caryophyllata by supercritical fluid extraction and high-speed counter-current chromatography. Sep Purif Technol. 2007;57:237–241. [Google Scholar]
- Gosse BK, Gnabre JN, Ito Y, et al. Isolation of saponins with viral entry inhibitory activity by combined chromatographic methods. J Liq Chromatogr Relat Technol. 2002;25:3199–3211. [Google Scholar]
- Graham AS, McConvey IF, Shering P. An evaluation of the performance of a preparative CCC machind for the separation of an active pharmaceutical ingredient. J Liq Chromatogr Relat Technol. 2001;24:1811–1825. [Google Scholar]
- Grancher D, Jaussaud P, Durix A, et al. Countercurrent chromatographic isolation of lolitrem B from endophyte infected ryegrass (Lolium perenne L) seed. J Chromatogr A. 2004;159:73–81. doi: 10.1016/j.chroma.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Graziose R, Rathinasabapathy T, Lategan C, et al. Antiplasmodial activity of aporphine alkaloids and sesquiterpene lactones from Liriodendron tulipifera L. J Ethnopharmacol. 2011;133:26–30. doi: 10.1016/j.jep.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, Zhang G, Su Z, et al. Identification of major active constituents in the fingerprint of Salvia miltiorrhiza Bunge developed by high speed counter current chromatography. J Chromatogr A. 2004;1041:239–243. doi: 10.1016/j.chroma.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Gu M, Su Z, Ouyang F. Fingerprinting of Salvia miltiorrhiza Bunge by thin-layer chromatography scan compared with high speed countercurrent chromatography. J Liq Chromatogr Relat Technol. 2006;29:1503–1514. [Google Scholar]
- Gutiérrez F, Estévez-Braun A, Ravelo AG, et al. Terpenoids from the medicinal plant Maytenus ilicifolia. J Nat Prod. 2007;70:1049–1052. doi: 10.1021/np070019g. [DOI] [PubMed] [Google Scholar]
- Ha YW, Kim YS. Preparative isolation of six major saponins from Platycodiradix by high-speed counter-current chromatography. Phytochem Anal. 2009;20:207–213. doi: 10.1002/pca.1116. [DOI] [PubMed] [Google Scholar]
- Ha YW, Lim SS, Ha IJ, et al. Preparative isolation of four ginsenosides from Korean red ginseng (steam-treated Panax ginseng C. A. Meyer), by high-speed counter-current chromatography coupled with evaporative light scattering detection. J Chromatogr A. 2007;1151:37–44. doi: 10.1016/j.chroma.2007.01.038. [DOI] [PubMed] [Google Scholar]
- Ha IJ, Kang M, Na YC, et al. Preparative separation of minor saponins from Platycodi Radix by high-speed counter-current chromatography. J Sep Sci. 2011;34:2559–2565. doi: 10.1002/jssc.201100326. [DOI] [PubMed] [Google Scholar]
- Hamzaoui M, Renault JH, Nuzillard JM, et al. Stepwise elution of a three-phase solvent system in centrifugal partition extraction: a new strategy for the fractionation and phytochemical screening of a crude bark e xtract. Phytochem Anal. 2013;24:367–373. doi: 10.1002/pca.2418. [DOI] [PubMed] [Google Scholar]
- Han X, Zhang T, Ito Y. Separation of high purity przewaquinone A by high speed countercurrent chromatography. J Liq Chromatogr Relat Technol. 2003;26:1267–1274. doi: 10.1080/10826076.2011.556965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han QB, Song JZ, Qiao CF, et al. Preparative isolation of cyclolanostane-type saponins from Astragalus membranaceus Bge. var. mongholicus (Bge.) Hsiao by TLC-MS/MS guided high-speed counter-current chromatography. J Sep Sci. 2007;30:135–140. doi: 10.1002/jssc.200600264. [DOI] [PubMed] [Google Scholar]
- Han QB, Wong L, Yang NY, et al. A simple method to optimize the HSCCC two-phase solvent system by predicting the partition coefficient for target compound. J Sep Sci. 2008;31:1189–1194. doi: 10.1002/jssc.200700582. [DOI] [PubMed] [Google Scholar]
- Han QB, Wong L, Lai F, et al. Preparative isolation of pseudolaric acids A and B and their glucosides from the root bark of Pseudolarix kaempferi using high-speed counter-current chromatography. J Sep Sci. 2009;32:309–313. doi: 10.1002/jssc.200800547. [DOI] [PubMed] [Google Scholar]
- He W, Van Puyvelde L, Maes L, et al. Antitrichomonas in vitro activity of Cussonia holstii Engl. Nat Prod Res. 2002;17:127–133. doi: 10.1080/1478641031000103713. [DOI] [PubMed] [Google Scholar]
- He F, Bai Y, Wang J, et al. Isolation and purification of oridonin from the whole plant of Isodon rubescens by high-speed counter-current chromatography. Molecules. 2011;16:7949–7957. doi: 10.3390/molecules16097949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Li S, Yang J, et al. Application of step-wise gradient high-performance counter-current chromatography for rapid preparative separation and purification of diterpene components from Pseudolarix kaempferi Gordon. J Chromatogr A. 2012;1235:34–38. doi: 10.1016/j.chroma.2012.01.040. [DOI] [PubMed] [Google Scholar]
- Hewitson P, Ignatova S, Ye H, et al. Intermittent counter-current extraction as an alternative approach to purification of Chinese herbal medicines. J Chromatogr A. 2009;1216:4187–4192. doi: 10.1016/j.chroma.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Hu J, Liang Y, Xie Y, et al. Isolation and purification of solanesol from potato leaves by high-speed counter-current chromatography and identification by atmospheric pressure chemical ionization mass spectrometry. Chin J Chromatogr. 2007;25:528–531. [PubMed] [Google Scholar]
- Huang XY, Fu JF, Di DL. Preparative isolation and purification of steviol glycosides from Stevia rebaudiana Bertoni using high-speed counter-current chromatography. Sep Purif Technol. 2010;71:220–224. [Google Scholar]
- Huang J, Xu X, Xie C, et al. Isolation and purification of paeoniflorin and albiflorin from radix Paeoniae rubra by high-speed counter-current chromatography. J Liq Chromatogr Relat Technol. 2013;36:419–427. [Google Scholar]
- Ikai Y, Oka H, Hayakawa J, et al. Isolation of colistin C and B using high-speed countercurrent chromatography. J Liq Chromatogr Relat Technol. 1998;21:143–155. [Google Scholar]
- Ito Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J Chromatogr A. 2005;1065:145–168. doi: 10.1016/j.chroma.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Ito Y, Weinstein M, Aoki I, et al. The coil planet centrifuge. Nature. 1966;212:985–987. doi: 10.1038/212985a0. [DOI] [PubMed] [Google Scholar]
- Ito Y, Oka H, Lee YW. Improved high speed counter current chromatograph with three multilayer coils connected in series. II. Separation of various biological samples with a semipreparative column. J Chromatogr. 1990;498:169–178. doi: 10.1016/s0021-9673(01)84245-x. [DOI] [PubMed] [Google Scholar]
- Ito Y, Goto T, Yamada S, et al. Application of dual counter-current chromatography for rapid sample preparation of N-methylcarbamate pesticides in vegetable oil and citrus fruit. J Chromatogr A. 2006;1108:20–25. doi: 10.1016/j.chroma.2005.12.070. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lu HT, Chen F. Preparative purification of glycyrrhizin extracted from the root of liquorice using high-speed counter-current chromatography. J Chromatogr A. 2004;1033:183–186. doi: 10.1016/j.chroma.2004.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Chen W, Liu S, et al. Preparation of crocin from Gardenia yellow pigment by slow rotary countercurrent chromatography. Chin J Chromatogr. 2011;29:277–280. doi: 10.3724/sp.j.1123.2011.00277. [DOI] [PubMed] [Google Scholar]
- Kim SM, Shang YF, Um BH. A preparative method for isolation of fucoxanthin from Eisenia bicyclis by centrifugal partition chromatography. Phytochem Anal. 2011;22:322–329. doi: 10.1002/pca.1283. [DOI] [PubMed] [Google Scholar]
- Knapp H, Straubinger M, Fornari S, et al. {S}-3,7-dimethyl-5-octene-1,7-diol and related oxygenated monoterpenoids from petals of Rosa damascena Mill. J Agric Food Chem. 1998;46:1966–1970. [Google Scholar]
- Koch A, Basar S, Richter R. TLC of mono- and sesquiterpenes. In: Waksmundzka-Hajnos M, Sherma J, Kowalska T, editors. Chromatography science series. Boca Raton: CRC Press; 2008. pp. 451–480. [Google Scholar]
- Lechtenberg M, Schepmann D, Niehues M, et al. Quality and functionality of saffron: quality control, species assortment and affinity of extract and isolated saffron compounds to NMDA and σ1 (Sigma-1) receptors. Planta Med. 2008;74:764–772. doi: 10.1055/s-2008-1074535. [DOI] [PubMed] [Google Scholar]
- Lee JH, Ha YW, Jeong CS, et al. Isolation and tandem mass fragmentations of an anti-inflammatory compound from Aralia elata. Arch Pharmacal Res. 2009;32:831–840. doi: 10.1007/s12272-009-1603-5. [DOI] [PubMed] [Google Scholar]
- Li HB, Chen F. Preparative isolation and purification of astaxanthin from the microalga Chlorococcum sp. by high-speed counter-current chromatography. J Chromatogr. 2001;925:133–137. doi: 10.1016/s0021-9673(01)01022-6. [DOI] [PubMed] [Google Scholar]
- Li HB, Chen F. Preparative isolation and purification of six diterpenoids from the Chinese medicinal plant Salvia miltiorrhiza by high-speed counter-current chromatography. J Chromatogr A. 2001;925:109–114. doi: 10.1016/s0021-9673(01)01026-3. [DOI] [PubMed] [Google Scholar]
- Li HB, Chen F, Zhang TY, et al. Preparative isolation and purification of lutein from the microalga Chlorella vulgaris by high-speed counter-current chromatography. J Chromatogr. 2001;905:151–155. doi: 10.1016/s0021-9673(00)00987-0. [DOI] [PubMed] [Google Scholar]
- Li A, Sun A, Liu R. Preparative isolation and purification of costunolide and dehydrocostuslactone from Aucklandia lappa Decne by high-speed counter-current chromatography. J Chromatogr A. 2005;1076:193–197. doi: 10.1016/j.chroma.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Li HB, Fan KW, Chen F. Isolation and purification of canthaxanthin from the microalga Chlorella zofingiensis by high-speed counter-current chromatography. J Sep Sci. 2006;29:699–703. doi: 10.1002/jssc.200500365. [DOI] [PubMed] [Google Scholar]
- Li XC, Zhang QW, Yin ZQ, et al. Preparative separation of patchouli alcohol from patchouli oil using high performance centrifugal partition chromatography. J Essent Oil Res. 2011;23:19–24. [Google Scholar]
- Liang J, Meng J, Guo M, et al. Conical coils counter-current chromatography for preparative isolation and purification of tanshinones from Salvia miltiorrhiza Bunge. J Chromatogr A. 2013;1288:35–39. doi: 10.1016/j.chroma.2013.02.079. [DOI] [PubMed] [Google Scholar]
- Liu S, Cao M, Li D, et al. Purification and anticancer activity investigation of pentacyclic triterpenoids from the leaves of Sinojackia sarcocarpa L.Q. Luo by high-speed counter-current chromatography. Nat Prod Res. 2011;25:1600–1606. doi: 10.1080/14786419.2010.496115. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu R, Sun A, et al. Connection of high-speed counter-current chromatography with evaporative light scattering detector by flow injection and its application for preparative isolation and purification of ginkgolide compounds from Ginkgo biloba L. J Liq Chromatogr Relat Technol. 2013;36:2317–2329. [Google Scholar]
- Liu M, Yang F, Shi H, et al. Preparative separation of triterpene alcohol ferulates from rice bran oil using a high performance counter-current chromatography. Food Chem. 2013;139:919–924. doi: 10.1016/j.foodchem.2013.01.106. [DOI] [PubMed] [Google Scholar]
- Lu HT, Jiang Y, Chen F. Preparative separation and purification of squalene from the microalga Thraustochytrium ATCC 26185 by high speed counter current chromatography. J Chromatogr. 2003;994:37–43. doi: 10.1016/s0021-9673(03)00454-0. [DOI] [PubMed] [Google Scholar]
- Lu Y, Sun C, Pan Y. Isolation and purification of oridonin from Rabdosia rubescens using upright counter-current chromatography. J Sep Sci. 2006;29:314–318. doi: 10.1002/jssc.200500326. [DOI] [PubMed] [Google Scholar]
- Lu Y, Sun C, Liu R, Pan Y. Effective two-dimensional counter-current chromatographic method for simultaneous isolation and purification of oridonin and ponicidin from the crude extract of Rabdosia rubescens. J Chromatogr A. 2007;1146:125–130. doi: 10.1016/j.chroma.2007.01.141. [DOI] [PubMed] [Google Scholar]
- Ma Y, Aisha HA, Liao L, et al. Preparative isolation and purification of rupestonic acid from the Chinese medicinal plant Artemisia rupestris L. by high-speed counter-current chromatography. J Chromatogr A. 2005;1076:198–201. doi: 10.1016/j.chroma.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Ma J, Chen Q, Lai D, et al. Separation and purification of triterpene saponins from roots of Radix Phytolaccae by high-speed countercurrent chromatography coupled with evaporative light scattering detection. J Liq Chromatogr Relat Technol. 2010;33:563–571. doi: 10.1080/10826070903574659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston A, Borel C, Hostettmann K. Separation of natural products by centrifugal partition chromatography. J Chromatogr. 1988;450:91–99. [Google Scholar]
- Martin AM, Queiroz EF, Marston A, et al. Labdane diterpenes from Juniperus communis L. berries. Phytochem Anal. 2006;17:32–35. doi: 10.1002/pca.878. [DOI] [PubMed] [Google Scholar]
- Maurya A, Srivastava SK. Preparative-scale separation of anticancer triterpenes from Eucalyptus hybrid by centrifugal partition chromatography. Sep Sci Technol. 2011;46:1189–1194. [Google Scholar]
- Medhi B, Patyar S, Rao RS, et al. Pharmacokinetic and toxicological profile of artemisinin compounds: an update. Pharmacology. 2009;84:323–332. doi: 10.1159/000252658. [DOI] [PubMed] [Google Scholar]
- Mudge E, Lopes-Lutz D, Brown PN, et al. Purification of phenylalkanoids and monoterpene glycosides from Rhodiola rosea L. roots by high-speed counter-current chromatography. Phytochem Anal. 2013;24:129–134. doi: 10.1002/pca.2391. [DOI] [PubMed] [Google Scholar]
- Nasser ALM, Mazzolin LP, Hiruma-Lima CA, et al. Preparative droplet counter-current chromatography for the separation of the new nor-seco-triterpene and pentacyclic triterpenoids from Qualea parviflora. Chromatographia. 2006;64:695–699. [Google Scholar]
- Oka F, Oka H, Ito Y. Systematic search for suitable two-phase solvent systems for high speed counter-current chromatography. J Chromatogr. 1991;538:99–108. doi: 10.1016/s0021-9673(01)91626-7. [DOI] [PubMed] [Google Scholar]
- Peng A, Li R, Hu J, et al. Flow rate gradient high-speed counter-current chromatography separation of five diterpenoids from Triperygium wilfordii and scale-up. J Chromatogr A. 2008;1200:129–135. doi: 10.1016/j.chroma.2008.05.041. [DOI] [PubMed] [Google Scholar]
- Peng J, Dong F, Qi Y, et al. Preparative separation of four triterpene saponins from Radix Astragali by high-speed counter-current chromatographycoupled with evaporative light scattering detection. Phytochem Anal. 2008;19:212–217. doi: 10.1002/pca.1011. [DOI] [PubMed] [Google Scholar]
- Pinel B, Audo G, Mallet S, et al. Multi-grams scale purification of xanthanolides from Xanthium macrocarpum. Centrifugal partition chromatography versus silica gel chromatography. J Chromatogr A. 2007;1151:14–19. doi: 10.1016/j.chroma.2007.02.115. [DOI] [PubMed] [Google Scholar]
- Puertas Mejia M, Hillebrand S, Stashenko E, et al. In vitro radical scavenging activity of essential oils from Columbian plants and fractions from oregano (Origanum vulgare L) essential oil. Flavour Fragr J. 2002;17:380–384. [Google Scholar]
- Qi X, Ignatova S, Luo G, et al. Preparative isolation and purification of ginsenosides Rf, Re, Rd and Rb1 from the roots of Panax ginseng with a salt/containing solvent system and flow step-gradient by high performance counter-current chromatography coupled with an evaporative light scattering detector. J Chromatogr A. 2010;1217:1995–2001. doi: 10.1016/j.chroma.2010.01.057. [DOI] [PubMed] [Google Scholar]
- Qiu F, Friesen J, McAlpinea JB, et al. Design of countercurrent separation of Ginkgo biloba terpene lactones by nuclear magnetic resonance. J Chromatogr A. 2012;1242:26–34. doi: 10.1016/j.chroma.2012.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha GG, Simões M, Lúcio KA, et al. Natural triterpenoids from Cecropia lyratiloba are cytotoxic to both sensitive and multidrug resistant leukemia cell lines. Bioorgan Med Chem. 2007;15:7355–7360. doi: 10.1016/j.bmc.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Rodrigues VF, Carmo HM, Oliveira RR, et al. Isolation of terpenoids from Trichilia quadrijuga (Meliaceae) by droplet counter-current chromatography. Chromatographia. 2009;70:1191–1195. [Google Scholar]
- Roscher R, Winterhalter P. 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN) formation in wine. Application of multilayer coil countercurrent chromatography for the study of Vitis vinifera cv. Riesling leaf glycosides. J Agric Food Chem. 1993;41:1452–1457. [Google Scholar]
- Severino VGP, Cazal CM, Forim MR, et al. Isolation of secondary metabolites from Hortia oreadica (Rutaceae) leaves through high-speed counter-current chromatography. J Chromatogr A. 2009;1216:4275–4281. doi: 10.1016/j.chroma.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Shehzad O, Jin Ha I, et al. Development of a rapid and convenient method to separate eight ginsenosides from Panax ginseng by high-speed counter-current chromatography coupled with evaporative light scattering detection. J Sep Sci. 2011;34:1116–1122. doi: 10.1002/jssc.201000932. [DOI] [PubMed] [Google Scholar]
- Shehzad O, Khan S, Ha IJ, et al. Rational development of a selection model for solvent gradients in single-step separation of ginsenosides from Panax ginseng using high-speed counter-current chromatography. J Sep Sci. 2012;35:1462–1469. doi: 10.1002/jssc.201200135. [DOI] [PubMed] [Google Scholar]
- Shehzad O, Kim HP, Kim YS. State-of-the-art separation of ginsenosides from Korean white and red ginseng by countercurrent chromatography. Anal Bioanal Chem. 2013;405:4523–4530. doi: 10.1007/s00216-012-6609-z. [DOI] [PubMed] [Google Scholar]
- Shi S, Jiang D, Zhao M, et al. Preparative isolation and purification of triterpene saponins from Clematis mandshurica by high-speed counter-current chromatography coupled with evaporative light scattering detection. J Chromatogr B. 2007;852:679–683. doi: 10.1016/j.jchromb.2007.02.056. [DOI] [PubMed] [Google Scholar]
- Shi SY, Zhou HH, Huang KL, et al. Application of high-speed counter-current chromatography for the isolation of antiviral eremophilenolides from Ligularia atroviolacea. Biomed Chromatogr. 2008;22:985–991. doi: 10.1002/bmc.1017. [DOI] [PubMed] [Google Scholar]
- Shi X, Wang X, Wang D, et al. Separation and purification of α-cyperone from Cyperus rotundus with supercritical fluid extraction and high-speed counter-current chromatography. Sep Sci Technol. 2009;44:712–721. [Google Scholar]
- Shirota O, Nagamatsu K, Sekita S. Simple preparative isolation of salvinorin a from the hallucinogenic sage, Salvia divinorum, by centrifugal partition chromatography. J Liq Chromatogr Relat Technol. 2007;30:1105–1114. [Google Scholar]
- Shirota O, Nagamatsu K, Sekita S, et al. Preparative separation of the saponin lancemaside A from Codonopsis lanceolata by centrifugal partition chromatography. Phytochem Anal. 2008;19:403–410. doi: 10.1002/pca.1065. [DOI] [PubMed] [Google Scholar]
- Silva JCT, Jham GN, Oliveira RDL, et al. Purification of the seven tetranortriterpenoids in neem (Azadirachta indica) seed by counter-current chromatography sequentially followed by isocratic preparative reversed-phase high-performance liquid chromatography. J Chromatogr A. 2007;1151:203–210. doi: 10.1016/j.chroma.2007.03.086. [DOI] [PubMed] [Google Scholar]
- Skalicka-Woźniak K, Walasek M, Ludwiczuk A, et al. Isolation of terpenoids from Pimpinella anisum essential oil by high-performance counter-current chromatography. J Sep Sci. 2013;36:2611–2614. doi: 10.1002/jssc.201300407. [DOI] [PubMed] [Google Scholar]
- Slacanin I, Marston A, Hostettmann K, et al. The isolation of Eleutherococcus senticosus constituents by centrifugal partition chromatography and their quantitative determination by high performance liquid chromatography. Phytochem Anal. 1991;2:137–142. [Google Scholar]
- Sun A, Zhang Y, Li A, et al. Extraction and preparative purification of tanshinones from Salvia miltiorrhiza Bunge by high-speed counter-current chromatography. J Chromatogr B Anal Technol Biomed Life Sci. 2011;879:1899–1904. doi: 10.1016/j.jchromb.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Sutherland IA, Fisher D. Dynamic extraction technology. Innov Pharm Technol. 2004;10:68–71. [Google Scholar]
- Sutherland IA, Fisher D. Role of counter-current chromatography in the modernisation of Chinese herbal medicines. J Chromatogr A. 2009;1216:740–753. doi: 10.1016/j.chroma.2008.11.095. [DOI] [PubMed] [Google Scholar]
- Sutherland IA, Brown L, Forbes S, et al. Countercurrent chromatography (CCC) and its versatile application as an industrial purification and production process. J Liq Chromatogr Relat Technol. 1998;21:279–298. [Google Scholar]
- Tian G, Zhang Y, Zhang T, et al. Separation of tanshinones from Salvia miltiorrbiza Bunge by high-speed counter-current chromatography. J Chromatogr. 2000;904:107–111. doi: 10.1016/s0021-9673(00)00916-x. [DOI] [PubMed] [Google Scholar]
- Tian G, Zhang T, Zhang Y, et al. Separation of tanshinones from Salvia miltiorrhiza Bunge by multidimensional counter current chromatography. J Chromatogr A. 2002;945:281–285. doi: 10.1016/s0021-9673(01)01495-9. [DOI] [PubMed] [Google Scholar]
- Tsao R, Yang R. Lutein in selected Canadian crops and agri-food processing by-products and purification by high-speed counter-current chromatography. J Chromatogr A. 2006;1112:202–208. doi: 10.1016/j.chroma.2005.09.088. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel R (2007) Investigation into the mechanics and feasibility of continuous counter-current extraction. PhD Thesis, Brunel Univeristy, London, UK
- Wang G, Tang W, Bidigare RR. Terpenoids as therapeutic drugs and pharmaceutical agents. In: Zhang L, Demain AL, editors. Natural products: drug discovery and therapeutic medicine. Totowa: Humana Press Inc.; 2005. pp. 197–227. [Google Scholar]
- Wang J, Bai HL, Liu CM, et al. Isolation and purification of ginsenosides from plant extract of Panax quinquefolium L. by high performance centrifugal partition chromatography coupled with ELSD. Chromatographia. 2010;71:267–271. [Google Scholar]
- Wang D, Fang L, Wang X, et al. Preparative separation and purification of sesquiterpenoids from Tussilago farfara L. by high-speed counter-current chromatography. Quim Nova. 2011;34:804–807. [Google Scholar]
- Wang J, Liu CM, Li L, et al. Isolation of four high-purity dammarane saponins from extract of Panax notoginseng by centrifugal partition chromatography coupled with evaporative light scattering detection in one operations. Phytochem Anal. 2011;22:263–267. doi: 10.1002/pca.1274. [DOI] [PubMed] [Google Scholar]
- Wang D, Bai A, Lin X, et al. Efficient method for extraction and isolation of shionone from Aster tataricus L. f. by supercritical fluid extraction and high-speed counter-current chromatography. Acta Chromatogr. 2012;24:615–625. [Google Scholar]
- Wang Y, Liu M, Zheng L, et al. Preparative purification of five bioactive components from Agrimonia pilosa Ledeb by high-speed counter-current chromatography. J Sep Sci. 2012;35:1977–1984. doi: 10.1002/jssc.201200011. [DOI] [PubMed] [Google Scholar]
- Wang J, Yu J, Li L, et al. Isolation of high purity ginsenosides from plant extract of Panax ginseng by high performance centrifugal partition chromatography coupled with evaporative light scattering detection. J Liq Chromatogr Relat Technol. 2013;36:583–590. [Google Scholar]
- Wei Y, Zhang T, Xu G, et al. Application of analytical and preparative high-speed counter-current chromatography for separation of lycopene from crude extract of tomato paste. J Chromatogr A. 2001;929:169–173. doi: 10.1016/s0021-9673(01)01177-3. [DOI] [PubMed] [Google Scholar]
- Wei Y, Zhang T, Xu G, et al. Application of CCC for the separation of lutein from a crude extract of marigold flower petals. J Liq Chromatogr Relat Technol. 2003;26:1659–1669. [Google Scholar]
- Wei Y, Du J, Lu Y. Preparative separation of bioactive compounds from essential oil of Flaveria bidentis (L.) Kuntze using steam distillation extraction and one step high-speed counter-current chromatography. J Sep Sci. 2012;35:2608–2614. doi: 10.1002/jssc.201200266. [DOI] [PubMed] [Google Scholar]
- Wu S, Sun C, Wang K, et al. Preparative isolation and purification of celastrol from Celastrus orbicularis Thunb. by a new counter current chromatography method with an upright coil planet centrifuge. J Chromatogr A. 2004;1028:171–174. doi: 10.1016/j.chroma.2003.11.072. [DOI] [PubMed] [Google Scholar]
- Wu H, Su Z, Yang Y, et al. Isolation of three sesquiterpene lactones from the roots of Cichorium glandulosum Boiss. et Huet. by high-speed counter-current chromatography. J Chromatogr A. 2007;1176:217–222. doi: 10.1016/j.chroma.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Wu S, Yang L, Gao Y, et al. Multi-channel counter-current chromatography for high-throughput fractionation of natural products for drug discovery. J Chromatogr A. 2008;1180:99–107. doi: 10.1016/j.chroma.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Wu D, Jiang X, Wu S. Direct purification of tanshinones from Salvia miltiorrhiza Bunge by high-speed counter-current chromatography without presaturation of the two-phase solvent mixture. J Sep Sci. 2010;33:67–73. doi: 10.1002/jssc.200900491. [DOI] [PubMed] [Google Scholar]
- Xiang F, Yu J, Yin R, et al. Structure-activity relationship of Taxol inferring from docking Taxol analogues to microtubule binding site. Z Naturforsch. 2009;64c:551–556. doi: 10.1515/znc-2009-7-814. [DOI] [PubMed] [Google Scholar]
- Xiao X, Si X, Yuan Z, et al. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J Sep Sci. 2012;35:2313–2317. doi: 10.1002/jssc.201200231. [DOI] [PubMed] [Google Scholar]
- Xiao XH, Yuan ZQ, Li GK. Preparation of phytosterols and phytol from edible marine algae by microwave-assisted extraction and high-speed counter-current chromatography. Sep Purif Technol. 2013;104:284–289. [Google Scholar]
- Xie J, Wang S, Sun B, et al. Preparative separation and purification of β-caryophyllene from leaf oil of Vitex negundo L. var. heterophylla (Franch.) Rehd. by high speed countercurrent chromatography. J Liq Chromatogr Relat Technol. 2008;31:2621–2631. [Google Scholar]
- Xie J, Sun B, Wang S, et al. Isolation and purification of nootkatone from the essential oil of fruits of Alpinia oxyphylla Miquel by high-speed counter-current chromatography. Food Chem. 2009;117:375–380. doi: 10.1016/j.foodchem.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Yang Y, Zhong J, et al. Preparative isolation and purification of isobenzofuranone derivatives and saponins from seeds of Nigella glandulifera Freyn by high-speed counter-current chromatography combined with gel filtration. J Chromatogr A. 2009;1216:4258–4262. doi: 10.1016/j.chroma.2009.03.050. [DOI] [PubMed] [Google Scholar]
- Xu J, Luo J, Kong L. Simultaneous separation of triterpenoid saponins and flavonoid glycosides from the roots of Glycyrrhiza uralensis Fisch by pH-zone-refining counter-current chromatography. J Sep Sci. 2013;36:3295–3301. doi: 10.1002/jssc.201300410. [DOI] [PubMed] [Google Scholar]
- Yan J, Chen G, Tong S, et al. Preparative isolation and purification of germacrone and curdione from the essential oil of the rhizomes of Curcuma wenyujin by high-speed counter-current chromatography. J Chromatogr A. 2005;1070:207–210. doi: 10.1016/j.chroma.2005.02.064. [DOI] [PubMed] [Google Scholar]
- Yan G, Ji L, Luo Y, et al. Preparative isolation and purification of three sesquiterpenoid lactones from Eupatorium lindleyanum DC. by high-speed counter-current chromatography. Molecules. 2012;17:9002–9009. doi: 10.3390/molecules17089002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Ou Q, Yu W. Semi-preparative separation of taraxeryl-acetate and coumarins from Artemesia dalailamae Kraschen by high-speed countercurrent chromatography. J Liq Chromatogr. 1995;18:395–403. [Google Scholar]
- Yang Y, Gu D, Yili A, et al. One-step separation and purification of rupestonic acid and chrysosptertin B from Artemisia rupestris L. by high-speed counter-current chromatography. Phytochem Anal. 2010;21:205–209. doi: 10.1002/pca.1169. [DOI] [PubMed] [Google Scholar]
- Yang Y, Bakri M, Gu D, et al. Separation of (S)-dehydrovomifoliol from leaves of Nitraria sibirica Pall. by high-speed counter-current chromatography. J Liq Chromatogr Relat Technol. 2013;36:573–582. [Google Scholar]
- Yao S, Liu R, Huang X, et al. Preparative isolation and purification of chemical constituents from the root of Adenophora tetraphlla by high-speed counter-current chromatography with evaporative light scattering detection. J Chromatogr A. 2007;1139:254–262. doi: 10.1016/j.chroma.2006.11.056. [DOI] [PubMed] [Google Scholar]
- Yao S, Luo J, Huang X, et al. Application of preparative high-speed counter-current chromatography/preparative high-performance liquid chromatography mode in rapid separation of saponins. J Chromatogr B. 2008;864:69–77. doi: 10.1016/j.jchromb.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Ye H, Chen L, Li Y, et al. Preparative isolation and purification of three rotenoids and one isoflavone from the seeds of Millettia pachycarpa Benth by high-speed counter-current chromatography. J Chromatogr A. 2008;1178:101–107. doi: 10.1016/j.chroma.2007.11.060. [DOI] [PubMed] [Google Scholar]
- Ye H, Ignatova S, Luo H, et al. Preparative separation of a terpenoid and alkaloids from Tripterygium wilfordii Hook. using high-performance counter-current chromatography. Comparison of various elution and operating strategies. J Chromatogr A. 2008;1213:145–153. doi: 10.1016/j.chroma.2008.09.116. [DOI] [PubMed] [Google Scholar]
- Yoo KY, Park SY. Terpenoids as potential anti-Alzheimer’s disease therapeutics. Molecules. 2012;17:3524–3538. doi: 10.3390/molecules17033524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KD, Kim J. Application of centrifugal partition chromatography coupled with evaporative light scattering detection for the isolation of saikosaponins-a and -c from Bupleurum falcatum roots. J Sep Sci. 2009;32:74–78. doi: 10.1002/jssc.200800544. [DOI] [PubMed] [Google Scholar]
- Yoon KD, Chin YW, Ahn MJ, et al. Preparative isolation of alisol B and alisol b 23-acetate from Alismatis rhizoma by centrifugal partition chromatography coupled with ELSD. Chromatographia. 2009;69:791–793. [Google Scholar]
- Yuan Y, Wang B, Chen L, et al. How to realize the linear scale-up process for rapid purification using high-performance counter-current chromatography. J Chromatogr A. 2008;1194:192–198. doi: 10.1016/j.chroma.2008.04.049. [DOI] [PubMed] [Google Scholar]
- Zhang H, Feng B, Liu K, et al. Isolation and purification of two triterpenoids from the Chinese medicinal plant Fomes officinalis Ames. Asian J Chem. 2013;25:6130–6132. [Google Scholar]
- Zhang Y, Liu C, Qi Y, et al. Application of accelerated solvent extraction coupled with counter-current chromatography to extraction and online isolation of saponins with a broad range of polarity from Panax notoginseng. Sep Purif Technol. 2013;106:82–89. [Google Scholar]
- Zhao Y, Du Q. Separation of solanesol in tobacco leaves extract by slow rotary counter-current chromatography using a novel non-aqueous two-phase solvent system. J Chromatogr A. 2007;1151:193–196. doi: 10.1016/j.chroma.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Zhao C, He C. Preparative isolation and purification of atractylon and atractylenolide III from the Chinese medicinal plant Atractylodes macrocephala by high-speed counter-current chromatography. J Sep Sci. 2006;29:1630–1636. doi: 10.1002/jssc.200500464. [DOI] [PubMed] [Google Scholar]
- Zhao D, Yan M, Huang Y, et al. Efficient protocol for isolation and purification of different soyasaponins from soy hypocotyls. J Sep Sci. 2012;35:3281–3292. doi: 10.1002/jssc.201200531. [DOI] [PubMed] [Google Scholar]