SUMMARY
Adrenals and gonads share a common primordium (AGP), but the molecular events driving differentiation are poorly understood. Here we demonstrate that the Wilms’ tumor suppressor WT1 is a key factor defining AGP identity by inhibiting the steroidogenic differentiation process. Indeed, ectopic expression of WT1 precludes differentiation into adreno-cortical steroidogenic cells by locking them into a progenitor state. ChIP experiments identify Tcf21 and Gli1 as direct targets of WT1. Moreover, cell lineage tracing analyses identify a long-living progenitor population within the adrenal gland, characterized by the expression of WT1, GATA4, GLI1 and TCF21 that can generate steroidogenic cells in vivo. Strikingly, gonadectomy dramatically activates these WT1+ cells and leads to their differentiation into gonadal steroidogenic tissue. Thus our data describes a previously unknown mechanism of response to organ loss by recreating hormone-producing cells at a heterotopic site.
INTRODUCTION
Adrenals and gonads are crucial steroidogenic organs required to maintain body homeostasis in mammals. Consistent with their overlapping functions, adrenal cortex and gonads share a common developmental origin, the adreno-gonadal primordium (AGP). In the mouse, the AGP can be first detected as a thickening of the coelomic epithelium at around embryonic day E9.5 (Hatano et al., 1996; Ikeda et al., 1994). By E10.5 the AGP splits into adrenal and gonadal primordia that will continue to differentiate along separate pathways. Neural crest derived cells invade the adrenal primordium at E12.5 and will ultimately differentiate into the neuroendocrine chromaffin cells (Anderson and Axel, 1986). Encapsulation of the adrenal occurs at E14.5, in a process that is believed to involve condensation of mesenchymal cells surrounding the developing organ (Mesiano and Jaffe, 1997; Uotila, 1940). However, definitive proof of this hypothesis is missing to date. After encapsulation, adrenal development proceeds with the development of the definitive cortex (Zubair et al., 2008) followed by the formation of specific steroidogenic zones (glomerulosa, fasciculata, X-zone), a process that is only completed after birth.
The Wilms’ tumour suppressor gene 1 (WT1) encodes a transcriptional regulator that plays key roles during the formation of many organs (Hohenstein and Hastie, 2006). More recent work highlights an equally important role in the maintenance and repair of several tissues (Chau et al., 2011; Smart et al., 2011). The molecular biology of WT1 is complex and at least 36 different isoforms can be produced by a combination of alternative transcription start sites, alternative splicing and RNA editing. Alternative splicing at the junction of exon 9 and 10 generates distinct isoforms that contain (+KTS) or lack (−KTS) the three aminoacids KTS, which gives rise to proteins that have different biochemical and biological properties. WT1-KTS shows high affinity to DNA (Bickmore et al., 1992) and a diffuse nuclear distribution (Larsson et al., 1995) that is typical of classic transcription factors. Indeed, several studies have demonstrated the ability of WT1 -KTS to directly regulate gene expression (Hohenstein and Hastie, 2006) and influence the chromatin state of target loci (Essafi et al., 2011). In contrast, WT1+KTS isoforms preferentially bind RNA (Caricasole et al., 1996; Kennedy et al., 1996) and have been suggested to play a role in RNA metabolism (Niksic et al., 2004). In vivo evidence for distinct roles of +KTS and −KTS comes from genetic analysis in mice and mutants lacking either of the variants display distinct phenotypes (Hammes et al., 2001).
Development of the AGP requires WT1 and its direct target SF1 (Luo et al., 1994; Val et al., 2007; Wilhelm and Englert, 2002) and targeted deletion of either of these genes results in AGP apoptosis and, as a consequence, agenesis of both gonads and adrenal glands (Kreidberg et al., 1993; Luo et al., 1994; Moore et al., 1999). Whereas both WT1 and SF1 are expressed in the AGP, WT1 is switched off within the adrenal primordium soon after separation (Moore et al., 1998; Vidal and Schedl, 2000). The functional significance of this repression is presently unknown.
In the present study we identify WT1 as an essential player in defining AGP cell identity. We show that ectopic expression of the transcriptionally active WT1−KTS isoform is sufficient to prevent differentiation of AGP cells into steroidogenic cells by directly regulating the expression of genes such as Gli1 and Tcf21. In addition, we identify AGP-like cells within the adrenal cortex that exhibit WT1 expression and maintain the ability to generate both adrenocortical and gonadal steroidogenic cells throughout life.
RESULTS
WT1 repression is essential for adrenocortical development
To study the dynamics of WT1 expression in adrenal development, we first carried out comparative expression analysis at various developmental stages. At E9.5 WT1 expression was found throughout the urogenital ridge, where it largely overlapped with GATA4 (fig. 1A). Appearance of first SF1 positive (WT1+/GATA4+/SF1+) cells occurred at E9.75 and was followed by rapid downregulation of WT1 and GATA4 in the presumptive adrenal progenitor compartment (fig. 1B). By E11.5 WT1 was no longer detectable in the majority of SF1+ adrenocortical progenitors (fig. 1B). Of note, rare WT1+/GATA4+ cells, some of which expressed SF1, persisted within the adrenal cortex throughout development (fig. 1B, arrows; fig. S1C). In contrast to the adrenal gland, cells within the developing gonad continued to co-express WT1 and SF1 until later stages (fig. 1C and data not shown).
Fig. 1. Repression of WT1 is required to allow adrenocortical differentiation.
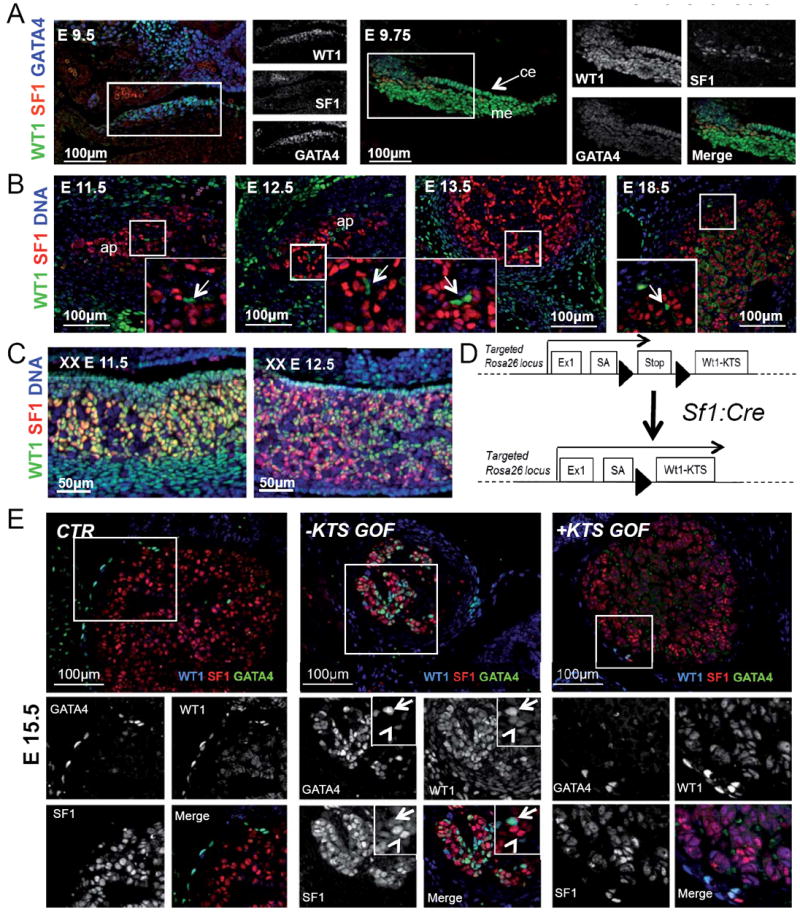
(A) Immunostaining against WT1 (green), SF1 (red) and GATA4 (blue) in early (E9.5) and late (E9.75) AGP. WT1 and GATA4 characterize AGP cells from the earliest stages, whereas SF1 can only be detected in late AGP cells. (B) WT1+ cells (green) are detected in the mesenchyme surrounding the adrenal primordium prior encapsulation (E11.5 and E12.5) and in the adrenal capsule at later developmental stages. Few WT1+, SF1- cells are also located within the adrenal cortex after encapsulation (arrow). (C) Coexpression of WT1 and SF1 persists in E11.5 and E12.5 gonads until later stages. (D) Schematic representation of the genetic approach used to ectopically express WT1−KTS isoforms in steroidogenic cells. Black arrow-heads indicate LoxP sites. An equivalent strategy was used to knock in and ectopically express Wt1+KTS isoforms. (E) WT1 (blue) SF1 (red) and GATA4 (green) immunofluorescence on adrenal sections from CTR, − and +KTSGOF E15.5 embryos. Two cell populations can be distinguished in −KTSGOF adrenal cortex (insets): WT1high, which have strong GATA4 and low SF1 expression (arrows); and WT1low, which instead have reduced or absent GATA4 and strong SF1 expression (arrowheads). Please note that the cytoplasmic WT1 staining in control adrenals represents background noise. me, mesonephros; ce, coelomic epithelium; ap, adrenal primordium, Co, adrenal cortex; Ca, adrenal capsule. See also Fig. S1.
To understand the functional significance of Wt1 repression during adrenocortical differentiation, we generated mice that permit Cre-mediated activation of WT1 + or −KTS isoforms in a tissue specific fashion (Rosa26:Wt1+KTS and Rosa26:Wt1-KTS lines fig S1A, B). Genetic crosses with the SF1:Crehigh line (Bingham et al., 2006), a transgenic line expressing high levels of CRE within the steroidogenic compartment, resulted in activation of WT1 in the developing adrenal cortex as early as E12.5 (fig. S1C). Heterozygous Rosa26+/Wt1+KTS; Sf1:Cre and Rosa26+/Wt1-KTS; Sf1:Cre embryos developed normal adrenal glands (data not shown). Since Rosa26 is known to be a relatively weak promoter, we crossed the targeted allele to the homozygous state to further increase transgene expression levels. Homozygous Rosa26Wt1+KTS/Wt1+KTS; Sf1:Cre embryos (from now on called +KTSGOF) displayed no dramatic changes in adrenal architecture (fig. 1E). In contrast, Rosa26Wt1-KTS/Wt1-KTS; Sf1:Cre mice (from now on called -KTSGOF) developed highly abnormal adrenal glands of reduced size and abnormal cellular morphology (fig. 1E, S1C-E).
To determine if WT1 repression was necessary for differentiation of progenitors into adrenocortical cells, we examined whether the expression of the AGP marker gene GATA4 persisted in transgenic animals. Indeed, activation of WT1-KTS, but not WT1+KTS, triggered expression of GATA4 in SF1 expressing cells (fig. 1E). Expression of GATA4 was evident as early as E12.5 (fig. S1C). At this early time point, both GATA4 and WT1 were expressed homogenously in all fetal adrenocortical (SF1+) cells of -KTSGOF mice (fig S1C). Later in development, two subtypes of cells became apparent that were distinguished by the levels of WT1 expression, perhaps as a result of stochastic/epigenetic factors. WT1high cells (high levels of WT1) showed ectopic activation of GATA4, but exhibited low levels of SF1 (fig. 1E, -KTSGOF, and S1C). In contrast, WT1low cells maintained strong SF1 expression, and showed reduced levels of GATA4. We conclude that high levels of WT1-KTS prevent complete differentiation of AGP cells into steroidogenic adrenocortical cells.
Compensation of hormonal production in adult WT1-KTS expressing adrenals
Adrenal glands from adult −KTSGOF animals were smaller than controls (table S1) and displayed cortical spindle-shaped cells, effectively dividing the cortex into lobular structures (fig. 2A). In each lobule the zonation of the gland was grossly conserved, as indicated by the expression of the general steroidogenic enzyme 3β-HSD2, and the zona fasciculata marker AKR1b7 (fig.S2A). The only affected adrenocortical area was the X-zone, which was dramatically reduced in −KTSGOF mice (fig. S2B). Despite the severe morphological changes, the adrenal glands from −KTSGOF animals appeared to be functional and transgenic animals showed normal circulating levels of corticosterone (fig. S2C). Expression levels of the main enzymes involved in steroidogenesis were also comparable to those found in control animals (fig. S2E). Maintenance of steroid production was likely achieved through raised ACTH levels in −KTSGOF mice (fig. S2C). ACTH is known to stimulate the expression of steroidogenic enzymes, and indeed increased cellular staining for AKR1b7 and 3β-HSD2 (fig. S2A) and 21-Hydroxylase could be observed (fig. S1E) together with a mild increase in steroidogenic cell size (fig. S2D).
Fig. 2. Cells ectopically expressing WT1-KTS are blocked in an AGP-like state throughout life.
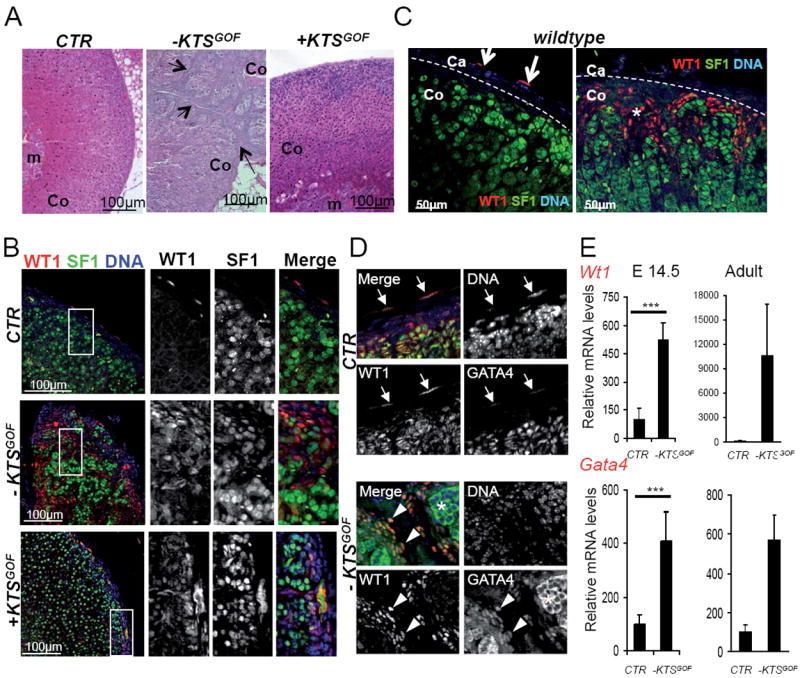
(A) Haematoxylin and eosin staining of adrenals from wild type, + and −KTSGOF adult mice. Arrows indicate capsular-like cells within the adrenal cortex of −KTSGOF animals. (B) Immunostaining against WT1 (red) and SF1 (green) on adrenals from wild type, + and −KTSGOF adult mice shows the persistence of WT1+ expressing cells within the adrenal cortex of −KTSGOF and +KTSGOF mice. (C) Immunostaining for WT1 (green) and SF1 (red) on adult adrenal glands reveals the presence of WT1+ cells within the adrenal capsule (left panel) and in rare patches located in the subcapsular cortex (right panel). (D) WT1 positive capsular and cortical cells (red) found in adult wildtype animals also express GATA4 (green). Note the cytoplasmic GATA4 signal in steroidogenic cells represents background (asterisk). (E) Quantification of Wt1 and Gata4 messenger RNAs reveals increased expression of both genes in −KTSGOF adrenals. E 14.5: Wt1, CTR: 100 ± 61.85, n=6; −KTSGOF: 522.28 ± 89.27, n=6; Gata4, CTR: 100 ± 35.38, n=6; −KTSGOF: 407.20 ± 109.67, n=6. Adult: Wt1, CTR: 100 ± 48.20, n=9; −KTSGOF: 10578.96 ± 12509.51, n=3; Gata4, CTR: 100 ± 69.51, n=6; −KTSGOF: 569.70 ± 309.06, n=4. *** P< 0.001 using student T test. Ca, adrenal capsule; Co, adrenal cortex; m, adrenal medulla. See also Fig. S2.
Immunostaining analysis revealed a similar association as observed during development and steroidogenic SF1 positive cells expressed only very low levels of WT1 (fig. 2B). In contrast cortical spindle-shaped cells in adult −KTSGOF animals showed strong expression of WT1 and reduced SF1 (fig. 2B). Surprisingly, we also occasionally observed spindle-shaped WT1+; SF1low cells in the adrenal cortex of wild type animals, but in contrast to −KTSGOF mice these were found in rare wedge shape patches located in the subcapsular region (fig. 2C). + KTSGOF animals did not show a dramatic phenotype (fig. 2A) and, although low expression of WT1 was present in steroidogenic cells, only few spindle-shaped WT1+ cells could be detected and these were restricted to the subcapsular region (fig. 2B).
-KTSGOF animals develop normal gonads and are fertile
The Sf1:Cre driver is not only expressed in adrenocortical, but also in gonadal cells (Bingham et al 2006) and we wondered whether gonads may also be affected in this transgenic strain. In males, gonadal steroid synthesis is initiated during development, whereas females only produce sex hormones after birth. The expression pattern p450scc, a functional marker of steroidogenic cells, was unchanged between wild type and −KTSGOF embryos (fig. S2F), suggesting that ectopic expression of WT1 did not interfere with steroid synthesis during development. ISH analysis for 21-hydroxylase did not reveal abnormal displacement of adrenocortical cells into the gonads of −KTSGOF embryos and only rare adrenal-like cells were detected (fig. S2G), as has been described previously in wild type testes (Val et al 2006). Finally, gonads from adult −KTSGOF animals displayed normal histology (fig. S2H, I) and both sexes were fertile. qPCR analysis for WT1 revealed only very mild overexpression of Wt1-KTS in the developing gonads (fig. S2J), which may explain the lack of a gonadal phenotype.
WT1 activates Tcf21 and Gli1
Given the similar morphology of WT1 expressing cells in −KTSGOF animals and those discovered in control animals (fig. 2C), we next asked whether they shared expression of common AGP markers. We first investigated the expression of the transcription factor GATA4 that we found co-expressed with WT1 in AGP cells, in a proportion of capsular and in few cortical cells in the developing adrenals (fig 1A, E, S1C). Indeed, GATA4 was expressed in both WT1+ capsular cells and spindle-shaped cells within the adult adrenal cortex in both wild type and −KTSGOF animals (fig. 2D). RT-Q-PCR analysis further confirmed the association of WT1 and GATA4 expression and the expression of both genes was strongly increased in −KTSGOF animals (fig. 2E).
Gli1 and Tcf21 encode two transcription factors expressed in capsular cells that have been recently suggested to mark adrenal progenitor cells (Huang et al., 2010; King et al., 2009; Kim and Hammer, 2007). To examine whether WT1 overlaps with the expression of these markers we performed staining against β-galactosidase in Gli1:LacZ mice (Bai et al., 2002) and RNA in situ hybridization for the Tcf21 transcript. Indeed, expression of both genes largely followed WT1 expression in cortical and capsular cells in wild type animals (fig. 3A-C, E), albeit a proportion of GLI1 expressing cells, primarily located at the interior side of the capsule, were negative for WT1 (fig. 3A, asterisk). To test whether WT1 induces expression of Tcf21 and Gli1, we next performed RT-qPCR analysis. Both, Tcf21 and Gli1 transcripts were dramatically increased in −KTSGOF adrenals (fig. 3D) and their expression was restricted to spindle-shaped WT1+ cells (fig. 3E). Similarly, Ptch1, a downstream target of GLI1, was increased in −KTSGOF, but not in +KTSGOF animals (fig. S3A, B). In contrast, expression of Shh, a gene marking a population of subcapsular cells in wild type animals (King et al., 2009; Huang et al., 2010), showed no significant change albeit Shh expressing cells were misplaced in −KTSGOF mice (fig. S3A, B).
Fig. 3. WT1-KTS ectopic expression directly upregulates Tcf21 and Gli1.
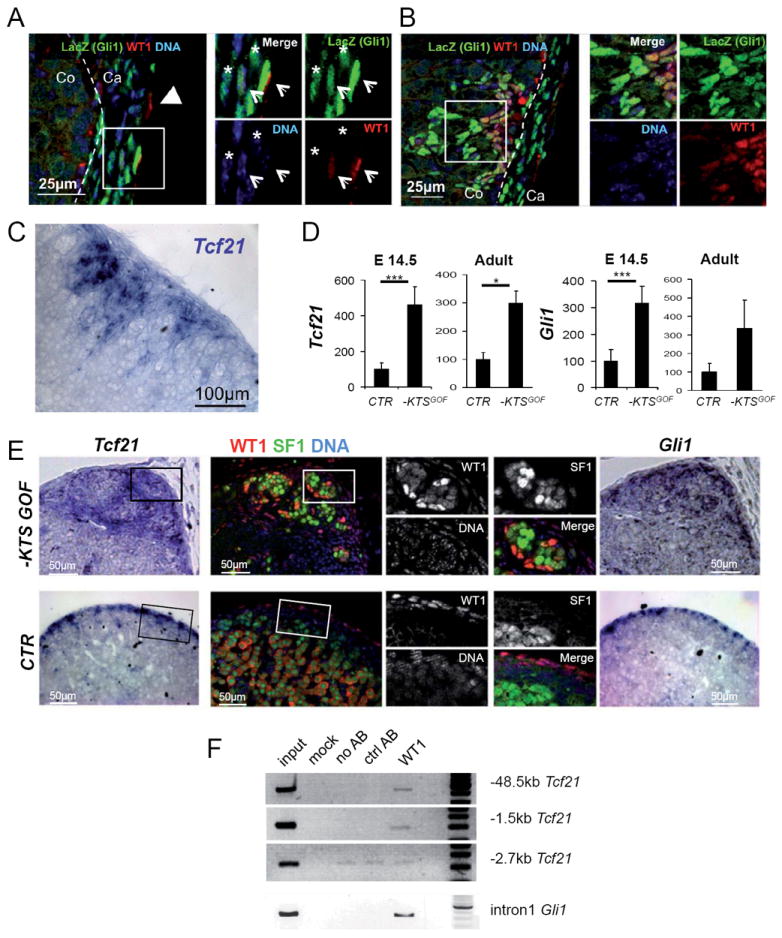
(A-B) LacZ (green) and WT1 (red) immunofluorescence on adrenal sections from adult Gli1:LacZ mice. (A) The adrenal capsule is composed of at least three cells types that can be distinguished by the presence or absence of WT1 and GLI1 (WT1+ GLI1-, arrowhead; WT1- GLI1+ asterisk; WT1+ GLI1+, arrows in insets). (B) WT1+ adrenocortical patches are also positive for GLI1 expression. (C) Tcf21 RNA in situ hybridization showing expression of this marker in the same cells. (D) Tcf21 and Gli1 in adrenal glands of E14.5 embryos or adult −KTSGOF mice show increased expression. E14.5: Tcf21, CTR: 100 ± 33.29, n=4; −KTSGOF: 461.52 ± 102.07, n=6; Gli1, CTR: 100 ± 42.58, n=4; −KTSGOF: 317.34 ± 63.02, n=6. Adult: Tcf21 CTR: 100 ± 110.91, n=20; −KTSGOF: 298.77 ± 123.12, n=8; Gli1, CTR: 100 ± 147.46, n=10; −KTSGOF: 334.15 ± 305.52, n=4. *** P< 0.001, * P< 0.05 using student T test. The data presented were normalized for Hprt1 expression. (E) WT1 (red) and SF1 (green) immunofluorescence on consecutive sections to Tcf21 (left panel) or Gli1 (right panel) RNA in situ hybridization on E18.5 adrenals, shows overlap between WT1 and the RNA for the two markers in both CTR and −KTSGOF. Please note that the cytoplasmic WT1 staining in control adrenals is background. (F) ChIP analysis on −KTSGOF adrenals shows binding of WT1-KTS to the proximal promoter of Tcf21 and to an intronic region of Gli1. A region located -2.7kb upstream of Tcf21 with background amplification is shown as a negative control. Ca, adrenal capsule; Co, adrenal cortex. See also Fig. S3.
Since WT1-KTS acts as a transcriptional regulator, we next asked whether it might directly activate the promoters of Gata4, Tcf21 and Gli1. Primers were designed according to the presence of evolutionary conserved regions and peaks identified in WT1 ChIP-Seq analysis carried out on E17.5 kidneys (fig. S3C). In vivo ChIP assays performed on adrenal glands from −KTSGOF animals (fig. 3F) demonstrated WT1 binding to two evolutionary conserved regions upstream from Tcf21 (-1.5 and -48.5Kb from the transcription start site) and an intronic region of Gli1 (fig. 3F). We were unable to detect any enriched region in the promoter of Gata4 (data not shown).
Taken together, these data indicate that ectopic expression of the transcriptionally active isoform of WT1 (WT1-KTS), but not WT1+KTS, in adrenal primordial cells is sufficient to prevent AGP cells from differentiation in a process that is likely to involve the WT1 target genes Gli1 and Tcf21 (fig. 3F).
WT1+ progenitors generate adrenocortical cells during development
To better understand the relationship between WT1-expressing cells and their descendants, we next carried out cell-lineage tracing experiments using Wt1:Cre-GFP; mTmG mice (Zhou et al., 2008). Cre-induced recombination activates the green fluorescent protein and thus irreversibly labels all Wt1-expressing cells as well as their descendants. Consistent with a direct descendance of steroidogenic cells from WT1+ AGP cells (fig.1A), the totality of the SF1+ adrenal cortical cells stained positive for GFP (fig. S4A). To address whether WT1 expressing mesenchymal cells maintain progenitor features at later stages of development, we resorted to the recently generated Wt1:Cre-ERT2 strain, which allows temporal control of Cre activity (Zhou et al., 2008) (fig. 4A). Activation of Cre recombinase by tamoxifen injection at E12.5 or E14.5 resulted in labelling of a large proportion of cells within the adrenal capsule at E18.5 (Fig. 4B, D), indicating that WT1+ mesenchymal cells directly participate to the generation of this tissue. Moreover, a small proportion of GFP cells was located within the adrenal cortex and some of them expressed the steroidogenic marker SF1 (fig. 4B). Importantly, these GFP+SF1+ cells had lost WT1, indicating a complete differentiation of WT1+ progenitors into adrenocortical cells (Fig. 4B, S4B).
Fig. 4. WT1 expressing cells are progenitors able to differentiate into steroidogenic cells.
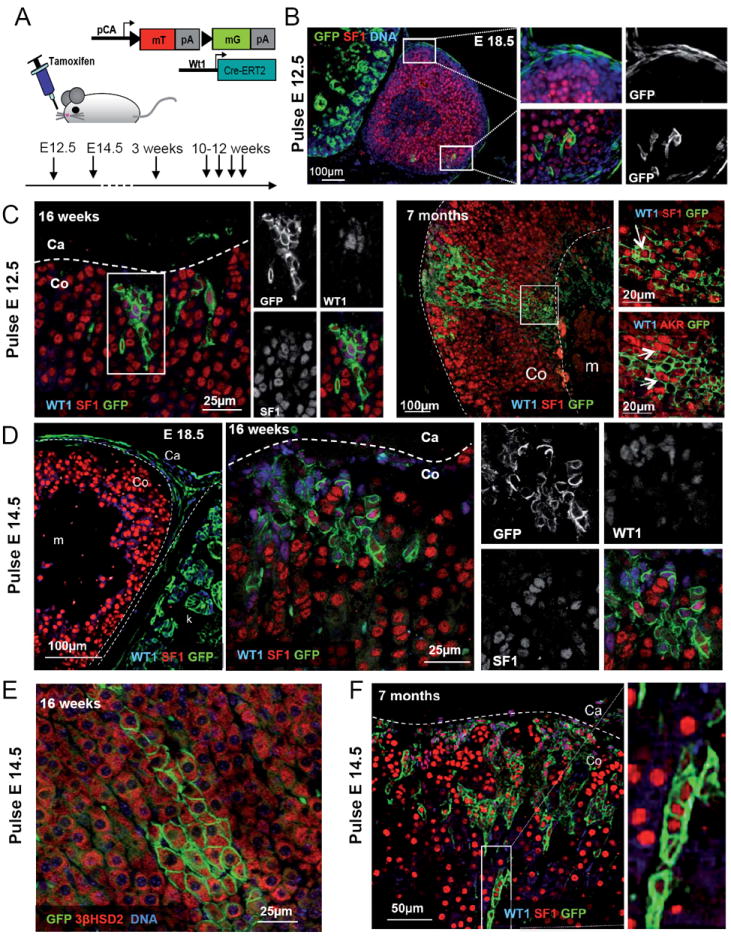
(A) Schematic representation of the cell lineage tracing experiments performed on Wt1:Cre-ERT2; mTmG mice or embryos indicating the time point when tamoxifen was administered. (B-F) WT1 or DNA (blue), SF1, 3βHSD2 or AKR1b7 (red) and GFP (green) immunofluorescence on samples of adrenal glands from Wt1:Cre-ERT2; mTmG mice of different ages treated with tamoxifen at E12.5 (B and C) or E14.5 (D to F). During development the majority of GFP+ cells are localized within the capsule (B and D E18.5). Postnatal patches of WT1+, GFP+ cells expand under the adrenal capsule and few cells acquire a steroidogenic phenotype. F) Quantification of the number of GFP+ patches of cells within the adrenal cortex of E18.5 embryos and 3 weeks old Wt1:Cre-ERT2; mTmG mice treated with tamoxifen at E14.5. The overall number of GFP+ clusters increases from 4.9 ± 2.42 (2.6 ± 1.84 WT1+, 2.3 ± 2.45 WT1-) (N=10) to 16.33 ± 4.78 (1.25 ± 0.84 WT1+, 14.0 ± 4.55 WT1-) (N=5). Co, adrenal cortex; Ca, adrenal capsule; m, adrenal medulla. See also Fig. S4.
Recently King and colleagues (King et al., 2009) described a progenitor cell population residing within the adrenal capsule that expresses the transcription factor GLI1. Given the fact that we identified Gli1 as a direct target of WT1, we decided to investigate the relationship between these two populations. Analysis of Wt1:Cre-ERT2; mTmG; Gli1:LacZ E18.5 embryos treated with tamoxifen at E12.5 allowed us to highlight a partial overlap of expression between the two factors (fig. S4E, F). Nevertheless, lineage tracing of WT1 expressing cells resulted in a substantially different pattern when compared to that obtained with Gli1:Cre-ERT2 mice (King et al., 2009). GFP was mainly detected in the external capsule, where WT1 expression is stronger, and did not cover the entire GLI1 population (fig. S4E and F).
From our data it appears that the activation of the Wt1:Cre-ERT2 only occurs in a subpopulation of cells expressing WT1 (and thus Cre-ERT2) at high levels (fig. S4E). Cells traced by King and colleagues are likely to be WT1low or WT1 negative precursors that may have arisen from AGP cells at an early time point of development. Indeed we could identify WT1-GLI1+ cells at the interior side of the capsule, which seem good candidates for such a population (fig. S4E, asterisk).
WT1+ cells are long living progenitors able to generate adrenocortical cells throughout life
Given the persistence of rare WT1+ cells in adult adrenals (fig. 2C), we next examined whether they might retain progenitor properties throughout life. Tamoxifen induction at E12.5 or E14.5 revealed GFP-labeled cells within the capsule (fig. 4D, E18.5), as well as a small number of GFP patches within the cortex at E18.5 (fig. 4F, S4B-D). The number (fig. S4G) and size of cortical patches of GFP+ cells significantly increased over time with some of the wedge-shaped patches spanning the entire adrenal cortex (fig. 4C, F). Interestingly, a small proportion of cells, generally located at the tips of the wedges, lost WT1 and acquired steroidogenic features, as evidenced by the expression of high levels of SF1, AKR1b7 and 3βHSD2 (fig. 4C, E, F).
To further investigate the role of WT1+ cells in adrenal homeostasis, we performed time course experiments in adult animals (tamoxifen induction at 3 or 10-12 weeks of age; fig. 4A). GFP+ cells were detected in the sub-capsular region as early as 3 days after tamoxifen induction. Over time, patches significantly increased in size (fig. 5A to C) and number (fig. 5E, F), although they remained a small proportion of the adrenal cortex in all samples analysed. Staining for the endothelial marker PECAM did not reveal an overlap with GFP positive cells (fig. S5C and D), suggesting that WT1+ progenitors do not give rise to endothelial cells within the adrenal. Instead we observed a small proportion of GFP+ cells that lost WT1 expression and acquired the morphology of differentiated steroidogenic cells and strong SF1 staining (fig. 5B, C, arrowheads).
Fig. 5. WT1+ cells maintain the ability to generate subcapsular patches and to differentiate into steroidogenic cells throughout life.
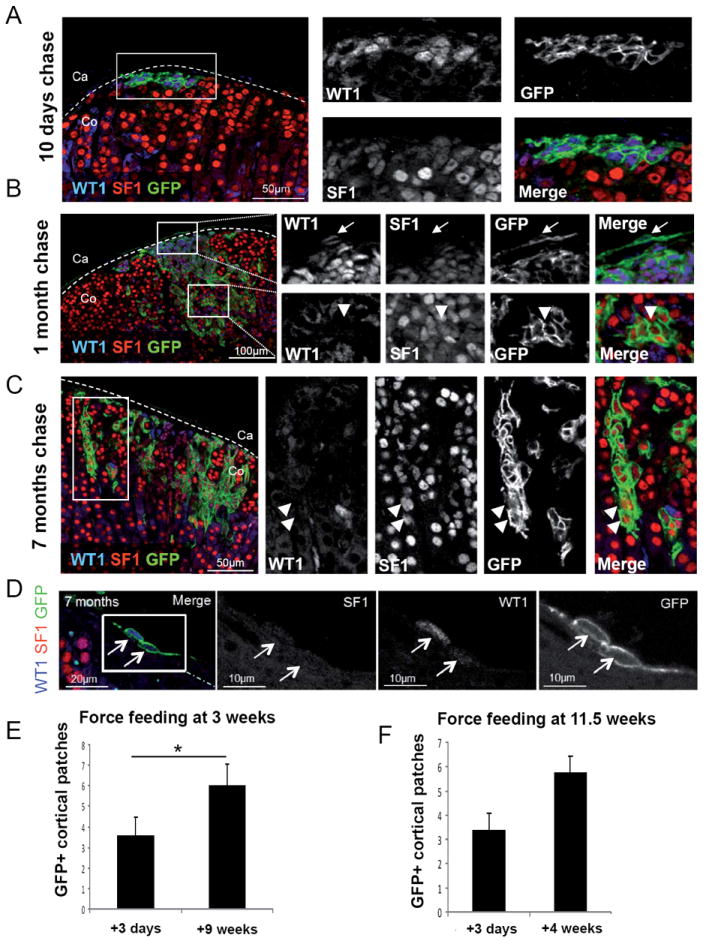
(A-D) Immunostaining against WT1 (blue), SF1 (red) and GFP (green) on adrenals from Wt1:Cre-ERT2; mTmG mice treated with 4 consecutive doses of tamoxifen between 10 and 12 weeks of age and collected after 10 days (A), 1 month (B) and 7 months (C and D) after the last administration. Almost all GFP+ cells also express WT1 and SF1 (A-C). GFP+ SF1+ WT1- cells can be detected in a subset of patches (B-C). GFP+ WT1+ SF1- capsular cells can be detected within the adrenal capsule up to 7 month after force-feeding (D). (E-F) Quantification of the number of GFP+ patches within the adrenal cortex of Wt1:Cre-ERT2; mTmG mice treated with tamoxifen at 3 weeks (E) or 11.5 weeks (F) of age. The number of GFP+ clusters increases significantly from 3.56 ± 2.70 (N=9) to 6.00 ± 2.35 (N=9), 3 days and 9 weeks after force-feeding at 3 weeks of age, respectively. No significant increase in the number of GFP+ clusters is detected in animals force-fed at 11.5 weeks, from 3.38 ± 2.00 (N=8) to 5.75 ± 1.98 (N=8). Co, adrenal cortex; Ca, adrenal capsule. See also Fig. S5.
Thus WT1+ cells in adult adrenals represent a progenitor cell population able to differentiate into adrenocortical steroidogenic cells throughout life. Interestingly, GFP positive cells that retained WT1 expression could be identified within the adrenal capsule as late as 7 months after tamoxifen injection (fig. 5D) suggesting that they represent a long-lived cell population.
WT1+ cells generate gonadal-like cells within the adrenal cortex upon gonadectomy
WT1+ adrenocortical and AGP cells appear to share a significant number of features and we wondered whether they might have retained gonadal potential in the adrenal throughout life. Gonadectomy has been shown to increase the appearance of subcapsular spindle-shaped cells (called ‘A cells’) and to promote their differentiation into gonadal-like cells (called ‘B’ cells) capable of sex hormone synthesis (Bielinska et al., 2003; Woolley et al., 1952, 1953), as a consequence of the increase in gonadotropins (Bielinska et al., 2005; Kero et al., 2000).
To address whether WT1+ cells can differentiate into gonadal steroidogenic cells, we subjected adult male and female mice (Wt1-CreERT2; mTmG) to gonadectomy shortly after mTmG activation (fig. 6A). Strikingly, 10 weeks after gonadectomy we observed a dramatic expansion of GFP+ cells invading the adrenal cortex (fig. 6B). Quantification also showed a rapid increase in the number of GFP+ patches, as early as 7 days after gonadectomy in males (fig. S6B-D). In non-gonadectomised mice, the majority of GFP+ cells are incapable of steroidogenesis (fig. 4 and 5), as evidenced by the absence of the steroidogenic enzymes p450scc and 21-hydroxylase (fig.S5A, B). In contrast, the majority of GFP+ cells in gonadectomized mice had lost WT1 expression and showed high levels of SF1 (fig. 6C) and GATA4 (fig. S6A). Moreover, GFP+ cells expressed the general (both adrenal and gonad) steroidogenic marker p450scc (fig. 6D) indicating that a massive process of differentiation had taken place. Interestingly, the same cells lacked the adrenocortical marker 21-hydroxylase (fig. 6D), but expressed the gonadal specific genes CYP17 and Lhr (fig. 6E, F, G). Importantly, no CYP17 and Lhr staining was found in sections from non-gonadectomized control animals (fig. 6E, F) indicating that gonadectomy oriented these GFP+ cells towards a gonadal steroidogenic fate.
Fig. 6. WT1+ cells generate cells with gonadal characteristics in the adrenal cortex upon gonadectomy.
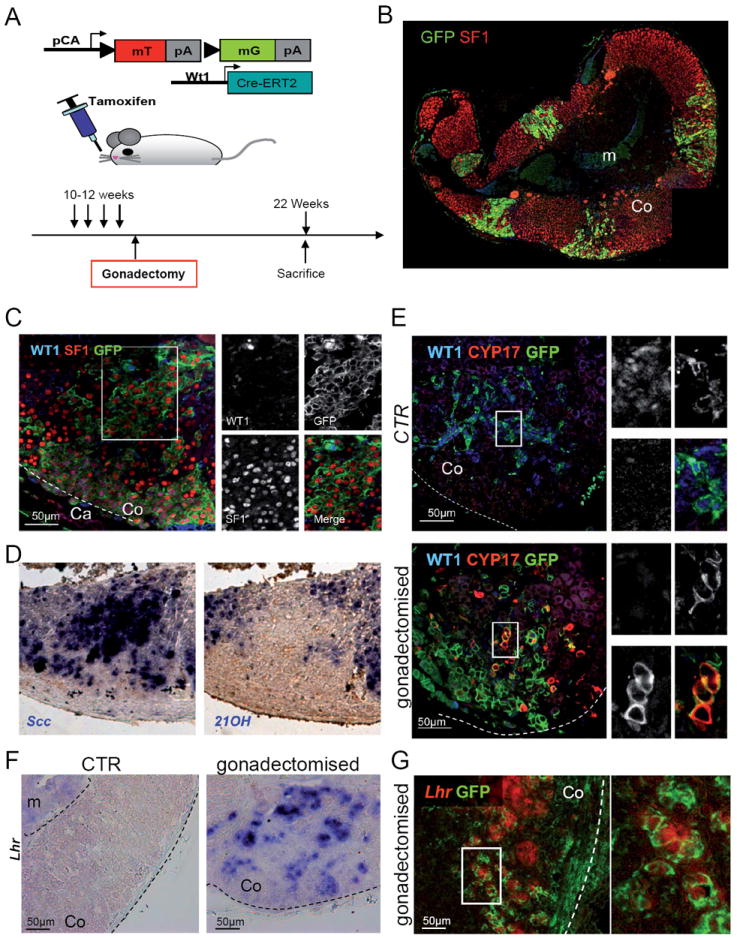
(A) Schematic representation of the experimental design. (B-E) Adrenal glands from mice sacrificed 10 weeks after gonadectomy. Gonadectomy induces massive invasion of the adrenal cortex by GFP+ cells, derived from WT1+ cells (B). Higher magnification shows that the majority of GFP+ cells have lost WT1 expression, whereas SF1 is present (C). (D) RNA in situ hybridization shows that cells expressing cytochrome P450scc, do not express the adrenocortical specific marker 21-hydroxylase. Please note that the three stainings in panels C to D were performed on consecutive sections, thus representing the same area. (E) CYP17 (red), a marker of cells producing sex hormones (gonadal cells) appears in some cells within the adrenal cortex of gonadectomised mice, but is never found in control animals. Coexpression of CYP17 and GFP in the same cells indicates that they are directly derived from WT1 expressing cells. (F-G) LH receptor RNA in situ hybridization shows expression of this gene in gonadectomised animals (F), where it colocalises with GFP+ cells (G). Co, adrenal cortex; Ca, adrenal capsule; m, adrenal medulla. See also Fig. S6.
Thus, WT1+ cells are able to respond to hormonal changes following gonadectomy by differentiating into sex hormone producing ‘B’ cells (Bielinska et al., 2005; Kero et al., 2000). More generally, our results suggest that WT1+ cells represent a progenitor population that maintains AGP-like features throughout life and as such have the ability to differentiate into steroidogenic cells of either adrenal or gonadal lineages.
DISCUSSION
High levels of WT1 expression are incompatible with differentiation into steroidogenic cells
The central role of WT1 in adreno-gonadal development has been established by a series of loss-of-function studies and is well documented in the literature (Kreidberg et al., 1993; Moore et al., 1999). Prior to activation of SF1 (E9.5), early AGP precursors express both WT1 and GATA4 (fig. 1A). WT1 plays a major role in this tissue by directly regulating expression of the steroidogenic factor Sf1 (Wilhelm and Englert, 2002) probably through association with CITED2 (Val et al., 2007). After separation, and upon activation of Sf1, adrenocortical cells lose WT1 before they differentiate into true steroidogenic cells.
Our gain-of-function analysis lends further support to the hypothesis that WT1 needs to be repressed for steroidogenesis to occur. Indeed, ectopic expression of high levels of WT1 effectively blocked the differentiation process. Interestingly, while all adrenal progenitor cells in −KTSGOF mice express WT1, two different types of cells that differ in WT1 expression levels could be distinguished. Although the molecular reason for this differential expression is presently unclear and may involve stochastic events, the outcome is highly instructive: while WT1low cells develop adrenocortical features including high levels of SF1 and fully differentiate into steroidogenic cells, WT1 expression in WT1high cells has crossed a certain threshold and as a consequence these cells express GATA4, Gli1 and Tcf21, have low levels of SF1 and fail to differentiate. This results in a composite organ containing adrenal steroidogenic cells and undifferentiated adreno-gonadal precursors.
On the molecular level our data reveal a direct regulation of Gli1 and Tcf21 by WT1, two genes that are upregulated in AGP-like cells in -KTSGOF animals. The finding that TCF21 can bind and inhibit the SF1 promoter (Franca et al., 2013; Tamura et al., 2001) provides a molecular explanation for the observed SF1 repression, although we cannot exclude that additional, Tcf21-independent mechanisms may also contribute to the observed phenotype. Consequently WT1 appears to have a dual function in the development of the adrenal cortex. Firstly, it is required for the activation of Sf1 thus determining the steroidogenic primordium. At the same time it limits the amount of Sf1 expression via the activation of Tcf21, thus effectively inhibiting full-blown differentiation towards the steroidogenic lineage (fig. 7). This second process probably occurs in a population of cells, which will give rise to the adrenal capsule. Thus WT1 acts as a molecular switch between steroidogenic or capsular identity playing a major role in regulating organ homeostasis.
Fig. 7. Schematic representation of the fate of WT1+ progenitors during development and adulthood.
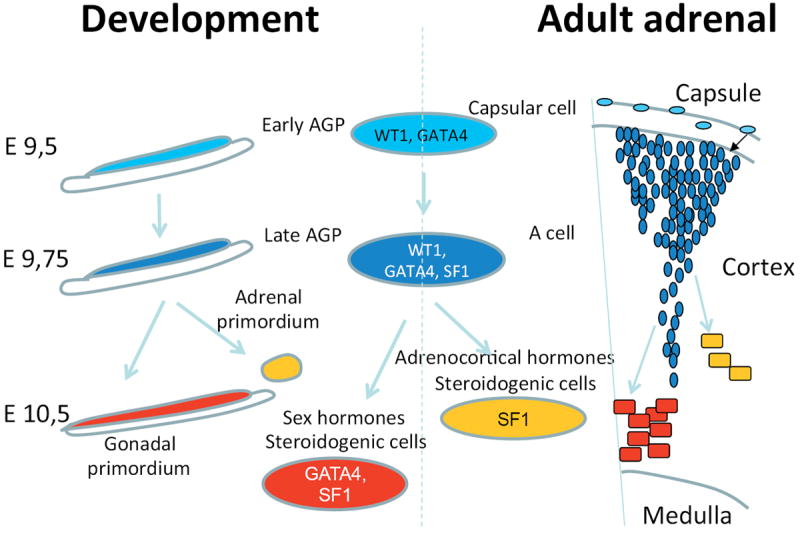
Our data expose clear parallels between mechanisms that drive adrenogonadal development and the differentiation of AGP-like cells in the adult adrenal cortex. Progenitor cells are characterized by WT1 and GATA4 expression. WT1 prevents differentiation by regulating expression of key genes such as GLI1 and TCF21. Suppression of WT1 is thus a key step to allow steroidogenic differentiation both during development and or adult AGP-like cells. Upon gonadectomy AGP-like cells respond by differentiating into gonadal steroidogenic cells.
In gonads, co-expression of WT1 and intermediate levels of SF1 persist within Sertoli cells until later stages, where they contribute to the expression of the non-steroidogenic hormone AMH (Nachtigal et al., 1998). Remarkably, also in gonads steroidogenesis is restricted to cells that lose WT1 expression and show high levels of SF1: the Leydig cells in testes and Theca cells in ovaries (Morohashi and Omura, 1996). We therefore hypothesize that high levels of WT1 are more generally incompatible with the steroidogenic program. Consistent with this notion, induced deletion of Wt1 in Sertoli cells leads to an increase in the number of steroidogenic (Leydig) cells (Gao et al., 2006), possibly by trans-differentiation. -KTSGOF mice do not show a gonadal phenotype, but the levels of WT1 expression from the Rosa26 promoter appear to be extremely low. Further studies with stronger promoters would be needed to evaluate the effect of WT1 overexpression in gonadal steroidogenic cells.
WT1 marks a progenitor cell population within the adrenal gland
After separation from the AGP, adrenal development proceeds through a series of well-defined steps. 1) Invasion of the adrenal primordium by chromaffin cells that will eventually form the adrenal medulla (Anderson and Axel, 1986); 2) Encapsulation of the adrenal primordium (Mesiano and Jaffe, 1997; Uotila, 1940); 3) Development of the definitive cortex (Zubair et al., 2008). Our cell lineage tracing experiments provide the first direct evidence for a mesenchymal origin of the adrenal capsule. In Wt1:Cre-ERT2; mTmG embryos that were induced at E11.5 and E12.5 - a time point when WT1 is absent from the adrenal cortex, but expressed within mesenchymal cells surrounding the forming adrenal – the majority of capsular cells were found to be GFP positive. These data indicate that a significant proportion of cells of the adrenal capsule are derived from condensing WT1+ mesenchymal precursors.
The lineage tracing experiments carried out in this study also allows us to draw important conclusions regarding the formation of the steroidogenic lineage. Using the constitutive Wt1:Cre line, all steroidogenic cells were GFP positive indicating that an early progenitor population - the AGP - must have expressed WT1. In contrast, activation of WT1:Cre-ERT2 at later stages (E11.5 and onwards) did not result in a significant number of labeled steroidogenic cells suggesting that these cells are derived from a population of progenitors that has lost WT1 expression. The recently identified GLI1+ cell population (King et al., 2009, Huang et al., 2010) is likely to fulfill this role. Our immunofluorescent analysis identified four cell populations defined by the expression pattern of WT1 and GLI1 in the developing adrenal (fig. S4E and F): WT1+ GLI1+ capsular, WT1+ GLI1- capsular, WT1+ GLI1- cortical and WT1- GLI1+ capsular cells. We thus propose that this last population constitute the main steroidogenic progenitors that give rise to the definitive cortex.
While not the major source of steroidogenic cells during development, WT1+ adrenal cells maintain the ability to differentiate into steroidogenic cells throughout life. Interestingly, the number of cortical GFP+ patches in Wt1:Cre-ERT2; mTmG mice increases over time. Whereas early on patches are probably generated by expansion of WT1+ cells located within the cortex at the time of activation, in adult life other progenitors must be responsible for their generation. Three lines of evidence suggest the adrenal capsule as a source of these cells. 1) The adrenal capsule forms a rich reservoir of WT1+ cells. 2) Capsular cells share many of the properties of ‘A’ cells including the expression of WT1, GATA4, Tcf21 and Gli1. 3) GFP+/WT1+ patches are always found in the subcapsular area and are in direct contact with the capsule.
Invading cells maintain AGP-like properties throughout life
Invasion of the subcapsular region by spindle shaped cells (called ‘A’ cells) is a frequent event in aged mice (Woolley et al., 1952, 1953). Our data identify WT1+ progenitors that are probably situated within the capsule, as the source of these cells. Interestingly, AGP-like cells have the ability not only to differentiate into adrenocortical, but also into gonadal steroidogenic lineages upon gonadectomy. As such they share features of adreno-gonadal progenitors. Indeed, their molecular signature appears to largely overlap with that of AGP cells as they express WT1, GATA4 and SF1.
The process of differentiation of capsular cells into adrenocortical or gonadal-like cells appears to recapitulate normal development (fig. 7). WT1+ capsular cells co-express GATA4, both of which are also found in pre-AGP cells (fig. 1A, E9.5). During the process of differentiation into ‘A’ cells, low levels of SF1 expression, a specific marker of the AGP, are acquired (fig. 1A, E9.75) (Hatano et al., 1996). The final step of differentiation involves loss of WT1 and an increase of SF1 expression that in turn activates the steroidogenic program.
‘A’ cells are generated in control and gonadectomized mice. In control mice, ‘A’ cells remain quiescent and only very few cells terminally differentiate into adrenocortical cells and express the steroidogenic markers SF1, AKR1b7 and 3 beta HSD. By contrast, gonadectomy induces proliferation and differentiation of ‘A’ cells towards the steroidogenic gonadal lineage, as demonstrated by expression of CYP17 and the LH receptor. Differentiation is likely to be a direct response to the increased levels of luteinizing hormone (LH) that are induced after removal of the gonads (Bielinska et al., 2005; Bielinska et al., 2003; Johnsen et al., 2006; Kero et al., 2000). The increased production of ‘A’ cells and their differentiation into gonadal cells can therefore be interpreted as an attempt of the mammalian body to balance hormone homeostasis.
Interestingly, a similar mechanism may be at work in gonads of children that suffer from congenital adrenal hyperplasia. Lack of glucocorticoid production in these patients disrupts the negative feedback regulation of ACTH secretion. The resulting high levels of ACTH induce so-called adrenal rests within gonadal tissue that have features of adrenal steroidogenic cells (Cabrera et al., 2001; Stikkelbroeck et al., 2001). It is tempting to speculate that AGP-like cells also persist in gonads that, upon hormonal challenge, can be activated to differentiate into adrenal steroidogenic precursors. Alternatively these cells are derived from developmental progenitors as suggested by (Val et al., 2006).
Based on the above findings, we propose that WT1+ capsular cells in the adrenal gland might represent a reserve stem/progenitor cell population with adrenal progenitor features that is mobilized only after severe loss of steroidogenic cells has occurred. The fact that the mammalian body responds by inducing differentiation of WT1+ progenitors into gonadal cells at a heterotopic site (the adrenal) is an impressive feat that exemplifies the remarkable adaptation of the mammalian body to stress.
In conclusion we identified WT1, and more specifically its −KTS isoforms, as an essential player in maintaining AGP cells in the undifferentiated state. Although vital for adrenal and gonadal development, its repression is a key step for differentiation of adrenocortical steroidogenic cells. As a further demonstration of this, undifferentiated AGP-like cells that maintain WT1 are found in the adrenal cortex throughout life, where they maintain the ability to generate both steroidogenic cell types.
EXPRIMENTAL PROCEDURE
Generation of the Rosa26:Wt1+ and −KTS mice
Rosa26:Wt1-KTS knock-in mice were generated by homologous recombination in ES cells as previously described (Hammes et al., 2001). The cDNA encoding WT1–KTS was cloned into pENTR11 (Invitrogen) and introduced into the Rosa26-DEST vector (Hohenstein et al., 2008), by in vitro recombination using the enzyme LR clonase II® (Gateway® technology, Invitrogen). The generated targeting vector was linearized with KpnI and electroporated into 129-derived murine ES cells. G-418 resistant clones were then screened by Southern blot using a 5’ end probe and double-checked with a neomycin probe.
Three positive clones for the construct were microinjected into C57BL6 blastocysts. Chimeric male founders were crossed with C57BL6 females to achieve germ-line transmission of the targeted allele.
Mice
All animal work was conducted according to national and international guidelines. The Sf1:Cre; mTmG, Wt1:Cre-ERT2 and Gli1:LacZ mouse lines were previously described (Bingham et al., 2006; Muzumdar et al., 2007; Zhou et al., 2008). Cell lineage tracing experiments were carried out by force-feeding the mice with 4mg of tamoxifen (Sigma-Aldrich) dissolved in corn oil (Sigma-Aldrich), per 20g of body weight. Cre activation during development was obtained by a single tamoxifen administration of pregnant female carrying either E 12.5 or E 14.5 embryos. Cre activation in adult mice was obtained by administrating tamoxifen twice at 3 weeks of age or twice a week for two weeks starting at 10 weeks of age. For quantification of the number of GFP+ patches, A single administration of tamoxifen was given to 11.5 weeks old animals.
Immunofluorescence and histological analysis
For immunofluorescence experiments, tissues were fixed overnight in 4% paraformaldehyde, progressively dehydrated, and paraffin embedded. 7 μm thick sections were rehydrated, boiled in a pressure cooker for 2 minutes with Antigen Unmasking Solution (Vector laboratories), and blocked in a PBS solution containing 10% normal donkey serum (Jackson immunoresearch) and 3% BSA. All primary antibodies were applied overnight at 4°C at the concentration listed in supplementary information. Secondary antibodies were diluted 1:200 and applied at room temperature for 1 hour. WT1 was detected using a biotinylated secondary antibody, followed by streptavidin-Cy3 conjugated (Sigma-Aldrich).
GFP immunohistochemistry after RNA in situ hybridization was performed without antigen retrieval. The primary GFP antibody was detected with a POD-conjugated secondary antibody antibody. DAB staining was performed using the Vector DAB substrate kit for peroxidise (cat. n. SK 4100) following manufacturer instructions.
For histological analysis, adrenal glands from embryos or adult mice were fixed overnight in Bouin’s solution (Sigma Aldrich) or 4% paraformaldehyde, were progressively dehydrated, and were embedded in paraffin embedding media. 7-μm-thick sections were then stained with haematoxylin and eosin.
RNA in situ hybridization
Tissues were fixed overnight in 4% paraformaldehyde, progressively dehydrated, and paraffin embedded. Seven-micrometer-thick sections were hybridized with Tcf21, Gli1, Ptch1, Shh, P450scc, 21-hydroxylase or LH receptor probes (details on request) according to previously described protocols (Comai et al., 2010). The Tcf21 probe was PCR amplified from embryonic kidney cDNA using the primers Tcf21 S and Tcf21 AS. The PCR fragment was cloned into the pCRII vector (Invitrogen). The Lhr probe was PCR amplified from adult mouse cDNA ovary using the primers LUPub Fr and LUPub Rev. pCR2.1 containing the Gli1 in situ probe was kindly provided by M. Studer (INSERM U636, Université de Nice-Sophia Antipolis).
RT-Q-PCR
RNA was extracted from E14.5 or adult adrenal glands using TRIzol® reagent (Invitrogen), following manufacture’s instructions. Reverse transcription was performed using M-MLV reverse transcriptase (Invitrogen) in combination with random primers. The cDNA obtained was then used as a template for quantitative PCR carried out using the TaqMan® Master Kit (Roche) and a Light Cycler 1.5® (Roche). Expression levels were normalized for Hprt1. Primers (see primers table) were designed on Roche Universal ProbeLibrary® website. For a complete list of primers see supplementary information.
Chromatin immunoprecipitation
Chromatin immunoprecipitation assays were conducted as described in Weinmann and Farnham 2002, with minor modifications. Adrenal glands from Rosa26Wt1-KTS/Wt1-KTS; Sf1:CRE adult mice were dissected and dissociated in 20 mM HEPES pH7.4, 1 mM EDTA, 150 mM NaCl, 1% SDS, 0.125 M Glycine, 0.5 mg/ml PMSF and nuclei were released by mechanical stress. Chromatin from nuclei was sheared by sonication to an average of 400-600bp in 20 mM HEPES pH 7.4, 1 mM EDTA, 150 mM NaCl, 0.4%SDS, 0.5 mg/ml PMSF. 15 μg of antibody directed against WT1 (C19, Santa Cruz) or Dicer (Santa Cruz) (used as a control) was incubated with chromatin in 20 mM HEPES pH7.4, 1 mM EDTA, 150 mM NaCl, 0.8% TritonX100, 0.1%SDS, 0.5 mg/ml PMSF. Controls included a mock reaction consisting of a chromatin sample in buffer and with no antibody. Protein A sepharose (Sigma) was used to precipitate chromatin IgG complexes. Protein A was washed in series; three times with 20 mM HEPES pH7.4, 1 mM EDTA, 500 mM NaCl, 0.8% TritonX100, 0.1% SDS, 0.5 mg/ml PMSF; three times with 20 mM Tris pH8.0, 1 mM EDTA, 250 mM LiCl, 0.5% NP40, 0.5% Deoxycholate, 0.5 mg/ml PMSF; and twice in 20 mM Tris pH 8.0, 1 mM EDTA 0.5 mg/ml PMSF. Chromatin was eluted with 50 mM NaHCO3, 1%SDS. Protein-DNA cross-links in chromatin were reversed by heating at 65 °C for 6 h, followed by phenol chloroform extraction and ethanol precipitation. DNA was resuspended in 20μl of water, and 0.5μl was used in each PCR reaction.
Hormone measurements
Hormone measurements were performed as previously described (Sahut-Barnola et al., 2010)
Cell size measurement
Cell size in wild type and −KTSGOF adrenals was measured using the program ‘cell profiler’ (BROAD institute).
Supplementary Material
Acknowledgments
We would like to thank the staff of the animal facility for their outstanding work and dedication; Alan J. Conley (University of California), Michael Thomas (Grenoble), Ken Morohashi (Japan) and Yacob Weinstein for the gift of the Cyp17, 3βHSD, SF1 and 20-αHSD antibodies respectively. We are grateful to Marie-Christine Chaboissier and Pierre Val for continuous scientific input. This work was supported by grants from ARC (Association pour la Recherche sur les Cancers), ANR (ADSTEM) and the FRM (Fondation de la Recherche Médicale) to A.S.; R.B. was supported by a fellowship from the Boehringer Ingelheim Foundation and ARC. A.M. was supported by the ANR (ANR08-GENOPAT-002). P.H. was supported by the Association for International Cancer Research (AICR) and the Medical Research Council (MRC).
Footnotes
Author contributions
R.B. and A.S. designed the project. R.B. carried out all experiments, if not otherwise stated. V.V. contributed to immune-histological experiments and RNA in situ hybridization. F.R. and R.B. generated the Rosa26:Wt1-KTS Rosa26:Wt1+KTS strains. V.V analysed the gonadal phenotype in −KTSGOF mice. F.R. performed gonadectomies. M.C. performed ChIP experiments. I.S-B. performed hormone dosage on WT1GOF mice. P.H. provided the backbone for the construction of the targeting construct. W.T.P. provided the WT1-Cre and WT1-CreERT2 strains. A.M. provided important intellectual input and helped analyze the gain-of-function phenotype. R.B. and A.S. wrote the manuscript, and all authors provided editorial input.
References
- Anderson DJ, Axel R. A bipotential neuroendocrine precursor whose choice of cell fate is determined by NGF and glucocorticoids. Cell. 1986;47:1079–1090. doi: 10.1016/0092-8674(86)90823-8. [DOI] [PubMed] [Google Scholar]
- Bickmore WA, Oghene K, Little MH, Seawright A, van Heyningen V, Hastie ND. Modulation of DNA binding specificity by alternative splicing of the Wilms tumor wt1 gene transcript. Science. 1992;257:235–237. doi: 10.1126/science.1321494. [DOI] [PubMed] [Google Scholar]
- Bielinska M, Genova E, Boime I, Parviainen H, Kiiveri S, Leppaluoto J, Rahman N, Heikinheimo M, Wilson DB. Gonadotropin-induced adrenocortical neoplasia in NU/J nude mice. Endocrinology. 2005;146:3975–3984. doi: 10.1210/en.2004-1643. [DOI] [PubMed] [Google Scholar]
- Bielinska M, Parviainen H, Porter-Tinge SB, Kiiveri S, Genova E, Rahman N, Huhtaniemi IT, Muglia LJ, Heikinheimo M, Wilson DB. Mouse strain susceptibility to gonadectomy-induced adrenocortical tumor formation correlates with the expression of GATA-4 and luteinizing hormone receptor. Endocrinology. 2003;144:4123–4133. doi: 10.1210/en.2003-0126. [DOI] [PubMed] [Google Scholar]
- Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- Cabrera MS, Vogiatzi MG, New MI. Long term outcome in adult males with classic congenital adrenal hyperplasia. The Journal of clinical endocrinology and metabolism. 2001;86:3070–3078. doi: 10.1210/jcem.86.7.7668. [DOI] [PubMed] [Google Scholar]
- Caricasole A, Duarte A, Larsson SH, Hastie ND, Little M, Holmes G, Todorov I, Ward A. RNA binding by the Wilms tumor suppressor zinc finger proteins. Proc Natl Acad Sci U S A. 1996;93:7562–7566. doi: 10.1073/pnas.93.15.7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau YY, Brownstein D, Mjoseng H, Lee WC, Buza-Vidas N, Nerlov C, Jacobsen SE, Perry P, Berry R, Thornburn A, et al. Acute multiple organ failure in adult mice deleted for the developmental regulator wt1. PLoS Genet. 2011;7:e1002404. doi: 10.1371/journal.pgen.1002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai G, Boutet A, Neirijnck Y, Schedl A. Expression patterns of the Wtx/Amer gene family during mouse embryonic development. Dev Dyn. 2010;239:1867–1878. doi: 10.1002/dvdy.22313. [DOI] [PubMed] [Google Scholar]
- Franca MM, Ferraz-de-Souza B, Santos MG, Lerario AM, Fragoso MC, Latronico AC, Kuick RD, Hammer GD, Lotfi CF. POD-1 binding to the E-box sequence inhibits SF-1 and StAR expression in human adrenocortical tumor cells. Mol Cell Endocrinol. 2013 doi: 10.1016/j.mce.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Maiti S, Alam N, Zhang Z, Deng JM, Behringer RR, Lecureuil C, Guillou F, Huff V. The Wilms tumor gene, Wt1, is required for Sox9 expression and maintenance of tubular architecture in the developing testis. Proc Natl Acad Sci U S A. 2006;103:11987–11992. doi: 10.1073/pnas.0600994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes A, Guo JK, Lutsch G, Leheste JR, Landrock D, Ziegler U, Gubler MC, Schedl A. Two splice variants of the Wilms’ tumor 1 gene have distinct functions during sex determination and nephron formation. Cell. 2001;106:319–329. doi: 10.1016/s0092-8674(01)00453-6. [DOI] [PubMed] [Google Scholar]
- Hatano O, Takakusu A, Nomura M, Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells. 1996;1:663–671. doi: 10.1046/j.1365-2443.1996.00254.x. [DOI] [PubMed] [Google Scholar]
- Hohenstein P, Hastie ND. The many facets of the Wilms’ tumour gene, WT1. Hum Mol Genet. 2006;15(Spec No 2):R196–201. doi: 10.1093/hmg/ddl196. [DOI] [PubMed] [Google Scholar]
- Hohenstein P, Slight J, Ozdemir DD, Burn SF, Berry R, Hastie ND. High-efficiency Rosa26 knock-in vector construction for Cre-regulated overexpression and RNAi. Pathogenetics. 2008;1:3. doi: 10.1186/1755-8417-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol. 1994;8:654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- Johnsen IK, Slawik M, Shapiro I, Hartmann MF, Wudy SA, Looyenga BD, Hammer GD, Reincke M, Beuschlein F. Gonadectomy in mice of the inbred strain CE/J induces proliferation of sub-capsular adrenal cells expressing gonadal marker genes. J Endocrinol. 2006;190:47–57. doi: 10.1677/joe.1.06750. [DOI] [PubMed] [Google Scholar]
- Kennedy D, Ramsdale T, Mattick J, Little M. An RNA recognition motif in Wilms’ tumour protein (WT1) revealed by structural modelling. Nat Genet. 1996;12:329–331. doi: 10.1038/ng0396-329. [DOI] [PubMed] [Google Scholar]
- Kero J, Poutanen M, Zhang FP, Rahman N, McNicol AM, Nilson JH, Keri RA, Huhtaniemi IT. Elevated luteinizing hormone induces expression of its receptor and promotes steroidogenesis in the adrenal cortex. The Journal of clinical investigation. 2000;105:633–641. doi: 10.1172/JCI7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci U S A. 2009;106:21185–21190. doi: 10.1073/pnas.0909471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Larsson SH, Charlieu JP, Miyagawa K, Engelkamp D, Rassoulzadegan M, Ross A, Cuzin F, van Heyningen V, Hastie ND. Subnuclear localization of WT1 in splicing or transcription factor domains is regulated by alternative splicing. Cell. 1995;81:391–401. doi: 10.1016/0092-8674(95)90392-5. [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocrine reviews. 1997;18:378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development. 1999;126:1845–1857. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]
- Moore AW, Schedl A, McInnes L, Doyle M, Hecksher-Sorensen J, Hastie ND. YAC transgenic analysis reveals Wilms’ tumour 1 gene activity in the proliferating coelomic epithelium, developing diaphragm and limb. Mech Dev. 1998;79:169–184. doi: 10.1016/s0925-4773(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Morohashi KI, Omura T. Ad4BP/SF-1, a transcription factor essential for the transcription of steroidogenic cytochrome P450 genes and for the establishment of the reproductive function. FASEB J. 1996;10:1569–1577. doi: 10.1096/fasebj.10.14.9002548. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Nachtigal MW, Hirokawa Y, Enyeart VanHouten DL, Flanagan JN, Hammer GD, Ingraham HA. Wilms’ tumor 1 and Dax-1 modulate the orphan nuclear receptor SF- 1 in sex-specific gene expression. Cell. 1998;93:445–454. doi: 10.1016/s0092-8674(00)81172-1. [DOI] [PubMed] [Google Scholar]
- Niksic M, Slight J, Sanford JR, Caceres JF, Hastie ND. The Wilms’ tumour protein (WT1) shuttles between nucleus and cytoplasm and is present in functional polysomes. Hum Mol Genet. 2004;13:463–471. doi: 10.1093/hmg/ddh040. [DOI] [PubMed] [Google Scholar]
- Sahut-Barnola I, de Joussineau C, Val P, Lambert-Langlais S, Damon C, Lefrancois-Martinez AM, Pointud JC, Marceau G, Sapin V, Tissier F, et al. Cushing’s syndrome and fetal features resurgence in adrenal cortex-specific Prkar1a knockout mice. PLoS Genet. 2010;6:e1000980. doi: 10.1371/journal.pgen.1000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stikkelbroeck NM, Otten BJ, Pasic A, Jager GJ, Sweep CG, Noordam K, Hermus AR. High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. The Journal of clinical endocrinology and metabolism. 2001;86:5721–5728. doi: 10.1210/jcem.86.12.8090. [DOI] [PubMed] [Google Scholar]
- Tamura M, Kanno Y, Chuma S, Saito T, Nakatsuji N. Pod-1/Capsulin shows a sex- and stage-dependent expression pattern in the mouse gonad development and represses expression of Ad4BP/SF-1. Mech Dev. 2001;102:135–144. doi: 10.1016/s0925-4773(01)00298-2. [DOI] [PubMed] [Google Scholar]
- Uotila UU. The early embryological development of the fetal and permanent adrenal cortex in man. The Anatomical Record. 1940;76:183–203. [Google Scholar]
- Val P, Jeays-Ward K, Swain A. Identification of a novel population of adrenal-like cells in the mammalian testis. Dev Biol. 2006;299:250–256. doi: 10.1016/j.ydbio.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Val P, Martinez-Barbera JP, Swain A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development. 2007;134:2349–2358. doi: 10.1242/dev.004390. [DOI] [PubMed] [Google Scholar]
- Vidal V, Schedl A. Requirement of WT1 for gonad and adrenal development: insights from transgenic animals. Endocrine research. 2000;26:1075–1082. doi: 10.3109/07435800009048640. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Englert C. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev. 2002;16:1839–1851. doi: 10.1101/gad.220102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley GW, Dickie MM, Little CC. Adrenal tumors and other pathological changes in reciprocal crosses in mice. I. Strain DBA × strain CE and the reciprocal. Cancer Res. 1952;12:142–152. [PubMed] [Google Scholar]
- Woolley GW, Dickie MM, Little CC. Adrenal tumors and other pathological changes in reciprocal crosses in mice. II. An introduction to results of four reciprocal crosses. Cancer Res. 1953;13:231–245. [PubMed] [Google Scholar]
- Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair M, Parker KL, Morohashi K. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Molecular and cellular biology. 2008;28:7030–7040. doi: 10.1128/MCB.00900-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


