Abstract
Knee soft tissue structures are frequently injured, leading to the development of osteoarthritis even with treatment. Understanding how these structures contribute to knee function during activities of daily living (ADLs) is crucial in creating more effective treatments. This study was designed to determine the role of different knee structures during a simulated ADL in both human knees and ovine stifle joints. A six degree-of-freedom robot was used to reproduce each species’ in vivo gait while measuring three-dimensional joint forces and torques. Using a semi-randomized selective cutting method, we determined the primary and secondary structures contributing to the forces and torques along and about each anatomical axis. In both species, the bony interaction, ACL, and medial meniscus provided most of the force contributions during stance, whereas the ovine MCL, human bone, and ACLs of both species were the key contributors during swing. This study contributes to our overarching goal of establishing functional tissue engineering parameters for knee structures by further validating biomechanical similarities between the ovine model and the human to provide a platform for measuring biomechanics during an in vivo ADL. These parameters will be used to develop more effective treatments for knee injuries to reduce or eliminate the incidence of osteoarthritis.
Keywords: Knee Kinetics, Anterior Cruciate Ligament, Meniscus, Activities of Daily Living, Ovine
1. Introduction
Current treatments for the frequently injured soft tissue structures in the knee do not fully restore normal biomechanical properties, which contribute to long term joint degeneration. After surgical treatment and rehabilitation for severe knee injuries (e.g. ACL and meniscus tears), patients still experience the early onset of osteoarthritis (Jomha, Borton et al. 1999; von Porat, Roos et al. 2004). One factor contributing to joint degeneration is the surgeon’s inability to restore the native knee kinematics (Andriacchi, Koo et al. 2009). This clinical problem stems from our limited understanding of normal knee biomechanics during actual activities of daily living (ADLs). Thus, surgeons must repair soft tissue structures with little knowledge of the function of these structures during ADLs.
Though investigators have provided important information about knee kinematics during ADLs (Lafortune, Cavanagh et al. 1992; Andriacchi and Dyrby 2005), our current understanding of knee loading is usually limited to non-physiologic motions such as simulated clinical examinations (e.g. pivot shift test). Historically, the restraining roles of knee structures have primarily been determined from laxity tests (Grood, Noyes et al. 1981; Sakane, Livesay et al. 1999; Kanamori, Woo et al. 2000; Robinson, Bull et al. 2006; Markolf, Park et al. 2008). Although investigators have also attempted to use strain gauges and force probes to estimate in vivo tissue forces (Henning, Lynch et al. 1985; Holden, Grood et al. 1994; Roberts, Cummings et al. 1994), the inability to isolate structures for sensor calibration has prevented researchers from accurately measuring soft tissue forces in humans. Recently, Gill et al. (2011) estimated changes in human anterior cruciate ligament (ACL) forces by recording in vivo ACL elongation measurements during a single leg lunge activity at various flexion angles. To determine the in vivo ACL force, the elongation data was matched to force-elongation curves acquired from uniaxial tensile tests performed on cadaveric human limbs at various flexion angles. While this study increases our understanding of knee ligament forces for more complex activities, the investigators simulated a quasi-static activity and utilized non-physiologic tensile tests to estimate in vivo ligament forces. The field lacks knowledge of the forces and torques in the knee for ADLs.
While determining the required functional tissue engineering parameters (FTEPs) (Butler, Shearn et al. 2004) in a human is not practical, establishing and characterizing an animal model provides a first step in determining the forces and torques in the intact knee and for individual structures during ADLs. We have chosen to use the ovine model because prior work has shown that the ovine stifle is a valid surgical model for the human knee (Radford, Amis et al. 1996; Allen, Houlton et al. 1998) and is a suitable experimental model for studying various orthopaedic conditions and treatments for the knee (Dürselen, Claes et al. 1996; Edwards, Whittle et al. 1996; Oakley, Lassere et al. 2004; Lu, Markel et al. 2009).
The objective of this study was to determine the role of various knee structures during simulated gait kinematics for both the ovine stifle and human knee joints and to compare the role of each structure across species. The results of this study will help in determining the utility of the ovine model as a biomechanical surrogate for the human knee. Our long term research goal is to establish FTEPs for ADLs to serve as design criteria and evaluation benchmarks for traditional and novel treatment strategies.
2. Materials and methods
2.1. Experimental Design
Three left and six right hind limbs (no pairs) from skeletally mature, mixed breed, female sheep (3–4 yrs; 50–78 kg) were included in the study along with four left and two right lower limbs (no pairs) from human cadavers (4 female, 2 male, 83±4 (SEM) yrs). However, two cadaver limbs transmitted no load through the ACL during testing, indicating abnormal physiology, and were excluded from the study. The remaining ovine (N=9) and human (N=4) limbs were testing using a six degree-of-freedom (DOF) robot (KR210; Kuka Robotics Corp., Clinton Township, MI) equipped with a six-axis load cell (Theta Model; ATI Industrial Automation, Apex, NC). The robot simulated 6 DOF species specific gait motions derived from ovine (Tapper, Fukushima et al. 2006) and human subjects (Lafortune, Cavanagh et al. 1992), while recording corresponding joint forces and torques. Once a specimen was fixed to the robot end effector, the knee joint was cycled through its species specific gait during a selective cutting protocol, as described in detail below. Resulting changes in force and torque allowed us to rank the loading contributions of each structure during simulated gait.
2.2. Sample Preparation and Setup in Robot
All limbs were stored at −20°C until the evening before testing. Each knee was dissected free of all muscles and tendons, leaving the joint capsule, the collateral and cruciate ligaments, and the two menisci intact. The tibia was rigidly attached and aligned with the load cell and robot end effector axes according to the tibial joint coordinate system (Grood and Suntay 1983). The tibial joint center point was digitized using a coordinate measurement machine (CMM, Faro Digitizer F04L2, FARO Technologies Inc., Lake Mary, FL), and all rotations, translations, forces, and torques were applied and recorded about this point. Landmarks on the collateral ligaments and along the mechanical axes of the tibia and femur were used to define and measure the position of the knee during setup and testing.
Due to differences in kinematics and information available in the Tapper (ovine) and Lafortune (human) manuscripts, the remaining test setup procedures to achieve the gait starting position were species dependent.
In ovine testing the knee was placed at a 60.5° flexion angle, corresponding to the midpoint of joint flexion during swing phase, as reported byTapper et al. (2006). This starting flexion angle was selected as we postulated that the forces and torques are minimal as the knee moves toward peak flexion. Small translational changes were made to minimize the forces and torques to <5 N and <1 Nm, respectively, to achieve an unloaded state. The ovine gait kinematics were cycled from this starting position in swing.
In human testing, the knee was placed at the position of peak flexion during stance, as reported byLafortune et al. (1992). The orientation of the knee was adjusted until all three rotations were within ±0.5° of the Lafortune motion. At this position, a 500N compressive force was applied to establish the starting pose. The limb was cycled several times to account for the viscoelastic effects, and the limb was again set to 500N. The 500N compressive load was based on unpublished data acquired from the Hewett biodynamics group, focused on knee injury prevention.
A more detailed description of how each limb was prepared, secured into test fixtures, and placed into its starting position can be found in previous reports (Boguszewski, Shearn et al. 2011; Herfat, Boguszewski et al. 2012).
2.3. Simulated 6 DOF In Vivo Motion Robot Testing
All tests were performed at room temperature with the joint wrapped in saline soaked gauze to prevent soft tissue dehydration. Ovine motions were applied to ovine specimens and human motions were applied to human specimens. To minimize viscoelastic effects, an initial set of 10 gait cycles was applied to each knee, followed by another 10 cycles to record the forces and torques of the intact joint. A structure was then selectively cut at random (e.g. MCL), and 10 cycles of simulated gait kinematics were repeated to record the new joint forces and torques. This process was repeated in random order to reduce the effect of tissue interactions until each of the posterior cruciate ligament (PCL), medial collateral ligament (MCL), lateral collateral ligament (LCL), lateral meniscus (LM), medial meniscus (MM), medial capsule (MedCap), and lateral capsule had been cut. Each test always concluded with the elimination of the bony interaction followed by the removal of the ACL. The ACL-isolated condition allowed us to measure forces and torques with only the ACL transmitting load across the joint. Finally, the ACL was removed, and the motions were applied with the tibia rotating freely in space. The resulting forces and torques due to gravity and robot inertia were then subtracted from each previously run test. The reduction in force and torque from the removal of a given structure was used to determine the primary and secondary structures contributing to loads in each anatomical DOF.
2.4. Data Analysis
Forces and torques were recorded in the tibial reference frame based on the knee joint coordinate system established by Grood and Suntay (1983). Forces correspond to the anterior-posterior, medial-lateral, and compression-distraction anatomical axes. Torques correspond to the adduction-abduction, flexion-extension, and internal-external moment convention. The 8th and 9th cycles of each 10 cycle test were used for analysis to reduce cycle effects. Data were averaged across specimen over a normalized gait cycle (%) for the intact condition and each selectively cut condition. The load contribution of each structure was determined by computing the changes in force and torque along and about each anatomical axis due to the removal of that structure. The average change in force or torque was then compared against the average intact condition to calculate the percent contribution in each anatomical DOF. The structures were then ranked according to the most significant contributors during both stance phase (heel strike, mid stance, and push-off) and swing phase (peak flexion). A primary or secondary contributor to load in our position controlled test translates to a primary or secondary restraint to in vivo motion.
2.5. Statistical Analysis
For each point of interest, a two-tailed Student’s t-test with a test value equal to zero was used to examine the contribution (% of intact load) of the individual structures between species. In all cases, to be considered a structural contributor, the force or torque value recorded after cutting the structure must have shown a statistically significant difference from the intact condition while also representing a physiologically significant change. Therefore, thresholds for physiologic significance were set at 10% of the intact force or torque and >5N of force or >1Nm of torque. Overall rankings for stance phase contribution were assigned for each structure by considering loads with the highest percent of intact contribution among the three stance phase points. Rankings for swing phase contribution were assigned by only considering loads at peak flexion. Structures were designated as secondary contributors if their percentage of intact load was more than 5% lower than the primary contributor(s). All data were normal. Significance level for all comparisons was set at p < 0.05.
3. Results
3.1. Stance Phase
The primary structural contributors during the stance phase of gait were similar between the human and ovine knees (Table 1: Primary). Both species demonstrated compression as the primary direction of load. The total force recorded in the ovine stifle during stance was approximately 300N, while in the human knee approximately 500N were recorded (Figures 1 and 2: Stance). Of note, the ACL was the primary restraint to anterior tibial translation and bony interaction provided primary restraint to posterior, medial and compressive translations as well as adduction and internal rotations.
Table 1.
Force and torque contributions of knee structures during the stance phase of gait. Ovine and human ACLs, MMs, and bony interactions have similar restraining roles, particularly in the translational degrees of freedom.
| Contributors in Swing |
Sheep | Human | |||
|---|---|---|---|---|---|
| Primary | Secondary | Primary | Secondary | ||
| Translations | Anterior | ACL* | MCL*** | ACL* | MM |
| Posterior | Bone, LM | Bone | |||
| Medial | ACL | LM | Bone | ACL | |
| Lateral | MCL | Bone | |||
| Compression | LM | Bone | Bone | MM, LM | |
| Distraction | MCL | ACL*, PCL | ACL | ||
| Rotations | Adduction | ACL* | Bone | MM, ACL* | |
| Abduction | MCL*** | Bone | |||
| Flexion | MCL***, ACL | Bone | |||
| Extension | Bone | ACL | |||
| Internal | |||||
| External | MCL*** | ||||
* denotes statistical difference between species
p < 0.05;
p < 0.01;
p < 0.001 in comparing % contribution to intact loads
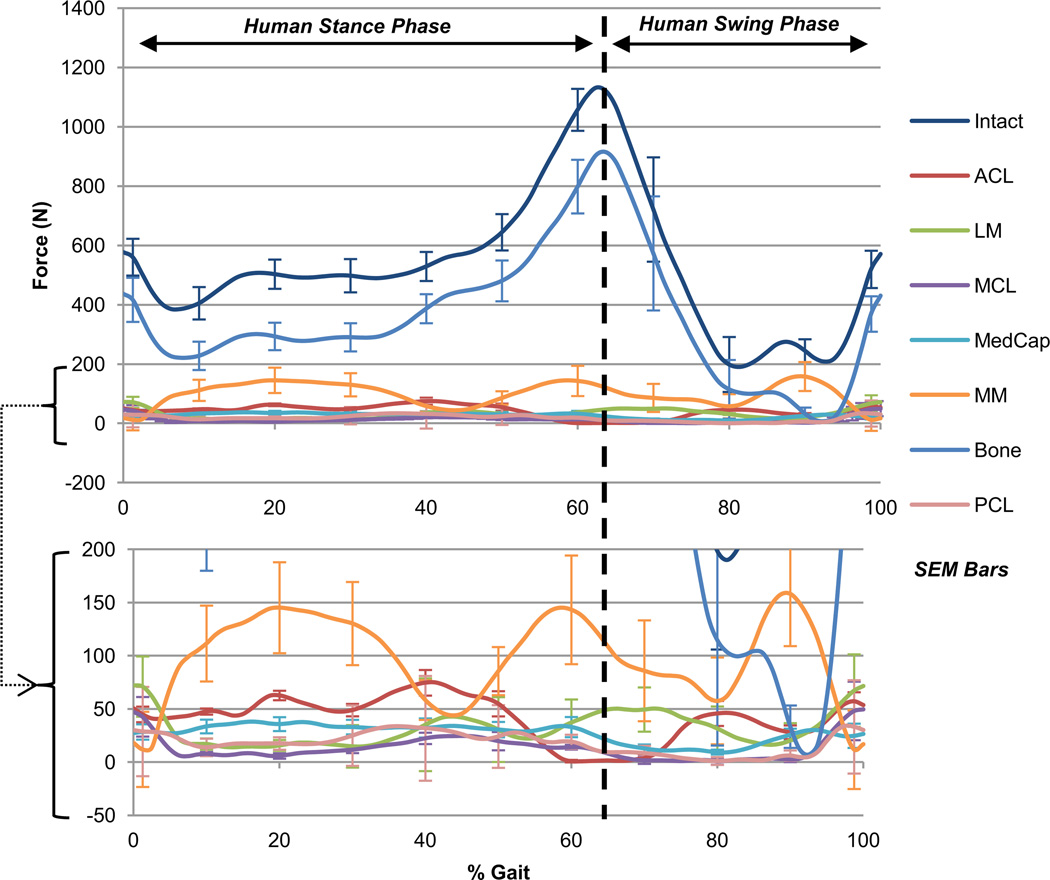
Figure 1.
Human total force during gait. Sum of the average translational components of intact knee force and the corresponding drop in all structurally deficient knee forces during the gait cycle in the human knee joint. Human toe-off occurs at 64% of gait, though loading continues to be compressive. Bony interaction accounts for the majority of total load in stance and swing, followed by the medial meniscus.
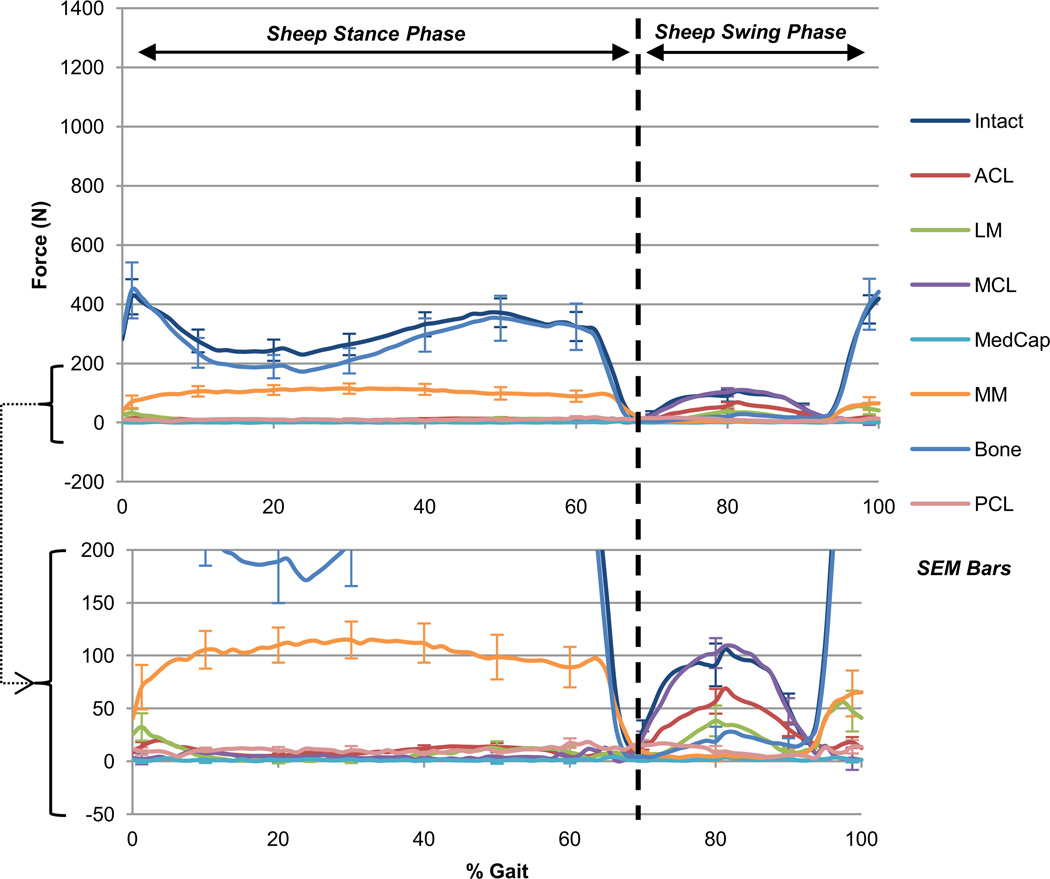
Figure 2.
Ovine total force during gait. Sum of the average translational components of intact knee force and the corresponding drop in all structurally deficient knee forces during the gait cycle in the ovine stifle joint. Ovine toe-off occurs at 68% of gait when loading is primarily distractive. Bony interaction accounts for the majority of total force in stance, followed by the medial meniscus. The MCL and ACL account for the majority in swing.
There were also similarities among the secondary contributors during the stance phase between the two species (Table 1: Secondary). Particularly, the ACL resisted medial translation while the medial meniscus was a secondary contributor to posterior and compressive translations. Interestingly, in the ovine knee, the only secondary structural contributors were the ACL and medial meniscus, while the human knee had many secondary contributors (Figure 1: Stance). In addition, there were no significant contributions from the PCL, LCL, or lateral capsule, nor did any structure function to resist distraction or external rotation during stance in either species.
3.2. Swing Phase
During the swing phase of gait, there were fewer similarities among the primary restraints between the human and ovine knees (Table 2: Primary). The primary loading direction in the ovine stifle was distraction, while the human knee remained overall compressed. The total force recorded in the ovine stifle during swing was approximately 100N, while in the human knee approximately 200N were recorded (Figures 1 and 2: Swing). In both species the ACL resisted anterior and medial tibial translation. However, in the human knee, only the bony interaction provided the remaining primary contributions, while in the ovine knee the menisci, bony interaction, and the MCL were all primary contributors.
Table 2.
Force and torque contributions of knee structures during the swing phase of gait. The ACL continues to act as a restraint to anterior translation and medial translation in both species throughout gait. The ovine MCL becomes a significant restraint in multiple degrees of freedom while the human MCL remains functionally absent.
| Contributors in Stance |
Sheep | Human | |||
|---|---|---|---|---|---|
| Primary | Secondary | Primary | Secondary | ||
| Translations | Anterior | ACL | ACL | MM | |
| Posterior | Bone | MM | Bone | MM, LM | |
| Medial | Bone | ACL | Bone | ACL | |
| Lateral | MM*** | MM***, LM | |||
| Compression | Bone*** | MM* | Bone*** | MM* | |
| Distraction | |||||
| Rotations | Adduction | Bone | MM* | Bone | MM* |
| Abduction | LM | LM | |||
| Flexion | Bone | MM | |||
| Extension | Bone | MM | ACL | Bone | |
| Internal | Bone | Bone | MedCap** | ||
| External | |||||
* denotes statistical difference between species
p < 0.05;
p < 0.01;
p < 0.001 in comparing % contribution to intact loads
There were no similarities between the two species related to secondary contributors (Table 2: Secondary). In addition, there were no significant contributions made by the LCL, medial, or lateral capsule, nor did any structure function to resist internal rotation during swing in either species.
4. Discussion
Analysis of the stance phase of gait reveals more similarities between species than in the swing phase of gait. The combination of the bony interaction, menisci, and ACL accounted for almost all of the primary and secondary stabilization in the knee during stance in both species. As expected, the ACL was a major restraint to anterior and medial tibial translations in both species, which is supported by previous studies (Markolf, Mensch et al. 1976; Butler, Noyes et al. 1980; Piziali, Seering et al. 1980; Sakane, Livesay et al. 1999). Similarly, the medial meniscus was a primary restraint to lateral translation in both species. This could have been caused by the superficial meniscal ridge contacting the medial condyle as the tibial moved laterally. The human medial meniscus also functioned to resist adduction, as the ovine medial meniscus did, and as has been previously reported (Levy, Torzilli et al. 1982; Shoemaker and Markolf 1986; Stürup, Iversen et al. 1987). The largest difference between the species during stance was the role of the lateral meniscus as a restraint to translation. Both the medial and lateral human menisci resisted posterior and lateral translations, while the sheep lateral meniscus did not resist any translation. This could possibly be due to differences in bone morphology.
The large load contributions from the bony interactions during stance phase demonstrate how the compressed state of the knee can shield soft tissue structures from loading. To our knowledge, only one previous biomechanical study investigated the bony interaction’s contribution to forces and torques in the knee. In a study bySakane et al. (1999), comparisons of anterior loads generated during anterior tibial translation showed that the bony interaction was a secondary restraint to anterior translation at flexion angles beyond 30°. This disputes our results that found bone resists posterior translations. However, this study applied pure anterior displacements in passive flexion positions at zero-load, which does not represent the native mechanical environment of the knee, as the hamstrings pull the tibia further posteriorly during flexion activities. In addition, this previous study, along with the majority of previous knee biomechanical studies, does not apply physiologic joint loads, minimizing compression and the role of bony interaction. Still, both studies suggest that load sharing in the knee joint occurs which can impact repair mechanics and design criteria.
During the swing phase of gait, the ACL remained the primary restraint to anterior tibial translation in both species, further demonstrating the ACL dependence of both human and ovine knees. As expected, in ovine knees, the bony interaction contributed less compared to stance phase, while human bony interactions played similar roles to stance phase. This was due to the compressive knee state measured for the human knee. Another key difference between the species during swing was that the ovine MCL became far more active than the human MCL while the ovine medial meniscus was less critical. This phenomenon could be explained by the sheep’s “knock-kneed” gait which naturally exposes the ovine knee to higher levels of abduction. Differences in knee laxity between species also became apparent during swing, as no structure of the human knee functioned to resist lateral translation, abduction, or external rotation. In each of these DOFs, the ovine MCL played the primary role.
One limitation of this study was the small sample size (N=4) for human cadaveric testing. Though the sample size would ideally be larger for cadaveric studies, results were consistent across specimen and the total load coefficient of variation was less than 22% during stance phase. Furthermore, the loads of the intact and ACL-only conditions (average 41.1N ± 20.6N) align closely with unpublished data from a separate study comparing intact and ACL-deficient conditions (N=4, average 42.9N ± 17.3N), also generated by our lab. Comparisons of these two unique groups of specimens showed that the total force in the ACLs of this study was not statistically different from the total force contribution of the unpublished ACL data, suggesting that the human specimens used in this study are representative of the population, not outliers, and that the contributions determined are representative of the population.
Other limitations of this study included the necessity to apply averaged kinematics from one study to a different set of knee specimens, and that among those knee specimens there is inherent population variability. We acknowledge that the application of an averaged motion to joints of different sizes could produce variability in the sample population. To examine the effect of this variability on study outcomes, the analysis of structural contribution to force and torque was repeated by internally ranking each specimen before combining the results, which did not affect our conclusions. Furthermore, there was minimal variability in measured loads between subjects (Figures 1 and 2). Therefore, it is unlikely that this limitation would invalidate resulting rank order of each restraint, though it may help explain the differences seen between ovine and human structures in contributing to percent total intact force. In future studies, we plan to use the ovine model to investigate this limitation further, by applying both subject-specific and averaged motions to each specimen to determine how averaged motion paths may affect the measured kinetics. One solution may be to utilize the robot’s load control capabilities to apply individualized kinematics based on specimen specific parameters and load boundaries. This knowledge would be useful to apply to human cadaveric testing, as we are currently unable to collect reliable in vivo load measurements and must always rely on in vitro testing using one averaged or representative motion for an entire cohort of specimens.
Results of this study support use of the ovine stifle joint as a biomechanical model for human knee dynamics, along with other previously reported studies (Radford, Amis et al. 1996; Osterhoff, Löffler et al. 2011), as the loaded structures in the knee during stance are similar between species. Concurrent studies in our lab may further validate the ovine model by investigating knee ligament strains and kinematic response to injury. By using the ovine model as a surrogate for measuring in vivo and in vitro biomechanical function, researchers may be able to further develop the functional standards needed for designing repair procedures. This model may also serve to evaluate the safety and efficacy of future repair strategies.
Normal knee function during gait depends on the restraining roles of the soft tissue structures and bony interaction during normal activities. While previous studies provide valuable information about the roles of knee structures, they have not presented critical information about their functions during an ADL. Knowledge of the contributions of each structure during ADLs will allow investigators to better understand the normal biomechanics of the knee joint. For example, as it applies to ACL research, ovine and human specimens both demonstrated that in addition to its primary role as a restraint to anterior movement the ACL also acts as a restraint to medial translation. This additional role may be key a consideration when designing materials and techniques for ACL repair. By exposing tissues to the mechanical context encountered during normal activities, researchers can better establish the patterns and limits of expected usage which govern functional tissue engineering parameters (FTEPs). These FTEPs will provide crucial design criteria for the development of more effective strategies for the prevention and treatment of knee injuries.
Acknowledgments
This work was supported by the National Institutes of Health (R21 EB004859 and R01 AR056660).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no potential conflicts of interest to this work.
References
- Allen MJ, Houlton JE, et al. The surgical anatomy of the stifle joint in sheep. Vet Surg. 1998;27(6):596–605. doi: 10.1111/j.1532-950x.1998.tb00536.x. [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Dyrby CO. Interactions between kinematics and loading during walking for the normal and ACL deficient knee. Journal of Biomechanics. 2005;38(2):293–298. doi: 10.1016/j.jbiomech.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Koo S, et al. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91(Suppl 1):95–101. doi: 10.2106/JBJS.H.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguszewski DV, Shearn JT, et al. Investigating the effects of anterior tibial translation on anterior knee force in the porcine model: Is the porcine knee ACL dependent? J Orthop Res. 2011;29(5):641–646. doi: 10.1002/jor.21298. [DOI] [PubMed] [Google Scholar]
- Butler DL, Noyes FR, et al. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am. 1980;62(2):259–270. [PubMed] [Google Scholar]
- Butler DL, Shearn JT, et al. Functional tissue engineering parameters toward designing repair and replacement strategies. Clin Orthop Relat Res. 2004;(427 Suppl):S190–S199. doi: 10.1097/01.blo.0000144858.65450.d2. [DOI] [PubMed] [Google Scholar]
- Dürselen L, Claes L, et al. Comparative animal study of three ligament prostheses for the replacement of the anterior cruciate and medial collateral ligament. Biomaterials. 1996;17(10):977–982. doi: 10.1016/0142-9612(96)84671-0. [DOI] [PubMed] [Google Scholar]
- Edwards DJ, Whittle SL, et al. Radiographic Changes in the Knee After Meniscal Transplantation An Experimental Study in a Sheep Model. The American Journal of Sports Medicine. 1996;24(2):222–226. doi: 10.1177/036354659602400219. [DOI] [PubMed] [Google Scholar]
- Gill TJ, Van de Velde SK, et al. Estimation of in vivo ACL force changes in response to increased weightbearing. Journal of Biomechanical Engineering. 2011;133:051004–051001. doi: 10.1115/1.4003780. [DOI] [PubMed] [Google Scholar]
- Grood ES, Noyes FR, et al. Ligamentous and capsular restraints preventing straight medial and lateral laxity in intact human cadaver knees. J Bone Joint Surg Am. 1981;63(8):1257–1269. [PubMed] [Google Scholar]
- Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105(2):136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- Henning CE, Lynch MA, et al. An in vivo strain gage study of elongation of the anterior cruciate ligament. The American Journal of Sports Medicine. 1985;13(1):22–26. doi: 10.1177/036354658501300104. [DOI] [PubMed] [Google Scholar]
- Herfat S, Boguszewski D, et al. Applying Simulated In Vivo Motions to Measure Human Knee and ACL Kinetics. Annals of Biomedical Engineering. 2012:1–9. doi: 10.1007/s10439-011-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden JP, Grood ES, et al. In vivo forces in the anterior cruciate ligament: direct measurements during walking and trotting in a quadruped. J Biomech. 1994;27(5):517–526. doi: 10.1016/0021-9290(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Jomha NM, Borton DC, et al. Long term osteoarthritic changes in anterior cruciate ligament reconstructed knees. Clinical Orthopaedics and Related Research. 1999;358:188–193. [PubMed] [Google Scholar]
- Kanamori A, Woo SL, et al. The forces in the anterior cruciate ligament and knee kinematics during a simulated pivot shift test: A human cadaveric study using robotic technology. Arthroscopy. 2000;16(6):633–639. doi: 10.1053/jars.2000.7682. [DOI] [PubMed] [Google Scholar]
- Lafortune MA, Cavanagh PR, et al. Three-dimensional kinematics of the human knee during walking. Journal of Biomechanics. 1992;25(4):347–357. doi: 10.1016/0021-9290(92)90254-x. [DOI] [PubMed] [Google Scholar]
- Levy IM, Torzilli P, et al. The effect of medial meniscectomy on anterior-posterior motion of the knee. The Journal of bone and joint surgery. American volume. 1982;64(6):883. [PubMed] [Google Scholar]
- Lu Y, Markel MD, et al. Comparison of Single-Versus Double-Tunnel Tendon-to-Bone Healing in an Ovine Model A Biomechanical and Histological Analysis. The American Journal of Sports Medicine. 2009;37(3):512–517. doi: 10.1177/0363546508327543. [DOI] [PubMed] [Google Scholar]
- Markolf KL, Mensch J, et al. Stiffness and laxity of the knee: the contributions of the supporting structures. J Bone Joint Surg [Am] 1976;58:583–594. [PubMed] [Google Scholar]
- Markolf KL, Park S, et al. Contributions of the posterolateral bundle of the anterior cruciate ligament to anterior-posterior knee laxity and ligament forces. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2008;24(7):805–809. doi: 10.1016/j.arthro.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Oakley S, Lassere M, et al. Biomechanical, histologic and macroscopic assessment of articular cartilage in a sheep model of osteoarthritis. Osteoarthritis and Cartilage. 2004;12(8):667–679. doi: 10.1016/j.joca.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Osterhoff G, Löffler S, et al. Comparative anatomical measurements of osseous structures in the ovine and human knee. The Knee. 2011;18(2):98–103. doi: 10.1016/j.knee.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Piziali RL, Seering WP, et al. The function of the primary ligaments of the knee in anterior-posterior and medial-lateral motions. J Biomech. 1980;13(9):777–784. doi: 10.1016/0021-9290(80)90239-0. [DOI] [PubMed] [Google Scholar]
- Radford WJP, Amis AA, et al. The ovine stifle as a model for human cruciate ligament surgery. Veterinary and Comparative Orthopaedics and Traumatology. 1996;9(3):134–139. [Google Scholar]
- Roberts C, Cummings J, et al. In vivo measurement of human anterior cruciate ligament forces during knee extension exercises. Trans. 40th Orthopaedic Research Society. 1994;19:15. [Google Scholar]
- Robinson JR, Bull AM, et al. The role of the medial collateral ligament and posteromedial capsule in controlling knee laxity. Am J Sports Med. 2006;34(11):1815–1823. doi: 10.1177/0363546506289433. [DOI] [PubMed] [Google Scholar]
- Sakane M, Livesay GA, et al. Relative contribution of the ACL, MCL, and bony contact to the anterior stability of the knee. Knee Surg Sports Traumatol Arthrosc. 1999;7(2):93–97. doi: 10.1007/s001670050128. [DOI] [PubMed] [Google Scholar]
- Shoemaker S, Markolf K. The role of the meniscus in the anterior-posterior stability of the loaded anterior cruciate-deficient knee. Journal of bone and joint surgery. American volume. 1986;68(1):71–79. [PubMed] [Google Scholar]
- Stürup J, Iversen BF, et al. Abnormal knee mobility and meniscal injury. Acta Orthopaedica. 1987;58(6):655–657. doi: 10.3109/17453678709146508. [DOI] [PubMed] [Google Scholar]
- Tapper JE, Fukushima S, et al. Dynamic in vivo kinematics of the intact ovine stifle joint. J Orthop Res. 2006;24(4):782–792. doi: 10.1002/jor.20051. [DOI] [PubMed] [Google Scholar]
- von Porat A, Roos EM, et al. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]