Abstract
BACKGROUND CONTEXT
A large percentage of back pain can be attributed to degeneration of the intervertebral disc (IVD). Bone morphogenetic protein-2 (BMP-2) is known to play an important role in chondrogenesis of the IVD. Simvastatin is known to up-regulate expression of BMP-2. Thus, we hypothesized that intradiscal injection of simvastatin in a rat model of degenerative disc disease (DDD) would result in retardation of DDD.
PURPOSE
To develop a novel conservative treatment for DDD and related discogenic back pain.
STUDY DESIGN/SETTING
Laboratory investigation.
METHODS
Disc injury was induced in 272 rats via 21-gauge needle puncture. After 6 weeks, injured discs were treated with simvastatin in a saline or hydrogel carrier. Rats were sacrificed at predetermined time points. Outcome measures assessed were radiologic, histologic, and genetic. Radiologically, the MRI index (number of pixels multiplied by corresponding image densities) was determined. Histologically, disc spaces were read by 3 blinded scorers employing a previously described histological grading scale. Genetically, nuclei pulposi were harvested and polymerase chain reaction was run to determine relative levels of aggrecan, collagen type II, and BMP-2 gene expression. This project was supported by Grant No. R01 AR056649 from NIAMS/NIH. There are no other financial conflicts of interest to report.
RESULTS
Radiologically, discs treated with 5 mg/mL simvastatin in hydrogel or saline demonstrated MRI indices that were normal through 8 weeks post-treatment, although this was more sustained when delivered in hydrogel. Histologically, discs treated with 5 mg/mL simvastatin in hydrogel demonstrated improved grades in comparison to discs treated at higher doses. Genetically, discs treated with 5 mg/mL of simvastatin in hydrogel demonstrated higher gene expression of aggrecan and collagen type II than control.
CONCLUSIONS
Degenerate discs treated with 5 mg/mL simvastatin in a hydrogel carrier demonstrated radiographic and histologic features resembling normal, non-injured IVDs. In addition, gene expression of aggrecan and collagen type II (important constituents of the IVD extracellular matrix) was up-regulated in treated discs. Injection of simvastatin into degenerate IVDs may result in retardation of disc degeneration and represents a promising investigational therapy for conservative treatment of DDD.
Keywords: Back pain, BMP-2, degenerative disc disease, rodent model, simvastatin
Introduction
While multifactorial, at least 40% of back pain cases may be associated with degeneration of the intervertebral disc (IVD), ie, discogenic back pain [1–5]. The normal IVD consists of a central nucleus pulposus (NP) and surrounding annulus fibrosus (AF). The NP has a matrix that consists of collagen type II embedded within the proteoglycan aggrecan in a ratio of 1:20 [6]. With degeneration, production of collagen type II and aggrecan decrease, while production of collagen type I is increased [7, 8]. Also, matrix metalloproteinases are up-regulated [9]. In this setting of degeneration, the normal balance between forces generated in the NP and AF is lost, resulting in decreased tension in the collagen fibers in the AF; this promotes shock-loading at the vertebral body endplates during normal movement, leading to microtrauma and pain [10]. This microtrauma damages both the AF and the bone into which the fibers of the AF insert, allowing blood vessels and nociceptive nerves a route into the typically avascular and aneural IVD [11].
Bone morphogenetic proteins (BMPs) comprise a group of numerous multipotent growth factors that belong to the transforming growth factor-beta (TGF-β) superfamily, and are involved in a wide range of developmental processes throughout the body. They regulate the growth, differentiation, and apoptosis of various cell types, including osteoblasts, chondroblasts, neurons, and epithelial cells [12]. While the literature regarding BMPs and the spine has focused primarily on osteogenesis and tumorigenesis, a growing number of researchers are examining the role BMPs play in chondrogenesis and production of the extracellular matrix in the IVD. In fact, BMP-2 [13, 14], -5 [15], -6 [16], -8 [17], -9 [14], and -14 [18] have all been shown to influence chondrogenesis [19]. BMPs and their receptors were first identified in mouse disc spaces in 1999 [20]. Since that time, studies have focused on BMP expression in the normal disc and animal models of degenerative disc disease (DDD), as well as the use of gene therapy and other mechanisms to up-regulate BMP as a means of initiating chondrogenesis and restoring normal disc morphology. Thus, BMPs present a promising therapeutic avenue for treating degenerative disc-related diseases such as discogenic back pain, adjacent segment disease, and disc herniation.
To date, BMP-based treatment in animal and human models of DDD has involved either direct administration of BMP or up-regulation of BMP via alternate routes (such as with adenoviruses) [21–32]. However, endogenous up-regulation of BMP may be more effective and less toxic than supraphysiologic exogenous administration. Exogenous administration of BMP at the time of spine surgery has been associated with seroma formation [33], epidural bone formation [34, 35], and perineural cyst formation [36–38]. Thus, it may be advantageous to develop strategies for endogenous up-regulation of this important pathway. It has been shown that the common cholesterol-lowering medications, statins, stimulate bone formation via up-regulation of the BMP-2 pathway [39]. Zhang and Lin revealed that simvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor, increases BMP-2 mRNA expression in rat [40], pig, as well as human IVD cells in vitro (unpublished data). Furthermore, aggrecan and type II collagen gene expression as well as proteoglycan products were up-regulated, indicating an anabolic effect on IVD cells. More importantly, these results provided the first evidence that increased mRNA expression of aggrecan and type II collagen induced by simvastatin is partially mediated by up-regulated BMP-2 through the mevalonate pathway. However, this prior study tested only one drug concentration (5 mg/mL) at one time point (2 weeks after drug delivery).
The finding that simvastatin can enhance chondrogenesis of IVD cells is of great clinical significance, because it sheds light on the real possibility and practicality of developing a non-surgical therapeutic method to achieve effective in situ repair or prevention of DDD. Since simvastatin is a widely prescribed drug, the availability and cost are very acceptable for its long-term clinical use. Since the mechanism and efficacy of statins have been well-defined and regulated, the translation of statins for use in the spine can be facilitated. To begin the process of potential eventual use in humans, in this study we hypothesized that intradiscal injection of simvastatin in a rat model of DDD would result in radiologic, histologic, and genetic evidence of disc regeneration. This is the first study to test the drug in various concentrations at various time points, using two different delivery vehicles.
Materials and methods
Animals
We obtained 272 Sprague-Dawley rats (3 months old) from Charles River Laboratories International, Inc. (Wilmington, MA, USA). Rats were initially housed in groups of 3 rats per cage (rats would be re-caged doubly then singly as they became larger). Experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals, and the experimental protocols were approved by the University Committee on the Use and Care of Animals at the University of Michigan.
Surgical technique
The surgical procedure was performed as previously described [41]. Briefly, anesthesia for all surgical procedures was achieved and maintained by inhalation of anesthetic isoflurane, and the operative field was prepared in sterile fashion. Palpation was used to determine the correct Co5/Co6 disc level, which in a rat is the level below the last palpable transverse process; this was also reliably found to be 2 cm from the rat’s anus. A 21-gauge (G) needle was used to induce injury at both the Co5/Co6 and Co7/Co8 levels; Co6/Co7 was left unperturbed to serve as a control. Live fluoroscopy was used to visualize needle penetration and to ensure that the stab injury went to the center of the NP, roughly at a distance of 5 mm from the tail surface (Figure 1). The needle was rotated 180 degrees and held in place for 5 seconds. Any bleeding following needle removal was stopped with the application of skin glue (GLUture, Abbott Laboratories, Abbott Park, IL, USA). The skin was marked circumferentially with a permanent marker at sites of puncture for ease of level localization at the time of drug treatment.
Figure 1.
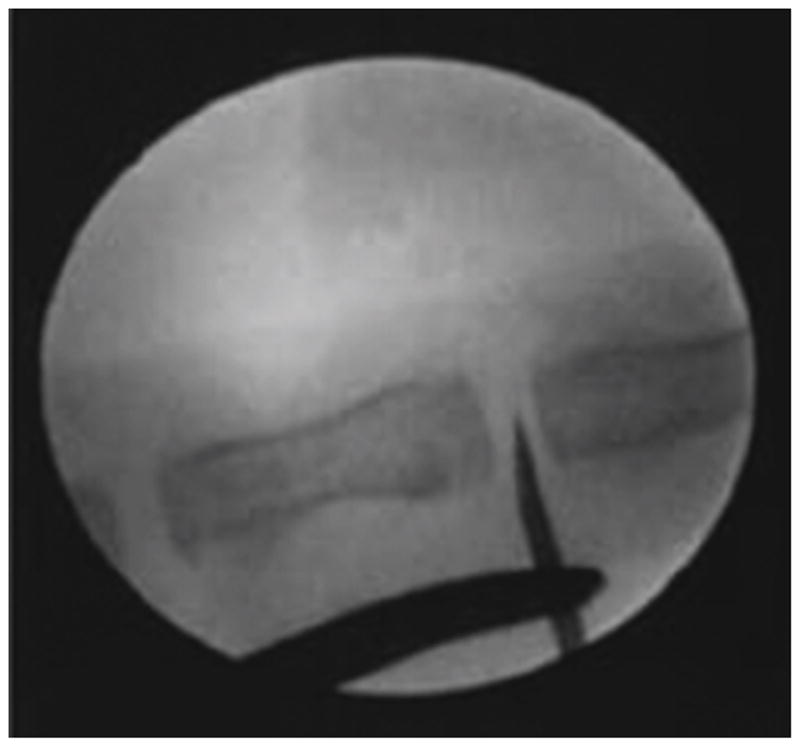
Disc injury was induced via 21-G needle puncture with fluoroscopic guidance.
Simvastatin treatment
Six weeks after stab injury, 2 μL simvastatin (LKT Laboratories, St. Paul, MN, USA) at 3 different doses (5, 10, or 15 mg/mL) in either a saline or hydrogel carrier was injected into injured discs using a micro-syringe attached to a 31-G needle. Based on previous trials, the 31-G needle does not cause significant injury leading to disc degeneration. Control groups included saline only (n = 90), hydrogel only (n = 64), and stab control (n = 64). Rats were then assessed 2, 4, 8, 12, and 24 weeks after drug delivery via imaging, histological, and microbiological endpoints.
Hydrogel was used in collaboration with investigators in the Republic of Korea [42]. This cross-linkable gelatin poly-(ethylene glycol)-tyramine hydrogel was formed rapidly using horseradish peroxidase (HRP) and hydrogen peroxide. The gelation time of this hydrogel can be controlled by adjusting the HRP concentration. In vitro and in vivo experiments found this hydrogel to have good bioactivity [43]. Thus, it serves as a potential delivery mechanism for injectable materials in tissue regenerative medicine. The release curve of the hydrogel carrier, as previously reported, is such that 50% of the drug is released after 1 week, and 100% after 4 weeks.
Magnetic resonance imaging (MRI) procedures and data processing
For radiological evidence of disc regeneration, rats were assessed via MRI at each follow-up time point. Briefly, rats were anesthetized with a 2% isoflurane/oxygen mixture. The animal was placed in the prone position (with tail straightened) in a 7.0 Tesla Agilent MR scanner (Agilent Technologies, Santa Clara, CA, USA). The appropriate disc spaces were marked superficially with Lacri-Lube (Allergan, Irvine, CA, USA) for ease of identification on the final images. A double-tuned volume radiofrequency coil was used to scan the tail region of the rats. Sagittal T2-weighted images covering the entire disc area were acquired using a spin-echo sequence with the following parameters: fat saturation on; repetition time/effective echo time, 3000/30 ms; field of view, 30 × 60 mm; matrix, 128 × 128; slice thickness, 0.5 mm; slice spacing, 0 mm; number of slices, 8; number of scans, 1 (total scan time 6.24 minutes).
We used a previously described procedure to quantitatively analyze the image slices using Analyze 6.0 software (AnalyzeDirect, Overland Park, KS, USA) [44]. The NP region was segmented from the sliced images, followed by image reconstruction and volume rendering procedures to generate volumetric images and, therefore, calculate each NP volume. These values were multiplied by the average signal intensity to determine the MRI index. For each treated disc, a normalized MRI index was calculated using the normal control disc as reference; values closer to 1 represented disc volume closer to that of normal control discs.
Histological analysis
After the animals were euthanized, roughly one-third of them were allotted for histological analysis. The disc spaces of interest were harvested. The discs, with adjacent vertebral bodies, were fixed in formaldehyde for at least 24 hours. They were subsequently decalcified in 22.5% formic acid and 10% sodium citrate for 5–7 days, on average. Decalcification was verified regularly using roentgenography. They were then processed for paraffin embedding and sagittal sectioning (10 μm thick) using a microtome. Sections were stained with hematoxylin and eosin (H & E), and then analyzed qualitatively using an Olympus BX51 light microscope (Olympus, Center Valley, PA, USA). The histological sections were graded by 3 blinded observers using a previously described histological grading scale (Table 1) [45].
Table 1.
| Grade | Grade Description | |
|---|---|---|
| I. Annulus fibrosus | 1 | Normal pattern of fibrocartilage lamellae (U-shaped in the posterior aspect and slightly convex in the anterior aspect) without ruptured fibers and without a serpentine appearance anywhere within the annulus. |
| 2 | Ruptured or serpentine-patterned fibers in less than 30% of the annulus. | |
| 3 | Ruptured or serpentine-patterned fibers in more than 30% of the annulus. | |
|
| ||
| II. Border between the annulus fibrosus and nucleus pulposus | 1 | Normal. |
| 2 | Minimally interrupted. | |
| 3 | Moderate/severe interruption. | |
|
| ||
| III. Cellularity of the nucleus pulposus | 1 | Normal cellularity with large vacuoles in the gelatinous structure of the matrix. |
| 2 | Slight decrease in the number of cells and fewer vacuoles. | |
| 3 | Moderate/severe decrease (>50%) in the number of cells and no vacuoles. | |
|
| ||
| IV. Matrix of the nucleus pulposus | 1 | Normal gelatinous appearance. |
| 2 | Slight condensation of the extracellular matrix. | |
| 3 | Moderate/severe condensation of the extracellular matrix. | |
Histological grading scale based on 4 categories of degenerative changes, with scores ranging from a normal disc with 4 points (1 point in each category) to a severely degenerated disc with 12 points (3 points in each category).
Quantitative real-time polymerase chain reaction (rtPCR)
Rats whose disc spaces were not utilized for histology instead had their NPs harvested for evaluation of gene expression. This was accomplished by making a small incision in the AF with micro-scissors, then scooping out the NP with a micro-scoop (Circon MicroSurgical, Santa Barbara, CA, USA). Total ribonucleic acid (RNA) was extracted from the NP using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA, USA). Reverse transcription was carried out at 42°C for 50 minutes using the SuperScript First-Strand Synthesis Kit (Invitrogen, Carlsbad, CA, USA). Types I and II collagen, aggrecan, BMP-2, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene expression were quantified by rtPCR using Gene Amp 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). A positive standard curve for each primer was obtained by rtPCR with serially-diluted complementary deoxyribonucleic acid (cDNA) sample mixtures. Quantities of gene expression of types I and II collagen, aggrecan, and BMP-2 were calculated using standard samples and normalized with GAPDH. The amounts of mRNA were presented as a ratio to the intact control group.
Statistical analysis
All data are summarized as mean ± standard deviation by treatment group and follow-up time. To standardize the histological data, two approaches were taken: in the first approach, we analyzed histological grades after subtracting the grade of the normal control disc of the same animal, and in the second approach, we included histological grades of the normal control discs as a referent group. Linear mixed-effects models were used to compare between-group means for MRI measurement, histological scores, and gene expression. Both the MRI and histology data analyses were adjusted for potential within-animal correlation from multiple discs using random intercepts. A p-value of less than 0.05 was considered statistically significant.
Results
MRI
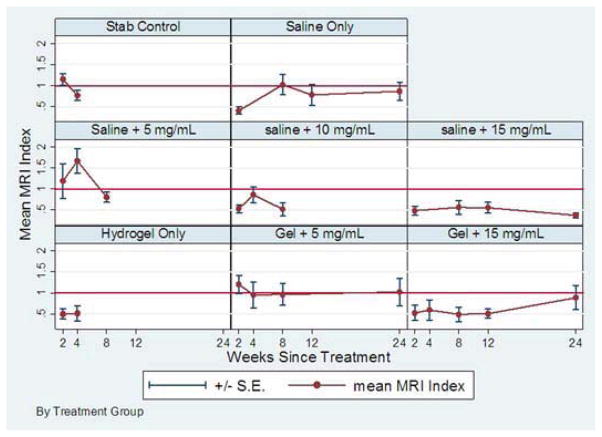
One hundred twenty-six rats underwent MRI (6 rats/group). Summary statistics for normalized MRI indices for the treatment groups are shown in Table 2 and Figure 2. The linear mixed-effects model for MRI indices did not show significant time trends during follow-up. Based on weeks 2 and 4 data, treatment with 5 mg/mL simvastatin in hydrogel and the same dose in saline showed higher time-averaged MRI indices than that of stab controls by 0.12 (p=0.57) and by 0.43 (p=0.05), respectively, showing potential early therapeutic benefit. On the other hand, the time-averaged mean MRI index was lower than that of stab controls for all other treatment groups, and significantly lower for saline alone (p=0.04) and hydrogel alone (p=0.02). Based on data from the treatment groups where follow-up data were assessed to week 24, long-term time-averaged MRI index was 0.78 (95% CI, 0.57–0.98) for saline only and was even lower for treatment with 15 mg/mL administered either in saline (p=0.03) or in hydrogel (p=0.28). However, these values were higher by 0.26 (p=0.07) for treatment with 5 mg/mL administered in hydrogel with an expected time-averaged MRI index of 1.03 (95% CI, 0.83–1.24). A representative example is demonstrated in Figure 3.
Table 2.
Normalized MRI indices. Six rats were imaged in each group (N = 126).
| Treatment Group | Time Since Treatment (Mean ± Standard Deviation) | ||||
|---|---|---|---|---|---|
| 2 Weeks | 4 Weeks | 8 Weeks | 12 Weeks | 24 Weeks | |
| Stab control | 1.15 ± 0.35 | 0.77 ± 0.30 | |||
| Saline only | 0.41 ± 0.23 | * | 1.03 ± 0.57 | 0.78 ± 0.55 | 0.87 ± 0.52 |
| Saline + 5 mg/mL simvastatin | 1.19 ± 1.00 | 1.67 ± 0.59 | 0.80 ± 0.25 | ||
| Saline + 10 mg/mL simvastatin | 0.53 ± 0.23 | 0.86 ± 0.27 | 0.52 ± 0.34 | ||
| Saline + 15 mg/mL simvastatin | 0.48 ± 0.27 | * | 0.56 ± 0.39 | 0.56 ± 0.29 | 0.37 ± 0.14 |
| Hydrogel only | 0.50 ± 0.29 | 0.51 ± 0.42 | * | * | |
| Hydrogel + 5 mg/mL simvastatin | 1.20 ± 0.53 | 0.95 ± 0.74 | 0.97 ± 0.58 | * | 1.02 ± 0.79 |
| Hydrogel + 15 mg/mL simvastatin | 0.53 ± 0.36 | 0.59 ± 0.59 | 0.49 ± 0.42 | 0.51 ± 0.26 | 0.89 ± 0.70 |
No value due to MRI scanner malfunction. Empty cells without an asterisk indicate groups that were not assessed radiographically at later time points.
Figure 2.
Line graph showing normalized MRI indices.
Figure 3.
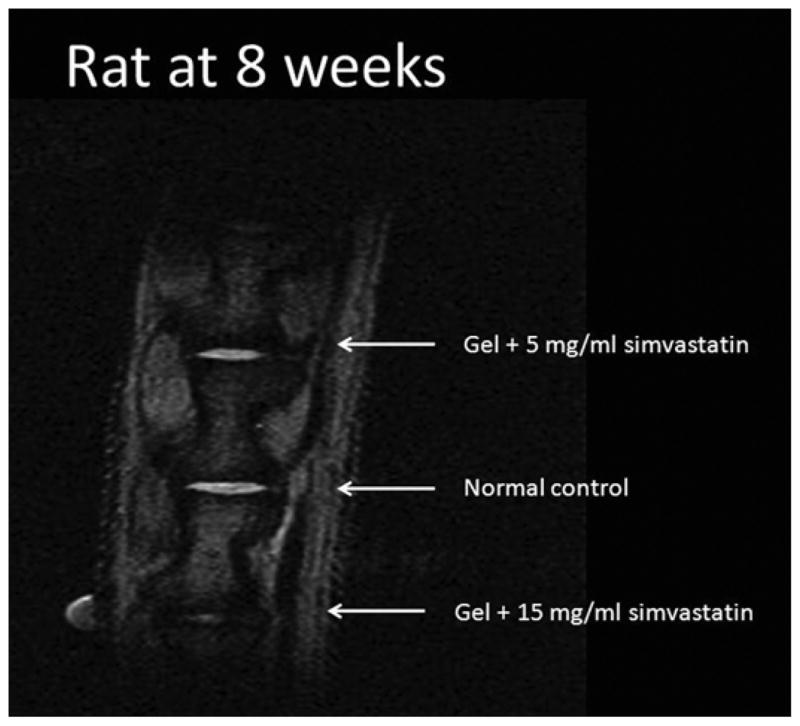
MRI of rat 8 weeks after treatment. The most-cranial disc was treated with 5 mg/mL simvastatin in hydrogel (normalized MRI index, 1.955), the middle disc is a normal control (1.0), and the most caudal disc was treated with 15 mg/mL simvastatin in hydrogel (0.144). Note the greater disc volume and signal intensity in the disc treated with 5 mg/mL simvastatin compared to the one treated with 15 mg/mL.
Histology
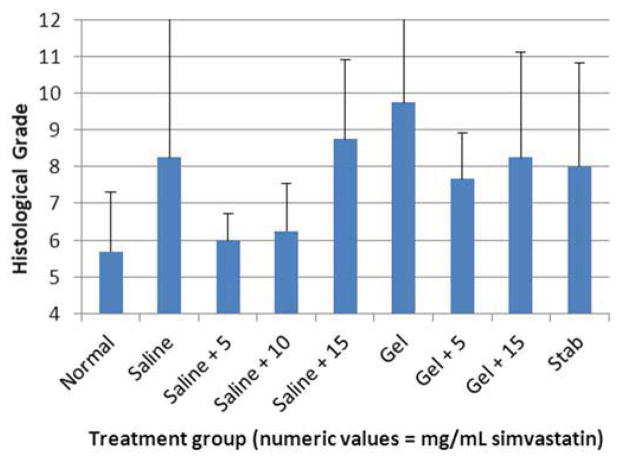
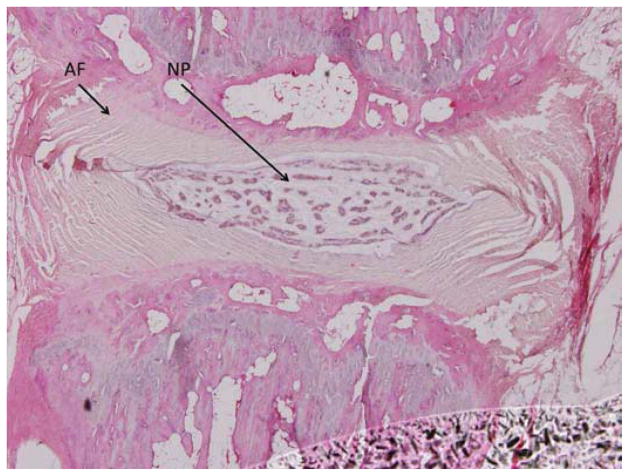
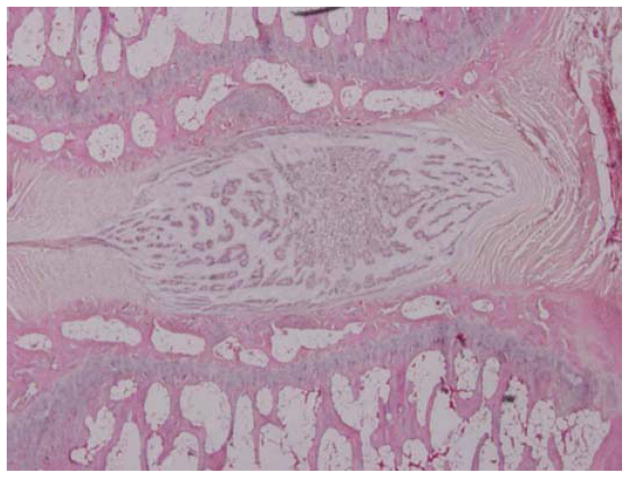
One hundred forty-four disc spaces were prepared for histological analysis. Histological scores based on the scale in Table 1 are summarized in Table 3 and graphically displayed in Figure 4A–D. Histological grades averaged over time showed significantly higher grades (worse outcomes) when compared with normal controls in the following groups: hydrogel only (p<0.01), 10 mg/mL and 15 mg/mL simvastatin in saline (both with p<0.01), and 15 mg/mL simvastatin in hydrogel (p<0.001). Although not statistically significant, within the saline and hydrogel groups, administration of 5 mg/mL simvastatin showed lower histological grades (better outcomes) compared with saline only or hydrogel only and also compared with the corresponding higher doses of 10 and 15 mg/mL. Of the treatment groups that were followed to week 24 (saline only, 15 mg/mL simvastatin in saline, 5 mg/mL simvastatin in hydrogel, and 15 mg/mL simvastatin in hydrogel), treatment with 5 mg/mL simvastatin in hydrogel was the only treatment that resulted in histological grades lower (better) than normal control at week 24 (lower by 0.98; p=0.32). Representative examples are displayed in Figure 5A–C.
Table 3.
Histological grades of disc spaces based on readings of 3 independent observers.
| Time (wks) | Treatment Groups (Mean ± Standard Deviation) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal Control | Stab | Saline Only | Saline + 5 mg/mL Simvastatin | Saline + 10 mg/mL Simvastatin | Saline + 15 mg/mL Simvastatin | Gel Only | Gel + 5 mg/mL Simvastatin | Gel + 15 mg/mL Simvastatin | |
| 2 | 5.8 ± 1.1 (n = 15) | 6.8 ± 1.9 (n = 4) | 5.3 ± 1.2 (n = 3) | 7.0 ± 0.8 (n = 4) | 6.5 ± 1.3 (n = 4) | 7.7 ± 3.5 (n = 3) | 6.3 ± 2.1 (n = 4) | 10.1 ± 3.5 (n = 4) | |
| 8 | 5.7 ± 1.7 (n = 16) | 8.0 ± 3.5 (n = 3) | 8.3 ± 4.4 (n = 4) | 6.0 ± 0.8 (n = 4) | 6.3 ± 1.5 (n = 4) | 8.8 ± 2.50 (n = 4) | 9.8 ± 3.3 (n = 4) | 7.7 ± 1.5 (n = 3) | 8.3 ± 3.3 (n = 4) |
| 12 | 5.9 ± 1.6 (n = 12) | 9.0 ± 2.7 (n = 3) | 6.3 ± 3.9 (n = 4) | 10.5 ± 3.0 (n = 4) | 9.0 ± 2.9 (n = 4) | 5.8 ± 1.0 (n = 4) | 6.0 ± 2.3 (n = 4) | ||
| 24 | 5.9 ± 1.1 (n = 8) | 6.7 ± 2.9 (n = 3) | 6.5 ± 1.0 (n = 4) | 5.5 ± 0.6 (n = 4) | 8.0 ± 2.7 (n = 3) | ||||
n = number of disc spaces graded
Figure 4.
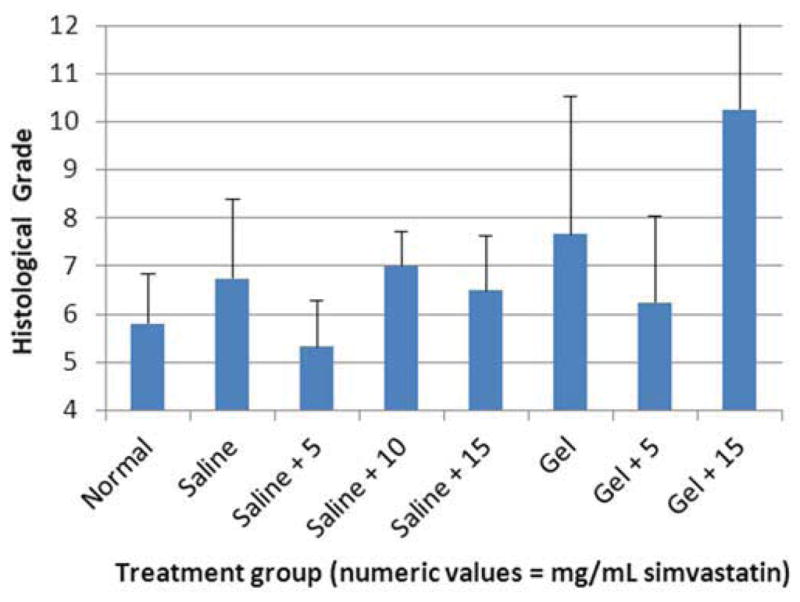
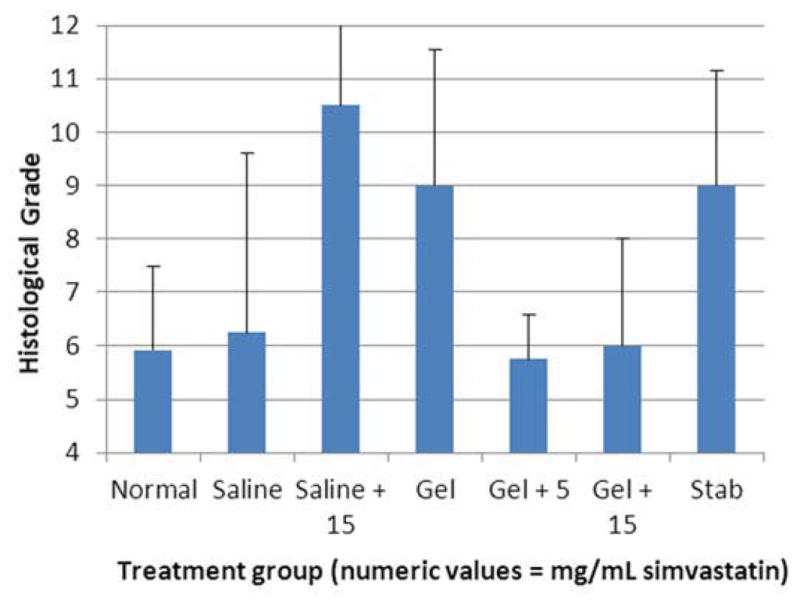
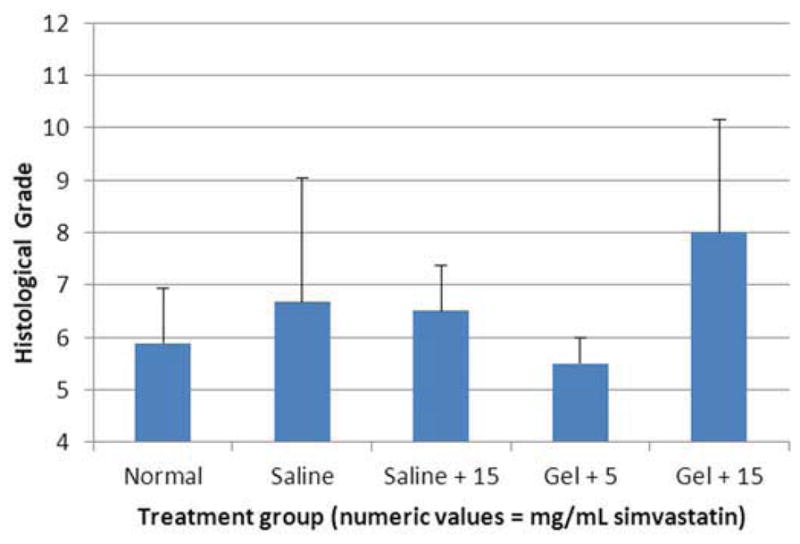
Bar graphs illustrating histological grades (sum of 4 categories from grading scale) at A) 2 weeks, B) 8 weeks, C) 12 weeks, and D) 24 weeks.
Figure 5.
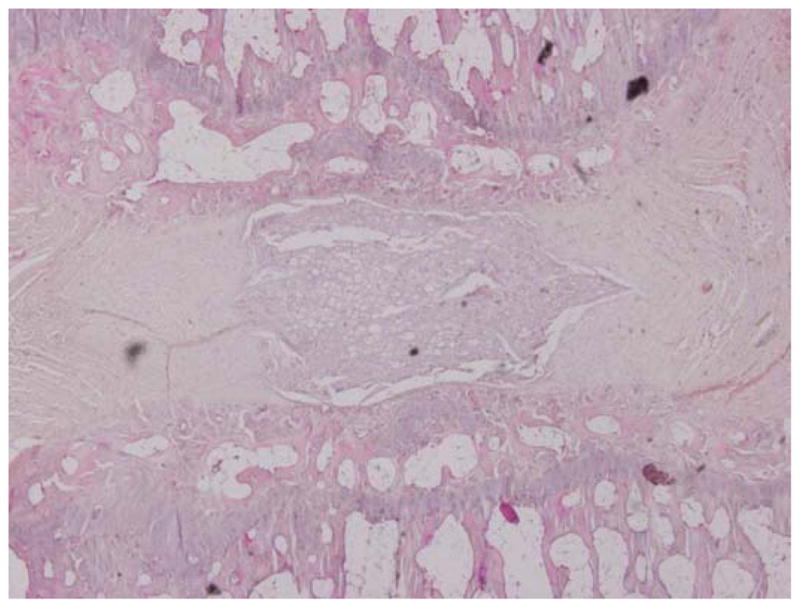
Representative histological specimens of rats sacrificed at 24 weeks. A) Normal control. B) Disc treated with 5 mg/mL simvastatin. C) Disc treated with 15 mg/mL simvastatin. Note in A and B the regular appearance of the AF, the intact border of the AF and NP, and the increased cellularity compared to C. (Images at 4x microscopic power.)
Quantitative rtPCR
As discussed above, genetic quantification of BMP-2, aggrecan, and collagen types I and II was performed using rtPCR.
Genetic expression of BMP-2 as a ratio to control is displayed numerically in Table 4 and graphically in Figure 6. The premise of the study was that administration of simvastatin up-regulates BMP-2 expression, and indeed it was up-regulated at all doses in both saline and hydrogel carriers. Note that BMP-2 expression decreased with time in the saline carrier groups, and increased with time in the (hydrogel + 5 mg/mL simvastatin) group.
Table 4.
Gene expression of BMP-2.
| Treatment Group | Time Point (Ratio to Control ± Standard Deviation) | |||
|---|---|---|---|---|
| 2 Weeks | 4 Weeks | 8 Weeks | 12 Weeks | |
| Saline + 5 mg/mL simvastatin | 9.14 ± 0.83 | 6.63 ± 0.79 | 2.20 ± 1.23 | |
| Saline + 15 mg/mL simvastatin | 27.41 ± 0.02 | 3.11 ±0.33 | 1.91 ± 0.88 | 1.22 ± 0.10 |
| Hydrogel + 5 mg/mL simvastatin | 1.84 ± 0.91 | 1.45 ± 0.12 | 7.82 ± 2.78 | |
| Hydrogel + 15 mg/mL simvastatin | 1.86 ± 0.20 | 1.21 ± 0.52 | ||
Figure 6.
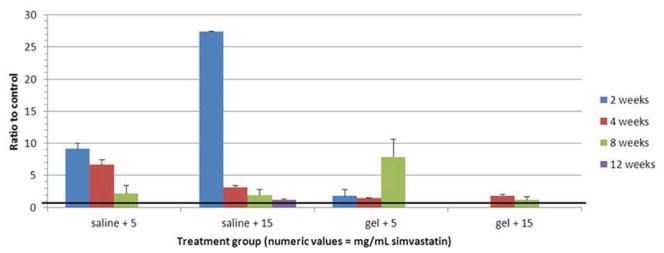
Bar graph illustrating gene expression of BMP-2. Heavy black line = control (1.0).
Genetic expression of aggrecan as a ratio to control is displayed numerically in Table 5 and graphically in Figure 7. Recall that aggrecan expression decreases in degenerated discs. In the saline carrier subgroups, mRNA expression of aggrecan was consistently elevated compared to control at a dose of 10 mg/mL; the effect was less consistent at doses of 5 and 15 mg/mL simvastatin. In the hydrogel carrier subgroups, aggrecan was consistently elevated at a drug dose of 5 mg/mL but not 15 mg/mL.
Table 5.
Gene expression of aggrecan.
| Treatment Group | Time Point (Ratio to Control ± Standard Deviation) | ||||
|---|---|---|---|---|---|
| 2 Weeks | 4 Weeks | 8 Weeks | 12 Weeks | 24 Weeks | |
| Saline only | 1.27 ± 0.29 | 0.20 ± 0.03 | |||
| Saline + 5 mg/mL simvastatin | 2.07 ± 1.35 | 0.84 ± 0.12 | 1.33 ± 0.11 | ||
| Saline + 10 mg/mL simvastatin | 1.41 ± 0.33 | 1.82 ± 0.27 | 1.57 ± 0.35 | ||
| Saline + 15 mg/mL simvastatin | 2.19 ± 0.47 | 0.86 ± 0.17 | 0.70 ± 0.10 | 2.29 ± 0.29 | |
| Hydrogel only | 0.83 ± 0.18 | 1.03 ± 0.14 | |||
| Hydrogel + 5 mg/mL simvastatin | 0.99 ± 0.18 | 1.85 ± 0.23 | 1.99 ± 0.48 | ||
| Hydrogel + 15 mg/mL simvastatin | 0.86 ± 0.35 | 0.97 ± 0.15 | 2.81 ± 0.17 | 3.09 ± 1.03 | |
Figure 7.
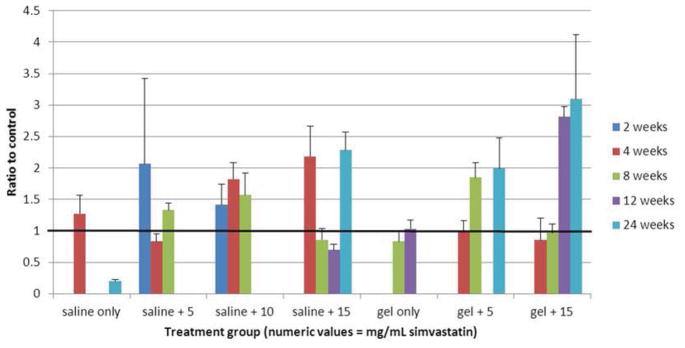
Bar graph illustrating gene expression of aggrecan. Heavy black line = control (1.0).
Genetic expression of collagen type II as a ratio to control is displayed numerically in Table 6 and graphically in Figure 8. Like aggrecan, collagen type II is elevated in healthy discs and diminished in degenerated ones. Here, up-regulation of this gene is consistently seen at all time points in the (saline + 5 mg/mL simvastatin) and (hydrogel + 15 mg/mL simvastatin) subgroups. In the (saline + 10 mg/mL simvastatin), (saline + 15 mg/mL simvastatin), and (hydrogel + 5 mg/mL simvastatin) subgroups, expression of collagen type II is generally elevated but less consistently so.
Table 6.
Gene expression of collagen type II.
| Treatment Group | Time Point (Ratio to Control ± Standard Deviation) | ||||
|---|---|---|---|---|---|
| 2 Weeks | 4 Weeks | 8 Weeks | 12 Weeks | 24 Weeks | |
| Saline only | 0.91 ± 0.20 | 0.59 ± 0.10 | |||
| Saline + 5 mg/mL simvastatin | 4.10 ± 0.70 | 1.62 ± 0.34 | 2.38 ± 1.21 | ||
| Saline + 10 mg/mL simvastatin | 0.77 ± 0.14 | 3.18 ± 0.45 | 3.87 ± 0.86 | ||
| Saline + 15 mg/mL simvastatin | 7.43 ± 1.23 | 0.74 ± 0.15 | 1.19 ± 0.24 | 1.78 ± 0.21 | |
| Hydrogel only | 0.64 ± 0.42 | 1.67 ± 0.37 | |||
| Hydrogel + 5 mg/mL simvastatin | 3.17 ± 0.59 | 0.48 ± 0.13 | 2.25 ± 0.32 | 0.94 ± 0.44 | |
| Hydrogel + 15 mg/mL simvastatin | 2.55 ± 0.83 | 1.59 ± 0.20 | 11.63 ± 1.55 | 2.22 ± 0.49 | |
Figure 8.
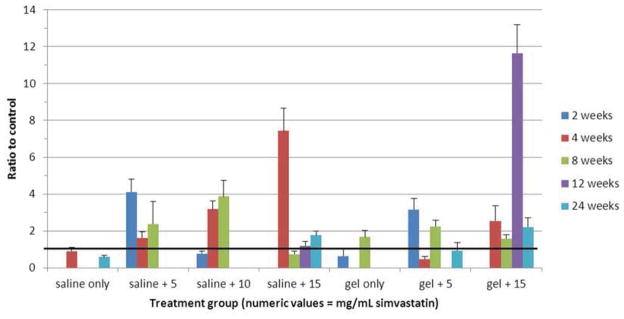
Bar graph illustrating gene expression of collagen type II. Heavy black line = control (1.0).
Genetic expression of collagen type I as a ratio to control is displayed numerically in Table 7. As opposed to aggrecan and collagen type II, collagen type I increases as discs become increasingly degenerated. Compared to aggrecan and collagen type II, the values of collagen type I are widespread with generally large standard deviations. A meaningful use of the collagen type I data is in calculating the differentiation index (collagen type II/collagen type I), which is a measure of active chondrogenesis. A table of calculated differentiation indices (DI) is provided in Table 8. The highest DI occurred in the (hydrogel + 5 mg/mL simvastatin) group at 24 weeks. Elevated DI also occurred in the (saline + 5 mg/mL simvastatin) group at 2 weeks and the (hydrogel + 15 mg/mL simvastatin) group at 4 weeks. This latter group was the only one to have elevated DI consistently across all time points.
Table 7.
Gene expression of collagen type I.
| Treatment Group | Time Point (Ratio to Control ± Standard Deviation) | |||
|---|---|---|---|---|
| 2 Weeks | 4 Weeks | 12 Weeks | 24 Weeks | |
| Saline only | 0.81 ± 0.71 | 65.80 ± 20.56 | 3.92 ± 0.70 | |
| Saline + 5 mg/mL simvastatin | 1.27 ± 0.42 | 216.09 ± 171.70 | ||
| Saline + 10 mg/mL simvastatin | 3.98 ± 0.84 | 49.26 ± 14.49 | ||
| Saline + 15 mg/mL simvastatin | 70.99 ± 56.75 | 28.19 ± 6.97 | 3.69 ± 0.40 | |
| Hydrogel only | 4.80 ± 0.72 | |||
| Hydrogel + 5 mg/mL simvastatin | 1.26 ± 0.95 | 0.17 ± 0.03 | ||
| Hydrogel + 15 mg/mL simvastatin | 1.12 ± 0.89 | 11.35 ± 9.17 | 1.84 ± 0.24 | |
Table 8.
Differentiation indices for collagen type II/collagen type I.
| Treatment Group | Time Point | |||
|---|---|---|---|---|
| 2 Weeks | 4 Weeks | 12 Weeks | 24 Weeks | |
| Saline only | 1.11 | 0.15 | ||
| Saline + 5 mg/mL simvastatin | 3.23 | 0.01 | ||
| Saline + 10 mg/mL simvastatin | 0.19 | 0.06 | ||
| Saline + 15 mg/mL simvastatin | 0.10 | 0.04 | 0.48 | |
| Hydrogel + 5 mg/mL simvastatin | 0.38 | 5.70 | ||
| Hydrogel + 15 mg/mL simvastatin | 2.28 | 1.03 | 1.20 | |
Values > 1 shown in bold text.
Discussion
Back pain is an enormous problem to an individual’s health and to society. Approximately 15–20% of adults have back pain during a single year, and 50–80% of adults will experience at least 1 episode of back pain during their lifetime [46]. Back pain is one of the most (if not the most) common reasons that individuals seek medical attention; there are 19 million office visits and 224,000 hospital admissions for low back pain in the United States each year, costing our country more than $100 billion annually [47]. The vast majority of these costs are indirect, resulting from lost wages and productivity. Only a small percentage of patients with back pain are surgical candidates, yet the conservative therapies of medications, physical therapy, and epidural injections do not result in analgesia for all patients. As such, there exists a continuing need for the development of novel treatments for back pain.
In this study, we utilized a rodent model of DDD in order to determine whether intradiscal injection of simvastatin results in retardation of the degenerative process, or even regeneration of the IVD. Two delivery vehicles were chosen: saline, which exposed the IVD to the entire concentration of drug at once, and a hydrogel, which allowed for delayed drug release. Outcome measures assessed included radiography (MRI), histology, and gene expression. On MRI, we found that rat discs treated with 5 mg/mL simvastatin in a hydrogel carrier were radiographically normal through the 24-week study period. That same dose had a short-term but not sustained benefit when delivered in saline. Discs treated with doses higher than 5 mg/mL simvastatin were degenerated in appearance regardless of the carrier or time point. Similar results were obtained histologically. In fact, at the later time points of 12 and 24 weeks, discs treated with 5 mg/mL simvastatin in hydrogel had average histologic grades even better than the normal controls. This data does suggest that the hydrogel served its purpose, namely delayed delivery of simvastatin.
Expression of the genes of interest was variable, likely due to the inherent challenges of PCR experiments. For BMP-2, we saw increased expression compared to normal control at all doses and time points regardless of carrier. Interestingly, in the saline carrier subgroups, BMP-2 expression was highest at 2 weeks and then gradually declined; contrarily, in the (hydrogel + 5 mg/mL simvastatin) group, expression of BMP-2 gradually increased and was highest at 8 weeks. Aggrecan was consistently elevated in the (hydrogel + 5 mg/mL simvastatin) and (saline + 10 mg/mL simvastatin) groups, and showed a gradual increase in the former with increasing time. Collagen type II was consistently elevated in the (saline + 5 mg/mL simvastatin) and (hydrogel + 15 mg/mL simvastatin) groups. The ratio of collagen type II/type I, or differentiation index, was consistently elevated in the (hydrogel + 15 mg/mL simvastatin) group. In general, PCR data does demonstrate up-regulation of BMP-2 and evidence for disc regeneration given the elevated expression of aggrecan and collagen type II.
Previous work on a smaller number of animals at one time point showed 5 mg/mL simvastatin to be a beneficial dose [48]; we used this dose as well as higher doses of 10 mg/mL and 15 mg/mL to determine whether greater benefit would be attained with more drug, which was not the case. One question that presents itself, then, is: Why did lower doses of simvastatin demonstrate greater benefit than higher doses? While further work will determine the optimal intradiscal dose of simvastatin, we surmise that higher doses were toxic to the disc secondary to altered cell membrane adipose metabolism, resulting in cell death. Other studies have found that statins decrease cell viability in a variety of cell types [49] due to interference of farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which are involved in control of cell adhesion, growth, and survival through protein prenylation [50, 51].
In the next phase of this project, experiments will be performed on larger animals, specifically Yorkshire pigs. One study has shown that, of non-primates, pig spines are most similar to that of humans [52]. To date, one porcine model of disc degeneration has been developed in miniature Yucatan pigs [53]. We have developed a similar model for Yorkshire pigs that utilizes a trans-psoas approach (manuscript in preparation). As we extend our investigations toward larger animals, an important question is whether the improved radiologic, histologic, and genetic outcomes seen in rats following simvastatin treatment of degenerated discs will correlate with improved functional outcomes. As such, future investigations will include measurements of inflammatory cell markers known to be elevated in the serum of back pain patients, as well as observational measures to determine if there is a difference in pain levels between treated and untreated pigs. This hopefully will provide some idea as to whether the treatment actually accomplishes its intended purpose: the reduction of discogenic back pain. If large animal investigations demonstrate benefit, consideration can then be given to testing the treatment in human subjects.
As with all studies, this one certainly possesses some limitations. First, the mechanism of disc injury (via needle puncture) differs from the chronic load-bearing etiology for disc degeneration in humans. However, this and previous studies [41, 48] have demonstrated that needle puncture results in radiographic, histologic, and genetic evidence of DDD akin to that seen in human disc degeneration. Second, while this study demonstrated that intradiscal injection of simvastatin led to beneficial results for our specific outcome measures, behavioral assessments were not performed. It is unknown whether regeneration of DDD in our model correlated with resolution of pain or function. Future large animal and human studies will certainly include such functional outcomes. Third, due to limitations in resources, certain subgroups were not extended through all time points. Specifically, the hydrogel-only (no drug) group was assessed only through 12 weeks, and the (saline + 5 mg/mL simvastatin) and (saline + 10 mg/mL simvastatin) groups were assessed only through 8 weeks. The results clearly demonstrated that injecting the hydrogel by itself did not result in disc regeneration radiographically, histologically, or genetically. Fourth and last, also due to resources, the stab control group was assessed only histologically at 8 and 12 weeks. However, the studies cited earlier in this paragraph have documented the radiographic and genetic changes that take place following needle puncture.
Footnotes
DISCLOSURES
The authors gratefully acknowledge funding support provided by the National Institutes of Health. The project described was supported by Grant Number R01 AR056649 from NIAMS/NIH awarded to Chia-Ying Lin.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson JAD. Back pain and occupation. In: Jayson MIV, editor. The lumbar spine and back pain. 3. Edinburgh: Churchill Livingstone; 1987. pp. 2–36. [Google Scholar]
- 2.Barrick WT, Schofferman JA, Reynolds JB, et al. Anterior lumbar fusion improves discogenic pain at levels of prior posterolateral fusion. Spine (Phila Pa 1976) 2000;25(7):853–7. doi: 10.1097/00007632-200004010-00014. [DOI] [PubMed] [Google Scholar]
- 3.Kellgren JH. The anatomical source of back pain. Rheumatol Rehabil. 1977;16(1):3–12. doi: 10.1093/rheumatology/16.1.3. [DOI] [PubMed] [Google Scholar]
- 4.Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22(2):181–7. [PubMed] [Google Scholar]
- 5.Luoma K, Riihimaki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25(4):487–92. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 6.Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:58–64. doi: 10.22203/ecm.v008a06. [DOI] [PubMed] [Google Scholar]
- 7.Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35(Pt 4):652–5. doi: 10.1042/BST0350652. [DOI] [PubMed] [Google Scholar]
- 8.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;(427 Suppl):S27–36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 9.Shen B, Melrose J, Ghosh P, Taylor F. Induction of matrix metalloproteinase-2 and -3 activity in ovine nucleus pulposus cells grown in three-dimensional agarose gel culture by interleukin-1beta: a potential pathway of disc degeneration. Eur Spine J. 2003;12(1):66–75. doi: 10.1007/s00586-002-0454-2. [DOI] [PubMed] [Google Scholar]
- 10.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48(1):5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 11.Hilton RC, Ball J. Vertebral rim lesions in dorsolumbar spine. Ann Rheum Dis. 1984;43(6):856–7. doi: 10.1136/ard.43.6.856-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddi AH. Bone and cartilage differentiation. Curr Opin Genet Dev. 1994;4(5):737–44. doi: 10.1016/0959-437x(94)90141-o. [DOI] [PubMed] [Google Scholar]
- 13.Gilbertson L, Ahn SH, Teng PN, Studer RK, Niyibizi C, Kang JD. The effects of recombinant human bone morphogenetic protein-2, recombinant human bone morphogenetic protein-12, and adenoviral bone morphogenetic protein-12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. Spine J. 2008;8(3):449–56. doi: 10.1016/j.spinee.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001;189(3):275–84. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- 15.Snelling SJ, Hulley PA, Loughlin J. BMP5 activates multiple signaling pathways and promotes chondrogenic differentiation in the ATDC5 growth plate model. Growth Factors. 2010;28(4):268–79. doi: 10.3109/08977191003752296. [DOI] [PubMed] [Google Scholar]
- 16.Sekiya I, Colter DC, Prockop DJ. BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem Biophys Res Commun. 2001;284(2):411–8. doi: 10.1006/bbrc.2001.4898. [DOI] [PubMed] [Google Scholar]
- 17.DiLeone RJ, King JA, Storm EE, Copeland NG, Jenkins NA, Kingsley DM. The BMP-8 gene is expressed in developing skeletal tissue and maps near the Achondroplasia locus on mouse chromosome 4. Genomics. 1997;40(1):196–8. doi: 10.1006/geno.1996.4533. [DOI] [PubMed] [Google Scholar]
- 18.Bolt P, Clerk AN, Luu HH, et al. BMP-14 gene therapy increases tendon tensile strength in a rat model of Achilles tendon injury. J Bone Joint Surg Am. 2007;89(6):1315–20. doi: 10.2106/JBJS.F.00257. [DOI] [PubMed] [Google Scholar]
- 19.Thawani JP, Wang AC, Than KD, Lin CY, La Marca F, Park P. Bone morphogenetic proteins and cancer: review of the literature. Neurosurgery. 2010;66(2):233–46. doi: 10.1227/01.NEU.0000363722.42097.C2. [DOI] [PubMed] [Google Scholar]
- 20.Takae R, Matsunaga S, Origuchi N, et al. Immunolocalization of bone morphogenetic protein and its receptors in degeneration of intervertebral disc. Spine (Phila Pa 1976) 1999;24(14):1397–401. doi: 10.1097/00007632-199907150-00002. [DOI] [PubMed] [Google Scholar]
- 21.Fei QM, Jiang XX, Chen TY, et al. Changes with age and the effect of recombinant human BMP-2 on proteoglycan and collagen gene expression in rabbit anulus fibrosus cells. Acta Biochim Biophys Sin (Shanghai) 2006;38(11):773–9. doi: 10.1111/j.1745-7270.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- 22.Wallach CJ, Sobajima S, Watanabe Y, et al. Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine (Phila Pa 1976) 2003;28(20):2331–7. doi: 10.1097/01.BRS.0000085303.67942.94. [DOI] [PubMed] [Google Scholar]
- 23.Moon SH, Nishida K, Gilbertson LG, et al. Biologic response of human intervertebral disc cells to gene therapy cocktail. Spine (Phila Pa 1976) 2008;33(17):1850–5. doi: 10.1097/BRS.0b013e31817e1cd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Anderson DG, Phillips FM, et al. Comparative effects of bone morphogenetic proteins and Sox9 overexpression on matrix accumulation by bovine anulus fibrosus cells: implications for anular repair. Spine (Phila Pa 1976) 2007;32(23):2515–20. doi: 10.1097/BRS.0b013e318158cc09. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Li Z, Thonar EJ, et al. Transduced bovine articular chondrocytes affect the metabolism of cocultured nucleus pulposus cells in vitro: implications for chondrocyte transplantation into the intervertebral disc. Spine (Phila Pa 1976) 2005;30(23):2601–7. doi: 10.1097/01.brs.0000187880.39298.f0. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Phillips FM, Thonar EJ, et al. Cell therapy using articular chondrocytes overexpressing BMP-7 or BMP-10 in a rabbit disc organ culture model. Spine (Phila Pa 1976) 2008;33(8):831–8. doi: 10.1097/BRS.0b013e31816b1f38. [DOI] [PubMed] [Google Scholar]
- 27.Boden SD. Biology of lumbar spine fusion and use of bone graft substitutes: present, future, and next generation. Tissue Eng. 2000;6(4):383–99. doi: 10.1089/107632700418092. [DOI] [PubMed] [Google Scholar]
- 28.Park JS, Nagata K. BMP and LMP-1 for intervertebral disc regeneration. Clin Calcium. 2004;14(7):76–8. [PubMed] [Google Scholar]
- 29.Tim Yoon S, Su Kim K, Li J, et al. The effect of bone morphogenetic protein-2 on rat intervertebral disc cells in vitro. Spine (Phila Pa 1976) 2003;28(16):1773–80. doi: 10.1097/01.BRS.0000083204.44190.34. [DOI] [PubMed] [Google Scholar]
- 30.Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine (Phila Pa 1976) 2004;29(23):2603–11. doi: 10.1097/01.brs.0000146103.94600.85. [DOI] [PubMed] [Google Scholar]
- 31.Kuh SU, Zhu Y, Li J, et al. The AdLMP-1 transfection in two different cells; AF cells, chondrocytes as potential cell therapy candidates for disc degeneration. Acta Neurochir (Wien) 2008;150(8):803–10. doi: 10.1007/s00701-008-1617-7. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Ruan DK, Zhang C, Wang DL, Xin H, Zhang Y. Effects of adeno-associated virus-2-mediated human BMP-7 gene transfection on the phenotype of nucleus pulposus cells. J Orthop Res. 2011;29(6):838–45. doi: 10.1002/jor.21310. [DOI] [PubMed] [Google Scholar]
- 33.Anderson DW, Burton DC, Jackson RS. Postoperative cervical myelopathy and cord compression associated with the use of recombinant bone morphogenetic protein-2 in posterior cervical decompression, instrumentation, and arthrodesis: a report of two cases. Spine (Phila Pa 1976) 2011;36(10):E682–6. doi: 10.1097/BRS.0b013e3181f8f4d3. [DOI] [PubMed] [Google Scholar]
- 34.Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine. 1976;32(25):2885–90. doi: 10.1097/BRS.0b013e31815b7596. [DOI] [PubMed] [Google Scholar]
- 35.Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2) Spine J. 2008;8(6):1011–8. doi: 10.1016/j.spinee.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Choudhry OJ, Christiano LD, Singh R, Golden BM, Liu JK. Bone morphogenetic protein-induced inflammatory cyst formation after lumbar fusion causing nerve root compression. J Neurosurg Spine. 2012;16(3):296–301. doi: 10.3171/2011.11.SPINE11629. [DOI] [PubMed] [Google Scholar]
- 37.Mannion RJ, Nowitzke AM, Wood MJ. Promoting fusion in minimally invasive lumbar interbody stabilization with low-dose bone morphogenic protein-2--but what is the cost? Spine J. 2011;11(6):527–33. doi: 10.1016/j.spinee.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Than KD, Rahman SU, McKeever PE, Wang AC, La Marca F, Park P. Symptomatic calcified perineural cyst after use of bone morphogenetic protein in transforaminal lumbar interbody fusion: a case report. Spine J. 2013;13(8):21. doi: 10.1016/j.spinee.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 39.Mundy G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286(5446):1946–9. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Lin CY. Simvastatin stimulates chondrogenic phenotype of intervertebral disc cells partially through BMP-2 pathway. Spine (Phila Pa 1976) 2008;33(16):E525–31. doi: 10.1097/BRS.0b013e31817c561b. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, La Marca F, Hollister SJ, Goldstein SA, Lin CY. Developing consistently reproducible intervertebral disc degeneration at rat caudal spine by using needle puncture. J Neurosurg Spine. 2009;10(6):522–30. doi: 10.3171/2009.2.SPINE08925. [DOI] [PubMed] [Google Scholar]
- 42.Park KM, Ko KS, Joung YK, Shin H, Park KD. In situ cross-linkable gelatin-poly(ethylene glycol)-tyramine hydrogel via enzyme-mediated reaction for tissue regenerative medicine. J Mater Chem. 2011;21:13180–7. [Google Scholar]
- 43.Park YS, David AE, Park KM, et al. Controlled release of simvastatin from in situ forming hydrogel triggers bone formation in MC3T3-E1 cells. AAPS J. 2013;15(2):367–76. doi: 10.1208/s12248-012-9442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sobajima S, Kompel JF, Kim JS, et al. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine (Phila Pa 1976) 2005;30(1):15–24. doi: 10.1097/01.brs.0000148048.15348.9b. [DOI] [PubMed] [Google Scholar]
- 45.Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976) 2005;30(1):5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 46.Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25(2):353–71. doi: 10.1016/j.ncl.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88 (Suppl 2):21–4. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Wang L, Park JB, et al. Intradiscal injection of simvastatin retards progression of intervertebral disc degeneration induced by stab injury. Arthritis Res Ther. 2009;11(6):R172. doi: 10.1186/ar2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dirks AJ, Jones KM. Statin-induced apoptosis and skeletal myopathy. Am J Physiol Cell Physiol. 2006;291(6):C1208–12. doi: 10.1152/ajpcell.00226.2006. [DOI] [PubMed] [Google Scholar]
- 50.Aznar S, Lacal JC. Rho signals to cell growth and apoptosis. Cancer Lett. 2001;165(1):1–10. doi: 10.1016/s0304-3835(01)00412-8. [DOI] [PubMed] [Google Scholar]
- 51.Coleman ML, Marshall CJ, Olson MF. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat Rev Mol Cell Biol. 2004;5(5):355–66. doi: 10.1038/nrm1365. [DOI] [PubMed] [Google Scholar]
- 52.McLain RF, Yerby SA, Moseley TA. Comparative morphometry of L4 vertebrae: comparison of large animal models for the human lumbar spine. Spine (Phila Pa 1976) 2002;27(8):E200–6. doi: 10.1097/00007632-200204150-00005. [DOI] [PubMed] [Google Scholar]
- 53.Yoon SH, Miyazaki M, Hong SW, et al. A porcine model of intervertebral disc degeneration induced by annular injury characterized with magnetic resonance imaging and histopathological findings. Laboratory investigation. J Neurosurg Spine. 2008;8(5):450–7. doi: 10.3171/SPI/2008/8/5/450. [DOI] [PubMed] [Google Scholar]