Abstract
The prevalence of diabetes mellitus (DM), a chronic disease with hyperglycemia and impaired immune function, is increasing worldwide. Progression from impaired glucose tolerance (IGT) to type 2 DM has recently become a target for early intervention. The fruiting bodies (FB) and submerged culture mycelium (CM) of Tremella mesenterica, an edible and medicinal mushroom, have been demonstrated to have antihyperglycemic and immunomodulatory activities in type 1 DM rats. Herein, we investigated the effects of acidic polysaccharide glucuronoxylomannan (GX) extracted from CM on the immunocyte responses. Male Wistar rats were injected with streptozotocin (65 mg/kg) plus nicotinamide (200 mg/kg) for the induction of IGT, and gavaged daily with vehicle, FB, CM, or GX (1 g/kg/day). Rats injected with saline and gavaged vehicle were used as controls. Two weeks later, peripheral blood leukocytes (PBLs) and splenocytes were collected. Ingestion of FB, CM, and GX significantly decreased blood glucose levels in the postprandial period and in oral glucose tolerance test, and partially reversed T-splenocytic proliferation in IGT rats. CM significantly decreased T-helper lymphocytes in the PBLs and B-splenocytes. In addition, FB, CM, and GX significantly reversed the IGT-induced decreases in tumor necrosis factor-α production; GX significantly increased interleukin-6 production in T-lymphocytes in the PBLs and splenocytes; and CM and GX significantly reversed IGT-induced decrease in interferon-γ production in T-lymphocytes in the spleen. In conclusion, FB, CM, and acidic polysaccharide GX of T. mesenterica may increase T-cell immunity via the elevation of proinflammatory and T-helper cytokine production in rats with impaired glucose tolerance.
Keywords: Cytokines, Diabetes, Immunocytes, Polysacchardies, Tremella mesenterica
INTRODUCTION
The World Health Organization (WHO) indicated that approximately 3.4 million people died from the consequences of high fasting blood glucose, the major symptom of diabetes mellitus (DM), in 2010 and 347 million people have DM in 2013 worldwide.[1,2] The economic costs of DM are increasing globally, which cause a heavy health and economic burden.[3] Therefore, several countries have made and are still making great efforts on preventing and delaying the onset of DM, especially type 2 DM. Type 2 DM comprises 90% of people with DM around the world.[1] Before being diagnosed, almost all of the type 2 DM patients suffer from impaired glucose tolerance (IGT) for years.[4]
DM patients have abnormal carbohydrate, fat, and protein metabolism.[2] It has been reported that type 2 DM and IGT patients have increased blood tumor necrosis factor alpha (TNF)-α[5] and interleukin (IL)-6,[6] suggesting a cytokine-mediated acute-phase reaction inflammation.[7,8] In addition, the peripheral blood leukocytes (PBLs) obtained from type 2 DM patients were found to have reduced proliferative response to mitogens, suggesting an impaired cell-mediated immunity.[9,10] T cells in type 2 DM patients were toward proinflammatory subsets that may promote chronic inflammation in type 2 DM through elevated cytokine production.[11] These results implied that patients with type 2 DM have altered immune function.
The use of dietary supplements, nutraceuticals, and functional foods has become a popular approach to prevent and delay the development of type 2 DM, which may have the potential to eliminate the health and economic burden of the disease. Growing evidence suggests that mushroom polysaccharides have various biological activities, such as antitumor,[12] antidiabetic,[13] anti-inflammatory,[14] immunomodulatory,[15,16] and steroidogenic activities.[17] Tremella mesenterica (Heterobasidiomycetes), a yellow brain jelly mushroom, is a popular edible and medicinal mushroom in Asia. Its pharmacologically active polysaccharides make up 60-70% structural fruiting body polysaccharides and up to 20% of polysaccharides are glucuronoxylomannan (GX).[18] We previously demonstrated that ingestion of T. mesenterica fruiting bodies (FB), submerged culture mycelium (CM), and CM derived GX significantly attenuated the increases of blood glucose levels in 2-h postprandial period and in an oral glucose tolerance test (OGTT) in IGT rats.[19] In addition, T. mesenterica FB may increase peripheral cell-mediated immunity and CM may decrease proinflammatory and T-helper (Th) 1 cytokine production in type 1 DM rats.[20] However, the effects of T. mesenterica on immune function in type 2 DM and IGT have not been investigated.
In the present study, we aimed to investigate whether T. mesenterica may attenuate the altered immunity in IGT using a rat model injected with nicotinamide and streptozotocin, a cytotoxic agent that specifically affects pancreatic β-cells.[21] To evaluate changes in systemic and tissue-specific immunity, the subset distribution of immunocytes, and immunocytic proliferation, the peripheral blood and spleen were collected from IGT rats that ingested FB, CM, and GX of T. mesenterica for 2 weeks. The levels of cytokine production by the PBLs and splenocytes with and without mitogen stimulation were also determined.
MATERIALS AND METHODS
Animals and experimental design
Male Wistar rats (Animal Center of National Taiwan University, Taipei, Taiwan) weighing approximately 200 g (~6 weeks old, n = 50) were housed in individual stainless steel cages with free access to water and a normal chow diet (AIN-76; ICN Biomedicals Inc., Cleveland, OH) in a room maintained at 22°C on a 12:12-h light: dark cycle. The animal facilities and protocols were approved by the Institutional Animal Care and Use Committee (IACUC) in Changhua Christian Hospital, Changhua, Taiwan.
After the rats were adapted for 7 days, they were fasted overnight, weighed, and divided into normal and IGT groups. Under light ether anesthetization, normal rats were intraperitoneally and intravenously injected with saline (n = 8). IGT rats (n = 42) were intraperitoneally injected with nicotinamide (200 mg/kg, Sigma Chemical Co., St. Louis, MO, USA) 15 min before the injection of streptozotocin (STZ, 65 mg/kg of body weight, Sigma Chemical Co., St. Louis, MO, USA) via tail vein. Five days later, animals were fed with a chow diet at 0900 h to 1100 h and blood was drawn via the tail vein at 1300 h to measure the 2-h postprandial blood glucose concentrations. Rats having 2-h postprandial blood glucose concentrations higher than 250 mg/100 ml were considered as IGT and were divided into NTZ, NFB, NCM, and NGX groups. The final sample size was nine rats per group, with 85.7% rate of success in the induction of IGT.
Animals in the CON, NTZ, NFB, NCM, and NGX groups were intragastrically treated with vehicle, vehicle, FB, CM, and acidic polysaccharide GX for 2 weeks (1 g/kg/day on days 1-14), respectively. The vehicle provided equal amounts of the major components of CM, including protein (22.7%, casein), carbohydrate (56%, corn starch), and fiber (16.3%, cellulose). In order to pass through the feeding syringe and needle smoothly, the pulverized FB, CM, GX, and vehicles were mixed with distilled water to form paste mixtures (0.25 g/2 ml) for bolus feeding. The intragastric treatments were administered before providing the chow diet.
During the experimental period, body weights of the rats were measured two times per week and the amounts of food and water intake were measured daily. On day 15, animals were executed after being anesthetized by intramuscular injections of ketamine (100 mg/kg/day) and xylazine (10 mg/kg/day). After the animals became thoroughly unconscious, blood was collected by cardiac puncture, and plasma and blood cells were isolated for various assays. The spleens were dissected, weighed, and processed for further analyses.
Preparation of FB, TM, and GX
T. mesenterica Retz. Fr. (CBS 101939) FB were collected in Israel on the dead wood of Quercus calliprinos Webb. The basidiospore prints were obtained from a fresh fruiting body placed under a sterile Petri dish in a moist chamber with slowly decreasing humidity and were used for the submerged culture, as described by Reshetnikov et al.[18] Culture mycelia were obtained from a submerged cultivation of fermentation, and acidic polysaccharide GX was extracted and purified by Amberlite IR-120 (H+-form) chromatography column, 10% aqueous cetyl pyridinium chloride, 10% sodium chloride, and ethanol.[18] The relative content of acidic polysaccharide GX in the TM was approximately 50%. The components of TM included glycogen (6%), dietary fiber (16.3%), mineral (5%), protein (20%), and free amino acid (2.7%).
Blood glucose and OGTT
The 2-h postprandial blood glucose concentrations were measured prior to the induction of IGT, 5 days after the induction, and on days 0 and 14. After overnight fasting on day 10, the OGTT was performed for the animals. Briefly, glucose (2 g/kg body weight) was intragastrically administered as a 30% solution to animals. Blood samples were collected sequentially from the tail vein before, and 10, 20, 30, 60, 90, and 120 min after glucose administration. The blood glucose concentrations were determined using MediSense Card Blood Glucose Testing Systems (Precision Plus Electrodes; Abbott Laboratories, Bedford, MA, USA), using an electrochemical detection technique accompanied with glucose oxidase method.
Serological analysis and plasma cytokines
The numbers of circulating white blood cells (WBC) were determined using a hematology analyzer (GEN; Coulter Inc., Miami, FL, USA). Plasma concentrations of TNF-α, interferon (IFN)-γ, and IL-6 were measured using commercially available enzyme-linked immunosorbent assays (ELISA; R&D System, Minneapolis, MN, USA) with sensitivities 5, 10, and 18 pg/ml, respectively. All the samples for ELISA measurements were analyzed in one assay in duplicate. The inter-assay coefficients of variance were below 10%.
Analysis of immunocyte subpopulation
For the immunocyte subpopulation in the PBLs, ethylenediaminetetraacetic acid (EDTA)-whole blood was reacted with appropriate antibodies directed against cell-surface antigens and analyzed by flow cytometer, as described in our previous study.[20] For splenocytes, single-cell suspensions of splenocytes were prepared and 1 × 106 splenocytes were used to be labeled with the appropriate antibodies.
The cell-surface markers for determination of immunocyte subpopulations included CD3-fluorescein isothiocyanate (FITC, clone G4.18), CD3-phycoerythrin (PE, clone G4.18), CD4-PE (clone OX-35), and CD8b-FITC (clone 341) for T cells and CD45RA-PE (clone OX-33) and IgM-FITC (clone G53-238) for B cells. FITC-conjugated mouse IgG1 (clone A112-2) and PE-conjugated mouse IgG3 (clone A112-3) were used as nonspecific isotype control antibodies. Immunofluorescence detection of cell subsets was performed using a Becton–Dickinson FASCScan flow cytometer with excitation capabilities at 488 nm for the measurements of FITC- and PE-conjugated mouse anti-rat monoclonal antibodies (BD Biosciences, San Jose, CA, USA).
Analysis of immunocyte function
The proliferation of splenocytes in vitro in response to mitogens such as concanavalin A (Con A, a T-cell mitogen) and lipopolysaccharide (LPS, a B-cell and macrophage/monocyte mitogen) was measured. Splenocytes (2.5 × 105 cells) were plated in triplicate with 50 μl of RPMI 1640 medium with or without Con A (5 μg/ml) and LPS (5 μg/ml). After incubation at 37°C in 5% CO2 for 36 h, cell proliferation was determined by the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] method (CellTiter 96® aqueous one solution; Promega, Madison, WI, USA). The stimulation index of cell proliferation was calculated from the absorbance of splenocytes cultured with mitogen at 490 nm divided by that of those cultured in RPMI 1640 medium alone.
The PBLs and splenocytes were plated in triplicate with RPMI 1640 medium with or without LPS (5 μg/ml) and Con A (5 μg/ml) and cultured at 37°C in 5% CO2 for 18 h and the supernatants were removed by centrifugation and stored at −80°C. The cytokine production of TNF-α, IFN-γ, and IL-6 in the PBLs and splenocytes (5 × 106 cells) was measured using ELISA. Samples were analyzed in one assay in duplicate.
Statistical analysis
The differences among all groups were compared by one-way analysis of variance (ANOVA) using the SAS general linear models program. Values were represented as the means ± SEM. The protective least-significant difference (LSD) technique was used as the post-hoc analysis to compare the differences between groups when ANOVA indicated an overall significant treatment effect.
RESULTS
Body weight, food and water intake, and blood glucose
The body weight gains during the experimental period were not significantly different among groups [Figure 1a]. However, the amount of weekly food intake was significantly increased in the NTZ (150.3 ± 6.9 g) and NCM (134.0 ± 5.9 g) groups compared to the CON group (117.9 ± 3.1 g). The amount of weekly water intake was significantly increased in the NTZ (344.9 ± 56.9 ml) group compared to the CON group (231.4 ± 17.4 ml). Neither FB nor GX significantly altered the amount of food and water intake in IGT rats.
Figure 1.
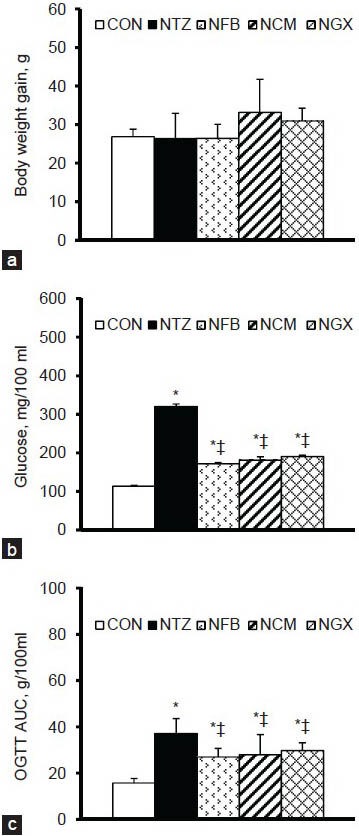
(a) Body weight gain from day 0 to day 14, (b) blood glucose concentrations in the 2-h postprandial period, and (c) blood glucose response in OGTT shown as area under the curve (AUC) in normal and IGT rats. Values are expressed as the means ± SEM (n = 8-9 per group). Differences were assessed using one-way ANOVA, followed by post-hoc tests of least significant difference (LSD). *P< 0.05 vs. CON group; ‡P< 0.05 vs. NTZ group in IGT rats
The 2-h postprandial blood glucose concentrations on day 0 were significantly higher in IGT rats (310.8 ± 15.1 mg/100 ml) than in normal rats (113.1 ± 4.2 mg/100 ml), but there was no significant difference among the NTZ, NFB, NCM, and NGX groups. The 2-h postprandial blood glucose levels on day 14 were significantly increased in the NTZ group compared to the CON group [Figure 1b]. Ingestion of FB, TM, and GX significantly attenuated the increases in the 2-h postprandial blood glucose levels.
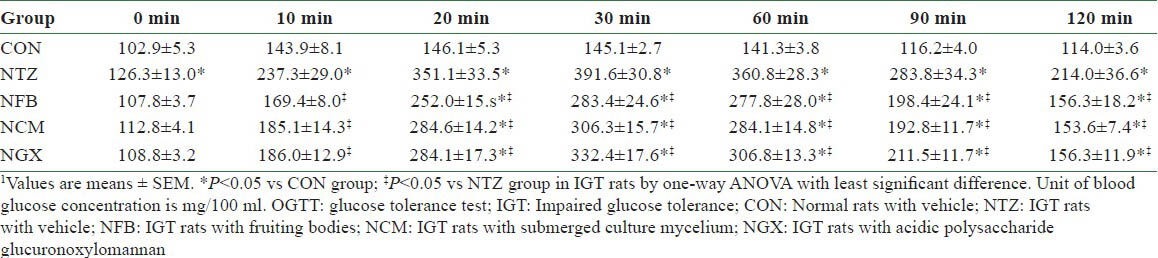
In the OGTT, blood glucose concentrations at 0 (i.e. the fasting blood glucose), 10, 20, 30, 60, 90, and 120 min were significantly higher in the NTZ group than those in the CON group [Table 1]. The NFB, NCM, and NGX groups had significantly lower blood glucose at 10, 20, 30, 60, 90, and 120 min, compared to the NTZ group. The blood glucose response in OGTT, as shown in area under the curve (AUC), was significantly increased in IGT rats and was significantly attenuated by ingestion of FB, TM, and GX [Figure 1c].
Table 1.
Blood glucose concentrations in the OGTI on day 10 in normal and IGT rats1
Plasma concentrations of cytokines
Plasma concentrations of TNF-α, IFN-γ, and IL-6 are shown in Figure 2. There were no significant differences in plasma TNF-α and IFN-γ concentrations among groups. The NFB and NGX groups had significantly lower plasma IL-6 concentrations, compared to the CON group. However, ingestion of FB, TM, and GX had no significant effect on the plasma IL-6 concentration in IGT rats.
Figure 2.
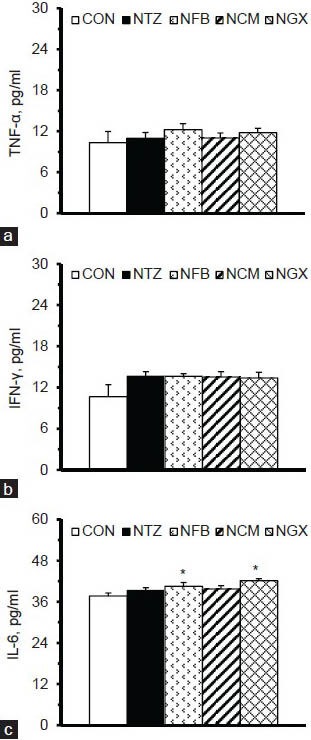
Plasma concentrations of (a) TNF-α, (b) IFN-γ, and (c) IL-6 in normal and IGT rats. Values are expressed as the means ± SEM (n = 8-9 per group). Differences were assessed using one-way ANOVA, followed by post-hoc tests of least significant difference (LSD). *P< 0.05 vs. CON group
Immunocyte subpopulation
There was no significant difference in the circulating numbers of WBC among groups (data not shown). In PBLs, there were no significant differences in the percentages of total T cells (CD3+, data not shown), Th cells (CD3+ CD4+), T-suppressor cells (CD3+ CD8b+), and mature B cells (CD45RA+IgM+) between the CON and NTZ groups [Table 2]. However, the NCM group had significantly decreased percentage of Th cells than the CON and NTZ groups. In addition, NCM and NGX groups had significantly decreased percentages of T-suppressor cells, compared to the CON group. Ingestion of FB, TM, and GX did not have significant impact on the mature B cells.
Table 2.
Percentages of lymphocyte subsets in the peripheral blood and spleen in normal and IGT rats1
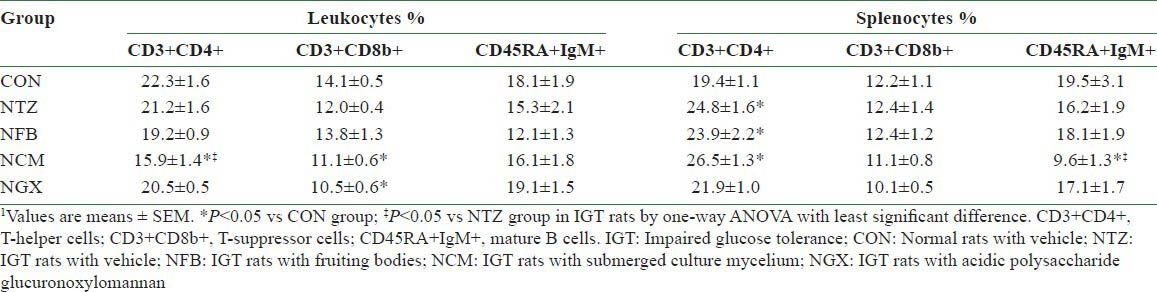
There was no significant difference in the spleen weights among groups (data not shown). In the splenocytes, the NTZ, NFB, and NCM groups had 20-25% increases in the percentages of Th cells, compared to the CON group. The percentages of T-suppressor cells in the splenocytes were not significantly different among groups. However, the NCM group had significantly decreased mature B cells in the splenocytes, compared to the CON and NTZ groups.
Splenocytic proliferation
The numbers of splenocytes were not significantly different among groups [Figure 3a]. To evaluate the function of splenocytes, Con A and LPS were used as mitogens to stimulate the proliferation of T and B cells/macrophages in the splenocytes. The results showed that the NTZ group had significantly decreased stimulation index in response to Con A [Figure 3b] but not LPS [Figure 3c], compared to the CON group. Ingestion of FB, TM, or GX had no significant impact on the proliferation of splenocytes derived from IGT rats.
Figure 3.
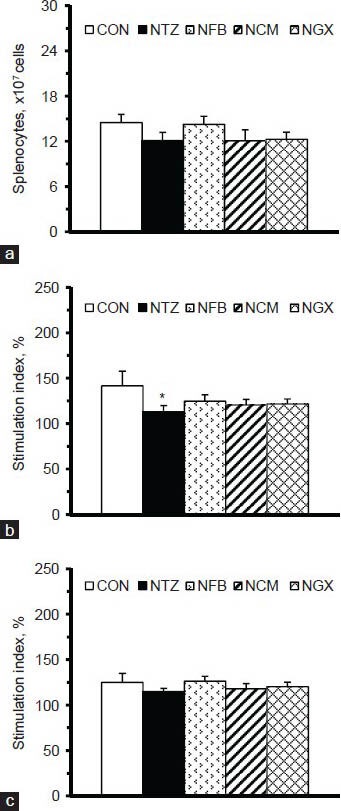
(a) The numbers of splenocytes and stimulation indices of cell proliferation in splenocytes (5 × 106 cells/ml) cultured with (b) Con A and (c) LPS. The stimulation index was calculated as the OD values (at 490 nm) of the splenocytes cultured with Con A or LPS divided by those cultured in medium alone, and multiplied by 100. Values are expressed as the means ± SEM (n = 8-9 per group). Differences were assessed using one-way ANOVA, followed by post-hoc tests of least significant difference (LSD). *P< 0.05 vs. CON group
Cytokine production by immunocytes
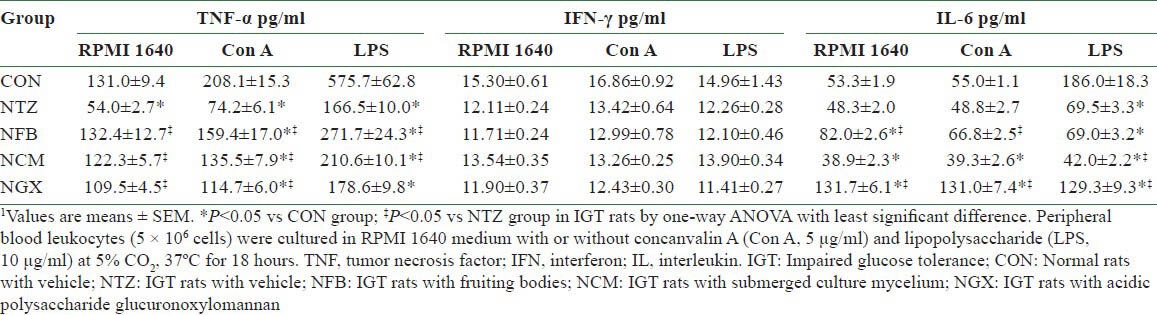
In the PBLs, the unstimulated and Con A- and LPS-stimulated production of TNF-α [Table 3] was significantly decreased in the NTZ group compared to the CON group. FB and TM ingestion significantly reversed these IGT-induced decreases in TNF-α production, and GX ingestion significantly reversed the decreases in the unstimulated and Con A-stimulated production. The production of IFN-γ with or without stimulations was not significantly different among groups. The unstimulated and Con A-stimulated production of IL-6 was significantly greater in the NFB and NGX groups than in the CON and NTZ groups. The IGT-decreased IL-6 production in LPS-stimulated PBLs was further decreased by the TM ingestion, whereas it was significantly reversed by the GX ingestion.
Table 3.
Cytokine productions of peripheral blood leukocytes in normal and IGT rats1
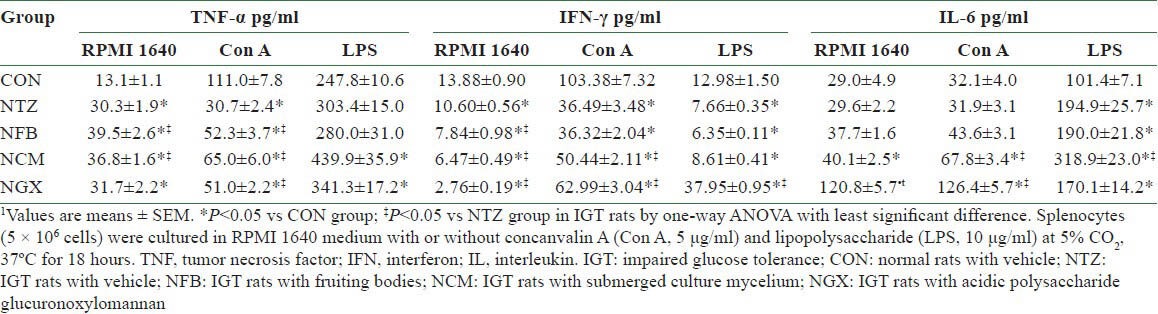
In the splenocytes, the unstimulated production of TNF-α [Table 4] was significantly increased and the Con A-stimulated production of TNF-α was significantly decreased in the NTZ group compared to the CON group. FB and TM ingestion further increased the unstimulated TNF-α production and reversed the Con A-stimulated TNF-α production in IGT rats. The NCM and NGX groups had significantly increased TNF-α production in LPS-stimulated splenocytes, compared to the CON group. The IFN-γ production of the splenocytes with or without stimulations was significantly decreased in the NTZ group compared to the CON group. FB, TM, and GX ingestion further decreased the unstimulated IFN-γ production; TM and GX ingestion significantly reversed the Con A-stimulated IFN-γ production; and GX ingestion not only reversed but further increased the LPS-stimulated IFN-γ production. In addition, the LPS-stimulated IL-6 production was significantly increased in the NTZ group compared to the CON group. The NCM group had 2- and 1.6-fold greater Con A- and LPS-stimulated IL-6 production, respectively, compared to the NTZ group. Moreover, the NGX group had approximately fourfold greater unstimulated and Con A-stimulated IL-6 production, compared to the NTZ group.
Table 4.
Cytokine productions of splenocytes in normal and IGT rats1
DISCUSSION
Accumulating evidence suggests that IGT and type 2 DM patients have altered or impaired innate immunity and elevated circulating proinflammatory cytokines that contributed to insulin resistance and infection susceptibility.[22] The antihyperglycemic and immunomodulatory effects of T. mesenterica have been demonstrated in animal studies.[16,19,20] In type 1 DM rats, ingestion of CM from T. mesenterica may improve the acute phase response, correct the shift in the cytokine profile of the PBLs, and decrease the splenocytic proinflammatory response.[20] In the present study, ingestion of FB, CM, and GX from T. mesenterica significantly reversed the IGT-induced decreases in TNF-α production in the PBLs and further decreased IFN-γ and increased IL-6 production in the splenocytes. These results imply that T. mesenterica has the potential to modulate innate and cell-mediated immunity in subjects with IGT or prediabetes.
The results of epidemiological studies indicated that IGT subjects have an elevated risk of cardiovascular morbidity and mortality[23] because they share a low-grade, proinflammatory state with altered immune function.[24,25,26] In our study, rats administered with nicotinamide and STZ had significantly increased blood glucose in the 2-h postprandial period [Figure 1b] and during the OGTT period [Table 1 and Figure 1c]. However, IGT rats did not have significantly increased plasma TNF-α, IFN-γ, or IL-6, unlike type 1 DM rats[20] and IGT patients.[24] These differences may be because the induction period of this study was not long enough, the severity of IGT was not strong enough, and/or the etiology of IGT in these rats was different from that of type 1 DM rats and IGT patients.
Cell-mediated immunity is crucial in host defense against intracellular pathogens. Chang and Shaio[9] reported that the production of Th1 cytokines, such as IL-1β, IL-2, and IFN-γ, and the percentages of total T (CD3+), Th (CD4+), and T-suppressor (CD8+) cells in PBLs were not different between type 2 DM patients and healthy subjects. However, recent evidence suggested that type 2 DM patients had impaired cell-mediated immunity as shown in the insufficient lymphocyte proliferation in PBLs.[27,28] In the present study, IGT rats had a significant decreased proliferative response in Con A-stimulated splenocytes [Figure 3b], with no significant changes in the percentages of T and B cells in PBLs [Table 2], whereas the percentages of Th splenocytes were significantly elevated. Whether this IGT-induced increase in Th splenocytes is a compensative reaction in response to the decreased T-cell proliferation deserves further investigation.
We also found that TNF-α production of spontaneous (unstimulated) and Con A- and LPS-stimulated PBLs was significantly decreased in IGT rats [Table 3]. This decrease implies that IGT rats had decreased acute phase reaction and inefficient systemic monocytes, T-lymphocytes, and natural killer cells. These changes may explain, at least partially, why IGT rats did not have significant changes in circulating cytokines as the type 1 DM rats did.[20] In terms of the splenic immunity, the Con A-stimulated TNF-α production [Table 4] and the spontaneous and Con A- and LPS-stimulated IFN-γ production were decreased in IGT rats compared to normal rats. These results suggest that IGT rats had impaired innate and T-cell mediated immunity in the spleen.
It has been found that mushrooms have antihyperglycemic activities because they are low in energy and carbohydrate, have no fat and cholesterol, and are high in protein, vitamins, and minerals, as well as they have abundant high-molecular-weight compounds, such as, polysaccharides, and a number of low-molecular-weight metabolites, such as lectins, lactones, terpenoids, alkaloids, sterols, and phenolic substances.[29] Our studies showed that FB, CM, and GX derived from T. mesenterica had significant antihyperglycemic activities in type 1 DM[19] and IGT rats. For example, ingestion of FB, CM, and GX significantly improved the glycemic response in IGT rats as shown by the decreased blood glucose levels in 2-h postprandial period and OGTT response and AUC [Figure 1 and Table 1].
Mushroom polysaccharides have various biological activities.[12,13,14,15,16,17] Polysaccharides of Tremella fuciformis have been reported to augment T-lymphocytes in the PBL proliferation and decrease the IL-2 activities in mouse splenocytes in vitro.[12] Jeong et al.[30] compared the immune-modulatory properties of exo- and endopolysaccharides extracted from seven mushroom species and showed that the exopolysaccharides of T. mesenterica may reduce IFN-γ, IL-2, IL-4, and IL-5 production in T-lymphocytes. In type 1 DM rats, FB from T. mesenterica may increase peripheral cell-mediated immunity and CM may decrease proinflammatory and Th1 cytokine production.[20] In this study, FB, CM, and GX of T. mesenterica did not have significant impact on plasma TNF-α, IFN-γ, and IL-6 concentrations and spontaneous and mitogen-stimulated splenocytic proliferation in IGT rats. In contrast, ingestion of CM significantly decreased the percentages of Th lymphocytes in the PBLs and B-splenocytes [Table 2]. These results reveal that the immune-modulatory effects of Tremella polysaccharides are different within the species in the same genus.
When evaluating the abilities of monocytes, macrophages, and T-cells by measuring TNF-α production, we previously showed that CM of T. mesenterica significantly decreased proinflammatory and Th1 cytokine production by PBLs and increased their production by splenocytes in type 1 DM rats.[20] In IGT rats, we found that FB and CM ingestion significantly reversed the IGT-induced decreases in unstimulated PBLs and Con A- and LPS-stimulated PBLs [Table 3] and Con A-stimulated splenocytes [Table 4] and further elevated the increases in unstimulated splenocytes. The acid polysaccharide, GX, significantly increased TNF-α production in spontaneous and Con A-stimulated PBLs. This altered cytokine production reveals that natural FB of T. mesenterica and its submerged culture mycelia and acid polysaccharides may elevate systemic innate immunity in IGT rats.
The IGT-decreased production of IFN-γ, a Th1 cytokine important for macrophage activation and critical for innate and adaptive immunity, in Con A-simulated splenocytes was significantly reversed by CM and GX, and this decrease in LPS-stimulated splenocytes was significantly increased approximately fivefold by GX. However, IGT-induced decrease in unstimulated splenocytes was further decreased by FB, CM, and GX. These results imply that T. mesenterica may inactivate T-splenocytes in normal condition and the submerged culture mycelia, especially the acidic polysaccharides, may improve IGT-induced impairment in T-cell mediated immunity when splenocytes are stimulated.
IL-6, a cytokine, acts as both pro- and anti-inflammatory cytokine, and is secreted by T cells and macrophages to stimulate immune response. T. mesenterica did not affect the IL-6 reduction of PBLs and splenocytes in type 1 DM rats.[20] In the present study, the IGT-induced decrease in IL-6 production in monocytes was significantly reversed by GC. In addition, ingestion of FB and GX significantly increased the spontaneous and Con A-stimulated IL-6 production in PBLs. In terms of tissue-specific immunity, IL-6 production in macrophages was increased twofold by CM and that in T-splenocytes was increased fourfold by GX. The elevated IL-6 production implies that T. mesenterica may increase innate and T-cell immunity in IGT rats.
In the present study, rats orally consumed approximately 250 mg/day of T. mesenterica, i.e., 1 g/kg body weight/day for 28 days. This dose corresponds to the recommended daily human dose which is 9.7 g in a person weighing 60 kg. These results suggest that 1000 mg/kg of T. mesenterica FB, CM, and GX could be categorized as no-observed-adverse-effect level (NOAEL) products, as it acts harmlessly under the current normal usage.
The present study has several limitations. First, the etiology of IGT induced by nicotinamide and STZ in rats is not the same as that in patients. Even though the gene knockout rat models are better than the chemical-induced models, the price and the source of the animals are not feasible for most of the researchers. Therefore, we used this chemical-induced animal model, as these rats had increased blood glucose in the postprandial and OGTT periods. The glycemic response is similar to that of IGT subjects. Second, we only performed this study for 14 days. Thus, we might have overlooked the long-term effects of T. mesenterica. This might explain partially why those rats did not have significant changes in plasma cytokines as shown in other studies. Third, we did not investigate the mechanisms of T. mesenterica in modulating systemic and splenic immunity. In this study, we could only show the changes and profiles of the immunocytic subsets and cytokine production. It is of interest to elucidate the detailed mechanisms of T. mesenterica in regulating splenocytic proliferation and activating the functions of PBLs and splenocytes.
In summary, the results presented here confirm that IGT is associated with elevation in Th splenocytes and suppression in proliferation of T-splenocytes, TNF-α production of PBLs, and IFN-γ production of splenocytes. Ingestion of FB, CM, and GX improved hyperglycemia and reversed the IGT-decreased peripheral and splenocytic innate immunities. In addition, CM and GX reversed IGT-decreased T-cell mediated immunity in splenocytes and GX increased IL-6 production in PBLs and splenocytes. Taken together, our findings reveal that FB, CM, and acidic polysaccharide GX of T. mesenterica may increase innate and T-cell mediated immunity via the elevation of proinflammatory and Th cytokine production in rats with IGT. The improvement in glycemic response and the elevation in innate and T-cell mediated immunity suggest that T. mesenterica may be used as an adjuvant treatment in subjects with IGT to prevent or to delay the onset of type 2 DM.
ACKNOWLEDGMENTS
This work was supported by a grant (CCH 4604) from Changhua Christian Hospital. We thank Ms. Fu-Ann Tsai, Su-Chen Lin, and Ya-Chi Lai for their technical support.
REFERENCES
- 1.World Health Organization. [Last accessed in 2013 Jul]. Available from: http://www.who.int/mediacentre/factsheets/fs312/en .
- 2.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 3.WHO (2010). Burden: mortality, morbidity and risk factors-Chapter 1 of the latest WHO report “Global status report on NCDs 2010”. [Last accessed on 2011 Apr]. Available from: http://www.who.int/nmh/publications/ncd_report_chapter 1.pdf .
- 4.American Diabetes Association. [Last accessed on 2013 Jul]. Available from: http://www.diabetes.org/diabetes-basics/prevention/pre-diabetes/?loc=DropDownDB-prediabetes .
- 5.Lechleitner M, Herold M, Dzien-Bischinger C, Hoppichler F, Dzien A. Tumour necrosis factor-alpha plasma levels in elderly patients with Type 2 diabetes mellitus-observations over 2 years. Diabet Med. 2002;19:949–53. doi: 10.1046/j.1464-5491.2002.00846.x. [DOI] [PubMed] [Google Scholar]
- 6.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–8. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, et al. Markers of inflammation and prediction of diabetes mellitus in adults (atherosclerosis risk in communities study): A cohort study. Lancet. 1999;15:1649–52. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 9.Chang FY, Shaio MF. Decreased cell-mediated immunity in patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1995;28:137–46. doi: 10.1016/0168-8227(95)00168-8. [DOI] [PubMed] [Google Scholar]
- 10.Müller S, Martin S, Koenig W, Hanifi-Moghaddam P, Rathmann W, Haastert B, et al. Impaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute-phase proteins but not TNF-alpha or its receptors. Diabetologia. 2002;45:805–12. doi: 10.1007/s00125-002-0829-2. [DOI] [PubMed] [Google Scholar]
- 11.Jagannathan-Bogdan M, McDonnell ME, Shin H, Rehman Q, Hasturk H, Apovian CM, et al. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol. 2011;186:1162–72. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma L, Lin ZB. Effect of Tremella polysaccharide on IL-2 production by mouse splenocytes. Yao Xue Xue Bao. 1992;27:1–4. [PubMed] [Google Scholar]
- 13.Ukai S, Kiriki H, Nagai K, Kiho T. Synthesis and antitumor activities of conjugates of mitomycin C-polysaccharide from Tremella fuciformis. Yakugaku Zasshi. 1992;112:663–8. doi: 10.1248/yakushi1947.112.9_663. [DOI] [PubMed] [Google Scholar]
- 14.Kiho T, Kochi M, Usui S, Hirano K, Aizawa K, Inakuma T. Antidiabetic effect of an acidic polysaccharide (TAP) from Tremella aurantia and its degradation product (TAP-H) Biol Pharm Bull. 2001;24:1400–3. doi: 10.1248/bpb.24.1400. [DOI] [PubMed] [Google Scholar]
- 15.Gao QP, Jiang RZ, Chen HQ, Jensen E, Seljelid R. Characterization and cytokine stimulating activities of heteroglycans from Tremella fuciformis. Planta Med. 1996;62:297–302. doi: 10.1055/s-2006-957888. [DOI] [PubMed] [Google Scholar]
- 16.Cui JY, Lin ZB. Effects of Tremella polysaccharide on cytoplasmic free calcium concentration in murine splenocytes. Yao Xue Xue Bao. 1997;32:561–4. [PubMed] [Google Scholar]
- 17.Lo HC, Yang JG, Liu BC, Chen YW, Huang YL, Poon SL, et al. The effects of Tremella aurantia on testosterone and corticosterone productions in normal and diabetic rats. Arch Androl. 2004;50:395–404. doi: 10.1080/01485010490484129. [DOI] [PubMed] [Google Scholar]
- 18.Reshetnikov SV, Wasser SP, Duckman I, Tsukor K. Regulation of growth and biosynthetic activity of the medicinal jelly mushroom Tremella mesenterica Retz.: Fr. pure culture. Int J Med Mushrooms. 2001;3:45–51. [Google Scholar]
- 19.Lo HC, Tsai FA, Wasser SP, Yang JG, Huang BM. Effects of ingested fruiting bodies, submerged culture biomass, and acidic polysaccharide glucuronoxylomannan of Tremella mesenterica Retz.: Fr. on glycemic responses in normal and diabetic rats. Life Sci. 2006;78:1957–66. doi: 10.1016/j.lfs.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Lo HC, Hsu TH, Lin FY, Wasser SP, Chen YH, Lee CH. Effects of yellow brain culinary-medicinal mushroom, Tremella mesenterica Ritz.:Fr. (higher Basidiomycetes), on immune function in normal and type diabetic rats. Int J Med Mushrooms. 2012;14:447–57. doi: 10.1615/intjmedmushr.v14.i5.20. [DOI] [PubMed] [Google Scholar]
- 21.Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, et al. Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998;47:224–9. doi: 10.2337/diab.47.2.224. [DOI] [PubMed] [Google Scholar]
- 22.Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Diabetes. 2003;52:812–7. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 23.Ceriello A. Impaired glucose tolerance and cardiovascular disease: The possible role of post-prandial hyperglycemia. Am Heart J. 2004;147:803–7. doi: 10.1016/j.ahj.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation. 2002;106:2067–72. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 25.Choi KM, Lee J, Lee KW, Seo JA, Oh JH, Kim SG, et al. Comparison of serum concentrations of C-reactive protein, TNF-alpha, and interleukin 6 between elderly Korean women with normal and impaired glucose tolerance. Diabetes Res Clin Pract. 2004;64:99–106. doi: 10.1016/j.diabres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Samaras K, Viardot A, Lee PN, Jenkins A, Botelho NK, Bakopanos A, et al. Reduced arterial stiffness after weight loss in obese type 2 diabetes and impaired glucose tolerance: The role of immune cell activation and insulin resistance. Diab Vasc Dis Res. 2013;10:40–8. doi: 10.1177/1479164112443375. [DOI] [PubMed] [Google Scholar]
- 27.Daoud AK, Tayyar MA, Fouda IM, Harfeil NA. Effects of diabetes mellitus vs. in vitro hyperglycemia on select immune cell functions. J Immunotoxicol. 2009;6:36–41. doi: 10.1080/15476910802604564. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Wen F, Zhang X, Su SB. Expression of T-helper-associated cytokines in patients with type 2 diabetes mellitus with retinopathy. Mol Vis. 2012;18:219–26. [PMC free article] [PubMed] [Google Scholar]
- 29.Lo HC, Wasser SP. Medicinal mushrooms for glycemic control in diabetes mellitus: History, current status, future perspectives, and unsolved problems. Int J Med Mushrooms. 2011;13:401–26. doi: 10.1615/intjmedmushr.v13.i5.10. [DOI] [PubMed] [Google Scholar]
- 30.Jeong SC, Koyyalamudi SR, Hughes J, Khoo C, Bailey T, Marripudi K, et al. Antioxidant and immunomodulating activities of exo-and endopolysaccharide fractions from submerged mycelia cultures of culinary-medicinal mushrooms. Int J Med Mushrooms. 2013;15:251–66. doi: 10.1615/intjmedmushr.v15.i3.30. [DOI] [PubMed] [Google Scholar]


