Abstract
The mechanisms that give rise to familiarity memory have received intense research interest. One current topic of debate concerns the extent to which familiarity is driven by the same fluency sources that give rise to certain implicit memory phenomena. Familiarity may be tied to conceptual fluency, given that familiarity and conceptual implicit memory can exhibit similar neurocognitive properties. However, familiarity can also be driven by perceptual factors, and its neural basis under these circumstances has received less attention. Here we recorded brain potentials during recognition testing using a procedure that has previously been shown to encourage a reliance on letter information when assessing familiarity for words. Studied and unstudied words were derived either from two separate letter pools or a single letter pool (“letter-segregated” and “normal” conditions, respectively) in a within-subjects contrast. As predicted, recognition accuracy was higher in the letter-segregated relative to the normal condition. Electrophysiological analyses revealed parietal old-new effects from 500–700 ms in both conditions. In addition, a topographically dissociable occipital old-new effect from 300–700 ms was present in the letter-segregated condition only. In a second experiment, we found that similar occipital brain potentials were associated with confident false recognition of words that shared letters with studied words but were not themselves studied. These findings indicate that familiarity is a multiply determined phenomenon, and that the stimulus dimensions on which familiarity is based can moderate its neural correlates. Conceptual and perceptual contributions to familiarity vary across testing circumstances, and both must be accounted for in theories of recognition memory and its neural basis.
Keywords: Implicit memory, explicit memory, familiarity, fluency, ERP
1. Introduction
The simplest expression of memory for a prior episode is the experience of recognizing something as familiar. Contemporary theories of recognition memory emphasize the distinction between familiarity and another mnemonic expression termed recollection (Aggleton & Brown, 2006; Eichenbaum, Yonelinas, & Ranganath, 2007; Mandler, 1980; Yonelinas, 2002). Recollection refers to a recognition experience accompanied by the ability to recall the spatiotemporal context or other specific features of a previous encounter—for example, when recognizing an acquaintance and recalling her name, or seeing a photograph and identifying the circumstances under which it was taken. By contrast, familiarity refers to the impression that a particular stimulus has been encountered previously without substantiation by the recall of relevant details.
The notion that distinct neurocognitive processes subserve recognition with and without recollection has been extremely influential in recent years. This distinction has been particularly useful in identifying properties that disproportionately characterize recollection. For example, there is now substantial evidence that recollection depends on hippocampal processing (Aggleton & Brown, 1999; Eichenbaum et al., 2007), that it is diminished when attentional resources are challenged (Troyer, Winocur, Craik, & Moscovitch, 1999; Yonelinas, 2001), and that it is susceptible to impairment in a variety of neurological and psychiatric disorders such as Alzheimer’s Disease, mild cognitive impairment (MCI; Anderson et al., 2008; Westerberg et al., 2006), and schizophrenia (Danion, Huron, Vidailhet, & Berna, 2007; Huron et al., 1995). By contrast, there is far less consensus on many of these issues with regard to familiarity (Algarabel, Escudero, et al., 2009; Cipolotti et al., 2006; Jacoby & Kelley, 1992; Libby, Yonelinas, Ranganath, & Ragland, in press; Weiss, Goff, Duff, Roffman, & Schacter, 2008; Wixted & Squire, 2004; Wolk et al., 2005). Moreover, disagreements abound regarding the neural correlates of familiarity in healthy individuals when measured with event-related potentials (ERPs; e.g., Paller, Voss, & Boehm, 2007; Rugg & Curran, 2007; Voss & Federmeier, 2011) and functional neuroimaging (fMRI; e.g., Cowell, Bussey, & Saksida, 2010; Ranganath et al., 2004; Wais, Wixted, Hopkins, & Squire, 2006).
Given that familiarity is notoriously difficult to measure and to separate from recollection, oftentimes discrepant findings in the literature may be attributable to differences in measurement techniques (Libby et al., in press; Paller et al., 2007; Wixted, Mickes, & Squire, 2010; Yonelinas, 2002) or, when applicable, in patient type or severity (Algarabel, Escudero, et al., 2009; Bastin, Willems, Genon, & Salmon, 2013; Yonelinas, Aly, Wang, & Koen, 2010). However, there is also evidence that familiarity performance—even when measured in the same individuals in the same manner—can vary according to the stimulus dimensions that are most relevant and/or salient during a particular task. For example, Embree, Budson, and Ally (2012) found that familiarity in a group of patients with MCI was intact for pictures, but impaired for words, a finding that roughly mirrors the general pattern in the literature (for review, see Ally, 2012). In addition, a patient with a left perirhinal cortex lesion who was initially characterized as having a general familiarity deficit (Bowles et al., 2007) was recently found to have intact familiarity for nonverbal stimuli, such as faces and abstract line drawings (Martin, Bowles, Mirsattari, & Köhler, 2011). Electrophysiological correlates of familiarity can also differ depending on the stimuli for which familiarity is measured. It has been widely assumed that familiarity can be generically indexed by a particular brain potential termed FN400 (e.g., Rugg & Curran, 2007). However, FN400 potentials usually correlate with familiarity when it occurs for words or nameable pictures but not when it occurs for nonverbalizable stimuli such as complex geometric patterns or faces (Danker et al., 2008; Voss, Lucas, & Paller, 2010; Voss & Paller, 2007, 2009b; Yovel & Paller, 2004).
Why might familiarity—which can be strikingly amodal from a phenomenological standpoint—appear to be heterogeneous on a neural level? Findings from our laboratory and others suggest that one key to deconstructing familiarity lies in a closer examination of its relationship to the implicit memory phenomena of priming (Dew & Cabeza, 2011; Leynes & Zish, 2012; Lucas, Taylor, Henson, & Paller, 2012; Wang, Lazzara, Ranganath, Knight, & Yonelinas, 2010; Wang & Yonelinas, 2012; Woollams, Taylor, Karayanidis, & Henson, 2008). Priming occurs when prior experience results in an increase in the fluency with which specific stimuli are processed and thereby facilitates certain responses or decisions about these stimuli. It has long been suggested that the same fluency signals thought to give rise to priming can also contribute to familiarity experiences (Jacoby & Dallas, 1981), though the extent and nature of these contributions remain an open topic of study.
Importantly, it is generally well-appreciated that priming is multifaceted in its neural underpinnings. The spatial and temporal loci of the repetition-related boosts in fluency that lead to priming are known to depend on the nature of the relevant stimulus representations. Repetition priming for visual stimuli, for example, can be registered at various points along a posterior-to-anterior gradient within the ventral visual processing stream, with posterior regions computing information about lower-level sensory information and anterior regions computing information about global form, structure, and meaning (e.g., Henson, 2003; Schacter, Wig, & Stevens, 2007). These regions also tend to be unevenly susceptible to the neuropathology of Alzheimer’s Disease and MCI, such that conceptual priming impairments are evident earlier and to a greater degree than perceptual priming impairments (Fleischman et al., 2005). Thus, insofar as familiarity can sometimes be an outcome of fluency, one would expect its neurocognitive basis to also show an amount of representational specificity. In particular, the research presented here examines the possibility that differential contributions of certain types of fluency to familiarity across experimental situations can account for discrepant findings in prior investigations.
We have previously argued (Lucas et al., 2012; Paller et al., 2007; Voss, Lucas, & Paller, 2012) that our understanding of familiarity and its neural basis is limited by the fact that most studies of familiarity have used stimuli that are replete with meaning, such as words and nameable pictures. As a result, neural measures such as FN400 potentials that appear to track stimulus familiarity per se may instead reflect increases in conceptual fluency that occur upon stimulus repetition. Indeed, conceptual fluency and familiarity may often be tightly correlated across trials—and their neural correlates thus highly confusable—because trial-to-trial fluctuations in factors such as attention and depth-of-encoding can exert parallel influences on both outcomes (Paller et al., 2007; Yonelinas, 2002). Moreover, evidence from our laboratory suggests that FN400 potentials covary more closely and reliably with conceptual priming than they do with familiarity (Voss et al., 2010; Voss & Paller, 2007; Voss, Schendan, & Paller, 2010). Whereas this research has generally been silent about whether conceptual fluency contributes to familiarity, findings using individual-difference and lesion-mapping approaches to compare the two phenomena (Wang et al., 2010; Wang & Yonelinas, 2012) are suggestive of a shared underlying mechanism when familiarity is based on conceptual stimulus dimensions. By comparison, the neural mechanisms of familiarity based on perceptual stimulus dimensions remain relatively unexplored. Given that many neurocognitive attributes of conceptual fluency do not apply to perceptual fluency, one might not expect findings about conceptually-driven familiarity to generalize to other situations. Investigations of familiarity in a more diverse set of circumstances—particularly circumstances in which familiarity is supported by relatively low-level stimulus features—will be necessary to gain a more precise and comprehensive view of familiarity memory and its neurocognitive properties.
A paradigm introduced by Parkin and colleagues (2001) seems promising in this regard. This paradigm capitalizes on the fact that processing fluency for words can be enhanced through exposure to their component lower-level elements, such as individual letters or letter clusters (Dehaene et al., 2004). Due to the limited number of letters and common letter combinations in most languages, fluency with letter information is inadequate for differentiating between studied and unstudied words in typical recognition tests. To investigate whether familiarity could be driven by these perceptual characteristics under certain circumstances, Parkin and colleagues investigated recognition for target and lure stimuli constructed from entirely separate pools of letters. In the key condition of this study—here termed the Letter-Segregated (LS) condition—target words were derived from a restricted set of letters and lures were derived from a different, non-overlapping letter set. In a second condition—here termed the Normal (N) condition—targets and lures were derived from the entire alphabet, as is normally the case in recognition memory experiments. The logic of this design was that information concerning low-level stimulus dimensions that correspond to letters would be available as a cue to recognition only in the LS condition. As predicted, Parkin and colleagues found that recognition memory was enhanced in the LS relative to the N condition. Moreover, this finding obtained even though subjects reported being unaware of the experimental manipulation, suggesting that they did not use a recollective strategy to discriminate the different letters that comprised targets and lures in the LS condition. Presumably, the memory improvement in the LS condition was due to familiarity derived from letter fluency (see also Algarabel, Pitarque, Tomás, & Mazón, 2009; Algarabel & Pitarque, 2010; Bastin et al., 2013; Keane, Orlando, & Verfaellie, 2006, for similar arguents).
In the present research, we combined the letter-segregation paradigm with recordings of ERPs in order to investigate the neural basis of familiarity driven by letter information. In Experiment 1, we neurally isolated contributions of letter fluency to recognition by analyzing ERPs for correctly recognized studied words (hits) and correctly rejected unstudied words (CRs) in the LS and N conditions. The logic of the analysis strategy was that fluency with conceptual information should differ to roughly the same extent between targets and lures in both conditions, whereas fluency with sublexical information—and its associated neural correlates—will correspond to prior exposure only in the LS condition. Because FN400 effects are generally observed only when familiarity co-occurs with conceptual fluency, we did not expect FN400 potentials to differ between the LS and N conditions. Rather, other ERPs should be associated with enhanced recognition due to letter fluency, perhaps including posterior ERPs associated with implicit memory for words or word components (Grainger & Holcomb, 2009; Paller & Gross, 1998; Rugg et al., 1998). In Experiment 2, we attempted to replicate and extend the findings obtained in Experiment 1 by altering the paradigm to examine ERPs to false alarms for words that were not presented in the study phase, but that shared letters with words that were presented in the study phase.
As in prior studies that have used this letter-segregation paradigm, data from participants who evinced any knowledge of the letter manipulation, and who could thereby have used a recollection-based strategy to improve recognition in the LS condition, were excluded from analysis. Thus, qualitatively different ERPs during recognition for words presented in the letter-segregated versus the normal condition would provide new insight into the range of electrophysiological signals that can support familiarity.
2. Experiment 1
2.1 Methods
2.1.1. Participants
Sixteen participants (12 female, 15 right-handed, ages 18–22) participated in the experiment. Data from an additional six participants were discarded due to excessive ocular artifacts and/or electrode drift (> 25% of trials, n = 3), participants’ suspicion about the letter manipulation (n = 2), or very poor performance on the memory test (false alarms greater than hits in both the N and LS conditions, n = 1). Also, data from one additional participant were excluded because key ERP comparisons were more than three standard deviations away from the mean.
2.1.2. Materials
Stimuli consisted of two lists of 240 English words. One of these word lists (List A) was derived using only the letters a, b, d, e, g, j, l, r, t, v, w, x, z. The second list (List B) contained words derived using the letters c, f, h, i, k, m, n, o, p, q, s, u, y. For each participant, the letters from one of these lists comprised the target stimuli for the LS blocks, and the letters from the other list comprised the lure stimuli. The stimuli were divided into four sets matched for mean word length, each of which contained 60 words from List A and 60 from List B (see Appendix A). The assignment of the sets to LS blocks versus N blocks was counterbalanced across participants, as was the frequency with which each word served as a target or a lure within either type of block. An additional eight words per list served only as buffers (primacy and recency buffers during each study phase and practice trials at the beginning of each test phase).
2.1.3. Procedure
Each participant completed four study-test blocks, two of which were presented as part of the N condition and two as part of the LS condition. Each participant saw both N blocks and LS blocks consecutively, and the frequency with which the N blocks were presented in the first versus the second half of the experiment was counterbalanced.
In each study phase, 60 words were presented in a random order bounded by one primacy buffer and one recency buffer. In the N blocks, 30 of these words came from List A and 30 from List B. In the LS blocks, all 60 of the words came from either List A or List B. During each test phase, 120 words were presented in a random order, 60 of which had been presented during the prior study phase and 60 of which were unstudied. In the N blocks, unstudied words were drawn equally from List A and List B. In the LS blocks, all unstudied words were drawn from the list that did not contain the studied words.
Each study trial began with a 50-ms presentation of the study word, followed by an additional 750 ms of fixation. Subjects were instructed to indicate using a button press whether the word had one syllable (Button 1) or more than one syllable (Button 2). Subjects were also told to try to remember the words for the upcoming memory test.
Each test block was preceded by one practice trial containing a buffer word. Data from practice trials were not included in analyses. Each test trial began with the message “Press Button 6 for the next trial.” Following a random delay between 1000–1500 ms after a participant’s key press, a test word was presented for 300 ms. Participants were instructed to indicate using a button press whether they were confident that the word was old (Button 1), believed the word was old without confidence (Button 2), believed the word was new without confidence (Button 3), or were confident that the word was new (Button 4). Both speed and accuracy were emphasized.
ERPs were extracted from scalp electroencephalographic recordings from 32 Ag/AgCl electrodes (BioSemi ActiveTwo system) at locations from the 10–20 system. Voltage was rereferenced offline to averaged mastoids. The electrooculogram was recorded from electrodes below the center of each eye and on each outer canthus. Signals were recorded with a band pass of 0–104 Hz, and sampled at a rate of 512 Hz. Signals were high-pass filtered offline at 0.1 Hz. Each 1100-ms averaging epoch began 200 ms prior to stimulus onset. Mean prestimulus amplitudes were subtracted to correct for baseline variability. Epochs containing electroocular or other artifacts were excluded from ERP analyses (mean = 15.1%, SE = 1.5). To detect blinks and eye-movement artifacts, two bipolar channels were created to represent the difference between each vertical EOG channel and the electrode located immediately above the corresponding eye. A third bipolar channel was created to represent the difference between the right and left horizontal EOG channels. A 200-ms moving window with 50-ms steps was used to identify trials that contained large voltage changes in the bipolar EOG channels. In addition, an absolute voltage threshold was applied to remaining channels to detect artifacts due to electrode drift, muscle tension, or head movement. Initial visual inspection of single epochs was used to tailor these thresholds to each participant in order to maximize the sensitivity and selectivity of artifact rejection (Luck, 2005). A second round of visual inspection blind to experimental conditions served to confirm and adjust the suggested rejections. Participants for whom artifacts were present on > 25% of trials were excluded from analysis (see Section 2.1.1). Averaged ERP waveforms were low-pass filtered at 30 Hz.
Statistical comparisons were performed on amplitudes averaged over three midline electrode clusters, and were made using repeated-measures ANOVA (criterion p = 0.05) with Greenhouse-Geisser correction for non-sphericity where appropriate. The clusters included the following electrodes: frontal cluster (F3, Fc1, Fc2, F4, Fz); parietal cluster (Cp1, P3, Pz, P4, Cp2); occipital cluster (Po3, O1, Oz, O2, Po4).
2.2. Results
2.2.1. Behavior
Table 1 shows the mean proportion of studied and unstudied items endorsed as “old” or “new” by participants in the N and LS conditions. Collapsing across the two confidence levels, mean values for hits minus false alarms (“old” for studied words minus “old” for unstudied words) were 26.5% (SE = 0.04) for the N condition and 48.5% (SE = 0.04) for the LS condition. Thus, as predicted, recognition was more accurate in the LS condition than in the N condition [t(15) = 5.66, p > .001].
Table 1.
Mean percentage of responses in each condition in Experiment 1 (SE in parentheses).
| “Old” High Confidence |
“Old” Low Confidence |
“New” Low Confidence |
“New” High Confidence |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Hits | Misses | ||||||||
| Studied | Normal | 42.4 | (4.7) | 28.7 | (4.0) | 19.8 | (2.9) | 9.1 | (2.5) |
|
Letter- Segregated |
51.2 | (6.3) | 23.9 | (4.8) | 18.6 | (3.2) | 6.4 | (1.6) | |
| False Alarms | Correct Rejections | ||||||||
| Unstudied | Normal | 20.4 | (3.9) | 24.2 | (3.3) | 36.8 | (4.9) | 18.7 | (4.3) |
|
Letter- Segregated |
9.1 | (1.6) | 17.5 | (3.0) | 45.4 | (5.8) | 28.1 | (6.4) | |
The data for proportion of “old” responses were submitted to a 2 (study status: studied/unstudied) × 2 (condition: N/LS) × 2 (confidence: high/low) repeated-measures ANOVA, which revealed a main effect of study status [F(1,15) = 100.79, p < .001], reflecting a higher proportion of “old” judgments for studied relative to unstudied words. A main effect of condition also emerged [F(1,15) = 11.66, p = .004], indicating more “old” responses in the N condition relative to the LS condition, particularly because false alarms were less prevalent in the LS condition. Most importantly, a study status × condition interaction [F(1,15) = 32.08, p < .001] confirmed the expected finding of better discrimination of old and new items in the LS condition relative to the N condition. This interaction was further qualified by a significant study status × condition × confidence interaction [F(1,15) = 4.73, p = .046], indicating that the improvement in discrimination accuracy for the LS relative to the N condition was greater for high-confidence relative to low-confidence trials1. Follow-up study status × condition ANOVAs performed separately for high- and low-confidence responses indicated that the difference in discrimination accuracy between the two conditions was significant for high-confidence responses [F(1,15) = 13.43, p = .002], but not for low-confidence responses [F(1,15) = 0.27, p = .61].
2.2.2. Electrophysiology
The primary purpose of this study was to identify neural measures that underlie the increased familiarity afforded to studied relative to unstudied words in the LS relative to the N condition. Accordingly, analyses were conducted only for correct trials. Visual inspection of the waveforms contrasting hits and correct rejections (CRs) revealed pronounced old/new effects maximal from 500–700 ms at centroparietal locations (Figure 1). The hit/CR contrast in the LS condition revealed an earlier and more occipital effect that appeared both at 300–500 ms and at 500–700 ms. Formal comparisons of ERP old/new effects in N and LS conditions were conducted over both of these intervals (300–500 ms and 500–700 ms). These statistical comparisons took the form of 3 (electrode cluster: frontal/parietal/occipital) × 2 (response: hit/CR) × 2 (condition: N/LS) ANOVAs. Participants varied widely in their use of the two confidence levels, likely due to a combination of individual differences in both memory strength and response criteria. Trials were thus collapsed across confidence levels for ERP analyses to ensure sufficient trial counts for all conditions. The mean number of artifact-free trials per participant was 77 for LS hits (range = 33–103), 73 for LS CRs (range = 51–97), 72 for N hits (range = 43–99), and 57 for N CRs (range = 25–86).
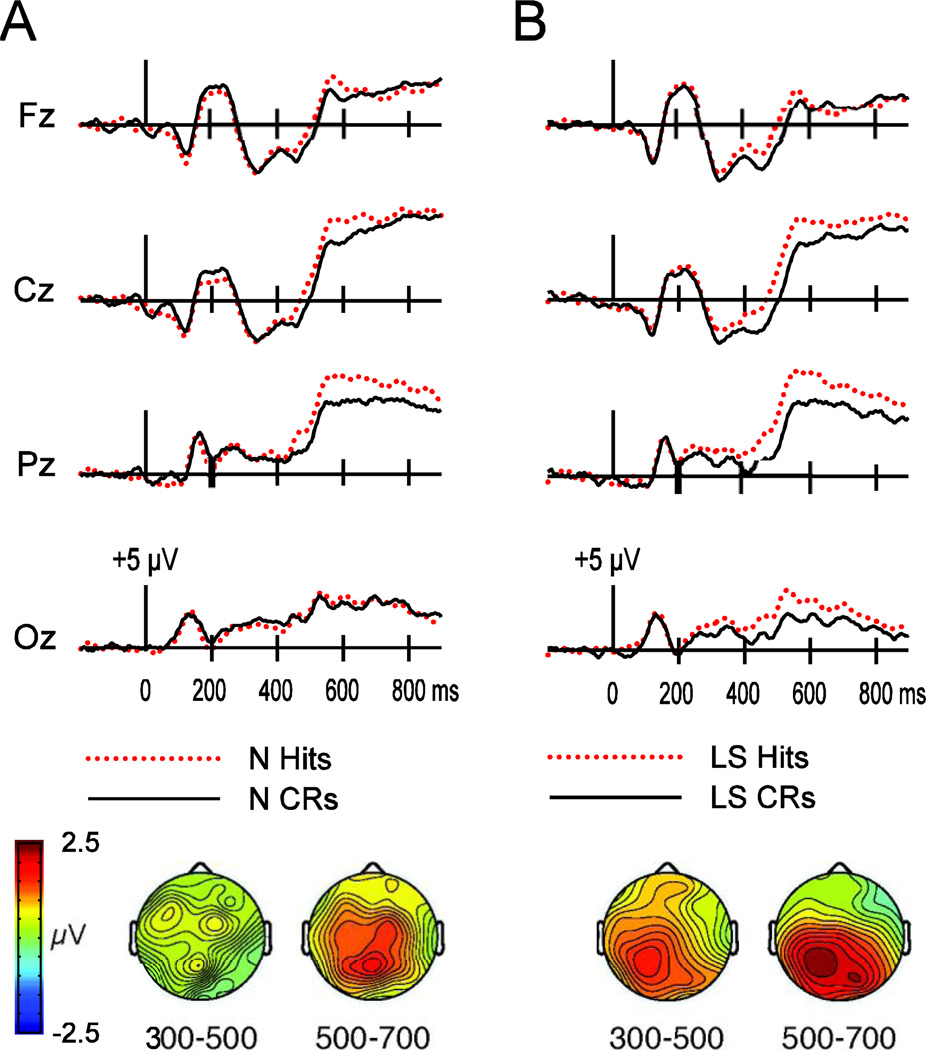
Figure 1.
ERP differences between hits and correct rejections (CRs) in the Normal (N) and Letter-Segregated (LS) conditions in Experiment 1. Waveforms are shown for four midline electrodes (Fz, Cz, Pz, Oz). Below, topographic plots (with colors corresponding to amplitude values illustrated on a view of the head from above) depict hit–CR differences from 300–500 ms and 500–700 ms. A) ERPs and topographic maps in the N condition. B) ERPs and topographic maps in the LS condition.
300–500 ms
Over the first interval, a marginal main effect of response emerged [F(1,15) = 4.11, p = .061] due to a tendency for more positive amplitudes for hits than CRs. Importantly, this difference was qualified by an interaction between response and condition [F(1,15) = 7.13, p = .017], reflecting a greater difference between hits and CRs in the LS condition relative to the N condition. Follow-up comparisons confirmed that the old/new effect was significant for the LS condition [F(1,15) = 8.9, p = .009], but not for the N condition [F(1,15) = 0.4, p = .54].
No interactions involving electrode cluster were significant [F’s < 2.23, p’s > .14]. However, given previous suggestions that frontal ERPs from 300–500 ms generically index familiarity, as well as prior indications that posterior ERPs over this latency may relate to forms of perceptual fluency for words (e.g., Lucas et al., 2012; Paller & Gross, 1998; Rugg et al., 1998) we performed additional planned analyses to better characterize the topography of the old/new effects present for the LS condition. We conducted separate response × electrode ANOVAs comparing LS hits to LS CRs over the frontal, parietal, and occipital electrode clusters. The main effect of response was significant for both the parietal cluster [F(1,15) = 9.91, p = .007], and the occipital cluster [F(1,15) = 10.33, p = .006], but not for the frontal cluster [F(1,15) = 2.15, p = .16], indicating that the difference between hits and CRs in the LS condition was robust at posterior rather than frontal electrodes (mean values of 0.86 µV for frontal, 1.43 µV for parietal, and 1.16 µV for occipital clusters). No response × electrode interactions emerged within any cluster [p’s > .20].
500–700 ms
Over the second interval, a main effect of response emerged [F(1,14) = 15.52, p = .001], indicating more positive ERPs for hits relative to CRs. No significant interaction between response and condition was present [F(1,15) = 0.94, p = .35]. However, there was a significant three-way interaction for response, condition, and electrode cluster [F(1.28,19.25) = 5.55, p = .022]. Thus, separate response × condition ANOVAs were conducted for each electrode cluster.
Main effects of response emerged for the parietal [F(1,15) = 28.18, p < .001] and occipital electrode clusters [F(1,15) = 32.93, p < .001] but not for the frontal electrode cluster [F(1,15) = 1.63, p = .22], indicating more positive ERPs for hits than CRs at parietal and occipital electrodes. Most importantly, a significant interaction between response and condition emerged for the occipital cluster [F(1,15) = 4.74, p = .046], indicating larger differences between hits and CRs over occipital electrodes in the LS relative to the N condition. Follow-up comparisons indicated that this occipital difference was significant for both the N [F(1,15) = 5.58, p < .032] and the LS conditions [F(1,15) = 23.98, p < .001]. Response × condition interactions at frontal and parietal clusters were nonsignificant [F ‘s < 2.4, p’s > .15]. For the LS condition, old/new ERP differences measured 0.48 µV for frontal, 2.21 µV for parietal, and 1.77 µV for occipital clusters. For the N condition, old/new ERP differences measured 1.03 µV for frontal, 1.52 µV for parietal, and 0.69 µV for occipital clusters.
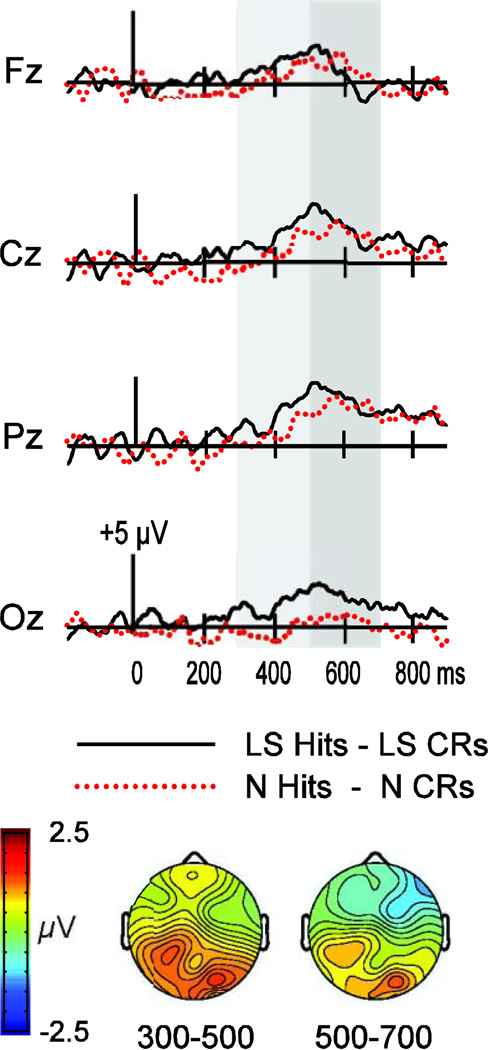
Formal assessments of differences in topographic distribution between the aforementioned N and LS hit/CR effects utilized the vector-normalization approach (McCarthy & Wood, 1985). Averaged amplitude values from each electrode were compared for the two conditions after overall amplitude differences were removed. This comparison sought to determine if the old/new ERP topography at 500–700 ms differed reliably between LS and N conditions. A significant electrode-by-condition interaction [F(6.77,101.53) = 2.4, p = .028] substantiated the observation that the LS old/new effect was more posterior than the N old/new effect. Thus, both conditions showed differences between hits and CRs from 500–700 ms, but the topographies suggest that partially distinct neural populations contributed to these effects. Difference waves (Figure 2) illustrate that old/new effects were more occipital for the LS condition than the N condition.
Figure 2.
Waveforms depicting the subtraction between hits and correct rejections (CRs) in the N and LS conditions in Experiment 1 (N condition, dotted red trace; LS condition, solid black trace). Waveforms are shown for four midline electrodes (Fz, Cz, Pz, Oz). Gray shading indicates the time intervals of interest (300–500 ms and 500–700 ms). Topographic maps depict the hit/CR effect in the LS condition minus that in the N condition over each time interval.
2.3. Discussion
We replicated previous findings of enhanced discrimination accuracy in a recognition test when targets and lures were derived from separate letter pools relative to when targets and lures shared letters (e.g., Parkin et al., 2001). This difference was robust despite excluding subjects who reported any awareness of the letter manipulation (i.e., two subjects for whom letter segregation may have enhanced recognition via explicit letter-based strategies).
ERP analyses were based on the assumption that recognition stemming from letter information would occur to a greater extent in the LS condition relative to the N condition. Thus, old/new ERP differences unique to the LS condition can be attributed to the additional contributions of letter information to accurate recognition. Comparisons between hits and CRs in the N condition revealed differences in positive-going, centroparietal ERPs from 500–700 ms. These ERP differences were similar to LPC potentials that have been widely associated with recollection in prior research, but are also sometimes associated with familiarity (Voss & Paller, 2008). Similar LPC differences were apparent in the LS condition; however, hits and CRs in this condition also differed in earlier, occipital ERPs that onset around 300 ms. These ERPs extended into the 500–700 ms interval where they overlapped with, but were topographically dissociable from the centroparietal LPC differences that were discernible in the N condition.
In support of the notion that familiarity can be associated with a plurality of neural correlates—and contradicting ideas that familiarity can be generically indexed by FN400 potentials—the added familiarity afforded to old relative to new words in the LS condition did not occur in conjunction with ERP differences at frontal electrodes, nor was it associated with a mere quantitative increase in the magnitude of old/new effects identified in the N condition. Instead, distinct occipitally-focused brain potentials were observed under conditions in which letter fluency facilitated accurate recognition decisions. Although a very large number of prior ERP studies have examined recognition memory for words, none to our knowledge has identified occipital old/new effects that resemble those found here in the LS condition. The most likely explanation for this difference is that recognition for words in prior studies was based strongly on conceptual stimulus dimensions, such that FN400 old/new effects reflected a role of conceptual fluency in facilitating accurate familiarity-based responding. By contrast, recognition in the present study was strongly influenced by sublexical information2. Indeed, similar ERPs have been identified in previous studies that have examined priming for visual word forms (Paller & Gross, 1998; Rugg et al., 1998) or position-independent orthographic representations (Grainger & Holcomb, 2009).
Given the assumption that all the common words in this experiment held conceptual meaning for the participants, and the link we have proposed between FN400 potentials and conceptual fluency (Voss et al., 2012), one might wonder why FN400 differences between old and new words were absent. Although we predicted that FN400 potentials would not differ between LS and N conditions, a complete lack of FN400 differences was unexpected. One plausible explanation is that the extremely fast pace and shallow encoding task used in the study phase provided minimal opportunities for semantic elaboration, such that conceptual fluency was not sufficiently enhanced when words were repeated. Consistent with this explanation are prior findings in which deep encoding instructions have resulted in larger FN400 differences than have shallow encoding instructions (Paller & Kutas, 1992; Rugg, Allan, & Birch, 2000; Ullsperger, Mecklinger, & Müller, 2000). Moreover, in a prior study in which recognition was tested for words that evoked minimal conceptual meaning, LPC potentials were found in association with both recollection and familiarity (Voss, Lucas, & Paller, 2010). Thus, it is possible that when conceptual fluency is not sufficiently diagnostic of recognition status, experiences of familiarity and recollection are indexed by a graded electrophysiological signal that corresponds to LPC potentials.
Interestingly, analyses of participants’ confidence ratings at test revealed that the beneficial effect of letter segregation on recognition accuracy was reflected primarily in more accurate high-confidence responding. This finding might raise questions as to whether the improvements in recognition memory in the LS condition are better characterized as recollection rather than familiarity, given that recollection is more often associated with higher levels of confidence. The fact that all included participants failed to catch on to the letter-segregation paradigm—and many expressed surprise when told about it during debriefing—suggests that recollection of letter cues to “oldness” per se are unlikely to account for the results. Nonetheless, we cannot rule out a contribution of some amount of recollective experience to the performance improvements seen in LS blocks. Indeed, while most prior studies that have manipulated stimulus fluency have found corresponding increases in familiarity, fluency effects on recollection have been reported occasionally (Kurilla & Westerman, 2008; Taylor & Henson, 2012b), and it has been suggested that all mnemonic experiences, including recollection, can result partially from the attribution of fluency to prior experience (Mayes, Gooding, & van Eijk, 1997; Mayes & Roberts, 2001). In addition, due to the orthographic similarity among studied words in the LS condition, it is possible that a proportion of the correct “old” responses stemmed from participants mis-recollecting words that were orthographically similar to target words. However, this explanation appeals to a mere quantitative increase in recollection in the LS relative to the N condition, and thus cannot easily account for the qualitatively different old/new effects that characterized the two conditions. Instead, these findings indicate that distinct retrieval mechanisms were at work in the LS condition. Future studies will be necessary to understand the extent to which these mechanisms can sometimes result in experiences of recollection in addition to familiarity. The potential for the novel electrophysiological signal described here to reflect contributions to multiple mnemonic experiences does not detract from the importance of the present findings, particularly since letter-fluency effects have been shown to be intact in multiple populations for whom recollection is typically impaired (Bastin et al., 2013; Keane et al., 2006; Parkin et al., 2001).
The finding that letter fluency preferentially influenced high-confidence responding is also of note because it distinguishes the pattern observed here from a phenomenon termed implicit recognition or recognition without awareness (Vargas, Voss, & Paller, 2012; Voss, Baym, & Paller, 2008; Voss & Paller, 2009a). Implicit recognition refers to a situation wherein fluency can support veridical recognition responses that are devoid of the subjective awareness that characterizes both familiarity and recollection. Instead, perceptual fluency in several studies has been found to contribute to recognition performance by producing qualitatively different effects on participants’ “guess” responses compared to confident responses. Such findings present an intriguing challenge to fluency-attribution accounts of familiarity, because implicit recognition hypothetically reflects a mechanism by which fluency can influence recognition performance with no involvement of experienced familiarity whatsoever. Moreover, prior attempts to empirically demonstrate effects of fluency on familiarity have seldom allowed for measures to separate familiarity from guessing, such that it is entirely possible that a portion of these effects stemmed from implicit recognition. In the present situation, however, analyses of participants’ confidence ratings indicate that an attributional explanation by which fluency contributes to familiarity experiences provides a better account than does an explanation based on recognition without awareness. Future research will be necessary to determine the circumstances under which fluency effects are more likely to manifest as guessing versus confident responding.
One limitation of Experiment 1 is that, although all repeated words in the LS condition differed from new words in the amount of prior exposure afforded to their component letters, repeated words also differed from new words in the amount of prior exposure afforded to the words themselves. Of course, word-level repetition occurred in the N conditions as well. However, the extent to which the ERP differences uniquely observed in the LS condition reflected interactive processing between neurocognitive outcomes of letter repetition and those of word repetition cannot be determined. In contrast, false alarms for words that share letters with studied words, but were not themselves studied, would provide a relatively isolated measure of contributions of letter fluency to familiarity. To attain this measure in Experiment 2, we tested recognition under circumstances that were similar to those of the LS condition in Experiment 1, but which also included new words that shared letters with studied words. Thus, Experiment 2 was an attempt both to replicate the hit/CR differences obtained for the LS condition in Experiment 1 and to extend these findings to false recognition driven by letter fluency.
3. Experiment 2
3.1. Methods
3.1.1. Participants
Fourteen participants (7 female, all right-handed, ages 18–24) participated in the experiment. Data from an additional 5 participants were discarded due to excessive ocular artifacts and/or electrode drift (> 25% of trials unusable, n = 2) or participants’ suspicion about the letter segregation (n = 3).
3.1.2. Materials
The materials were the same as those used in Experiment 1. However, no N blocks were presented in Experiment 2. Thus, for each participant, words from either List A or List B served as the target words for all blocks. Lures were comprised of an equal number of words from List A and List B for all participants.
3.1.3. Procedure
Each participant completed four study-test blocks. In each study phase, 30 words were presented in a random order bounded by one primary buffer and one recency buffer. For each participant, the 30 words were chosen at random for each block from each one of the four word sets (see Appendix A), with the constraint that studied words came from List A for half of the participants and from List B for the remaining participants. During each test phase, 90 words were presented in a random order, 30 of which had been presented during the study phase and 60 of which were unstudied. Of the unstudied words, 30 came from the same list as did the studied words, and the remaining 30 came from the other list. In this way, the unstudied words could be subdivided depending on whether or not they shared letters with studied items. The stimulus timing and instructions provided to participants in Experiment 2 were the same as in Experiment 1.
The procedures for recording and extracting ERPs in Experiment 2 were identical to those used in Experiment 1. Epochs containing electroocular or other artifacts were excluded from ERP analyses (mean = 10.3%, SE = 1.5). Unless otherwise noted, statistical comparisons were performed on amplitudes averaged over three midline electrode clusters, and were made using repeated-measures ANOVA (criterion p = 0.05) with Greenhouse-Geisser correction for non-sphericity where appropriate. As in Experiment 1, clusters included the following electrodes: frontal cluster (F3, Fc1, Fc2, F4, Fz); parietal cluster (Cp1, P3, Pz, P4, Cp2); occipital cluster (Po3, O1, Oz, O2, Po4).
3.2. Results
3.2.1. Behavior
Table 2 shows recognition response rates for studied words, as well as for unstudied words that shared letters with studied words (termed related lures) and for unstudied words that did not share letters with studied words (termed unrelated lures). To assess overall discrimination accuracy, a study status (studied/unstudied) × confidence (high/low) repeated-measures ANOVA was performed on the proportion of “old” responses after collapsing across related and unrelated lures. A main effect of study status emerged [F(1,13) = 696.18, p < .001], as well as an interaction between study status and confidence [F(1,13) = 104.36, p < .001]. These findings reflect a tendency for participants to correctly endorse studied items as “old” more often than they falsely endorsed unstudied items as “old” and further indicate that this tendency was stronger for high-confidence relative to low-confidence responses.
Table 2.
Mean percentage of responses in each condition in Experiment 2 (SE in parentheses).
| “Old” High Confidence |
“Old” Low Confidence |
“New” Low Confidence |
“New” High Confidence |
|||||
|---|---|---|---|---|---|---|---|---|
| Studied | 54.3 | (3.4) | 23.5 | (3.1) | 16.3 | (1.9) | 6.0 | (1.0) |
| Related Lures | 17.6 | (2.5) | 25.5 | (3.3) | 37.3 | (3.6) | 19.6 | (2.9) |
| Unrelated Lures | 3.9 | (1.4) | 10.5 | (1.9) | 42.9 | (4.4) | 42.7 | (4.6) |
False alarm rates for related versus unrelated lures were analyzed with a relatedness (related/unrelated) × confidence (high/low) repeated-measures ANOVA. A main effect of relatedness [F(1,13) = 239.67, p < .001] reflected a higher proportion of false alarms for related relative to unrelated lures. A main effect of confidence [F(1,13) = 5.4, p = .037] reflected more false alarms registered with low confidence than with high confidence. The relatedness × confidence interaction was nonsignificant [F(1,13) = 0.15, p = .70]. Thus, as predicted, participants produced more false alarms for unstudied items that shared letters with studied items than they did for unstudied items that did not share letters with studied items.
3.2.2. Electrophysiology, Collapsed Across Confidence Levels
Electrophysiological analyses served two main purposes. The first purpose was to determine whether occipital ERP differences were again found between hits and unrelated CRs, as in the LS condition in Experiment 1. The second purpose was to determine whether the same ERPs present for hits were also present for related false alarms (FAs), which would more convincingly tie these effects to recognition based on letter fluency. Note that the comparison between hits and related FAs is complicated by a larger proportion of high-confidence “old” responses for hits (see Table 2). Although analyses will first be presented collapsed across confidence levels, subsequent analyses will be presented that are limited to high-confidence “old” responses. For confidence-collapsed analyses, the mean number of artifact-free trials per participant was 84 for hits (range = 66–99), 47 for related FAs (range = 28–66), and 92 for unrelated CRs (range = 68–113).
Visual inspection of the waveforms corresponding to hits, related FAs, and unrelated CRs (Figure 3a) revealed that parieto-occipital ERPs from 300–500 ms were similar for hits and related FAs, but were more positive for both of those conditions compared to unrelated CRs. By contrast, ERPs at frontal electrodes over this latency appeared to be more positive for hits than for either related FAs or unrelated CRs. From 500–700 ms, broadly-distributed positive ERPs appeared to be greater for hits relative to either related FAs or unrelated CRs. Formal statistical comparisons of these differences took the form of electrode cluster (frontal/parietal/occipital) × response (hit/related FA/unrelated CR) ANOVAs conducted over the 300–500 ms and 500–700 ms intervals.
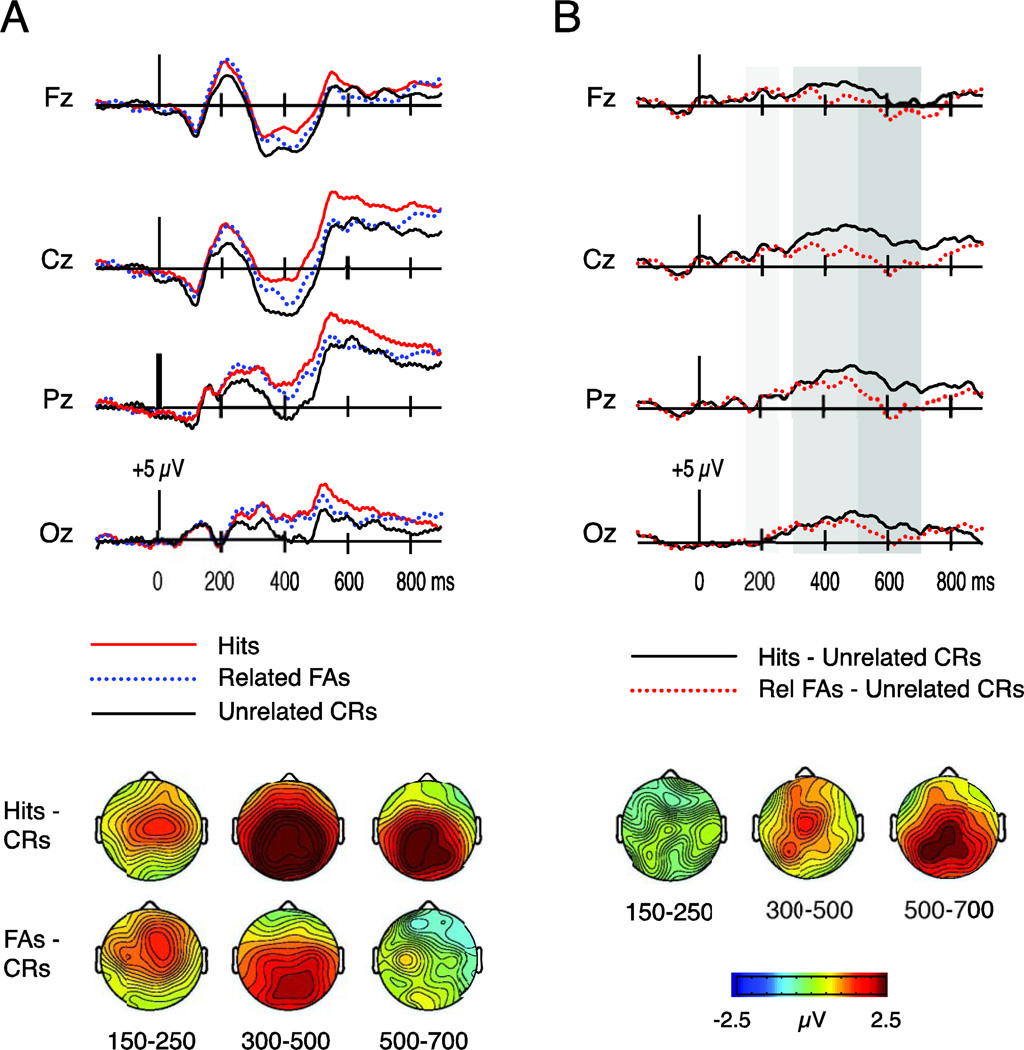
Figure 3.
ERPs to hits, related false alarms (FAs), and unrelated correct rejections (CRs) in Experiment 2. A) Waveforms at four midline electrodes (Fz, Cz, Pz, Oz) and topography of differences among conditions at 150–250 ms, 300–500 ms, and 500–700 ms. B) Waveforms depicting the subtraction between hits and unrelated CRs along with the subtraction between related FAs and unrelated CRs. Waveforms are shown for four midline electrodes (Fz, Cz, Pz, Oz). Topographic maps depict the hit/unrelated CR effect minus the related FA/unrelated CR effect over each time interval. Gray shading on the waveforms indicates the time intervals of interest (150–250 ms, 300–500 ms, 500–700 ms).
In addition, the waveforms included an earlier, frontocentral ERP difference from 150–250 ms that was not present in Experiment 1. ERPs over this time interval appeared to be more positive for both hits and related FAs relative to unrelated CRs. Thus, an additional electrode cluster × response ANOVA was conducted from 150–250 ms.
150–250 ms
A main effect of response emerged over the 150–250 ms interval [F(1.28,16.58) = 4.13, p = .050]. Follow-up paired comparisons revealed that ERPs were more positive for hits versus unrelated CRs [t(13) = 3.25, p = .006], and for related FAs versus unrelated CRs [t(13) = 3.05, p = .009]. No significant difference was present between hits and related FAs [t(13) = 0.19, p = .86]. Visual inspection indicated that these ERPs differences displayed a fronto-central topography; however, the condition × electrode cluster interaction did not reach significance [F(2.17,28.23) = 1.98, p = .15] We refer to this ERP difference as a P200 effect, and we address this unexpected finding in the Discussion.
300–500 ms
Over this interval, a main effect of response emerged [F(1.8,23.42) = 17.12, p < .001] as well as a marginal interaction between response and electrode cluster [F(1.67,21.71) = 3.06, p = .076]. Follow-up comparisons at each electrode cluster revealed that the main effect of response was significant for all three clusters [frontal F(1.81,23.53) = 5.81, p = .01; parietal F(1.72,22.34) = 26.65, p < .001; occipital F(1.85, 24.11) = 16.98, p < .001]. Thus, additional paired comparisons among hits, related FAs, and unrelated CRs were conducted separately at each electrode cluster.
At frontal electrodes, ERPs were significantly more positive for hits compared to unrelated CRs [t(13) = 3.84, p = .002], and marginally more positive for hits compared to related FAs [t(13) = 1.97, p = .07]. ERP differences between unrelated CRs and related FAs were nonsignificant [t(13) = 1.35, p = .20].
At parietal electrodes, ERPs were significantly more positive for hits compared to both related FAs [t(13) = 3.20, p = .007] and unrelated CRs [t(13) = 6.70, p < .001]. In addition, ERPs were also significantly more positive for related FAs compared to unrelated CRs [t(13) = 4.21, p = .001].
At occipital electrodes, ERPs did not differ between hits and related FAs [t(13) = 1.17, p = .26]. However, ERPs to hits differed from those to unrelated CRs [t(13) = 4.96, p < .001], and ERPs to related FAs also differed from those to unrelated CRs [t(13) = 5.01, p < .001].
500–700 ms
Over the 500–700 ms interval, a main effect of response emerged [F(1.88,24.4) = 5.68, p = .01], as did an interaction between electrode cluster and response [F(2.74,35.59) = 3.82, p = .02]. Additional comparisons revealed main effects of response at the parietal cluster [F(1.73,22.46) = 9.60, p = .001] and the occipital cluster [F(1.97,25.59) = 5.55, p = .01] but not at the frontal cluster [F(1.77,23.03) = 1.79, p = .19].
Follow-up comparisons among the three response types were conducted separately for the parietal and occipital clusters. Parietal ERPs were more positive for hits than for related FAs [t(13) = 4.60, p = .001] or unrelated CRs [t(13) = 3.76, p = .002], but did not differ between related FAs and unrelated CRs [t(13) = 0.32, p = .76]. Likewise, occipital ERPs were more positive for hits than for related FAs [t(13) = 2.35, p = .035] or unrelated CRs [t(13) = 3.23, p = .007], but did not differ between related FAs and unrelated CRs [t(13) = 0.69, p = .50].
Summary
Central ERPs from 150–250 ms and occipital ERPs from 300–500 ms were of equal amplitude for hits and related FAs, and these amplitudes were greater than those for unrelated CRs (hits = related FAs > unrelated CRs). On the other hand, frontal ERPs from 300–500 ms and posterior LPC-like potentials from 500–700 ms were greater for hits than for the other two response types (hits > related FAs = unrelated CRs). These patterns are illustrated in Figure 3b, which contrasts the difference waves between hits and unrelated CRs with the difference waves between related FAs and unrelated CRs.
These findings are broadly consistent with the results of Experiment 1, and further demonstrate that ERPs that reflect the contributions of letter fluency to recognition are distinct from the early frontal and late parietal old/new effects (FN400 and LPC) that are typically described in studies of recognition memory for words. As previously mentioned, however, differences between ERPs elicited by hits and by related FAs could also reflect the different proportions of high-confidence responses that are present in these comparisons. To investigate this possibility, additional analyses were limited to high-confidence responses.
3.2.3. Electrophysiology, High-Confidence Recognition
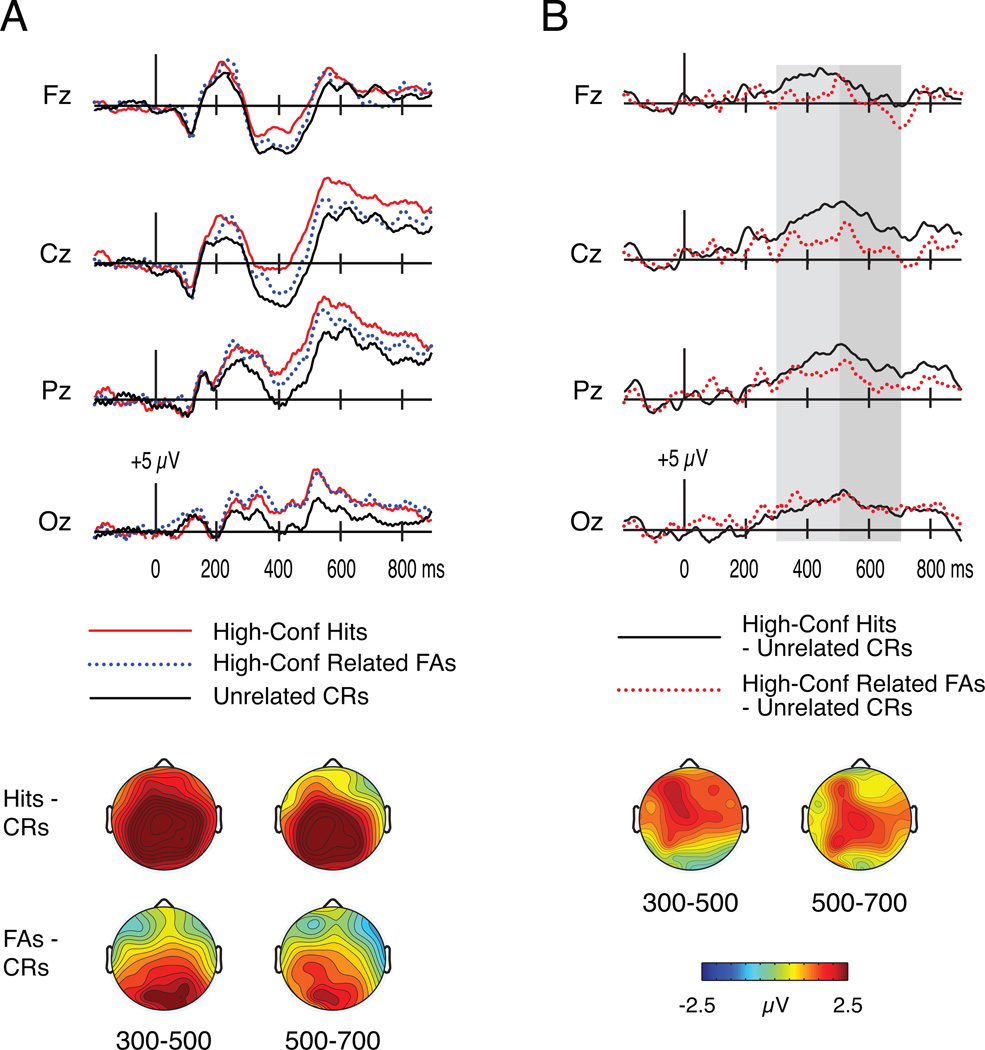
Analyses of the 300–500 ms and 500–700 ms intervals were repeated after excluding low-confidence “old” responses. These analyses necessitated the exclusion of data from four participants who had fewer than 15 artifact-free high-confidence related FAs. For the remaining 10 participants, the mean number of artifact-free, high-confidence trials per participant was 61 for hits (range = 37–92) and 23 for related FAs (range = 15–41), whereas there were 93 trials for unrelated CRs (including both confidence levels; range = 79–113). The resulting waveforms for all three conditions (high-confidence hits, high-confidence related FAs, and unrelated CRs) are depicted in Figure 4a. Figure 4b contrasts the difference waves between high-confidence hits and unrelated CRs with the difference waves between high-confidence related FAs and unrelated CRs.
Figure 4.
ERPs to high-confidence hits, high-confidence related false alarms (FAs), and unrelated correct rejections (CRs) in Experiment 2. A) Waveforms at four midline electrodes (Fz, Cz, Pz, Oz) and topography of differences among conditions at 300–500 ms and 500–700 ms. B) Waveforms depicting the subtraction between high-confidence hits and unrelated CRs along with the subtraction between high-confidence related FAs and unrelated CRs. Waveforms are shown for four midline electrodes (Fz, Cz, Pz, Oz). Topographic maps depict the hit/unrelated CR effect minus the related FA/unrelated CR effect over each time interval. Gray shading on the waveforms indicates the time intervals of interest (150–250 ms, 300–500 ms, 500–700 ms).
300–500 ms - Confident Recognition
Analyses for this interval revealed a main effect of response [F(1.88,16.89) = 6.71, p = .008] and an interaction between response and electrode cluster [F(2.14,19.27) = 4.71, p = .02]. Follow-up comparisons revealed a main effect of response at each electrode cluster [frontal F(1.86,16.71) = 4.18, p = .036; parietal F(1.78,16.01) = 8.75, p = .003; occipital F(1.67, 15) = 6.81, p = .01]. Thus, paired comparisons among the three response types were conducted separately at each electrode cluster.
Frontal ERPs were more positive for hits relative to unrelated CRs [t(9) = 3.21, p = .01], and marginally more positive for hits relative to related FAs [t(9) = 2.06, p = .07]. The difference between related FAs and unrelated CRs was nonsignificant [t(9) = 0.50, p = .63].
Parietal ERPs were more positive for hits relative to unrelated CRs [t(9) = 4.98, p = .001], and more positive for related FAs relative to unrelated CRs [t(9) = 2.55, p = .03]. The difference between hits and related FAs was nonsignificant [t(9) = 1.39, p = .20].
Occipital ERPs were more positive for hits relative to unrelated CRs [t(9) = 3.31, p = .009], and more positive for related FAs relative to unrelated CRs [t(9) = 3.96, p = .003]. The difference between hits and related FAs was nonsignificant [t(9) = 0.02, p = .98].
500–700 ms - Confident Recognition
Analyses for this interval revealed a marginal main effect of response [F(1.98,17.79) = 3.5, p = .053], along with a significant interaction between response and electrode cluster [F(3.20,28.84) = 2.94, p = .047]. Follow-up comparisons at each electrode cluster revealed a main effect of response at parietal [F(1.95, 17.53) = 5.3, p = .016] and occipital electrodes [F(1.74,15.64) = 3.85, p = .049], but not at frontal electrodes [F(1.99,17.95) = 1.29, p = .30]. Comparisons among hits, related FAs, and unrelated CRs were thus conducted separately at the parietal and occipital electrode clusters.
Parietal ERPs were more positive for hits relative to unrelated CRs [t(9) = 3.56, p = .006]. No significant differences emerged between related FAs and either hits [t(9) = 1.66, p = .13] or unrelated CRs [t(9) = 1.48, p = .17].
Occipital ERPs were more positive for hits relative to unrelated CRs [t(9) = 2.76, p = .02], as well as for related FAs relative to unrelated CRs [t(9) = 2.40, p = .04]. No significant difference emerged between hits and related FAs [t(9) = 0.57, p = .59].
Summary
When only high-confidence “old” responses were considered, comparisons between related FAs and unrelated CRs yielded occipital ERP differences from 300–700 ms that are strikingly similar to those associated with recognition in the LS condition of Experiment 1. These findings join to identify these occipital brain potentials as a signal that can support familiarity based on letter information.
3.3. Discussion
In Experiment 2, we replicated the pattern of occipital ERP differences that were obtained in the LS condition of Experiment 1 when hits were compared to unrelated CRs. In addition, we extended these findings by showing that the same occipital ERP differences were present when related FAs were compared to unrelated CRs. Importantly, these ERPs are markedly different from FN400 potentials, which have been associated with familiarity for words in other situations, typically when encoding conditions emphasize conceptual information. In fact, frontocentral ERPs resembling FN400 potentials differed in this experiment between hits and unrelated CRs, whereas contrasts between related FAs and unrelated CRs did not evince differences at frontal electrodes even when limited to high-confidence “old” responses. Thus, it appears that even in the context of the same experiment, early frontal old/new effects can occur in conjunction with some expressions of familiarity but not others3 (see also Leynes & Zish, 2012; Lucas et al., 2012; Wang, de Chastelaine, Minton, & Rugg, 2011). Together, these findings suggest that the neural mechanisms by which familiarity is expressed depend in part on the stimulus dimensions that are most relevant to familiarity assessment.
Interestingly, old/new effects in Experiment 2 were also evident at an earlier latency. Frontocentral P200 potentials were more positive for both hits and related FAs relative to unrelated CRs. These ERPs resemble P2 potentials that are considered to be part of the typical sensory-evoked response to visual stimuli, and have been linked to attention and target-detection processes during visual feature analysis (Luck & Hillyard, 1994). Although P200 differences are not common among ERP studies of recognition memory, they have sometimes been reported in tasks that involve implicit or explicit perceptual matching processes, by which an incoming stimulus is compared to stored perceptual representations in memory (e.g., Curran & Dien, 2003; Evans & Federmeier, 2007; Misra & Holcomb, 2003). For example, Evans and Federmeier (2007) observed repetition-related P200 increases during a continuous recognition task for visual words that were initially presented to the left visual field/right hemisphere, but not for words initially presented to the right visual field/left hemisphere, potentially reflecting a right-hemisphere superiority in form-based memory. P200 old/new effects were also identified in a recent study in which recognition memory was tested for abstract kaleidoscope stimuli (Voss & Paller, 2009a) and have been found in masked priming studies involving visual word forms (Misra & Holcomb, 2003). Thus, repetition-related P200 increases during recognition tests may reflect some form of interaction between visual memory and visual feature analysis, although their precise functional significance remains unclear. It is also unclear why P200 differences were found in Experiment 2 but not in the LS blocks of Experiment 1. One possibility is that the exclusive use of LS blocks and the 2:1 ratio of letter-fluent stimuli in the test phases of Experiment 2 caused participants to adopt a recognition strategy that emphasized visual perceptual analysis to a greater extent.
4. General Discussion
The vast majority of human electrophysiological studies that have examined familiarity to date have used recognition tests with words or nameable pictures. Most often, these studies have found differences in FN400 potentials between items judged to be more familiar and those judged to be less familiar, and these findings have been taken to support the notion that FN400 represents a neural index of a generic familiarity signal. However, because the type of processing typically emphasized in these studies was at the level of meaning, such studies cannot discriminate between neural patterns that reflect familiarity per se and those that specifically reflect outcomes of prior conceptual processing, such as conceptual fluency (Paller et al., 2007). Furthermore, conceptual fluency from prior processing could sometimes occur in such studies without being a precursor to recognition.
In two experiments, we found that testing recognition for common words in a manner that emphasized letter-level information resulted in qualitatively different neural patterns from those obtained in relation to familiarity under more conventional testing conditions. These findings indicate that—despite the homogeneous and amodal nature of familiarity as a subjective state—the neural mechanisms that can give rise to this state are diverse and depend on the nature of the relevant stimulus representations (for similar arguments see Lucas et al., 2012; Taylor & Henson, 2012a).
This focus on deconstructing the origins of familiarity memory shows promise for reconciling controversies over its electrophysiological basis. The past several years have witnessed a polarizing debate over whether FN400 potentials are better characterized as a neural correlate of familiarity (e.g., a form of explicit memory; Rugg & Curran, 2007) or as a neural correlate of conceptual fluency (a form of implicit memory; Paller et al., 2007). The present findings strongly suggest that these accounts should not be viewed as mutually exclusive, because the relationship between a particular neural signal of repetition and conscious awareness of repetition is not immutable or fixed. This perspective converges nicely with recent indications from fMRI and patient studies that brain regions once thought to be uniquely associated with conscious expressions of memory are also important for certain forms of memory that occur without awareness (for review, see Hannula & Greene, 2012). In addition, the present findings agree with domain-general accounts of the role of fluency as a meta-cognitive cue in judgment and decision making, which emphasize that the same behavioral outcome can arise from a potentially wide range of fluency subtypes with diverse neural origins (Alter & Oppenheimer, 2009).
The letter-segregation technique employed here has been used in several prior behavioral investigations of recognition driven by perceptual fluency (Algarabel, et al., 2009a, 2009b, 2010; Bastin et al., 2013; Keane et al., 2006; Parkin et al., 2001), although the present study is the first to our knowledge to examine the neural basis of recognition under these circumstances. One caveat to this body of research is that, thus far, no direct measures of letter fluency have been obtained in conjunction with this paradigm. Instead, we and others have inferred based on the nature of the manipulation that the performance benefits enjoyed by the LS condition stem from the greater diagnostic value of letter fluency. However, it is noteworthy that the occipital ERP old/new effects observed in the LS condition do closely resemble ERPs attributed to nonconscious perceptual repetition effects for words and word components in several previous studies (Grainger & Holcomb, 2009; Paller & Gross, 1998; Rugg et al., 1998; Woollams et al., 2008; Yu & Rugg, 2010). In this way, the present electrophysiological findings can be viewed as converging evidence that performance improvements in the LS condition reflect the greater diagnostic value of fluency with perceptual features such as letters. Nonetheless, a demonstration that these ERPs are also present in a task designed to directly measure fluency with letter information would constitute stronger evidence that this specific form of fluency was operative in producing the recognition benefit in the LS condition.
As another important caveat, we do not wish to imply that fluency is always the source of familiarity, nor do we believe that the two constructs should be directly equated even in situations in which the latter is derived from the former. The extent to which a given amount of fluency is attributed to and experienced as familiarity depends on several factors, such as whether this fluency exceeds the amount that would be expected for a given stimulus within a given context (Westerman, Lloyd, & Miller, 2002; Westerman, Miller, & Lloyd, 2003; Whittlesea, Jacoby, & Girard, 1990). When expectations regarding fluency are high—such as when a word is extremely common or is easily predicted by its context—participants may be less likely to attribute fluency to recent exposure. Indeed, some research suggests that the attributional stage of the fluency-attribution process may be electrophysiologically dissociable from the fluency itself (Kurilla & Gonsalves, 2012; Wolk et al., 2004). For example, Kurilla and Gonsalves (2012) compared ERPs in response to a manipulation of perceptual stimulus fluency under circumstances in which task conditions either did or did not encourage participants to monitor fluency as a cue to familiarity. While fluency effects were evident in posterior ERPs that occurred from 300–500 ms regardless of task conditions, ERPs from 500–700 ms differed according to whether or not participants were encouraged to attribute fluency to prior exposure. Interestingly, in Experiment 2 of the present study, ERPs during the 500–700 ms interval for related FAs were more sensitive to participants’ confidence levels than were ERPs from 300–500 ms. It is possible that a distinction between fluency per se and the attribution of fluency to familiarity can explain this pattern; for example, the degree of attribution may have covaried with participants’ recognition confidence to a greater degree than the experienced fluency itself. However, no firm conclusions of this nature can be drawn since the present study was not designed to separate neural activity related to fluency from that related to attributional processing.
The results of this study make close contact with current controversies about the neuroanatomical basis of recognition memory. The notion that recollection and familiarity might each be associated with a particular electrophysiological signal was initially developed alongside suggestions that these memory experiences are supported by separate subregions of the medial temporal region, namely the hippocampus and perirhinal cortex, respectively (Aggleton & Brown, 1999). These medial temporal regions have been further distinguished from more posterior cortical regions—such as those comprising the ventral visual stream—which support visual perception and priming (Tulving & Schacter, 1990). However, other recent accounts (e.g., the perceptual-mnemonic feature conjunction model of Bussey, Saksida, & Murray, 2005; Bussey & Saksida, 2002; Cowell et al., 2010) reject the notion that distinct cognitive processes are supported by medial temporal relative to ventral visual stream structures. Rather, the model suggests that these regions should be viewed as a hierarchical continuum in that they differ from one another in the complexity of the stimulus representations that they compute but not with respect to the cognitive processes that they support.
The letter-segregation technique used in this study may be a promising means to test these neuroanatomical predictions, because the letter-level information that is sufficient to discriminate between old and new items in LS blocks is less complex and more stimulus-bound relative to the word and/or concept information necessary to achieve accurate recognition in N blocks. Visual word perception is thought to rely on a hierarchy of increasingly complex neuronal detectors—from those that detect individual letters to detectors for bigrams, letter strings, and whole words—that resides predominantly in the posterior-to-anterior expanse of a region of left occipitotemporal cortex termed the visual word form area (Dehaene et al., 2004; Dehaene, Cohen, Sigman, & Vinckier, 2005; Vinckier et al., 2007). To the extent that fluency signals relating to letter-based information are also produced in these regions, one might predict that activity in the more posterior aspect of this area would correlate with word familiarity in the LS blocks, rather than or in addition to activity in perirhinal cortex. Recent findings linking perirhinal activity to conceptual priming for words (Voss, Hauner, & Paller, 2009; Wang et al., 2010) are also consistent with this account.
More generally, the present data underscore the need to test a wider variety of stimulus materials in order to attain a more thorough understanding of familiarity. Future research using this paradigm and others that vary the content and complexity of the relevant stimulus representations (e.g., Cleary, 2004; Ko, Duda, Hussey, & Ally, 2013; Leynes & Zish, 2012) can provide a broader and more representative picture of familiarity, its neural basis, and its relationships to other forms of memory such as priming. Such research may also shed light on the discrepancies that are rampant in the literature concerning the status of familiarity memory in patients diagnosed with mild cognitive impairment, Alzheimer’s Disease, or other diseases affecting memory (Algarabel, Escudero, et al., 2009; Cipolotti et al., 2006; Jacoby & Kelley, 1992; Libby et al., in press; Weiss et al., 2008; Westerberg et al., 2006, 2013; Wixted & Squire, 2004; Wolk et al., 2005). Interestingly, Bastin and colleagues (2013) recently reported that individuals with mild Alzheimer’s Disease were able to elevate their recognition performance by an amount comparable to control participants when recognition for words was tested under letter-segregated conditions like those used here. Thus, this form of perceptual fluency, as well as the inferential processes that allow this fluency to be experienced as familiarity, may be spared in some patients with memory disorders (but see Algarabel, Escudero, et al., 2009). By contrast, impairments in familiarity in such patients have most often been found under conditions that emphasize conceptual processing (e.g., Ally, 2012; Embree et al., 2012). Perhaps impoverished conceptual stimulus processing due to atrophy of anterior temporal regions can contribute to deficits in the use of conceptual information to discriminate between studied and unstudied items, even when the ability to use perceptual information in this manner remains unaffected. We hope that the present findings will spawn additional research to address these and other open questions about the deceptively complex origins of familiarity memory.
Research Highlights.
We examined ERP correlates of familiarity for words driven by letter fluency.
Target and lure words were derived from either the same or different letter pools.
Recognition improved when targets and lures contained different letters.
Recognition driven by letter fluency was associated with occipital ERPs.
Familiarity memory is determined by multiple neurocognitive mechanisms.
Acknowledgements
We wish to thank Susan Florczak for assistance with stimulus preparation, Megan Ichinose for assistance with data collection, and Kara Federmeier for helpful discussions of these data. This material is based upon work supported by National Science Foundation Grant BCS1025697, as well as by NIH Grant T32 NS047987 and a Beckman Institute Postdoctoral Fellowship Award to HDL.
Appendix A
List of experimental stimuli. The words in List A were derived using only the letters a, b, d, e, g, j, l, r, t, v, w, x, z. The words in list B contained the letters c, f, h, i, k, m, n, o, p, q, s, u, y. The stimuli were divided into four sets matched for word length, each of which contained an equal number of words from both lists.
| Set 1 | Set 2 | Set 3 | Set 4 | ||||
|---|---|---|---|---|---|---|---|
| List A | List B | List A | List B | List A | List B | List A | List B |
| advert | chin | able | cook | alder | chimp | adverb | chick |
| agree | chip | age | cosy | alert | chomp | algae | chump |
| ale | choosy | aware | coupon | algebra | chop | alter | comfy |
| area | chum | bad | cousin | are | chunk | ate | conk |
| art | con | badger | cuss | award | coif | aver | coop |
| avert | cony | bag | fin | axe | coin | average | copy |
| bade | cop | bead | finicky | axle | coo | awe | cosmic |
| bale | cumin | bear | fish | badge | cuckoo | bald | coup |
| barge | cup | beard | funny | bagel | cuff | bard | coy |
| beat | cusp | beaver | fuss | bar | fumy | bare | cushion |
| beet | finch | beer | his | bat | fusion | bed | fun |
| beg | finish | blare | hiss | beadle | honk | bee | hick |
| belt | focus | bleat | hocus | beagle | hoofs | begat | him |
| beret | funk | brat | homy | beta | hop | blew | hips |
| bet | hock | bread | hum | blade | hump | brag | hon |
| blazer | hook | breed | ink | brawl | icy | dare | huff |
| bled | hoop | deal | kick | braze | kimono | dart | hunk |
| brave | huck | debate | kiosk | bred | miff | date | husk |
| brew | hymn | delta | kiss | bree | miss | deer | inn |
| daze | icon | drab | mick | data | monk | delve | ion |
| drag | impish | drat | mink | dear | moon | deter | kin |
| dwelt | inch | drew | moo | debt | mousy | dew | mimic |
| earl | kip | eat | mop | drawl | muff | draw | minimum |
| eel | mission | elate | moss | ear | mumps | eagle | minus |
| elder | mom | ere | muck | edge | musk | eave | mock |
| era | mooch | ever | mummy | ewe | nip | eve | mono |
| evade | mopy | exalt | mush | gavel | nom | extra | mucus |
| exert | much | garb | music | gear | nosy | gab | munch |
| glad | mum | gaze | non | glare | off | gable | muss |
| grade | noisy | gel | nosh | glee | opium | gala | myopic |
| greed | nook | get | opus | graze | opossum | garble | nicks |
| jab | nun | glaze | phi | jeer | pinky | gate | noon |
| jade | nymph | grab | phony | lard | pip | gave | noun |
| jar | onus | gravel | physic | large | poky | glade | ominous |
| lab | oomph | great | pick | law | pons | grate | ouch |
| latex | ossify | java | pin | lax | pop | grave | pink |
| lead | pinon | jaw | pinch | laze | psychic | greet | pinny |
| leave | piny | lag | pock | ledge | puck | grew | pom |
| led | pious | lava | poison | rag | puff | jet | posh |
| ledger | piss | level | pomp | rage | pup | jewel | puss |
| leg | poncho | raze | pouch | raw | push | lad | quick |
| rat | pony | reave | pouf | real | scoop | larvae | shimmy |
| rate | poppy | regal | pun | rebel | scuff | later | shock |
| ravage | posy | revel | punch | red | scum | lear | shoo |
| rave | quin | tab | punish | reed | shin | leer | shop |
| ravel | shiny | tar | punk | teal | shook | let | shun |
| reel | ship | tea | puny | trade | sin | lewd | shy |
| reveal | sic | tear | quip | tree | sink | read | sip |
| tag | sick | tee | schism | twelve | ski | relax | skunk |
| tale | skim | tweed | scion | valet | skimp | table | sky |
| tread | skinny | veer | shuck | vertex | skin | tax | smoky |
| verb | smock | warble | snip | vet | skip | travel | snoop |
| wage | spicy | ward | snuff | wade | smooch | tzar | sock |
| wale | spiky | wave | son | wager | sonic | veal | soup |
| waltz | spy | weal | soon | wart | sop | verbal | soy |
| war | suck | web | spiny | wax | spook | vex | spick |
| ware | sun | wedge | spoof | wear | spoon | wad | spin |
| water | sup | weed | such | wed | summon | waver | spunk |
| weave | union | weld | sum | welt | sunk | wet | unison |
| zeal | you | zebra | sunny | were | unify | zeta | upon |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note that interactions involving confidence should be interpreted with caution given that the two confidence levels were not independent. Because participants could choose only one confidence level per trial, any factor that increases high confidence responding will necessarily decrease low confidence responding, potentially biasing the statistical outcome of interactions involving confidence.
Note that it is also possible that the higher proportion of high-confidence trials registered in the LS relative to the N condition led to the LS-specific occipital old/new effects. We would argue that a confidence-based account of these results is unlikely given that numerous studies have examined the effects of confidence on old/new ERPs (e.g., Curran, 2004; Finnigan, Humphreys, Dennis, & Geffen, 2002; Voss & Paller, 2009b) and none to our knowledge has reported occipital ERPs similar to those described here. Nonetheless, we conducted exploratory analyses of ERP differences from 300–700 ms over the occipital cluster in a subgroup of 9 participants who had at least 10 artifact-free trials corresponding to both high-confidence hits and high-confidence CRs in both the LS and N conditions. A significant difference between high-confidence hits and high-confidence CRs was present for the LS condition [t(8) = 3.53, p < 0.008], but not for the N condition [t(8) = .066, p = .53]. These results provide additional evidence against a confidence-based account of the occipital hit/CR effects.
It is unclear why differences in FN400 potentials between Hits and CRs were found in Experiment 2, but not in Experiment 1. It is possible that this difference stemmed from the fact that fewer words were studied in Experiment 2 relative to Experiment 1 (120 over the course of the four study blocks instead of 240 in Experiment 2). This smaller number of words might have resulted in less priming of related concepts that corresponded to words that were presented as lures, leading to a larger difference between the conceptual fluency of targets and lures.
REFERENCES
- Aggleton JP, Brown M. Interleaving brain systems for episodic and recognition memory. Trends in Cognitive Sciences. 2006;10:455–463. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behavioral and Brain Sciences. 1999;22:425–489. [PubMed] [Google Scholar]
- Algarabel S, Escudero J, Mazón JF, Pitarque A, Fuentes M, Peset V, et al. Familiarity-based recognition in the young, healthy elderly, mild cognitive impaired and Alzheimer’s patients. Neuropsychologia. 2009;47:2056–2064. doi: 10.1016/j.neuropsychologia.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Algarabel S, Pitarque A. Familiarity changes as a function of perceptual shifts. The Spanish Journal of Psychology. 2010;13:518–524. doi: 10.1017/s1138741600002213. [DOI] [PubMed] [Google Scholar]
- Algarabel S, Pitarque A, Tomás J, Mazón JF. Explorations of familiarity produced by words with specific combinations of letters. European Journal of Cognitive Psychology. 2009;22:265–285. [Google Scholar]
- Algarabel S, Rodríguez L, Escudero J, Fuentes M, Peset V, Pitarque A, et al. Recognition by familiarity is preserved in Parkinson’s without dementia and Lewy-Body disease. Neuropsychology. 2010;24:599–607. doi: 10.1037/a0019221. [DOI] [PubMed] [Google Scholar]
- Ally BA. Using pictures and words to understand recognition memory deterioration in amnestic Mild Cognitive Impairment and Alzheimer’s Disease: A review. Current Neurology and Neuroscience Reports. 2012;12:687–694. doi: 10.1007/s11910-012-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter AL, Oppenheimer DM. Uniting the tribes of fluency to form a metacognitive nation. Personality and Social Psychology Review. 2009;13:219–235. doi: 10.1177/1088868309341564. [DOI] [PubMed] [Google Scholar]
- Anderson ND, Ebert PL, Jennings JM, Grady CL, Cabeza R, Graham SJ. Recollection- and familiarity-based memory in healthy aging and amnestic mild cognitive impairment. Neuropsychology. 2008;22:177–187. doi: 10.1037/0894-4105.22.2.177. [DOI] [PubMed] [Google Scholar]
- Bastin C, Willems S, Genon S, Salmon E. Enhancing the salience of fluency improves recognition memory performance in mild Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2013;33:1033–1039. doi: 10.3233/JAD-2012-121678. [DOI] [PubMed] [Google Scholar]
- Bowles B, Crupi C, Mirsattari SM, Pigott SE, Parrent AG, Pruessner JC, et al. Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proceedings of the National Academy of Sciences. 2007;104:16382–16387. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. The organization of visual object representations: a connectionist model of effects of lesions in perirhinal cortex. The European Journal of Neuroscience. 2002;15:355–364. doi: 10.1046/j.0953-816x.2001.01850.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. The perceptual-mnemonic/feature conjunction model of perirhinal cortex function. The Quarterly Journal of Experimental Psychology. B, Comparative and Physiological Psychology. 2005;58:269–282. doi: 10.1080/02724990544000004. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Bird C, Good T, Macmanus D, Rudge P, Shallice T. Recollection and familiarity in dense hippocampal amnesia: a case study. Neuropsychologia. 2006;44:489–506. doi: 10.1016/j.neuropsychologia.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Cleary AM. Orthography, phonology, and meaning: word features that give rise to feelings of familiarity in recognition. Psychonomic Bulletin & Review. 2004;11:446–451. doi: 10.3758/bf03196593. [DOI] [PubMed] [Google Scholar]
- Cowell RA, Bussey TJ, Saksida LM. Components of recognition memory: dissociable cognitive processes or just differences in representational complexity? Hippocampus. 2010;20:1245–1262. doi: 10.1002/hipo.20865. [DOI] [PubMed] [Google Scholar]
- Curran T. Effects of attention and confidence on the hypothesized ERP correlates of recollection and familiarity. Neuropsychologia. 2004;42:1088–1106. doi: 10.1016/j.neuropsychologia.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Curran T, Dien J. Differentiating amodal familiarity from modality-specific memory processes: An ERP study. Psychophysiology. 2003;40:979–988. doi: 10.1111/1469-8986.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danion J, Huron C, Vidailhet P, Berna F. Functional mechanisms of episodic memory impairment in schizophrenia. Canadian Journal of Psychiatry. 2007;52:693–701. doi: 10.1177/070674370705201103. [DOI] [PubMed] [Google Scholar]
- Danker JF, Hwang GM, Gauthier L, Geller A, Kahana MJ, Sekuler R. Characterizing the ERP Old-New effect in a short-term memory task. Psychophysiology. 2008;45:784–793. doi: 10.1111/j.1469-8986.2008.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: a proposal. Trends in Cognitive Sciences. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline J-B, Le Bihan D, et al. Letter binding and invariant recognition of masked words: Behavioral and neuroimaging evidence. Psychological Science. 2004;15:307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Dew ITZ, Cabeza R. The porous boundaries between explicit and implicit memory: behavioral and neural evidence. Annals of the New York Academy of Sciences. 2011;1224:174–190. doi: 10.1111/j.1749-6632.2010.05946.x. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embree LM, Budson AE, Ally BA. Memorial familiarity remains intact for pictures but not for words in patients with amnestic mild cognitive impairment. Neuropsychologia. 2012;50:2333–2340. doi: 10.1016/j.neuropsychologia.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KM, Federmeier KD. The memory that’s right and the memory that’s left: Event-related potentials reveal hemispheric asymmetries in the encoding and retention of verbal information. Neuropsychologia. 2007;45:1777–1790. doi: 10.1016/j.neuropsychologia.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnigan S, Humphreys MS, Dennis S, Geffen G. ERP “old/new” effects: memory strength and decisional factor(s) Neuropsychologia. 2002;40:2288–2304. doi: 10.1016/s0028-3932(02)00113-6. [DOI] [PubMed] [Google Scholar]
- Fleischman DA, Wilson RS, Gabrieli JDE, Schneider JA, Bienias JL, Bennett DA. Implicit memory and Alzheimer’s disease neuropathology. Brain. 2005;128:2006–2015. doi: 10.1093/brain/awh559. [DOI] [PubMed] [Google Scholar]
- Grainger J, Holcomb PJ. An ERP investigation of orthographic priming with relative-position and absolute-position primes. Brain Research. 2009;1270:45–53. doi: 10.1016/j.brainres.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Greene AJ. The hippocampus reevaluated in unconscious learning and memory: at a tipping point? Frontiers in Human Neuroscience. 2012;6:80. doi: 10.3389/fnhum.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA. Neuroimaging studies of priming. Progress in Neurobiology. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Huron C, Danion JM, Giacomoni F, Grangé D, Robert P, Rizzo L. Impairment of recognition memory with, but not without, conscious recollection in schizophrenia. The American Journal of Psychiatry. 1995;152:1737–1742. doi: 10.1176/ajp.152.12.1737. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Dallas M. On the relationship between autobiographical memory and perceptual learning. Journal of Experimental Psychology: General. 1981;110:306–340. doi: 10.1037//0096-3445.110.3.306. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Kelley CM. A process-dissociation framework for investigating unconscious influences: Freudian slips, projective tests, subliminal perception, and signal detection theory. Current Directions in Psychological Science. 1992;1:174–179. [Google Scholar]
- Keane MM, Orlando F, Verfaellie M. Increasing the salience of fluency cues reduces the recognition memory impairment in amnesia. Neuropsychologia. 2006;44:834–839. doi: 10.1016/j.neuropsychologia.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko PC, Duda B, Hussey EP, Ally BA. Electrophysiological distinctions between recognition memory with and without awareness. Neuropsychologia. 2013;51:642–655. doi: 10.1016/j.neuropsychologia.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilla BP, Gonsalves BD. An ERP investigation into the strategic regulation of the fluency heuristic during recognition memory. Brain Research. 2012;1442:36–46. doi: 10.1016/j.brainres.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilla BP, Westerman DL. Processing fluency affects subjective claims of recollection. Memory & Cognition. 2008;36:82–92. doi: 10.3758/mc.36.1.82. [DOI] [PubMed] [Google Scholar]
- Leynes PA, Zish K. Event-related potential (ERP) evidence for fluency-based recognition memory. Neuropsychologia. 2012;50:3240–3249. doi: 10.1016/j.neuropsychologia.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Libby LA, Yonelinas AP, Ranganath C, Ragland JD. Recollection and Familiarity in Schizophrenia: A Quantitative Review. Biological Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas HD, Taylor JR, Henson RN, Paller KA. Many roads lead to recognition: Electrophysiological correlates of familiarity derived from short-term masked repetition priming. Neuropsychologia. 2012;50:3041–3052. doi: 10.1016/j.neuropsychologia.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Martin CB, Bowles B, Mirsattari SM, Köhler S. Selective familiarity deficits after left anterior temporal-lobe removal with hippocampal sparing are material specific. Neuropsychologia. 2011;49:1870–1878. doi: 10.1016/j.neuropsychologia.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Gooding PA, van Eijk R. A new theoretical framework for explicit and implicit memory. Psyche. 1997;3:1–43. [Google Scholar]
- Mayes AR, Roberts N. Theories of episodic memory. Philosophical Transactions of the Royal Society of London: Biological sciences. 2001;356:1395–1408. doi: 10.1098/rstb.2001.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: an ambiguity associated with analysis of variance models. Electroencephalography and Clinical Neurophysiology. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Misra M, Holcomb PJ. Event-related potential indices of masked repetition priming. Psychophysiology. 2003;40:115–130. doi: 10.1111/1469-8986.00012. [DOI] [PubMed] [Google Scholar]
- Paller KA, Gross M. Brain potentials associated with perceptual priming vs explicit remembering during the repetition of visual word-form. Neuropsychologia. 1998;36:559–571. doi: 10.1016/s0028-3932(97)00132-2. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M. Brain potentials during memory retrieval provide neurophysiological support for the distinction between conscious recollection and priming. Journal of Cognitive Neuroscience. 1992;4:375–392. doi: 10.1162/jocn.1992.4.4.375. [DOI] [PubMed] [Google Scholar]
- Paller KA, Voss JL, Boehm SG. Validating neural correlates of familiarity. Trends in Cognitive Sciences. 2007;11:243–250. doi: 10.1016/j.tics.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Ward J, Squires EJ, Furbear H, Clark A, Townshend J. Data-driven recognition memory: a new technique and some data on age differences. Psychonomic Bulletin & Review. 2001;8:812–819. doi: 10.3758/bf03196222. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Allan K, Birch CS. Electrophysiological evidence for the modulation of retrieval orientation by depth of study processing. Journal of Cognitive Neuroscience. 2000;12:664–678. doi: 10.1162/089892900562291. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends in Cognitive Sciences. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Mark RE, Walla P, Schloerscheidt AM, Birch CS, Allan K. Dissociation of the neural correlates of implicit and explicit memory. Nature. 1998;392:595–598. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Current Opinion in Neurobiology. 2007;17:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Henson RN. You can feel it all over: Many signals potentially contribute to feelings of familiarity. Cognitive Neuroscience. 2012a;3:209–210. doi: 10.1080/17588928.2012.689966. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Henson RN. Could masked conceptual primes increase recollection? The subtleties of measuring recollection and familiarity in recognition memory. Neuropsychologia. 2012b;50:3027–3040. doi: 10.1016/j.neuropsychologia.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer AK, Winocur G, Craik FIM, Moscovitch M. Source memory and divided attention: Reciprocal costs to primary and secondary tasks. Neuropsychology. 1999;13:467–474. [PubMed] [Google Scholar]
- Tulving E, Schacter DL. Priming and human memory systems. Science. 1990;247:301–306. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Mecklinger A, Müller U. An electrophysiological test of directed forgetting: The role of retrieval inhibition. Journal of Cognitive Neuroscience. 2000;12:924–940. doi: 10.1162/08989290051137477. [DOI] [PubMed] [Google Scholar]
- Vargas IM, Voss JL, Paller KA. Implicit recognition based on lateralized perceptual fluency. Brain Sciences. 2012;2:22–32. doi: 10.3390/brainsci2010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: Dissecting the inner organization of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Voss JL, Baym CL, Paller KA. Accurate forced-choice recognition without awareness of memory retrieval. Learning & Memory. 2008;15:454–459. doi: 10.1101/lm.971208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Federmeier KD. FN400 potentials are functionally identical to N400 potentials and reflect semantic processing during recognition testing. Psychophysiology. 2011;48:532–546. doi: 10.1111/j.1469-8986.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Hauner KKY, Paller KA. Establishing a relationship between activity reduction in human perirhinal cortex and priming. Hippocampus. 2009;19:773–778. doi: 10.1002/hipo.20608. [DOI] [PubMed] [Google Scholar]
- Voss JL, Lucas HD, Paller KA. Conceptual priming and familiarity: Different expressions of memory during recognition testing with distinct neurophysiological correlates. Journal of Cognitive Neuroscience. 2010;22:2683–2651. doi: 10.1162/jocn.2009.21341. [DOI] [PubMed] [Google Scholar]
- Voss JL, Lucas HD, Paller KA. More than a feeling: Pervasive influences of memory without awareness of retrieval. Cognitive Neuroscience. 2012;3:193–207. doi: 10.1080/17588928.2012.674935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Neural correlates of conceptual implicit memory and their contamination of putative neural correlates of explicit memory. Learning & Memory. 2007;14:259–267. doi: 10.1101/lm.529807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Brain substrates of implicit and explicit memory: The importance of concurrently acquired neural signals of both memory types. Neuropsychologia. 2008;46:3021–3029. doi: 10.1016/j.neuropsychologia.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. An electrophysiological signature of unconscious recognition memory. Nature Neuroscience. 2009a;12:349–355. doi: 10.1038/nn.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Remembering and knowing: Electrophysiological distinctions at encoding but not retrieval. NeuroImage. 2009b;46:280–289. doi: 10.1016/j.neuroimage.2009.01.048. [DOI] [PubMed] [Google Scholar]
- Voss JL, Schendan HE, Paller KA. Finding meaning in novel geometric shapes influences electrophysiological correlates of repetition and dissociates perceptual and conceptual priming. NeuroImage. 2010;49:2879–2889. doi: 10.1016/j.neuroimage.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Wais PE, Wixted JT, Hopkins RO, Squire LR. The hippocampus supports both the recollection and the familiarity components of recognition memory. Neuron. 2006;49:459–466. doi: 10.1016/j.neuron.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TH, de Chastelaine M, Minton B, Rugg MD. Effects of age on the neural correlates of familiarity as Indexed by ERPs. Journal of Cognitive Neuroscience. 2011;24:1055–1068. doi: 10.1162/jocn_a_00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Lazzara MM, Ranganath C, Knight RT, Yonelinas AP. The medial temporal lobe supports conceptual implicit memory. Neuron. 2010;68:835–842. doi: 10.1016/j.neuron.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yonelinas AP. Familiarity is related to conceptual implicit memory: An examination of individual differences. Psychonomic Bulletin & Review. 2012;19:1154–1164. doi: 10.3758/s13423-012-0298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss AP, Goff DC, Duff M, Roffman JL, Schacter DL. Distinguishing familiarity-based from source-based memory performance in patients with schizophrenia. Schizophrenia Research. 2008;99:208–217. doi: 10.1016/j.schres.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg C, Mayes A, Florczak SM, Chen Y, Creery J, Parrish T, Weintraub S, Mesulam MM, Reber PJ, Paller KA. Distinct medial temporal contributions to different forms of recognition in amnestic mild cognitive impairment and Alzheimer’s disease. Neuropsychologia. 2013;51(12):2450–2461. doi: 10.1016/j.neuropsychologia.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam MM, Holdstock JS, Mayes AR, et al. When memory does not fail: Familiarity-based recognition in mild cognitive impairment and Alzheimer’s disease. Neuropsychology. 2006;20:193–205. doi: 10.1037/0894-4105.20.2.193. [DOI] [PubMed] [Google Scholar]
- Westerman DL, Lloyd ME, Miller JK. The attribution of perceptual fluency in recognition memory: The role of expectation. Journal of Memory and Language. 2002;47:607–617. [Google Scholar]
- Westerman DL, Miller JK, Lloyd ME. Change in perceptual form attenuates the use of the fluency heuristic in recognition. Memory & Cognition. 2003;31:619–629. doi: 10.3758/bf03196102. [DOI] [PubMed] [Google Scholar]
- Whittlesea BWA, Jacoby LL, Girard K. Illusions of immediate memory: Evidence of an attributional basis for feelings of familiarity and perceptual quality. Journal of Memory and Language. 1990;29:716–732. [Google Scholar]
- Wixted JT, Mickes L, Squire LR. Measuring recollection and familiarity in the medial temporal lobe. Hippocampus. 2010;20:1195–1205. doi: 10.1002/hipo.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. Recall and recognition are equally impaired in patients with selective hippocampal damage. Cognitive, Affective & Behavioral Neuroscience. 2004;4:58–66. doi: 10.3758/cabn.4.1.58. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Schacter DL, Berman AR, Holcomb PJ, Daffner KR, Budson AE. An electrophysiological investigation of the relationship between conceptual fluency and familiarity. Neuroscience Letters. 2004;369:150–155. doi: 10.1016/j.neulet.2004.07.081. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Schacter DL, Berman AR, Holcomb PJ, Daffner KR, Budson AE. Patients with mild Alzheimer’s disease attribute conceptual fluency to prior experience. Neuropsychologia. 2005;43:1662–1672. doi: 10.1016/j.neuropsychologia.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Woollams AM, Taylor JR, Karayanidis F, Henson RNA. Event-related potentials associated with masked priming of test cues reveal multiple potential contributions to recognition memory. Journal of Cognitive Neuroscience. 2008;20:1114–1129. doi: 10.1162/jocn.2008.20076. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Consciousness, control, and confidence: the 3 Cs of recognition memory. Journal of Experimental Psychology. General. 2001;130:361–379. doi: 10.1037//0096-3445.130.3.361. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Aly M, Wang W, Koen JD. Recollection and familiarity: Examining controversial assumptions and new directions. Hippocampus. 2010;20:1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovel G, Paller KA. The neural basis of the butcher-on-the-bus phenomenon. NeuroImage. 2004;21:789–800. doi: 10.1016/j.neuroimage.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Yu SS, Rugg MD. Dissociation of the electrophysiological correlates of familiarity strength and item repetition. Brain Research. 2010;1320:74–84. doi: 10.1016/j.brainres.2009.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]