Over the last decade, greater understanding of the pathophysiology of diarrhea-predominant irritable bowel syndrome (IBS-D) has resulted in exploration of newer treatment targets, including tryptophan hydroxylase inhibitor (LX-1031), newer M3 muscarinic antagonists (otilonium, darifenacin, solifenacin), oral carbon adsorbents (AST-120), mast cell stabilizers (disodium cromoglycate, ketotifen), and amino acids (glutamine)1. Only a handful of these have been approved (e.g., otilonium by European Medicine Agency), many are being used off-label, and a few are in an investigational pipeline. Thus, there is a significant unmet need for treatment of disorders associated with chronic diarrhea such as IBS-D.
This editorial addresses three questions: First, are 5-HT3 antagonists as effective in males as in females with IBS-D, and what might be potential reasons for the differences? Second, are newer generation 5-HT3 antagonists also rarely associated with ischemic colitis? Third, were the endpoints used in the most recent studies consistent with regulatory guidelines for drugs in development for the treatment of IBS-D?
Antagonism of serotonin (5-HT) type 3 receptor has been an established treatment strategy for chronic diarrhea related to IBS, 2, 3 with occasional application to other disorders mediated by serotonin such as carcinoid syndrome4. Over 90% of the body’s 5-HT is located in the enterochromaffin cells in the mucosa of the GI tract, and these mucosal 5-HT receptors are involved in secretion, motility, and nociception. Initial studies showed that ondansetron decreased colonic transit in healthy volunteers5. Granisetron impeded the reflex activation of colonic motility in response to upper gastrointestinal stimuli, including mechanical or chemical stimuli, such as antral distension or intraduodenal lipid6 or a meal7. These observations with older 5-HT3 antagonists led to development of second generation, more potent and specific 5HT3 receptor antagonists (alosetron and cilansetron), which were highly efficacious in global endpoints (e.g. adequate relief), specific symptoms2, 3, quality of life, and reversing the restriction of daily activities8. However, these were either withdrawn from the market (alosetron) or never marketed (cilansetron) due to concerns of complications of severe constipation and reports of ischemic colitis (0.6 and 1.1 per 1000 patient-years respectively); these rates of complications were confirmed in the analysis of data in the risk management program9. However, the pathobiological mechanisms of the ischemic colitis are unclear10, 11, and it is still not completely resolved whether the risk is entirely due to the medication or related to IBS per se (independent of serotonergic therapies), since several population-based studies demonstrated that a diagnosis of IBS increases the risk of developing ischemic colitis 2- to 4-fold (reviewed in12). Currently, alosetron is available under a risk management program for women with severe IBS-D who are not responding to other therapies. Therefore, despite the efficacy of these agents, their restricted use has resulted in an unmet clinical need.
In this issue of Clinical Gastroenterology and Hepatology, Fukudo et al. present results of a randomized, double-blind, placebo-controlled trial of ramosetron in 296 male IBS-D patients recruited across 52 centers in Japan13. Ramosetron, a tetrahydrobenzimidazole derivative, is a potent and selective 5-HT3 receptor antagonist and has been marketed in Japan since 1996, predominantly as an antiemetic for patients receiving chemotherapy14. Ramosetron dose-dependently suppressed restraint stress-induced defecation disturbance in rats15 and was also found to suppress corticotropin-releasing hormone [CRH (intracerebroventricular injection)] induced accelerated defecation16. Moreover, ramosetron significantly increased the threshold of pain induced by colonic distension in rats17. In a previous trial from Japan, involving both men and women with IBS-D, ramosetron, 5μg once daily, was found to be effective, based on patient reported global relief of IBS, and ramosetron was well tolerated18. In Japan, the male IBS population is of particular relevance because of the higher prevalence of IBS in males as compared to the females. In the current trial, which was also conducted exclusively in males, there was a sizeable (30.7%) difference in the responder rates (50.3% ramosetron vs. 19.6% placebo) for the improvement in stool consistency in the first month (primary outcome). Additionally, monthly responder rates at all evaluation points were significantly higher in the ramosetron group than in the placebo group. The ramosetron group achieved significantly higher responder rates for global relief of overall IBS symptoms and abdominal pain/discomfort at all evaluation points. Greater improvements in overall IBS quality of life scores were observed in the ramosetron group at week 4 and at the last time-point. Significantly more people in the ramosetron group (8.2%) reported “hard stools” as compared to the placebo group (1.3%). However, the incidence of constipation was not significantly higher (3.4% vs. 0.7%, relative risk: 5.07, 95% CI: 0.6–42.3). The authors state that, between the current study and the previous studies with ramosetron, a total of 901 IBS-D patients have been recruited, and no cases of ischemic colitis have been reported. This is consistent with a summary of 28–52 weeks of follow-up of 957 patients who participated in ramosetron treatment trials of 12 weeks’ duration; in these patients, no serious adverse events of severe constipation or ischemic colitis were reported for long-term treatment with ramosetron19.
In the FDA guidance statement for clinical trial endpoints for IBS-D20, it is recommended that the defecation component of the proposed primary endpoint can be evaluated by assessing stool consistency using Bristol Stool Form Scale. It is also recommended to have abdominal pain intensity as a second primary pain assessment in IBS trials. However, the authors in the current article suggest that, if a drug is expected to act on either pain or defecation as its primary target, it is reasonable to use that as the primary endpoint, with the second symptom being a secondary endpoint. Fukudo et al. selected improvement in stool consistency as their primary outcome endpoint, which is reasonable given the demonstration of the effects of this class of medication on colonic transit, as described above. However, one could also make a good case for using improvement in pain as a primary endpoint in studies of the efficacy of a 5-HT3 antagonist, as it can be expected to improve pain and nociception7, 21; a previous trial with ramosetron showed improvement in pain18. Moreover, 5-HT3 receptors are located on visceral and central terminal of spinal afferents in animal models and play a role in visceral sensitivity22, 23.
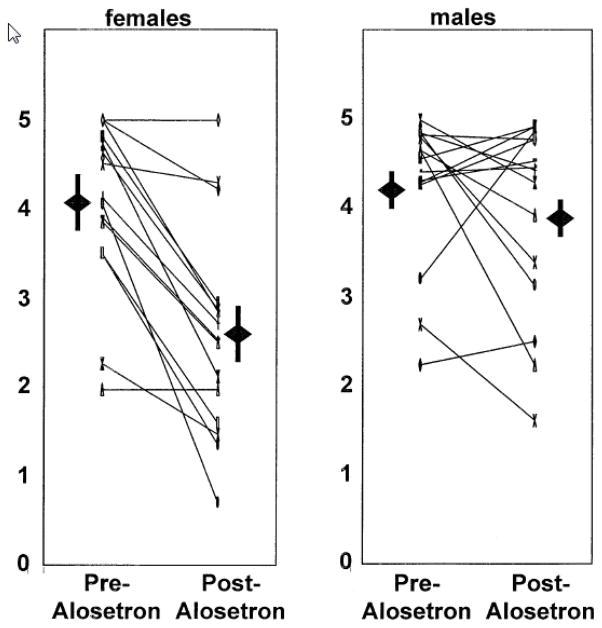
The evaluation of efficacy in males alone in this trial is noticeable. In another, dose-ranging, randomized, double-blind, placebo-controlled study in males with IBS-D, alosetron,1mg twice a day, resulted in adequate relief of pain and discomfort during weeks 5–12 of the treatment phase (53% in alosetron group vs. 40% in placebo). All doses (0.5, 1, 2, and 4mg) of alosetron showed significantly reduced stool consistency scores within 1 week, and these effects were maintained throughout the treatment until the first week following treatment. The stool frequency, bloating, or pain and discomfort free days did not improve statistically over placebo in any of the treatment groups24. Direct efficacy comparisons with 5-HT3 antagonists among male and female gender have not been made; however, in trials of women with IBS-D, each of these symptoms improved with alosetron compared to placebo. We have found that treatment with alosetron resulted in a significantly greater retardation of small bowel and colonic transit in 15 females with IBS-D as compared to 15 males25. In this pharmacodynamic study, the individual transit profiles (Fig.) showed that 2 of 15 males had retardation of colonic transit at 24h that was equal to or greater than the mean change (1.45 geometric center units) in females observed. Moreover, in 4 of the males, there was a slowing of colonic transit at 24h that was equivalent to at least one geometric center unit; that is, the geometric center in the evaluation with alosetron treatment was at least one region more proximal than it was at baseline, suggesting that the medication was having an effect on colonic function in these individuals. Conversely, alosetron had no effect on colonic transit in 2 females.
Figure.
Scatter plot of geometric centers of colonic transit at 24h pre- and post-alosetron in females and males with diarrhea-predominant irritable bowel syndrome. The range of geometric center is 0 for location of isotope at the ileocecal valve, and 5 for all isotope located in stool. Also shown are the mean + SEM for each data set.
The greater overall efficacy in women is plausibly due to differences in pharmacokinetics (e.g., differences in CYP1A2 activity)26, gender-related differences in serotonin-mediated modulation of gut sensorimotor responses25, differences in serotonin reuptake transported protein (SERT) polymorphisms among women and men with IBS27, and differential central effects (inhibitory effects on limbic areas which are more abnormally activated in response to visceral events in women than in men)28, 29. Thus, it is plausible that ramosetron will have equal or greater efficacy in females.
One of the key hopes in the development of newer 5-HT3 antagonists for IBS-D is development of an agent that would be less likely to cause serious effects from constipation and ischemic colitis. This will play a significant role in development and approval of a drug in this class. Although, a good portion of participants in this trial reported “hard stool” and “constipation” in comparison to the placebo, this did not reach significance, and the authors report that constipation was mild and easy to manage in most participants. Considering the reported ischemic colitis incidence of ~1/1000 patient years, it is not possible to be sure that ramosetron will be safer than alosetron. Overall, a successful trial of a new 5-HT3 antagonist for IBS-D is welcome news, but whether it holds a new promise for both safety and efficacy is yet to be fully established.
Acknowledgments
Funding: Dr. Camilleri is supported by NIH RO1-DK92179.
Footnotes
Conflict of interest: The authors have no conflict of interest relevant to the subject matter of this editorial.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Camilleri M. Current and future pharmacological treatments for diarrhea-predominant irritable bowel syndrome. Expert opinion on pharmacotherapy. 2013;14:1151–60. doi: 10.1517/14656566.2013.794223. [DOI] [PubMed] [Google Scholar]
- 2.Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2008;6:545–55. doi: 10.1016/j.cgh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford AC, Brandt LJ, Young C, Chey WD, Foxx-Orenstein AE, Moayyedi P. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: systematic review and meta-analysis. The American journal of gastroenterology. 2009;104:1831–43. doi: 10.1038/ajg.2009.223. quiz 1844. [DOI] [PubMed] [Google Scholar]
- 4.Saslow SB, Scolapio JS, Camilleri M, Forstrom LA, Thomforde GM, Burton DD, Rubin J, Pitot HC, Zinsmeister AR. Medium-term effects of a new 5HT3 antagonist, alosetron, in patients with carcinoid diarrhoea. Gut. 1998;42:628–34. doi: 10.1136/gut.42.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talley NJ, Phillips SF, Haddad A, Miller LJ, Twomey C, Zinsmeister AR, MacCarty RL, Ciociola A. GR 38032F (ondansetron), a selective 5HT3 receptor antagonist, slows colonic transit in healthy man. Digestive diseases and sciences. 1990;35:477–80. doi: 10.1007/BF01536922. [DOI] [PubMed] [Google Scholar]
- 6.Bjornsson ES, Chey WD, Ladabaum U, Woods ML, Hooper FG, Owyang C, Hasler WL. Differential 5-HT3 mediation of human gastrocolonic response and colonic peristaltic reflex. The American journal of physiology. 1998;275:G498–505. doi: 10.1152/ajpgi.1998.275.3.G498. [DOI] [PubMed] [Google Scholar]
- 7.Prior A, Read NW. Reduction of rectal sensitivity and post-prandial motility by granisetron, a 5 HT3-receptor antagonist, in patients with irritable bowel syndrome. Alimentary pharmacology & therapeutics. 1993;7:175–80. doi: 10.1111/j.1365-2036.1993.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 8.Cremonini F, Nicandro JP, Atkinson V, Shringarpure R, Chuang E, Lembo A. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Alimentary pharmacology & therapeutics. 2012;36:437–48. doi: 10.1111/j.1365-2036.2012.05208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong K, Nicandro JP, Shringarpure R, Chuang E, Chang L. A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Therapeutic advances in gastroenterology. 2013;6:344–57. doi: 10.1177/1756283X13491798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camilleri M. Is there an experimental basis for the development of ischaemic colitis as a result of 5-HT3 antagonist treatment? Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2007;19:77–84. doi: 10.1111/j.1365-2982.2006.00861.x. [DOI] [PubMed] [Google Scholar]
- 11.Painsipp E, Shahbazian A, Holzer P. Alosetron, cilansetron and tegaserod modify mesenteric but not colonic blood flow in rats. British journal of pharmacology. 2009;158:1210–26. doi: 10.1111/j.1476-5381.2009.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis JH. The risk of ischaemic colitis in irritable bowel syndrome patients treated with serotonergic therapies. Drug Saf. 2011;34:545–65. doi: 10.2165/11590690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Fukudo S, Ida M, Akiho H, Nakashima Y, Matsueda K. Effect of Ramosetron on Stool Consistency in Male Patients with Irritable Bowel Syndrome with Diarrhea. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2013 doi: 10.1016/j.cgh.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Miyata K, Kamato T, Nishida A, Ito H, Katsuyama Y, Iwai A, Yuki H, Yamano M, Tsutsumi R, Ohta M, et al. Pharmacologic profile of (R)-5-[(1-methyl-3-indolyl)carbonyl]-4,5,6,7-tetrahydro-1H- benzimidazole hydrochloride (YM060), a potent and selective 5-hydroxytryptamine3 receptor antagonist, and its enantiomer in the isolated tissue. The Journal of pharmacology and experimental therapeutics. 1991;259:15–21. [PubMed] [Google Scholar]
- 15.Miyata K, Kamato T, Nishida A, Ito H, Yuki H, Yamano M, Tsutsumi R, Katsuyama Y, Honda K. Role of the serotonin3 receptor in stress-induced defecation. The Journal of pharmacology and experimental therapeutics. 1992;261:297–303. [PubMed] [Google Scholar]
- 16.Miyata K, Ito H, Fukudo S. Involvement of the 5-HT3 receptor in CRH-induce defecation in rats. The American journal of physiology. 1998;274:G827–31. doi: 10.1152/ajpgi.1998.274.5.G827. [DOI] [PubMed] [Google Scholar]
- 17.Hirata T, Keto Y, Nakata M, Takeuchi A, Funatsu T, Akuzawa S, Sasamata M, Miyata K. Effects of serotonin 5-HT3 receptor antagonists on stress-induced colonic hyperalgesia and diarrhoea in rats: a comparative study with opioid receptor agonists, a muscarinic receptor antagonist and a synthetic polymer. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2008;20:557–65. doi: 10.1111/j.1365-2982.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 18.Matsueda K, Harasawa S, Hongo M, Hiwatashi N, Sasaki D. A phase II trial of the novel serotonin type 3 receptor antagonist ramosetron in Japanese male and female patients with diarrhea-predominant irritable bowel syndrome. Digestion. 2008;77:225–35. doi: 10.1159/000150632. [DOI] [PubMed] [Google Scholar]
- 19.Chiba T, Yamamoto K, Sato S, Suzuki K. Long-term efficacy and safety of ramosetron in the treatment of diarrhea-predominant irritable bowel syndrome. Clinical and experimental gastroenterology. 2013;6:123–8. doi: 10.2147/CEG.S32721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S Department of Health and Human services Food and Drug Administration Center for Drug Evalution and research (CDER) [Accessed December 11, 2013.];Guidance for Industry. Irritable bowel syndrome – Clinical Evaluation of Drugs for Treatment. 2012 Available at: www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm205269.pdf.
- 21.Delvaux M, Louvel D, Mamet JP, Campos-Oriola R, Frexinos J. Effect of alosetron on responses to colonic distension in patients with irritable bowel syndrome. Alimentary pharmacology & therapeutics. 1998;12:849–55. doi: 10.1046/j.1365-2036.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- 22.Hicks GA, Coldwell JR, Schindler M, Ward PA, Jenkins D, Lynn PA, Humphrey PP, Blackshaw LA. Excitation of rat colonic afferent fibres by 5-HT(3) receptors. The Journal of physiology. 2002;544:861–9. doi: 10.1113/jphysiol.2002.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradesi S, Lao L, McLean PG, Winchester WJ, Lee K, Hicks GA, Mayer EA. Dual role of 5-HT3 receptors in a rat model of delayed stress-induced visceral hyperalgesia. Pain. 2007;130:56–65. doi: 10.1016/j.pain.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Chang L, Ameen VZ, Dukes GE, McSorley DJ, Carter EG, Mayer EA. A dose-ranging, phase II study of the efficacy and safety of alosetron in men with diarrhea-predominant IBS. The American journal of gastroenterology. 2005;100:115–23. doi: 10.1111/j.1572-0241.2005.40365.x. [DOI] [PubMed] [Google Scholar]
- 25.Viramontes BE, Camilleri M, McKinzie S, Pardi DS, Burton D, Thomforde GM. Gender-related differences in slowing colonic transit by a 5-HT3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. The American journal of gastroenterology. 2001;96:2671–6. doi: 10.1111/j.1572-0241.2001.04138.x. [DOI] [PubMed] [Google Scholar]
- 26.Koch KM, Corrigan BW, Manzo J, James CD, Scott RJ, Stead AG, Kersey KE. Alosetron repeat dose pharmacokinetics, effects on enzyme activities, and influence of demographic factors. Alimentary pharmacology & therapeutics. 2004;20:223–30. doi: 10.1111/j.1365-2036.2004.02031.x. [DOI] [PubMed] [Google Scholar]
- 27.Camilleri M, Atanasova E, Carlson PJ, Ahmad U, Kim HJ, Viramontes BE, McKinzie S, Urrutia R. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425–32. doi: 10.1053/gast.2002.34780. [DOI] [PubMed] [Google Scholar]
- 28.Berman S, Munakata J, Naliboff BD, Chang L, Mandelkern M, Silverman D, Kovalik E, Mayer EA. Gender differences in regional brain response to visceral pressure in IBS patients. European journal of pain. 2000;4:157–72. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- 29.Naliboff BD, Berman S, Chang L, Derbyshire SW, Suyenobu B, Vogt BA, Mandelkern M, Mayer EA. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–47. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]