Abstract
Apoptosis is a fundamental process for metazoan development. It is also relevant to the pathophysiology of immune diseases and cancers and to the outcome of cancer chemotherapies, as well as being a target for cancer therapies. Apoptosis involves intrinsic pathways typically initiated by DNA damaging agents and engaging mitochondria, and extrinsic pathways typically initiated by “death receptors” and their ligands TRAIL and TNF at the cell surface. Recently, we discovered the apoptotic ring, which microscopically looks like a nuclear annular staining early in apoptosis. This ring is, in three-dimensional space, a thick intranuclear shell consisting of epigenetic modifications including histone H2AX and DNA damage response (DDR) proteins. It excludes the DNA repair factors usually associated with γ-H2AX in the DDR nuclear foci. Here, we summarize our knowledge of the apoptotic ring, and discuss its biological and pathophysiological relevance, as well as its value as a potential pharmacodynamic biomarker for anticancer therapies.
Keywords: Chromatin, Epigenetics, Biomarkers
Introduction
Programmed cell death by apoptosis is a physiological process by which cells are eliminated and their components recycled. It is essential for embryogenesis, organ development, and tissue homeostasis, providing a balance between survival and cellular destruction at the organism level. Deregulation of apoptosis is involved in many diseases, including cancers and autoimmune disorders [1]. Apoptosis is also a critical determinant of response to anticancer treatments and has become the focus of targeted therapies. Our understanding has evolved remarkably since apoptosis was discovered ~50 years ago as “a basic biological phenomenon with wide-ranging implications” [2]. The research focus went from morphological [2] and biochemical studies, centered on the typical internucleosomal DNA fragmentation and the nucleases implicated in its genesis [3], to the discovery of the caspases [4, 5], and the importance of mitochondria [6] and Bcl-2 family members [7, 8]. Apoptosis is now well understood genetically, culminating with the Nobel prize of Robert Horvitz 10 years ago [9]. Over the past years, the scientific interest in nuclear apoptosis has faded in spite of the fact that we still do not understand clearly how cells degrade their nucleus and chromatin. Such interest remains justified by the fact that lack of orderly and complete degradation of nuclear proteins and nucleic acids is potentially a cause for autoimmune disorders.
The apoptotic ring proteins
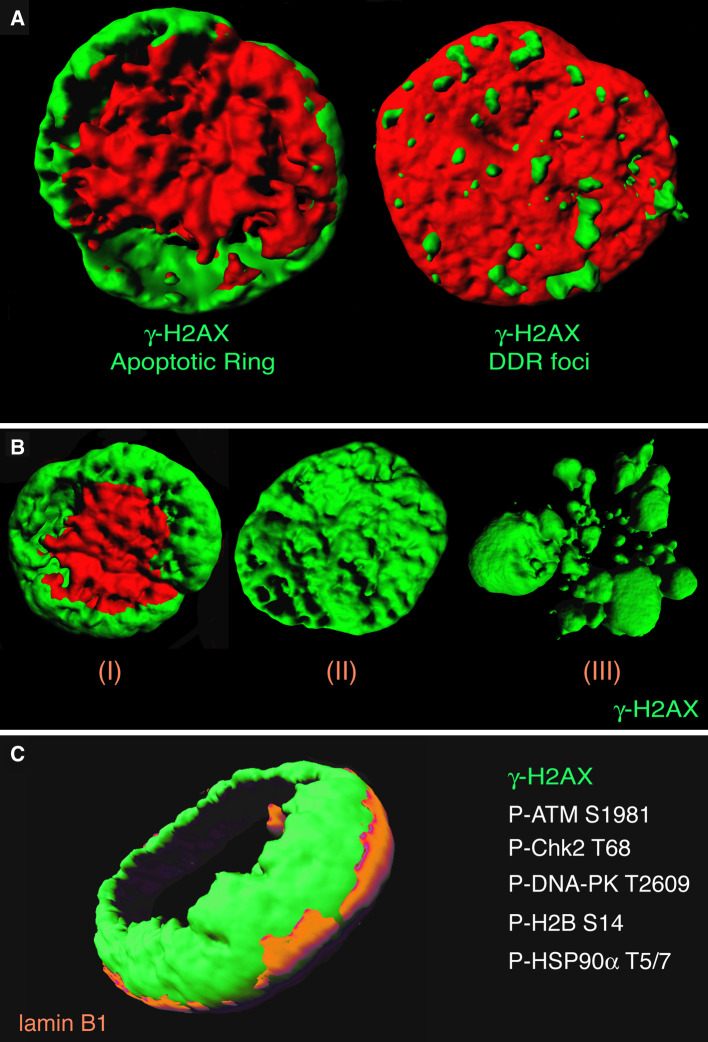
Histone H2AX phosphorylated on serine 139 (γ-H2AX) is a hallmark of DNA damage and DNA double-strand breaks (DSB) [10–12]. Soon after the discovery of γ-H2AX, we showed its involvement as the earliest epigenetic modification during apoptosis [13]. Then, in 2009, careful examination of single cells by confocal immunofluorescence microscopy revealed a previously unnoticed staining pattern for γ-H2AX during apoptosis induced by TRAIL (Fig. 1) [14, 15]. TRAIL (TNF-related apoptosis-inducing ligand) is an exquisitely well-characterized pro-apoptotic agonist that activates death receptors at the cell membrane, leading to caspase-8 activation and mitochondrial apoptosome formation with downstream activation of initiator caspase-9 and effector caspase-3 [16–18] (Fig. 2c). The γ-H2AX staining produced by TRAIL could be divided in three successive patterns: first a ring staining, which is best detected in early apoptotic cells without massive alteration of nuclear size (see Figs. 2, 3), then a pan-staining of the nucleus, which retains its overall morphology and size, and finally a pan-staining that persists within apoptotic bodies (Fig. 1b) [14, 15]. The ring constitutes an epigenetic landmark of early apoptosis and differs from the focal patterns of DNA damage foci produced by DNA damaging agents (Fig. 1a, right panel). It is worth noting that γ-H2AX forms at all phases of the cell cycle, although with some preference for S-phase cells [15].
Fig. 1.
Characterization of the apoptotic ring. a The γ-H2AX apoptotic ring versus the γ-H2AX DDR foci. Representative 3D images of a TRAIL-treated and an irradiated human colon carcinoma HCT116 cell. γ-H2AX is labeled in green; nuclei were stained with propidium iodide (red). b 3D single-cell analyses showing typical γ-H2AX apoptotic patterns. γ-H2AX is labeled in green; nuclei were stained with propidium iodide (red). c Proteins in the apoptotic ring. 3D picture of a TRAIL-treated HCT116 cell (0.1 μg/mL, 1 h). γ-H2AX is labeled in green; nuclear envelope was stained with lamin B1 (orange). P-S1981-ATM, P-T68-Chk2, P-T2609-DNA-PK, P-S14-H2B, and P-T5/7-HSP90α have been identified in the apoptotic ring. Confocal images were sequentially acquired with Zeiss AIM software on a Zeiss LSM 510 NLO confocal system (Carl Zeiss, Thornwood, NY, USA). Three-dimensional (3D) pictures were realized with the Bitplane (Zurich, Switzerland) Imaris software v.6.0
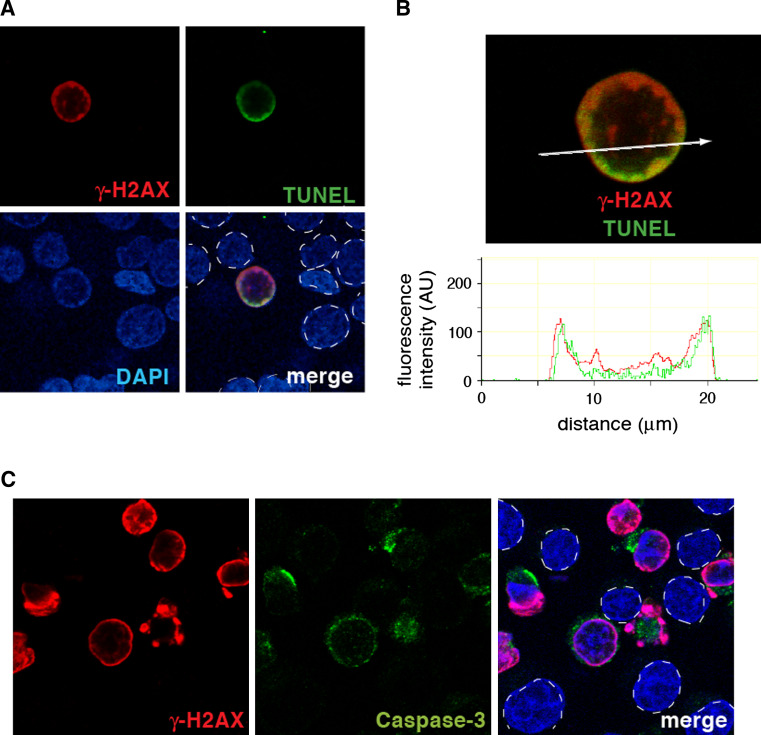
Fig. 2.
The ring coincides with DNA breaks in caspase-activated cells. a Representative TUNEL experiment showing colocalization between γ-H2AX and DNA breaks. HCT116 cells were treated with TRAIL (0.1 μg/mL, 1 h). γ-H2AX is labeled in red and DNA breaks in green; nuclei are stained in blue with DAPI. b Relative distribution of γ-H2AX and DNA breaks in a representative single cell. (Top) confocal microscopy image. (Bottom) Intensity tracing. c. Activated caspase-3 is stained in green (middle panel) and takes place in the cells that undergo apoptotic γ-H2AX conversion. In the merged images, nuclei are outlined with dashed lines to indicate the cells that do not stain for γ-H2AX and caspase-3
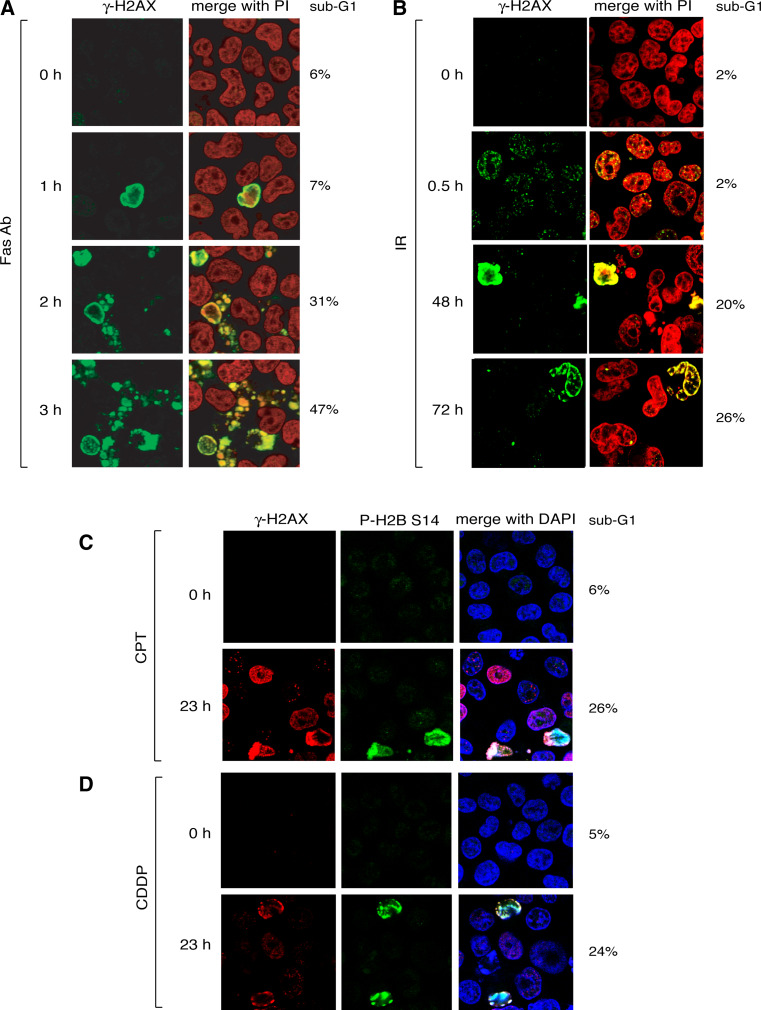
Fig. 3.
The ring is a general feature of apoptosis. a γ-H2AX confocal immunofluorescence staining of Burkitt’s lymphoma Jurkat cells (clone A3) treated with Fas antibody (0.1 μg/mL). γ-H2AX is labeled in green; nuclei are stained in red with propidium iodide. The percentage of (apoptotic) cells with sub-G1 DNA is indicated to the right of each panel. b γ-H2AX confocal immunofluorescence staining of irradiated HCT116 cells (15 Gy). γ-H2AX is labeled in green; nuclei are stained in red with propidium iodide. Percentages of cells with sub-G1 DNA are indicated at right. c γ-H2AX confocal immunofluorescence staining in HCT116 cells treated with camptothecin (CPT; 25 μM, 23 h). γ-H2AX is labeled in red and P-S14-H2B in green; nuclei are stained in blue with DAPI. The percentage of cells with sub-G1 DNA is indicated at right. d γ-H2AX confocal immunofluorescence staining in HCT116 cells treated with cisplatin (CDDP; 50 μM, 23 h). γ-H2AX is labeled in red and P-S14-H2B in green; nuclei are stained in blue with DAPI. The percentage of cells with sub-G1 DNA is indicated at right
Although total H2AX is diffuse in the nucleus due to the apparently random incorporation of H2AX in nucleosomes, γ-H2AX is initially limited to the outer portion of the nucleus, thereby forming the annular staining (“the ring”) in confocal immunofluorescence microscopy [14, 15]. The ring is located inside the nuclear envelope (Fig. 1c) and its peripheral distribution coincides with the peripheral heterochromatic nuclear region [15]. Importantly, the apoptotic γ-H2AX ring colocalizes with broken DNA ends labeled by TUNEL, indicating that it is initiated by the early wave of DNA breaks at the nuclear periphery [19] (Fig. 2a, b).
In addition to γ-H2AX, the ring contains phosphorylated histone H2B on serine 14 [14], activated/phosphorylated DNA damaging responsive proteins (Chk2, ATM, DNA-PK) [14, 15], and the heat shock protein HSP90α [19]. H2AX is primarily phosphorylated by DNA-PK, while Chk2 is phosphorylated by both ATM and DNA-PK [15, 20, 21]. Cross-talks between ATM and DNA-PK are likely, as the phosphorylation of DNA-PK on T2609 is partially dependent on ATM and the phosphorylation of ATM on S1981 and HSP90α on T5/7 is under the control of DNA-PK [15, 19]. DNA-PK is a client of HSP90α and HSP90α required for full DNA-PK activation, γ-H2AX formation, DNA fragmentation, and apoptotic body formation [19]. It is likely that a large number of macromolecules remain to be identified in the apoptotic ring.
Ubiquity of the apoptotic ring
We initially described and focused on the apoptotic ring generated by TRAIL because TRAIL initiates apoptosis starting with the well-defined plasma membrane death receptors DR4 and DR5, thereby avoiding the complexity of DNA damaging agents, which by themselves directly activate γ-H2AX [10] (see below and Fig. 4b).
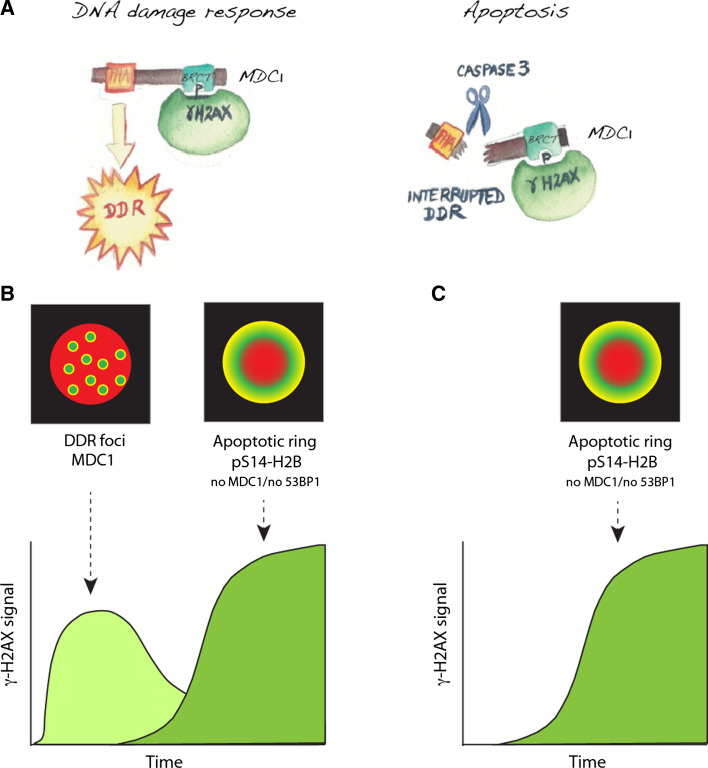
Fig. 4.
The two γ-H2AX phases in DDR-induced apoptosis. a Illustration of MDC1 interactions during DDR and apoptosis. b γ-H2AX response with high doses of DNA damage. DNA double-strand breaks induced the rapid and reversible formation of γ-H2AX DDR foci (left). Then, cells initiate apoptosis, and form apoptotic rings (right). Global measurement of γ-H2AX results in two waves of γ-H2AX. It is therefore important to consider time and morphology to score γ-H2AX signal in cells treated with DNA damaging agents. c Apoptotic ring induced by non-damaging apoptosis-inducing agents such as TRAIL and TNF. In addition to the morphological differences between the apoptotic and DDR γ-H2AX signals, the DDR foci contain MDC1 and 53BP1 but not phosphorylated histone H2B on Ser14, whereas the apoptotic γ-H2AX signal contains pS14-H2B but neither MDC1 nor 53BP1
However, the apoptotic ring is not limited to extrinsic apoptotic pathways, such as TRAIL or Fas ligand (FasL or CD95L) [15], which belong to the tumor necrosis factor (TNF) family and play critical roles in the regulation of the immune system. The apoptotic ring is also induced by anticancer agents including the broad spectrum kinase inhibitor and ubiquitous apoptosis inducer staurosporine [22, 23], as well as classical chemotherapeutic DNA damaging agents [10, 15, 19, 24] (Fig. 3). In the case of DNA damaging agents, the γ-H2AX response consists of two consecutive waves: the γ-H2AX damage foci and the γ-H2AX ring. For instance, within the first hours following 15-gray irradiation, a unique population of cells with DDR foci is present, whereas at later times (up to several days), cells harboring the apoptotic staining are mostly detected (Figs. 3b, 4b). High doses DNA damaging agents can simultaneously produce the two populations of cells: one harboring foci and the other apoptotic staining (Fig. 3c, d).
The apoptotic ring has been observed in all the cancer cells lines so far examined (HCT116 colon carcinoma cells, HeLa cervix carcinoma cells, Jurkat T cell leukemia cells, M059 J glioblastoma cells, HL60 promyelocytic leukemia cells, and MDA-MB-231 breast cancer cells), as well as in primary cells (prostate epithelial cells, lymphocytes, monocytes). Therefore, the apoptotic ring is an ubiquitous process.
Caspase-mediated inactivation of DNA repair proteins in the apoptotic ring
Immediately following DNA double-strand breaks, γ-H2AX initiates a fundamental epigenetic response, serving as a molecular platform for the docking of protein complexes to chromatin and the amplification of the DDR and DNA repair [10]. Direct binding of MDC1 (mediator of DNA damage checkpoint protein 1) to γ-H2AX via its BRCT (breast cancer C-terminal) domain [25] is essential for DDR because it leads to the subsequent recruitment and activation of DDR complexes including 53BP1 that promotes DNA repair and regulates cell cycle checkpoints [26, 27]. But, 53BP1, which is a landmark of γ-H2AX in DDR foci, is not present in the apoptotic ring [15, 24], indicating that the DDR proteins act differently during apoptosis and DNA damage/repair. The fact that DDR proteins are not fully recruited following an apoptotic signal such as TRAIL or TNF suggested the inactivation of DNA repair at the chromatin break sites during apoptosis [15, 24].
An important clue for the apoptotic γ-H2AX response is that it does not include MDC1, which normally binds to γ-H2AX and amplifies the DDR [25–27], because MDC1 is cleaved by activated caspase-3 (Fig. 4a) [24]. MDC1 cleavage by caspase-3, at the early phase of apoptosis, separates the MDC1 FHA (fork-head-associated) domain from its BRCT domains and therefore abrogates the interactions between the different DDR factors, consequently aborting DDR activation and DNA repair [28] (Fig. 4a). On the other hand, integrity of MDC1 is critical for DNA repair and DDR, as γ-H2AX binds the BRCT domain [25] and ATM the FHA domain [29, 30] of MDC1. Caspase-mediated inactivation of MDC1 is consistent with the inactivation DDR and repair proteins by caspases during apoptosis, a molecular event which was initially discovered for poly(ADPribose) polymerase 1 (PARP1) [31] and later extended to other DNA repair enzymes including DNA-PK [32].
In short, the apoptotic ring is an epigenetic mark of apoptotic DNA breaks (see Fig. 2a), initiating the recruitment of γ-H2AX, P-Chk2, P-ATM, and P-DNA-PK. Yet DNA repair is curtailed because of caspase-mediated cleavage and inactivation of key DDR mediators (such as MDC1; see Fig. 4a) and effectors (such as PARP1). Along the same lines, caspase-3 also inactivates DNA replication by cleaving Cdc6 [33].
Differentiating the apoptotic ring from other γ-H2AX pan-staining patterns
The apoptotic γ-H2AX ring evolves into nuclear pan-staining before nuclei undergo pyknosis (irreversible condensation and shrinkage) and ultimately form apoptotic bodies as the cells fragment into small corpses giving a sub-G1 flow cytometry signal (see Fig. 1b) [34]. Yet, diffuse apoptotic γ-H2AX pan-staining must be differentiated from other sources of γ-H2AX pan-staining, which are listed in Table 1 and discussed below.
Table 1.
Nuclear γ-H2AX pan-staining
| Type | References |
|---|---|
| Apoptotic ring and bodies | [14, 15, 19, 24] |
| UV-induced pan-staining | [35, 36] |
| Replicative stress; checkpoint abrogation | [40, 41] |
| Hypotonic treatment | [43] |
| Premature chromosome condensation | [45] |
| Heavy ion irradiation | [47] |
| Small DNA fragments (Dbaits) | [49] |
| Viral infections | [51, 52] |
Not including very high levels of genomic DNA double-strand breaks that produce confluent γ-H2AX DDR foci
Ultraviolet-C (UVC) light can induce γ-H2AX pan-staining independently of apoptosis and DSB [35, 36]. Cleaver and colleagues reported that, in replicating cells, UV irradiation induces three γ-H2AX responses: a minority of foci colocalizing with 53BP1 that represent DSB at replication sites, a majority of foci that do not colocalize with 53BP1 foci, and a pan-staining without foci, which appears to depend on nucleotide excision repair (NER) [35, 36]. This UV-induced pan-staining is ATM- and JNK-dependent, does not include 53BP1 and phosphorylated NBS1 at S343 [35, 36], and appears to be related to S phase progression [36]. Contrary to the apoptosis-induced γ-H2AX pan-staining [15], UV-induced γ-H2AX pan-staining is not abrogated by caspase inhibition [36]. One possibility is that UV-induced pan-staining occurs as a response to JNK-mediated phosphorylation of H2AX, which then stimulates caspase-activated DNase (CAD)-mediated nucleosomal DNA fragmentation [37], in which case, UV-induced pan-nuclear γ-H2AX staining may represent a pre-apoptotic process, initiated before CAD-mediated nucleosomal DNA fragmentation. Moreover, after UV irradiation, Karreman et al. [38] described new RNA-containing nuclear structures in the γ-H2AX pan-staining cells. These nuclear structures sometimes connect with the nucleolus, suggesting a possible nucleolar origin and some similarities with the nuclear stress bodies that contain RNA and RNA-splicing factors [39]. Pan-nuclear γ-H2AX may correspond to genome-wide chromatin modifications with conformational changes that increase the accessibility of DNA to transcription and repair, and possibly to the CAD endonuclease and additional H2AX kinases. Yet, during apoptosis induced by TRAIL or by high doses of DNA damaging agents, our data indicate that the phosphorylation of histone H2AX is due to DNA-PK without involvement of JNK.
γ-H2AX pan-staining has also been reported in association with replicative stress and cell cycle checkpoint abrogation [40, 41] (Table 1). The exact mechanism by which this response is elicited remains to be elucidated both in term of the kinase(s) responsible (ATR?) and the functional relevance at the chromatin level. During checkpoint abrogation in gemcitabine-treated cells, γ-H2AX pan-staining occurs but does not appear due to stalled DNA replication [42]. After Cdc25A or cyclin E overexpression, a significant fraction of cells also present γ-H2AX pan-staining in association with altered replicons and compromised EdU incorporation, suggesting replicative arrest [41]. In the absence of significant DNA breaks, γ-H2AX pan-staining induced by replicative responses might be related to the induction of γ-H2AX pan-staining in response to hypotonic shock, which acutely and reversibly alters chromatin structure [43]. Yet alterations of chromatin structure by DNA intercalating agents such as chloroquine, which activate ATM, do not elicit γ-H2AX pan-staining response [44].
γ-H2AX pan-staining is equally present during premature chromosome condensation (PCC), and it precedes the caspase activation and the induction of apoptosis [45] (Table 1). However, lasonolide A, a potent and reversible inducer of PCC [46], does not induce γ-H2AX and decreases the γ-H2AX staining induced by TRAIL (our unpublished data).
Recently, clustered DNA damage resulting from heavy ion irradiation (such as proton therapy) has been reported to induce γ-H2AX pan-nuclear staining mediated by ATM and DNA-PK [47] (Table 1). This nuclear-wide H2AX phosphorylation is not related to apoptosis and results from direct focal nuclear damage, as it does not occur after cytoplasmic irradiation. It does not affect cells adjacent to the irradiated ones [47], and therefore cannot be attributed to a bystander effect, which moreover tends to induce γ-H2AX foci [48]. It is not excluded that this pan-nuclear response to focal heavy ion radiation could be initiated by the diffusion of small DNA fragments generated at the irradiation site. In which case, this γ-H2AX pan-staining might share the same mechanism as the γ-H2AX pan-staining induced by the cellular introduction of short DNA oligonucleotides, which are referred to as “Dbait” [49] (Table 1), and formulated in nanoparticles as anticancer agents [50]. Similarly, DNA virus infections have been shown to induce γ-H2AX pan-staining [51, 52] (Table 1). Further studies are warranted to determine whether activation of the innate immune response could elicit the γ-H2AX pan-staining response [53].
Importance of complete nuclear apoptosis
The physiologic importance of complete nuclear apoptosis should not be overlooked because this process might be critical to avoid the persistence and release of toxic and potentially immunogenic cellular components, including nucleic acids and nuclear proteins from apoptotic cells and tissues. This concept has been put forward to explain auto-immune responses and potentially auto-immune diseases resulting from antiviral immune responses that become erroneously directed toward nuclear auto-antigens [54]. For instance, a working hypothesis for lupus nephritis is that genetic variants allow the persistence of nuclear particles corresponding to incomplete nuclear apoptosis and which persist in the extracellular space or inside lysosomes, mimicking viral particles [54]. The DNA could then activate viral nucleic acid recognition receptors in antigen-presenting cells, acting as auto-adjuvants to promote autoimmune responses to nuclear antigens. Such nuclear autoantigen are indeed common in autoimmune diseases. Among the first to be discovered was Scl-70, which is directed against topoisomerase I (Top1) in scleroderma patients [55]. The generation of Top1 antibodies might be related to the formation of a large number of Top1 cleavage complexes, which are covalent Top1-DNA complexes that form during apoptosis and contribute to the initial stages of global nuclear disorganization [56–58]. Such complexes, if not degraded, could serve as potent immunogens. The protein chaperone HSP90α, which is present in the ring, can be viewed as a facilitator of apoptosis by enabling robust activation of DNA-PK and allowing the completion of the apoptotic program leading to DNA fragmentation and formation of apoptotic bodies [19].
γ-H2AX as clinical biomarker
Because γ-H2AX is among the most sensitive and selective biomarkers of DNA damage [10], it is widely used in cell biology to monitor DNA damage by western blotting, fluorescence-activated cell sorting (FACS), and immunofluorescence microscopy [10, 59]. It should be pointed out, however, that western blotting cannot differentiate γ-H2AX elicited by DDR from apoptotic γ-H2AX, whereas FACS and immunofluorescence microscopy do. By FACS, the γ-H2AX-positive apoptotic bodies come in the sub-G1 fraction [34], and by immunofluorescence microscopy, the apoptotic nuclei show the ring and pan-staining patterns described above (see Figs. 1, 2, 3, 4) [15, 19, 24].
γ-H2AX is also used in clinical practice [10, 59–62]. Assays have been developed to detect γ-H2AX in tumor biopsies, circulating tumor cells (CTC), and hair follicles (in this case as surrogate marker) [60–62] for patients in clinical trials with Top1 inhibitors and PARP inhibitors [63]. The concept is to identify as early as possible and with a minimal number of patients the drugs that are active in order to select only the active compounds for development, thereby eliminating the inactive drugs and trimming the drug developmental process [64, 65]. This approach also allows the identification of early responders and lends itself to the investigation of the molecular and actionable determinants that underlie individual patient responses to the drug.
As illustrated in Fig. 4b, sample timing and morphological single cell analyses are critical to optimize the assays, and clarify whether DNA damage and/or apoptosis is being measured by γ-H2AX. Coupling γ-H2AX with additional biomarkers could sharpen the differences. Colocalization of γ-H2AX with histone H2B phosphorylation on serine 14 [15] (Fig. 3c, d), phosphorylated Hsp90α [19], and MDC1 (or 53-BP1) [24] could be potentially useful to differentiate DDR γ-H2AX foci from apoptotic γ-H2AX (see Fig. 4b, c). Ultimately, putting together several apoptotic biomarkers could lead to an “epigenetic apoptotic code”. Further studies are warranted to determine whether apoptotic γ-H2AX could be a useful biomarker for TRAIL-based therapies, in which case, animal models should be tested to determine whether and when the apoptotic γ-H2AX cells should be optimally detected. The apoptotic “ring pattern” with dual reactivity to γ-H2AX and P-H2B should provide new tools for basic cellular biology research, and also to score apoptosis versus DNA damage response in clinical samples to monitor the efficacy of anticancer regimens.
Perspectives
Apoptosis is usually associated with the activation of one or several nucleases that degrade nuclear DNA first into large and subsequently into nucleosomal fragments [66–68]. The initiation of the γ-H2AX signal at the nuclear periphery suggests that the nucleases responsible for the generation of the ring first act at the periphery of the nucleus; possibly where they are activated or transported from the cytosol. Therefore, the apoptotic γ-H2AX ring might revive general interest in the field of nuclear apoptosis and apoptotic nucleases. It will also be important to determine whether the nuclear genome could be initially damaged by reactive oxygen species. The fact that the protein chaperone HSP90α is present in the ring [19] suggests that some of other clients beside DNA-PK [69] (http://www.picard.ch/downloads/Hsp90facts.pdf) are also in the ring, which might give clues for novel regulators and effectors of nuclear apoptosis.
Acknowledgments
We wish to thank our close laboratory colleagues for their commitment to γ-H2AX basic research: Dr. William Bonner, Dr. Christophe Redon, Dr. James H. Doroshow, and Dr. Kurt W. Kohn. We also wish to thank our NCI colleagues from the DCTD, PADIS, for the development of γ-H2AX pharmacodynamics biomarker assays: Dr. James H. Doroshow, Dr. Joseph E. Tomaszewski, Dr. Raph E. Parchment, and Dr. Robert Kinders. Our studies are supported by the NCI Intramural Program, Center for Cancer Research, NIH.
References
- 1.Zhivotovsky B, Kroemer G. Apoptosis and genomic instability. Nat Rev Mol Cell Biol. 2004;5:752–762. doi: 10.1038/nrm1443. [DOI] [PubMed] [Google Scholar]
- 2.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enari M, Shahira H, Yokoyama H, et al. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 4.Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284:21777–21781. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alnemri ES, Livingston DJ, Nicholson DW, et al. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/S0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 6.Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 7.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/S0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 8.Hockenbery D, Nunez G, Milliman C, et al. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 9.Horvitz HR. Worms, life, and death (Nobel lecture) Chembiochem. 2003;4:697–711. doi: 10.1002/cbic.200300614. [DOI] [PubMed] [Google Scholar]
- 10.Bonner WM, Redon CE, Dickey JS, et al. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogakou EP, Pilch DR, Orr AH, et al. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 12.Rogakou EP, Boon C, Redon C, et al. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogakou EP, Nieves-Neira W, Boon C, et al. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem. 2000;275:9390–9395. doi: 10.1074/jbc.275.13.9390. [DOI] [PubMed] [Google Scholar]
- 14.Solier S, Pommier Y. The apoptotic ring: a novel entity with phosphorylated histones H2AX and H2B and activated DNA damage response kinases. Cell Cycle. 2009;8:1853–1859. doi: 10.4161/cc.8.12.8865. [DOI] [PubMed] [Google Scholar]
- 15.Solier S, Sordet O, Kohn KW, et al. Death receptor-induced activation of the Chk2- and histone H2AX-associated DNA damage response pathways. Mol Cell Biol. 2009;29:68–82. doi: 10.1128/MCB.00581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taieb J, Chaput N, Menard C, et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 17.Wang S. The promise of cancer therapeutics targeting the TNF-related apoptosis-inducing ligand and TRAIL receptor pathway. Oncogene. 2008;27:6207–6215. doi: 10.1038/onc.2008.298. [DOI] [PubMed] [Google Scholar]
- 18.Yagita H, Takeda K, Hayakawa Y, et al. TRAIL and its receptors as targets for cancer therapy. Cancer Sci. 2004;95:777–783. doi: 10.1111/j.1349-7006.2004.tb02181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solier S, Kohn KW, Scroggins B, et al. Feature Article: heat shock protein 90alpha (HSP90alpha), a substrate and chaperone of DNA-PK necessary for the apoptotic response. Proc Natl Acad Sci USA. 2012;109:12866–12872. doi: 10.1073/pnas.1203617109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukherjee B, Kessinger C, Kobayashi J, et al. DNA-PK phosphorylates histone H2AX during apoptotic DNA fragmentation in mammalian cells. DNA Repair (Amst) 2006;5:575–590. doi: 10.1016/j.dnarep.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T, Huang X, Halicka HD, et al. Cytometry of ATM activation and histone H2AX phosphorylation to estimate extent of DNA damage induced by exogenous agents. Cytometry Part A. 2007;71:648–661. doi: 10.1002/cyto.a.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertrand R, Solary E, Kohn KW, et al. Induction of a common pathway to apoptosis by staurosporine. Exp Cell Res. 1994;211:314–321. doi: 10.1006/excr.1994.1093. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson MD, Burne JF, Raff MC. Programmed cell death and Bcl-2 protection in the absence of a nucleus. EMBO J. 1994;13:1899–1910. doi: 10.1002/j.1460-2075.1994.tb06459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solier S, Pommier Y. MDC1 cleavage by caspase-3: a novel mechanism for inactivating the DNA damage response during apoptosis. Cancer Res. 2011;71:906–913. doi: 10.1158/0008-5472.CAN-10-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stucki M, Clapperton JA, Mohammad D, et al. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell. 2005;123:1213–1226. doi: 10.1016/j.cell.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 26.Eliezer Y, Argaman L, Rhie A, et al. The direct interaction between 53BP1 and MDC1 Is required for the recruitment of 53BP1 to sites of damage. J Biol Chem. 2009;284:426–435. doi: 10.1074/jbc.M807375200. [DOI] [PubMed] [Google Scholar]
- 27.Stewart GS, Wang B, Bignell CR, et al. MDC1 is a mediator of the mammalian DNA damage checkpoint. Nature. 2003;421:961–966. doi: 10.1038/nature01446. [DOI] [PubMed] [Google Scholar]
- 28.Xie A, Hartlerode A, Stucki M, et al. Distinct roles of chromatin-associated proteins MDC1 and 53BP1 in mammalian double-strand break repair. Mol Cell. 2007;28:1045–1057. doi: 10.1016/j.molcel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lou Z, Minter-Dykhouse K, Franco S, et al. MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol Cell. 2006;21:187–200. doi: 10.1016/j.molcel.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 30.Dimitrova N, De Lange T. MDC1 accelerates nonhomologous end-joining of dysfunctional telomeres. Genes Dev. 2006;20:3238–3243. doi: 10.1101/gad.1496606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazebnik YA, Kaufmann SH, Desnoyers S, et al. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 32.Song Q, Lees-Miller SP, Kumar S, et al. DNA-dependent protein kinase catalytic subunit: a target for an ICE-like protease in apoptosis. EMBO J. 1996;15:3238–3246. [PMC free article] [PubMed] [Google Scholar]
- 33.Yim H, Hwang IS, Choi J-S, et al. Cleavage of Cdc6 by caspase-3 promotes ATM/ATR kinase, Äìmediated apoptosis of HeLa cells. J Cell Biol. 2006;174:77–88. doi: 10.1083/jcb.200509141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka T, Halicka HD, Traganos F, et al. Induction of ATM activation, histone H2AX phosphorylation and apoptosis by etoposide: relation to cell cycle phase. Cell Cycle. 2007;6:371–376. doi: 10.4161/cc.6.3.3835. [DOI] [PubMed] [Google Scholar]
- 35.Marti TM, Hefner E, Feeney L, et al. H2AX phosphorylation within the G1 phase after UV irradiation depends on nucleotide excision repair and not DNA double-strand breaks. Proc Natl Acad Sci USA. 2006;103:9891–9896. doi: 10.1073/pnas.0603779103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Feraudy S, Revet I, Bezrookove V, et al. A minority of foci or pan-nuclear apoptotic staining of gammaH2AX in the S phase after UV damage contain DNA double-strand breaks. Proc Natl Acad Sci USA. 2010;107:6870–6875. doi: 10.1073/pnas.1002175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu C, Zhu F, Cho YY, et al. Cell apoptosis: requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol Cell. 2006;23:121–132. doi: 10.1016/j.molcel.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karreman MA, Agronskaia AV, Verkleij AJ, et al. Discovery of a new RNA-containing nuclear structure in UVC-induced apoptotic cells by integrated laser electron microscopy. Biol Cell. 2009;101:287–299. doi: 10.1042/BC20080076. [DOI] [PubMed] [Google Scholar]
- 39.Chiodi I, Biggiogera M, Denegri M, et al. Structure and dynamics of hnRNP-labelled nuclear bodies induced by stress treatments. J Cell Sci. 2000;113(Pt 22):4043–4053. doi: 10.1242/jcs.113.22.4043. [DOI] [PubMed] [Google Scholar]
- 40.Murga M, Bunting S, Montana MF, et al. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat Genet. 2009;41:891–898. doi: 10.1038/ng.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neelsen KJ, Zanini IM, Herrador R, et al. Oncogenes induce genotoxic stress by mitotic processing of unusual replication intermediates. J Cell Biol. 2013;200:699–708. doi: 10.1083/jcb.201212058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ewald B, Sampath D, Plunkett W. H2AX phosphorylation marks gemcitabine-induced stalled replication forks and their collapse upon S-phase checkpoint abrogation. Mol Cancer Ther. 2007;6:1239–1248. doi: 10.1158/1535-7163.MCT-06-0633. [DOI] [PubMed] [Google Scholar]
- 43.Baure J, Izadi A, Suarez V, et al. Histone H2AX phosphorylation in response to changes in chromatin structure induced by altered osmolarity. Mutagenesis. 2009;24:161–167. doi: 10.1093/mutage/gen064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 45.Huang X, Kurose A, Tanaka T, et al. Sequential phosphorylation of Ser-10 on histone H3 and ser-139 on histone H2AX and ATM activation during premature chromosome condensation: relationship to cell-cycle phase and apoptosis. Cytometry Part A. 2006;69:222–229. doi: 10.1002/cyto.a.20257. [DOI] [PubMed] [Google Scholar]
- 46.Zhang YW, Ghosh AK, Pommier Y. Lasonolide A, a potent and reversible inducer of chromosome condensation. Cell Cycle. 2012;11:4424–4435. doi: 10.4161/cc.22768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyer B, Voss KO, Tobias F, et al. Clustered DNA damage induces pan-nuclear H2AX phosphorylation mediated by ATM and DNA-PK. Nucl Acids Res. 2013;41:6109–6118. doi: 10.1093/nar/gkt304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sokolov MV, Smilenov LB, Hall EJ, et al. Ionizing radiation induces DNA double-strand breaks in bystander primary human fibroblasts. Oncogene. 2005;24:7257–7265. doi: 10.1038/sj.onc.1208886. [DOI] [PubMed] [Google Scholar]
- 49.Quanz M, Chassoux D, Berthault N, et al. Hyperactivation of DNA-PK by double-strand break mimicking molecules disorganizes DNA damage response. PLoS ONE. 2009;4:e6298. doi: 10.1371/journal.pone.0006298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devun F, Bousquet G, Biau J, et al. Preclinical study of the DNA repair inhibitor Dbait in combination with chemotherapy in colorectal cancer. J Gastroenterol. 2012;47:266–275. doi: 10.1007/s00535-011-0483-x. [DOI] [PubMed] [Google Scholar]
- 51.Fragkos M, Breuleux M, Clement N, et al. Recombinant adeno-associated viral vectors are deficient in provoking a DNA damage response. J Virol. 2008;82:7379–7387. doi: 10.1128/JVI.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz RA, Carson CT, Schuberth C, et al. Adeno-associated virus replication induces a DNA damage response coordinated by DNA-dependent protein kinase. J Virol. 2009;83:6269–6278. doi: 10.1128/JVI.00318-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burdette DL, Vance RE. STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol. 2013;14:19–26. doi: 10.1038/ni.2491. [DOI] [PubMed] [Google Scholar]
- 54.Migliorini A, Anders HJ. A novel pathogenetic concept-antiviral immunity in lupus nephritis. Nature reviews. Nephrology. 2012;8:183–189. doi: 10.1038/nrneph.2011.197. [DOI] [PubMed] [Google Scholar]
- 55.Shero JH, Bordwell B, Rothfield NF, et al. High titers of autoantibodies to topoisomerase I (Scl-70) in sera from scleroderma patients. Science. 1986;231:737–740. doi: 10.1126/science.3003910. [DOI] [PubMed] [Google Scholar]
- 56.Sordet O, Liao Z, Liu H, et al. Topoisomerase I-DNA complexes contribute to arsenic trioxide-induced apoptosis. J Biol Chem. 2004;279:33968–33975. doi: 10.1074/jbc.M404620200. [DOI] [PubMed] [Google Scholar]
- 57.Sordet O, Goldman A, Redon C, et al. Topoisomerase I requirement for death receptor-induced apoptotic nuclear fission. J Biol Chem. 2008;34:23200–23208. doi: 10.1074/jbc.M801146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 59.Ivashkevich A, Redon CE, Nakamura AJ, et al. Use of the gamma-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 2012;327:123–133. doi: 10.1016/j.canlet.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang LH, Pfister TD, Parchment RE, et al. Monitoring drug-induced gammaH2AX as a pharmacodynamic biomarker in individual circulating tumor cells. Clin Cancer Res. 2010;16:1073–1084. doi: 10.1158/1078-0432.CCR-09-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Redon CE, Nakamura AJ, Sordet O, et al. gamma-H2AX detection in peripheral blood lymphocytes, splenocytes, bone marrow, xenografts, and skin. Meth Mol Biol. 2011;682:249–270. doi: 10.1007/978-1-60327-409-8_18. [DOI] [PubMed] [Google Scholar]
- 62.Redon CE, Nakamura AJ, Zhang YW, et al. Histone gammaH2AX and poly(ADP-ribose) as clinical pharmacodynamic biomarkers. Clin Cancer Res. 2010;16:4532–4542. doi: 10.1158/1078-0432.CCR-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pommier Y. Drugging topoisomerases: lessons and challenges. ACS Chem Biol. 2013;8:82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kummar S, Kinders R, Rubinstein L, et al. Compressing drug development timelines in oncology using phase ‘0’ trials. Nat Rev Cancer. 2007;7:131–139. doi: 10.1038/nrc2066. [DOI] [PubMed] [Google Scholar]
- 65.Kummar S, Kinders R, Gutierrez ME, et al. Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. J Clin Oncol. 2009;27:2705–2711. doi: 10.1200/JCO.2008.19.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Earnshaw WC. Nuclear changes in apoptosis. Curr Opin Cell Biol. 1995;7:337–343. doi: 10.1016/0955-0674(95)80088-3. [DOI] [PubMed] [Google Scholar]
- 67.Yoshida A, Pourquier P, Pommier Y. Purification and characterization of a Mg2 + -dependent endonuclease (AN34) from etoposide-treated human leukemia HL-60 cells undergoing apoptosis. Cancer Res. 1998;58:2576–2582. [PubMed] [Google Scholar]
- 68.Zhivotovsky B, Wade D, Nicotera P, et al. Role of nucleases in apoptosis. Int Arch All Immunol. 1994;105:333–338. doi: 10.1159/000236778. [DOI] [PubMed] [Google Scholar]
- 69.Falsone SF, Gesslbauer B, Tirk F, et al. A proteomic snapshot of the human heat shock protein 90 interactome. FEBS Lett. 2005;579:6350–6354. doi: 10.1016/j.febslet.2005.10.020. [DOI] [PubMed] [Google Scholar]