Abstract
Adjuvants can be used to enhance the immunogenicity of antigens and improve the efficacy of vaccines. Potent adjuvant action is known to often correlate with the activation of the transcription factor, nuclear factor-κB (NF-κB). Specific plant polysaccharides and a variety of phytochemicals from foods and traditional medicinal herbs have been shown to modulate NF-κB activation. In the present study, selected plant polysaccharides and phytochemicals were evaluated for use as a DNA vaccine adjuvant in a murine melanoma model. We observed that a specific ethanol extract fraction (DsCE-I) from the tuber of a key Traditional Chinese Medicine plant, Dioscorea (山藥 Shān Yào), enhanced the protection against melanoma after immunization with a gene-based vaccine. A number of anti-inflammatory phytochemicals tested were able to partially diminish the inflammation-associated tumorigenesis elicited by LPS. Among the several phytochemical combinations investigated, the use of an adjuvant containing LPS in combination with emodin resulted in smaller tumors and higher survival rate in test mice than the use of other adjuvant treatments and the control sets in this DNA cancer vaccine model. A Dioscorea polysaccharide fraction (DsCE-I) and several specific phytochemicals warrant further exploration as useful adjuvants for anticancer vaccines.
Keywords: Adjuvants, Anticancer vaccine, Dioscorea, Phytochemicals
INTRODUCTION
Traditional Chinese medicines (TCM), or as part of so-called “complementary and alternative medicines” (CAM) by Western medical communities, are being increasingly recognized and used in public health care throughout the world. Government- and industry-funded research and development have started to focus on the use of scientific evidence-based approaches to verify the traditionally claimed medicinal efficacies, specific bioactivities, and related pharmacological mechanisms of such remedies. In Asia, Dioscorea spp. (山藥 Shān Yào) are very popularly used as a health food/supplement and/or as a TCM herb that can be taken alone or in multiple-herb formulations. They are used for a broad range of ailments or health care measures. Some specific biological effects have been reported for Dioscorea spp., including antitumor,[1] induction of hypoglycemia in experimental mice and rabbits,[2,3] antibacterial,[4] as well as antioxidative and hypolipidemic activities.[5] Anecdotal evidences suggest that Dioscorea tubers taken as a food supplement may promote human health by regulating and upgrading the immune responses[6] and promoting antitumor activities;[1] however, credible experimental results and related mechanisms are still very limited.
Suppression of tumor progression by functional bioactivities of secondary metabolites from plants has been shown to confer anticancer or chemoprevention activities. Previously, we reported that co-treatment with a 50-75% ethanol-partitioned fraction of the tuber crude extract of Dioscorea batatas (DsCE-II) and interleukin-2 (IL-2) resulted in a significantly higher rate of murine splenocyte cell proliferation ex vivo than treatment with DsCE-II or IL-2 alone. This DsCE-II fraction, which contains a polysaccharide with a high proportion of β-1,4 linkage mannose (≥64%), also promoted the regeneration of specific progenitor cell populations in damaged bone marrow tissues of 5-fluorouracil–treated mice.[7] In addition, DsCE-I, a 50% ethanol-insoluble fraction of D. batatas, significantly increased granulocyte-macrophage colony stimulating factor (GM-CSF) promoter activity in normal and inflamed skin,[8] suggesting that DsCE-I may be useful as an adjuvant for use alongside chemotherapy in cancer.
Potent adjuvant action often correlates with nuclear factor-κB (NF-κB) activation, as exemplified by monophosphoryl lipid A (MPL).[9] However, NF-κB activation is also known to play an important role in the development and/or maintenance of various types of cancer. NF-κB has been linked to cell proliferation, invasion, angiogenesis, metastasis, suppression of apoptosis, and chemoresistance in multiple tumor systems.[10,11] Most carcinogens, inflammatory agents, and tumor promoters, including tar components from cigarette smoking, phorbol ester, okadaic acid, H2O2, and tumor necrosis factor-alpha (TNF-α), have all been shown to activate NF-κB. In addition, NF-κB has been shown to regulate the expression of a number of genes whose products are involved in tumorigenesis. Therefore, the balance of NF-κB in anticancer immune responses and tumorigenesis is a critical and tricky issue in cancer therapy.
The present study investigated the adjuvant effect of DsCE-I in protecting against B16-hgp100 melanoma in a DNA vaccine model. In addition, several anti-inflammatory phytochemicals known for their anti–NF-κB activity were investigated for their ability to diminish the inflammation-associated tumorigenesis in this vaccine model study.
MATERIALS AND METHODS
Preparation of Dioscorea plant tuber crude extract (DsCE-I) using ethanol partition
Tuber tissues of the Dioscorea plant, D. batatas Decne (山藥 Shān Yào), were used to prepare the ethanol extract DsCE-I. The authenticity of all plant materials and species verification was validated by Dr. Sin-Yie Liu, Taiwan Agricultural Research Institute. Cultivation, growth, taxonomy, and agricultural practice details have been previously reported.[12] The preparation of DsCE-I was conducted as described previously.[8] In brief, 10 g tuber powder was mixed with 100 ml Milli-Q water, stirred for 1 h at room temperature, and centrifuged at 24,000 g for 20 min at 4°C. The supernatant was filtered through glass wool. The pellet was resuspended with another 100 ml water, stirred, centrifuged, and re-extracted as above. The supernatants from two extractions were then pooled to yield a crude extract (CE) fraction. The CE fraction was further extracted stepwise with 50% (V/V) ethanol. The ethanol-insoluble fractions were collected by centrifugation at 24,000 g for 20 min at 4°C; the pellet was lyophilized and then dissolved in sterilized water at 10 mg/ml. The fractions were named DsCE-I. Limulus amoebocyte lysate (LAL) assays (associates of Cape Cod, Falmouth, MA, USA) were performed to detect possible endotoxin contamination.
Reagents
Pyrrolidine dithiocarbamate (PDTC), SB203580, Lipopolysaccharide (LPS, Escherichia coli 055:B5), polymyxin B, forskolin, friedelin, oleanolic acid, resveratrol, and nidosamide were purchased from Sigma-Aldrich (St. Louis, MO, USA). Emodin was purchased from ACROS Organics (Fair Lawn, NJ, USA). Liquiritigenin was purchased from Extrasynthese (Lyon, France). Honokiol was purchased from Pharmaceutical Industry Technology Development Center (New Taipei City, Taiwan).
Mice
Female C57BL/6JNarl mice (6-8 weeks old), which were purchased from the National Laboratory Animal Breeding and Research Center, Taipei, Taiwan, were maintained under standard pathogen-free conditions. All facilities were approved by the Academia Sinica Institutional Animal Care and Utilization Committee (IACUC), and all animal experiments were conducted under the institutional guidelines established by the Animal Core Facility and IACUC in Academia Sinica, Taipei, Taiwan.
Cell lines and construction of cDNA expression vectors, stable gene transfection, and transgene studies
The mouse B16F10 (B16) melanoma cell line was obtained from American Type Culture Collection (ATCC; Manassas, VA, USA). B16 cells stably transfected with hgp100 cDNA vector, designated as B16-hgp100, were obtained as reported previously.[13] pNiFty-secreted embryonic alkaline phosphatase (SEAP) plasmid was purchased from InvivoGen (San Diego, CA, USA). The hgp100 cDNA expression plasmid pWRG1644 was constructed as reported previously.[14]
Transfection and SEAP reporter assay
B16-hgp100 melanoma cells were grown in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified 5% CO2/95% air incubator. The cells were seeded as 2 × 104 cells/well in a 96-well plate. After 24 h, B16-hgp100 melanoma cells were transfected with pNiFty-SEAP plasmid in the presence of lipofectin for 24 h. The transfected cells were then incubated with DsCE-I for 24 h and the supernatants were collected for chemiluminescent SEAP reporter gene assay. Chemiluminescent reporter gene assay was conducted by Phospha-Light™ system (AB Applied Biosystems, Bedford, MA, USA).
Sugar composition and sugar linkage analysis
Sugar composition determined by gas chromatography mass spectroscopy (GC-MS) has been described in an earlier report.[15] Hakomori methylation analysis used to examine carbohydrate linkage has also been clearly described in an earlier report.[16]
Gene-based immunization and challenge of mice with tumor cells
Skin of C57BL/6JNarl mice (5 mice/group) was transfected at two sites with hgp100 DNA (2.5 mg/mouse) using a Bio-Rad Helios gene gun delivery system as described previously.[13] Seven days later (day 0), vaccinated mice were challenged intradermally (i.d.) with 5 × 104 B16-hgp100 cells and were administered DsCE-I subcutaneously. Transfected mice were examined twice weekly for tumor appearance, and tumor volumes were determined form the length and width of test tumors; survival time of mice was also observed.
Statistical analysis
Statistical analyses were carried out with STATGRAPHICS Plus version 3.0 (Statistical Graphics Corp., Princeton, NJ, USA). Groups were compared by one-way analysis of variance (ANOVA). P values less then 0.05 were considered statistically significant.
RESULTS
DsCE-I confers efficacious adjuvant activity on suppression of melanoma mediated by hgp100 DNA vaccination
GM-CSF is an important hematopoietic growth factor and a multi-functional immune modulator. It has been extensively studied for use as an adjuvant in various cancer vaccine approaches. Our previous study showed that DsCE-I can significantly increase GM-CSF promoter activity in normal and inflamed skin.[8] Hence, we assessed the adjuvant effect of DsCE-I on protection against B16-hgp100 melanoma. The skin tissue of each mouse was transfected at two abdominal sites with hgp100-encoding plasmid DNA, by using a gene gun–mediated transgene delivery. Different concentrations of DsCE-I were emulsified in incomplete Freund's adjuvant (IFA) and then subcutaneously injected into test mice. One week later (Day 0), vaccinated mice (N = 9) were challenged i.d. with 5 × 104 B16-hgp100 cells. At Day 15 after tumor challenge, tumor size and survival time of the unvaccinated or vaccinated mice were measured. As seen in Figure 1a, in comparison with mice receiving a high dose of DsCE-I (i.e. 100 μg/mouse) treatment, mice receiving a low dose of DsCE-I (1 μg/mouse) treatment showed significantly smaller tumor sizes (P < 0.05, as compared to the treatment with a medium or high dose of DsCE-I). Furthermore, as shown in Figure 1b, survival time of the vaccinated mice was considerably longer than that of the control mice. Results from mice tested with a medium dose (10 μg/mouse) showed a level of tumor burden and mouse survival time in between that of the high and low DsCE-I doses tested [Figure 1a and b]. These results together demonstrated that the low dose of DsCE-I tested (1 μg/mouse) effectively protected vaccinated mice from the tumor burden of B16-hgp100 melanoma.
Figure 1.
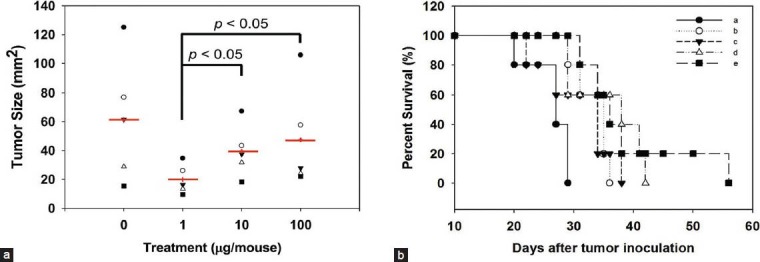
Effect of DsCE-I dosage on hgp100 gene-based vaccination, measured as protection against B16-hgp100 melanoma. Abdominal skin of C57BL/6 mice was transfected at two tissue sites with hgp100 cDNA expression plasmid pWRG1644 (2.5 mg/mouse) using a Helios gene gun. Seven days later (Day 0), the vaccinated mice were challenged i.d. with 5 × 104 B16-hgp100 cells and administered DsCE-I subcutaneously at the indicated concentrations. Tumor size (in diameter) on Day 15 (a) and the percentage of survival for test mice that developed tumors (b) are indicated. (a: No vaccination, b: hgp100, c: hgp100 + 100 μg/mouse DsCE-I, d: hgp100 + 10 μg/mouse DsCE-I, e: hgp100 + 1 μg/mouse DsCE-I)
Characterization of the chemical composition of DsCE-I
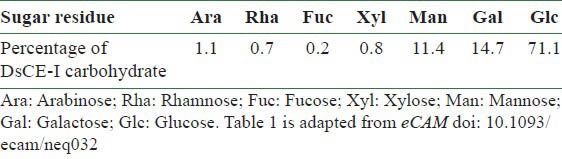
Since DsCE-I can exhibit potent immunomodulatory activities, we characterized the chemical content of DsCE-I by gel filtration and GC-MS analysis. As shown in Figure 2, symmetrical peaks exhibiting a relatively high absorbance in refractive index (RI) detector as well as at UV280 in size exclusion column were obtained for DsCE-I, prepared and presented as a watery, non-viscous aqueous extract. Based on the sugar composition and linkage analysis, a high content of glucose polymer (≥71%) with β-1,4 linkage was detected in DsCE-I [Table 1], as we have previously reported.[7]
Figure 2.
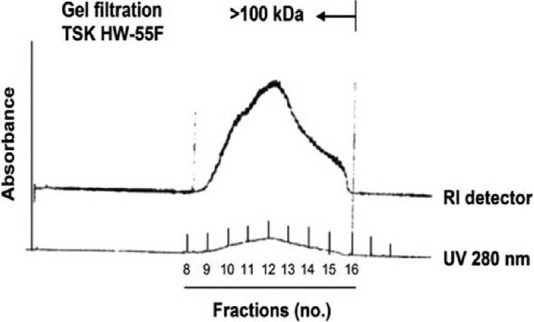
TSK HW-55F gel filtration and profile analysis of polysaccharides of DsCE-I fraction from D. batatas. Figure 2 is adapted from eCAM doi: 10.1093/ecam/neq032
Table 1.
GC-MS sugar linkage analysis of DsCE-I polysaccharides extracted from D. batatas
Effect of DsCE-I on NF-κB activation in B16-hgp100 melanoma
To investigate the possible effect of DsCE-I on NF-kB activation in tumor cells, the activity of transgenic NF-κB-inducible endothelial-leukocyte adhesion molecule 1 (ELAM-1) composite promoter was examined in B16-hgp100 melanoma. B16-hgp100 melanoma cells were first tranfected with NF-κB–inducible reporter plasmid pNiFty-SEAP in the presence of lipofectin for 24 h. The transfected cells were then incubated with DsCE-I for 24 h and chemiluminescent reporter gene assays were conducted. LPS is known to be a strong stimulator for activation of NF-κB and mitogen-activated protein kinases (MAPKs). Therefore, LPS was used as a positive control and the corresponding luciferase activity was normalized as 100%. To illustrate the specificity, the effects of inhibitors of the LPS-induced activation were also examined. The results showed that DsCE-I stimulated the transgenic composite promoter activity of NF-κB–inducible ELAM-1 in B16-hgp100 melanoma cells. As seen in Figure 3, DsCE-I effectively induced NF-κB activation, and this activity was significantly suppressed by SB203580 (a known inhibitor of MAPK p38) and PDTC (a known inhibitor of NF-κB) (P < 0.001, as compared to DsCE-I only treatment). These results indicate that the immunomodulatory effect of DsCE-I may be via the p38 and NF-κB cascade signaling pathways. Furthermore, polymyxin B was able to inhibit LPS-induced activation completely, but it only partially inhibited DsCE-I–induced NF-κB activation, suggesting that the NF-κB activation of DsCE-I was not likely mediated via toll-like receptor 4 (TLR4) receptor binding.
Figure 3.
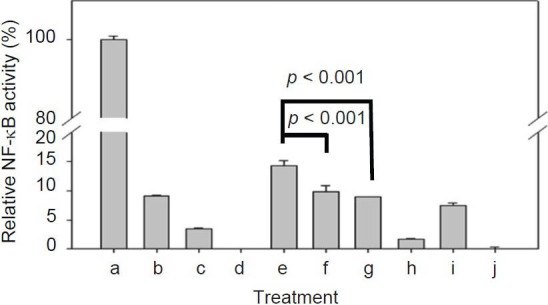
Effect of inhibitors on LPS and DsCE-I induced NF-κB activation in B-16 cells. Data are represented as the mean ± SD. (a: 0.1 μg/ml LPS, b: 0.1 μg/ml LPS + 15 μM SB203580, c: 0.1 μg/ml LPS + 30 μM PDTC, d: 0.1 μg/ml LPS + 400 units polymyxin B, e: 100 μg/ml DsCE-I, f: 100 μg/ml DsCE-I + 15 μM SB203580, g: 100 μg/ml DsCE-I + 30 μM PDTC, h: 100 μg/ml DsCE-I + 15 μM SB203580 + 30 μM PDTC, i: 100 μg/ml DsCE-I + 400 units polymyxin B, j: control)
NF-κB inhibitors can diminish inflammation-associated tumorigenesis elicited by DsCE-I or LPS
DsCE-I enhanced protection against B16-hgp100 melanoma in a DNA vaccine model. However, higher doses of DsCE-I did not result in more protection, but instead resulted in less protection. In addition, our results also showed that DsCE-I effectively stimulated NF-κB activation in B16-hgp100 melanoma cells. These results imply that DsCE-I–induced NF-κB activation in B16-hgp100 cells may impair the adjuvant effect on protection against B16-hgp100 melanoma in test DNA vaccine model. To further investigate this possibility, we performed an experiment using NF-κB inhibitors to determine whether inhibition of NF-κB activation could result in enhancement of the adjuvant effect of DsCE-I or LPS on vaccine protection against B16-hgp100 melanoma. The skin tissues of each mouse were transfected at two abdominal sites with hgp100-encoding plasmid DNA, delivered via gene gun. Test mice were then administered subcutaneously with DsCE-I or LPS emulsified in IFA. One week later (Day 0), the vaccinated mice were challenged i.d. with 5 × 104 B16-hgp100 cells. After the tumor challenge, survival time of the mice was recorded. At Day 15 after the tumor challenge, tumor size and mouse survival rate of the test mice were measured. Figure 4a and b shows that treatments with NF-κB inhibitors combined with DsCE-I or LPS prolonged the survival of test mice and led to tumor regression, suggesting that a balanced activation level of NF-κB is very important for the progression of tumorigenesis and the gene-based vaccination against test melanoma tumor.
Figure 4.
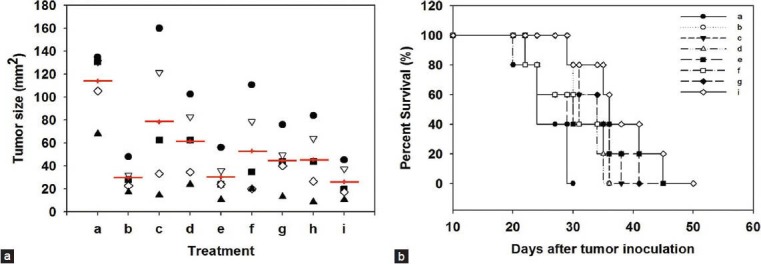
Effect of NF-κB inhibitors on B16-hgp100 melanoma in DNA vaccine model. C57BL/6 mice were skin vaccinated by transfection with hgp100 DNA (2.5 mg/mouse) using a gene gun. NF-κB inhibitors were administered subcutaneously. Seven days later (Day 0), the vaccinated mice were challenged i.d. with 5 × 104 B16-gp100 cells. Tumor size (in diameter) (a) and the percent survival of test mice that developed tumors (b) were serially measured. (a: No vaccination, b: hgp100, c: hgp100 + 100 μg/mouse DsCE-I, d: hgp100 + 100 μg/mouse DsCE-I + PDTC, e: hgp100 + 100 μg/mouse DsCE-I + PDTC + SB203580, f: hgp100 + 1 μg/mouse LPS, g: hgp100 + 1 μg/mouse LPS + PDTC, h: hgp100 + 1 μg/mouse LPS + SB203580, i: hgp100 + 1 μg/mouse LPS + PDTC + SB203580)
Specific anti-inflammatory phytochemicals can diminish inflammation-associated tumorigenesis elicited by LPS
We showed that inhibition of NF-κB activation can enhance the regression of melanoma induced by hgp100 DNA vaccine accompanied by treatment with DsCE-I or LPS. We next tested whether a group of anti-inflammatory phytochemicals that are known to confer anti–NF-κB activity may be useful as anticancer adjuvants in the current DNA vaccine model. The skin tissues of test mice were gene gun–transfected at two abdominal sites with hgp100-encoding plasmid DNA. LPS combined with test phytochemicals were emulsified in IFA and then subcutaneously injected into test mice. One week later (Day 0), the vaccinated mice were challenged i.d. with 5 × 104 B16-hgp100 cells. Our results showed that adjuvants containing LPS in combination with emodin had conferred the highest anti–NF-κB activity among all the tested phytochemicals (data not shown), and it also resulted in a much smaller tumor size (P < 0.05, as compared to hgp100 + LPS treatment) and significantly higher survival rate than the other tested adjuvant treatments and control in the melanoma DNA vaccine model [Figure 5a and 5b]. Hence, emodin has stood out as the utmost effective one amongst all tested phytochemicals for potential application as adjuvant for gene-based cancer vaccines in this comparative assay.
Figure 5.
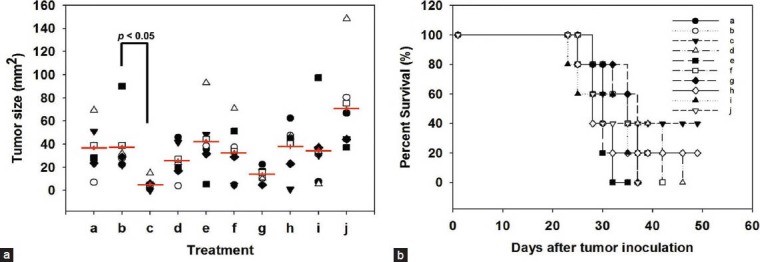
Effect of anti-inflammatory phytochemicals as vaccine adjuvant against B16-hgp100 melanoma cells in a DNA vaccine model. Skin of C57BL/6 mice was transfected with transgenic hgp100 cDNA (2.5 mg/mouse) using a gene gun. Anti-inflammatory phytochemicals in combination with LPS were then administered subcutaneously. Seven days later (Day 0), the vaccinated mice were challenged i.d. with 5 × 104 B16-gp100 cells. Tumor size (in diameter) (a) and the percent survival of test mice that developed tumors (b) were serially measured. (a: No vaccination, b: hgp100 + LPS, c: hgp100 + LPS + emodin, d: hgp100 + LPS + liquiritigenin, e: hgp100 + LPS + honokiol, f: hgp100 + LPS + forskolin, g: hgp100 + LPS + friedelin, h: hgp100 + LPS + oleanolic acid, i: hgp100 + LPS + resveratrol, j: hgp100 + LPS + niclosamide)
DISCUSSION
Various Traditional Chinese herbal medicinal literatures, such as the Ben Cao Gang Mu (本草綱目 Běn Cǎo Gāng Mù)[6] recognized as a classical TCM pharmacopoeia, have repeatedly related the various homeostatic, health care, and immune-regulatory effects to Dioscorea (山藥 Shān Yào) tuber. It is widely used as a functional food and/or phytomedicine in a number of East Asian cultures.[7] It is often served either as a fresh vegetable or in the form of stewed tubers in tonic soups. D. batatus tuber slices are also popularly used as a key component in many TCM formulations.[7] Hence, we investigated here one of the essential components of D. batatas Decne, DsCE-I, in terms of its effect on specific immunomodulatory activities. We have previously reported the isolation of two different ethanol fractions, DsCE-I and DsCE-II.[7] We not only have characterized their chemical contents but also distinguished their very different (even opposite) immunomodulatory activities for different and specific potential clinical applications. Therefore, our previous[7,8] and present studies may help strengthen the knowledge-based understanding on the historical claims of D. batatas Decne.
Gene-based (e.g. DNA) vaccines have been shown to not only induce antigen-specific CD8+ T lymphocytes but also activate CD4+ T cells for antitumor immunities.[14] However, for many cases, the efficacy of DNA vaccines is still limited and they need technical improvement. Therefore, a good combination of antigen-specific DNA vaccines with a potent adjuvant (s) has been considered as an important strategy for immunotherapy or therapeutic vaccination against cancers. GM-CSF has been extensively studied for use as an adjuvant in various cancer vaccine approaches.[17,18,19] It can not only can increase specific immune responses[20] but also alter the balance of Th1/Th2 cytokines.[21] In addition, recent studies also demonstrated a novel role for GM-CSF in enhancing the expansion and survival of Th17 cells via the regulation of IL-6 and IL-23 in vivo.[22] Since we demonstrated recently that DsCE-I can significantly increase the transgenic GM-CSF promoter activity in vivo,[8] we hypothesized in this study that DsCE-I may also be employed as an adjuvant when combined with hgp100 DNA vaccine in fighting against B16-hgp100 melanoma. In this study, we showed that lower dosages (e.g. 1 μg/ml) of DsCE-I administered along with hgp100 DNA vaccine can effectively suppress test melanoma tumor growth and enhance mouse survival rate. However, this antitumor effect of DsCE-I is decreased when applied at 10-100 (higher) doses. Compared to control treatment (hgp100 DNA only), our data showed that at the higher test dosage (100 μg/ml), DsCE-I has no effect on the suppression of melanoma growth. Hence, this result led us to further characterize the chemical composition and feature of DsCE-I.
Our result showed that polysaccharides constitute a major part (≥71%) of DsCE-I, with a β-1,4 linkage of the glucose polymer [Table 1]. Some β-glucans are known to confer potent immunomodulatory activity with an effect on both innate and adaptive immunities.[23] Some of these activities were considered to correlate with NF-κB activation and signaling.[24] However, growing evidences are also suggesting that constitutive NF-κB activation is closely correlated to human malignancies.[10,11] Hence, we investigated the possibility if at high dosage (e.g. 100 μg/dose), DsCE-I can drastically induce NF-kB activation in test B16-hgp100 melanoma and result in enhanced tumor progression. This possibility was demonstrated by the result that the high dosage of DsCE-I indeed stimulated the transgenic composite promoter activity of NF-κB–inducible ELAM-1 in B16-hgp100 melanoma cells. Our in vivo experiment conducted with NF-κB inhibitors [Figure 4] further confirmed this hypothesis. Due to the above considerations, we further investigated whether specific anti-inflammatory phytochemicals, abundant in a number of TCM herbs, were also able to partially diminish the inflammation-associated tumorigenesis elicited by high dose of DsCE-I or LPS. Our results shown in Figure 5 demonstrate a test that adjuvant containing LPS in combination with emodin exhibited the highest antitumor effects, resulting in smaller tumor size and higher survival rate in test mice, than those from other adjuvant treatments and control mice in this melanoma DNA vaccine model. Our results correlate well with a previous finding in which a combination of DNA vaccination and treatment with epigallocatechin-3-gallate (EGCG) generated synergistic antitumor therapeutic effects that were more effective than a single therapy modality.[25] These results are also in agreement with studies that indicated the suppression of NF-κB activities led to suppression of tumor growth and enhanced survival rate in treated animals. Pandey et al., reported that different dosages of berberine suppressed NF-κB inhibitors and, thus, suppressed the growth of different carcinoma cells under in vitro conditions.[26] Curcumin has been shown to potentiate the antitumor effect of Bacillus calmette-guerin against bladder cancer through the inhibition of NF-κB and induction of TNF-related apoptosis-inducing ligand (TRAIL) receptors in bladder cancer cells.[27] In addition, previous studies also showed that emodin induced apoptosis in several types of cancer cells[28,29] and strongly inhibited tumor cell migration[30] and invasion.[31] Similarly, friedelin[32] and oleanolic acid[33] were also found to possess antitumor activity and suppress tumor growth. Therefore, we suggest that emodin as well as other anti-inflammatory phytochemicals also could have the potential to serve as a useful adjuvant for (gene-based) cancer vaccine approaches.
CONCLUSION
In conclusion, we present in this study evidence that low dosages of Dioscorea (山藥 Shān Yào) may be used as an adjuvant with DNA cancer vaccine, and it can suppress tumor growth and prolong survival in B16-hgp100 melanoma-challenged mice. In addition, some phytochemicals, especially emodin, followed by friedelin to a lesser extent, may also show good antitumor adjuvant activities when used alongside a DNA vaccination regimen. Further experimental studies are warranted to evaluate both the mechanisms of action and the potential clinical applications of these phytochemicals under in vitro and in vivo conditions.
ACKNOWLEDGMENT
We thank Ms. Miranda Loney for editing the manuscript. This work was supported by grants (CCMP99-RD-067 and CCMP100-RD-019) from the Committee on Chinese Medicine and Pharmacy, Department of Health, Executive Yuan, Taiwan, ROC.
REFERENCES
- 1.Gao H, Kuroyanagi M, Wu L, Kawahara N, Yasuno T, Nakamura Y. Antitumor-promoting constituents from Dioscorea bulbifera L. in JB6 mouse epidermal cells. Biol Pharm Bull. 2002;25:1241–3. doi: 10.1248/bpb.25.1241. [DOI] [PubMed] [Google Scholar]
- 2.Hsu JH, Wu YC, Liu IM, Cheng JT. Dioscorea as the principal herb of Die-Huang-Wan, a widely used herbal mixture in China, for improvement of insulin resistance in fructose-rich chow-fed rats. J Ethnopharmacol. 2007;25:577–84. doi: 10.1016/j.jep.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Iwu MM, Okunji CO, Ohiaeri GO, Akah P, Corley D, Tempesta MS. Hypoglycaemic activity of dioscoretine from tubers of Dioscorea dumetorum in normal and alloxan diabetic rabbits. Planta Med. 1990;56:264–7. doi: 10.1055/s-2006-960952. [DOI] [PubMed] [Google Scholar]
- 4.Hriram V, Jahagirdar S, Latha C, Kumar V, Puranik V, Rojatkar S, et al. A potential plasmid-curing agent, 8-epidiosbulbin E acetate, from Dioscorea bulbifera L. against multidrug-resistant bacteria. Int J Antimicrob Agents. 2008;32:405–10. doi: 10.1016/j.ijantimicag.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Son IS, Kim JH, Sohn HY, Son KH, Kim JS, Kwon CS. Antioxidative and hypolipidemic effects of diosgenin, a steroidal saponin of yam (Dioscorea spp.) on high-cholesterol fed rats. Biosci Biotechnol Biochem. 2007;71:3063–71. doi: 10.1271/bbb.70472. [DOI] [PubMed] [Google Scholar]
- 6.Li SC, editor. Beijing: China Press; 1996. Ben-Cao-Gang-Mu. Principles and Use of Medicinal Herbs and Plants. [Google Scholar]
- 7.Su PF, Li CJ, Hsu CC, Benson S, Wang SY, Aravindaram K, et al. Dioscorea phytocompounds enhance murine splenocyte proliferation ex vivo and improve regeneration of bone marrow cells in vivo. Evid Based Complement Alternat Med 2011. 2011 doi: 10.1093/ecam/neq032. 731308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su PF, Staniforth V, Li CJ, Wang CY, Chiao MT, Wang SY, et al. Immunomodulatory effects of phytocompounds characterized by in vivo transgenic human GM-CSF promoter activity in skin tissues. J Biomed Sci. 2008;15:813–22. doi: 10.1007/s11373-008-9266-7. [DOI] [PubMed] [Google Scholar]
- 9.Ismaili J, Rennesson J, Aksoy E, Vekemans J, Vincart B, Amraoui Z, et al. Monophosphoryl lipid A activates both human dendritic cells and T cells. J Immunol. 2002;168:926–32. doi: 10.4049/jimmunol.168.2.926. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal BB. Nuclear factor-κB: The enemy within. Cancer Cell. 2004;6:203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Arlt A, Gehrz A, Muerkoster S, Vorndamm J, Kruse ML, Fölsch UR, et al. Role of NF-κB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–51. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 12.Liu SY, Wang JY, Shyu YT, Song LM. Studies on yams (Dioscorea spp.) in Taiwan. J Chin Med. 1995;6:111–26. [Google Scholar]
- 13.Rakhmilevich AL, Imboden M, Hao Z, Macklin MD, Roberts T, Wright KM, et al. Effective particle-mediated vaccination against mouse melanoma by coadministration of plasmid DNA encoding gp100 and granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 2001;7:952–61. [PubMed] [Google Scholar]
- 14.Aravindaram K, Yu HH, Lan CW, Wang PH, Chen YH, Chen HM, et al. Transgenic expression of human gp100 and RANTES at specific time points for suppression of melanoma. Gene Ther. 2009;16:1329–39. doi: 10.1038/gt.2009.90. [DOI] [PubMed] [Google Scholar]
- 15.Yang FL, Lu CP, Chen CS, Chen MY, Hsiao HL, Su Y, et al. Structural determination of the polar glycoglycerolipids from thermophilic bacteria Meiothermus taiwanensis. Eur J Biochem. 2004;271:4545–51. doi: 10.1111/j.1432-1033.2004.04415.x. [DOI] [PubMed] [Google Scholar]
- 16.Waeghe TJ, Darvill AG, McNeil M, Albersheim P. Determination, by methylation analysis, of the glycosyl-linkage compositions of microgram quantities of complex carbohydrates. Carbohydr Res. 1983;123:281–304. [Google Scholar]
- 17.Timmerman JM, Levy R. Dendritic cell vaccines for cancer immunotherapy. Annu Rev Med. 1999;50:507–29. doi: 10.1146/annurev.med.50.1.507. [DOI] [PubMed] [Google Scholar]
- 18.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 19.Rini BI, Small EJ. The potential for prostate cancer immunotherapy. Crit Rev Oncol Hematol. 2003;46(Suppl):117–25. doi: 10.1016/s1040-8428(03)00069-6. [DOI] [PubMed] [Google Scholar]
- 20.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer n patients. Ann Oncol. 2007;18:226–32. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: What we do and don’t know. Cell Res. 2006;16:126–33. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 22.Sonderegger I, Iezzi G, Maier R, Schmitz N, Kurrer M, Kopf M. GM-CSF mediates autoimmunity by enhancing IL-6-dependent Th17 cell development and survival. J Exp Med. 2008;205:2281–94. doi: 10.1084/jem.20071119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan GC, Chan WK, Sze DM. The effects of beta-glucan on human immune and cancer cells. J Hematol Oncol. 2009;2:25–36. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin YL, Liang YC, Lee SS, Chiang BL. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NF-kappaB and p38 mitogen-activated protein kinase pathways. J Leukoc Biol. 2005;78:533–43. doi: 10.1189/jlb.0804481. [DOI] [PubMed] [Google Scholar]
- 25.Kang TH, Lee JH, Song CK, Han HD, Shin BC, Pai Si, et al. Epigallocatechin-3-Gallate enhances CD8+T cell–mediated antitumor immunity induced by DNA vaccination. Cancer Res. 2007;67:802–11. doi: 10.1158/0008-5472.CAN-06-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey MK, Sung B, Kunnumakkara AB, Sethi G, Chaturvedi MM, Aggarwal BB. Berberine modifies cysteine 179 of IκBα kinase, suppresses nuclear factor-κB–regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 2008;68:5370–9. doi: 10.1158/0008-5472.CAN-08-0511. [DOI] [PubMed] [Google Scholar]
- 27.Kamat AM, Tharakan ST, Sung B, Aggarwal BB. Curcumin potentiates the antitumor effects of Bacillus calmette-guerin against bladder cancer through the downregulation of NF-κB and upregulation of TRAIL receptors. Cancer Res. 2009;69:8958–66. doi: 10.1158/0008-5472.CAN-09-2045. [DOI] [PubMed] [Google Scholar]
- 28.Srinivas G, Anto RJ, Srinivas P, Vidhyalakshmi S, Senan VP, Karunagaran D. Emodin induces apoptosis of human cervical cancer cells through poly (ADP-ribose) polymerase cleavage and activation of caspase-9. Eur J Pharmacol. 2003;473:117–25. doi: 10.1016/s0014-2999(03)01976-9. [DOI] [PubMed] [Google Scholar]
- 29.Yi J, Yang J, He R, Gao F, Sang H, Tang X, et al. Emodin enhances arsenic trioxide-induced apoptosis via generation of reactiveoxygen species and inhibition of survival signaling. Cancer Res. 2004;64:108–16. doi: 10.1158/0008-5472.can-2820-2. [DOI] [PubMed] [Google Scholar]
- 30.Huang Q, Shen HM, Ong CN. Emodin inhibits tumor cell migration through suppression of the phosphatidylinositol 3-kinase-Cdc42/Rac1 pathway. Cell Mol Life Sci. 2005;62:1167–75. doi: 10.1007/s00018-005-5050-2. [DOI] [PubMed] [Google Scholar]
- 31.Huang Q, Shen HM, Ong CN. Inhibitory effect of emodin on tumor invasion through suppression of activator protein-1 and nuclear factor-κB. Biochem Pharmacol. 2004;68:361–71. doi: 10.1016/j.bcp.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Lu B, Liu L, Zhen X, Wu X, Zhang Y. Anti-tumor activity of triterpenoid-rich extract from bamboo shavings (Caulis bamfusae in Taeniam) Afr J Biotech. 2010;9:6430–6. [Google Scholar]
- 33.Shyu MH, Kao TC, Yen GC. Oleanolic acid and ursolic acid induce apoptosis in huh7 human hepatocellular carcinoma cells through a mitochondrial-dependent pathway and downregulation of XIAP. J Agric Food Chem. 2010;58:6110–8. doi: 10.1021/jf100574j. [DOI] [PubMed] [Google Scholar]


