Abstract
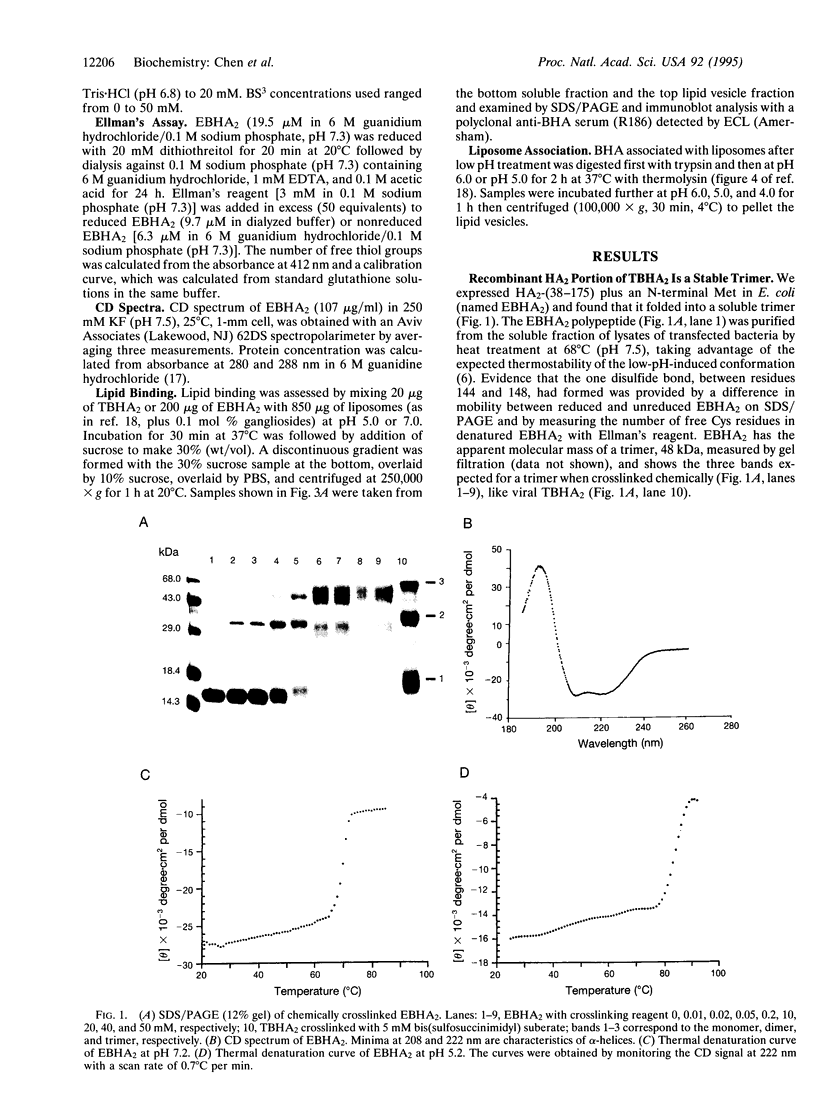
The extensive refolding of the membrane-anchoring chain of hemagglutinin (HA) of influenza virus (termed HA2) in cellular endosomes, which initiates viral entry by membrane fusion, suggests that viral HA is meta-stable. HA2 polypeptide residues 38-175 expressed in Escherichia coli are reported here to fold in vivo into a soluble trimer. The structure appears to be the same as the low-pH-induced conformation of viral HA2 by alpha-helical content, thermodynamic stability, protease dissection, electron microscopy, and antibody binding. These results provide evidence that the structure of the low-pH-induced fold of viral HA2 (TBHA2) observed crystallographically is the lowest-energy-state fold of the HA2 polypeptide. They indicate that the HA2 conformation in viral HA before low pH activation of its fusion potential is metastable and suggest that removal of the receptor-binding chain (HA1) is enough to allow HA2 to adopt the stable state. Further, they provide direct evidence that low pH is not required to form the membrane-fusion conformation but acts to make this state kinetically accessible in viral HA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brand C. M., Skehel J. J. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972 Aug 2;238(83):145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Bullough P. A., Hughson F. M., Skehel J. J., Wiley D. C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994 Sep 1;371(6492):37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- Carr C. M., Kim P. S. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993 May 21;73(4):823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- Daniels P. S., Jeffries S., Yates P., Schild G. C., Rogers G. N., Paulson J. C., Wharton S. A., Douglas A. R., Skehel J. J., Wiley D. C. The receptor-binding and membrane-fusion properties of influenza virus variants selected using anti-haemagglutinin monoclonal antibodies. EMBO J. 1987 May;6(5):1459–1465. doi: 10.1002/j.1460-2075.1987.tb02387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R. S., Douglas A. R., Skehel J. J., Wiley D. C. Analyses of the antigenicity of influenza haemagglutinin at the pH optimum for virus-mediated membrane fusion. J Gen Virol. 1983 Aug;64(Pt 8):1657–1662. doi: 10.1099/0022-1317-64-8-1657. [DOI] [PubMed] [Google Scholar]
- Daniels R. S., Downie J. C., Hay A. J., Knossow M., Skehel J. J., Wang M. L., Wiley D. C. Fusion mutants of the influenza virus hemagglutinin glycoprotein. Cell. 1985 Feb;40(2):431–439. doi: 10.1016/0092-8674(85)90157-6. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Gething M. J., Henneberry J., White J., Helenius A. Variant influenza virus hemagglutinin that induces fusion at elevated pH. J Virol. 1986 Feb;57(2):603–613. doi: 10.1128/jvi.57.2.603-613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Maeda T., Ohnishi S. Activation of influenza virus by acidic media causes hemolysis and fusion of erythrocytes. FEBS Lett. 1980 Dec 29;122(2):283–287. doi: 10.1016/0014-5793(80)80457-1. [DOI] [PubMed] [Google Scholar]
- Rott R., Orlich M., Klenk H. D., Wang M. L., Skehel J. J., Wiley D. C. Studies on the adaptation of influenza viruses to MDCK cells. EMBO J. 1984 Dec 20;3(13):3329–3332. doi: 10.1002/j.1460-2075.1984.tb02299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok R. W., Aitken A., Calder L. J., Martin S. R., Skehel J. J., Wharton S. A., Weis W., Wiley D. C. Studies on the structure of the influenza virus haemagglutinin at the pH of membrane fusion. J Gen Virol. 1988 Nov;69(Pt 11):2785–2795. doi: 10.1099/0022-1317-69-11-2785. [DOI] [PubMed] [Google Scholar]
- Ruigrok R. W., Martin S. R., Wharton S. A., Skehel J. J., Bayley P. M., Wiley D. C. Conformational changes in the hemagglutinin of influenza virus which accompany heat-induced fusion of virus with liposomes. Virology. 1986 Dec;155(2):484–497. doi: 10.1016/0042-6822(86)90210-2. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Bayley P. M., Brown E. B., Martin S. R., Waterfield M. D., White J. M., Wilson I. A., Wiley D. C. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci U S A. 1982 Feb;79(4):968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Waterfield M. D. Studies on the primary structure of the influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):93–97. doi: 10.1073/pnas.72.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C. W., Dopheide T. A. Influenza virus haemagglutinin. Structural predictions suggest that the fibrillar appearance is due to the presence of a coiled-coil. Aust J Biol Sci. 1980 Aug;33(4):441–447. doi: 10.1071/bi9800441. [DOI] [PubMed] [Google Scholar]
- Wharton S. A., Calder L. J., Ruigrok R. W., Skehel J. J., Steinhauer D. A., Wiley D. C. Electron microscopy of antibody complexes of influenza virus haemagglutinin in the fusion pH conformation. EMBO J. 1995 Jan 16;14(2):240–246. doi: 10.1002/j.1460-2075.1995.tb06997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton S. A., Skehel J. J., Wiley D. C. Studies of influenza haemagglutinin-mediated membrane fusion. Virology. 1986 Feb;149(1):27–35. doi: 10.1016/0042-6822(86)90083-8. [DOI] [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Yu Y. G., King D. S., Shin Y. K. Insertion of a coiled-coil peptide from influenza virus hemagglutinin into membranes. Science. 1994 Oct 14;266(5183):274–276. doi: 10.1126/science.7939662. [DOI] [PubMed] [Google Scholar]