Abstract
The ethnomedical uses of Piper (胡椒 Hú Jiāo) plants as anticancer agents, in vitro cytotoxic activity of both extracts and compounds from Piper plants, and in vivo antitumor activity and mechanism of action of selected compounds are reviewed in the present paper. The genus Piper (Piperaceae) contains approximately 2000 species, of which 10 species have been used in traditional medicines to treat cancer or cancer-like symptoms. Studies have shown that 35 extracts from 24 Piper species and 32 compounds from Piper plants possess cytotoxic activity. Amide alkaloids account for 53% of the major active principles. Among them, piplartine (piperlongumine) shows the most promise, being toxic to dozens of cancer cell lines and having excellent in vivo activity. It is worthwhile to conduct further anticancer studies both in vitro and in vivo on Piper plants and their active principles.
Keywords: Amide alkaloids, Anticancer, Cytotoxicity, Piper, Piperaceae
INTRODUCTION
Natural products from plants are important sources of new drugs.[1] The genus Piper (胡椒 Hú Jiāo) (Piperaceae), which contains approximately 2000 plant species distributed mainly in tropical areas,[2] is a potential source of drugs based on the use of some Piper species in traditional medicine. For example, nearly 30 out of 60 indigenous Chinese Piper species are used medically.[3,4,5,6,7,8] Our recent ethnobotanical and medicinal chemistry research focused on Piper plants led to the discovery of cytotoxic amides from Piper boehmeriifolium Wall.,[8,9,10,11] an anticancer medicine used in India.[12] In China, Piper plants are also used in some formulae to treat cancers.[13,14] The present paper reviews the traditional uses and scientific evidence for Piper natural products as anticancer agents. We reviewed the scientific articles that were published between 1970 and 2013 from Web of Science, SciFinder, and Google Scholar. We used the following search terms: Piperaceae, Piper, anticancer, antitumor, cytotoxicity, and ethnobotany. No restrictions regarding the language of publication were imposed, but most of the relevant studies were published in English and Chinese. Both plant extracts and compounds were found to show good in vitro cytotoxic activity with concentration giving 50% inhibition (IC50) values less than 30 μg/ml and 4 μg/ml, respectively, and some compounds showed significant in vivo antitumor activity with 50% inhibition of tumor growth at concentrations less than 15 mg/kg body weight in mice.[15]
PIPER PLANTS WITH TRADITIONAL ANTICANCER APPLICATION
In the literature, 10 Piper (胡椒 Hú Jiāo) species have been reported to treat cancer or cancer-like symptoms, as summarized in Table 1.
Table 1.
List of Piper plants used traditionally against cancer or cancer-like symptoms
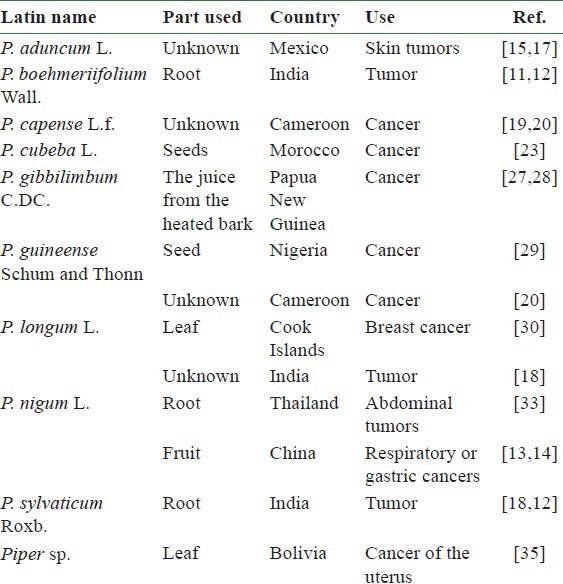
In Mexico, Piper aduncum L. is traditionally used to treat urological problems, dermatological conditions, and skin tumors.[15] Dichloromethane extracts of P. aduncum leaf were marginally cytotoxic to glioma (SF-268), human large cell lung carcinoma (H-460), and human breast carcinoma (MCF-7) cell lines with IC50 values of 23, 25, and 27 μg/ml, respectively [Table 2].[16] Piperaduncin A [27 in Figure 1] a dihydrochalcone from this plant, showed growth inhibitory activity against human nasopharynx carcinoma (KB) cells (IC50 = 2.3 μg/ml) [Table 3].[17]
Table 2.
Cytotoxic crude extracts from Piper plants
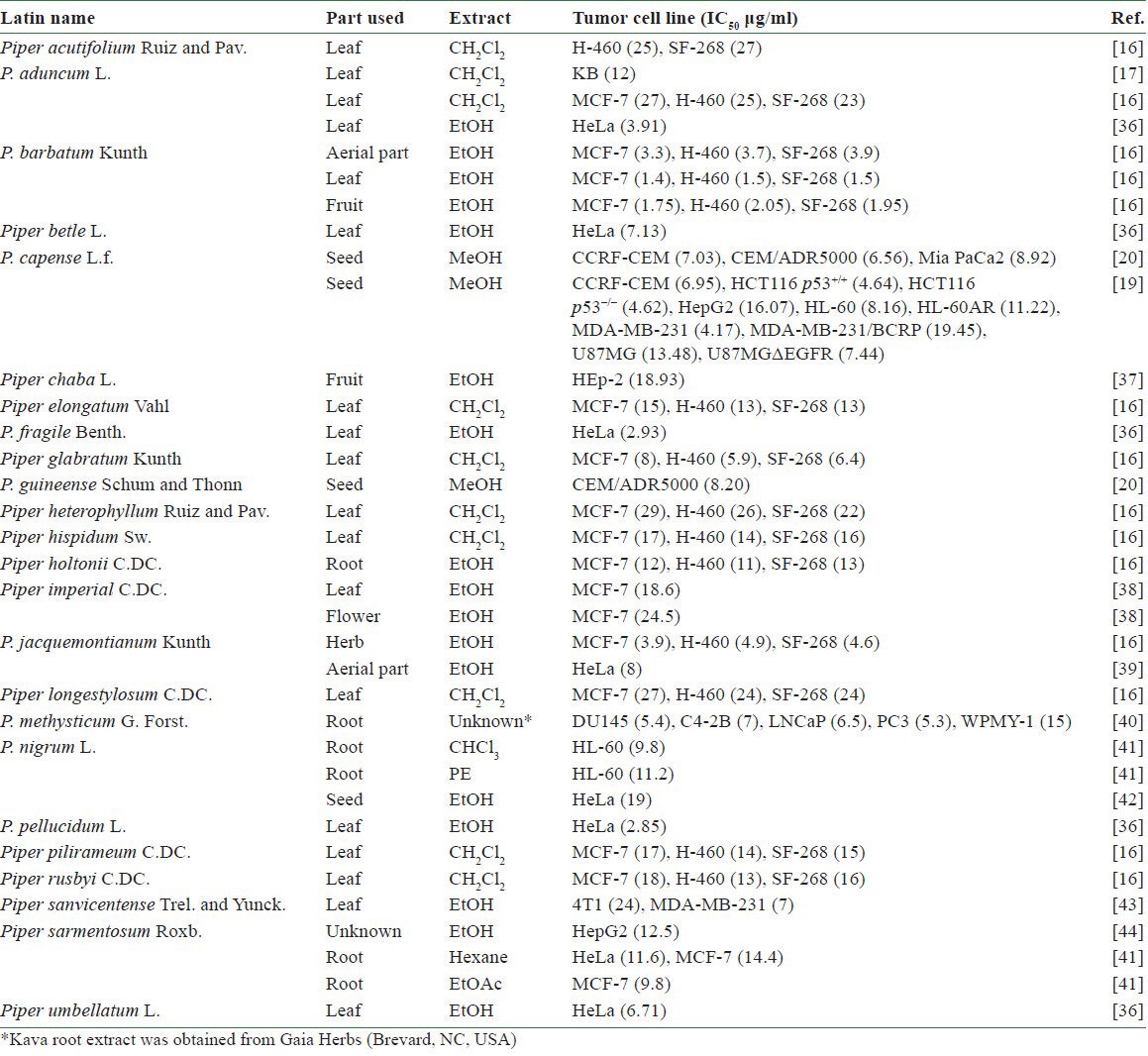
Figure 1.
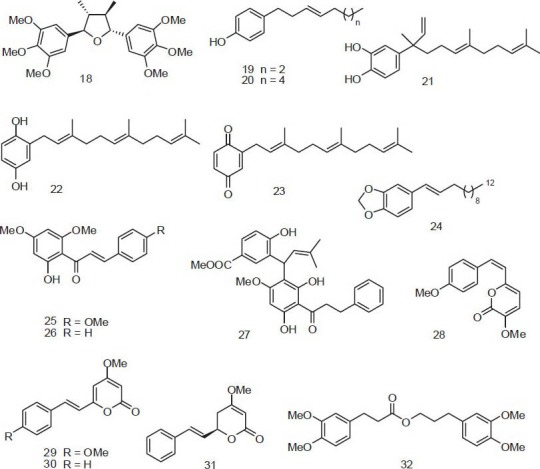
Cytotoxic non-alkaloid constituents from Piper plants
Table 3.
Cytotoxic principles from Piper plants
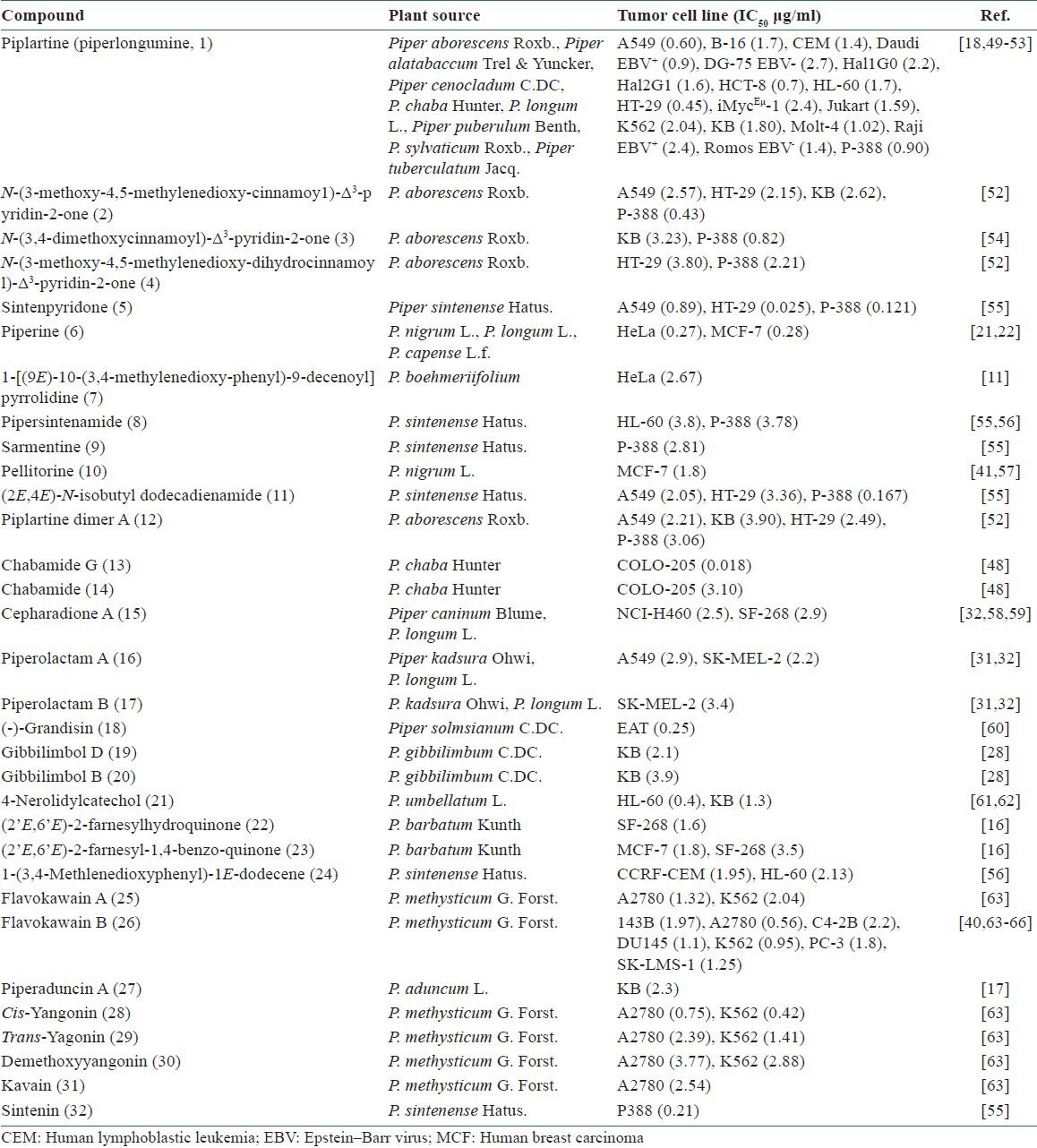
In the Ayurvedic system of Indian medicine, the roots of P. boehmeriifolium Wall. and Piper sylvaticum Roxb. are used for their laxative, anthelmintic, and carminative properties, as well as to treat bronchitis, diseases of the spleen, and tumors.[12] Recently, a cytotoxic amide alkaloid, 1-[(9E)-10-(3,4-methylenedioxyphenyl)- 9-decenoyl] pyrrolidine [7 in Figure 2], was isolated from the whole plant of P. boehmeriifolium. This compound exhibited an IC50 of 2.7 μg/ml against human cervical carcinoma human cervix adenocarcinoma (HeLa) cells [Table 3].[11] The amide alkaloid piplartine [1 in Figure 2, Tables 3 and 4] might be responsible for the anticancer effect of P. sylvaticum.[18]
Figure 2.
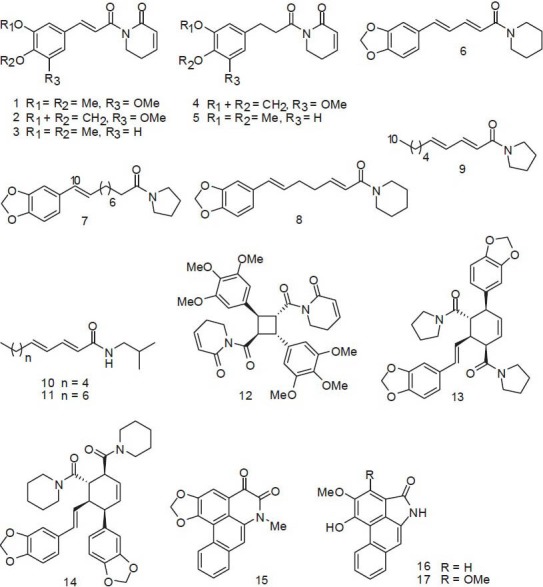
Cytotoxic amide alkaloids from Piper plants
Table 4.
Compounds from Piper plants with antitumor activity in vivo
Piper capense L.f. is reported to treat cancer in Cameroon; however, details about its ethnomedical uses are not included in the literature references.[19,20] Methanolic extracts of the seed are cytotoxic toward many tumor cell lines, including human leukemic lymphoblast (CCRF-CEM; IC50 = 7.03 μg/ml), human acute T-lymphoblastic leukemia (CEM/ADR5000; IC50 = 6.56 μg/ml), human pancreatic adenocarcinoma (Mia PaCa2; IC50 = 8.92 μg/ml), p53-expressing human colon cancer cell (HCT116 p53+/+; IC50 = 4.64 μg/ml), p53-knockout human colon cancer cell (HCT116 p53−/−; IC50 = 4.62 μg/ml), human hepatocarcinoma (Hep-G2; IC50 = 16.07 μg/ml), human myeloid leukemia (HL-60; IC50 = 8.16 μg/ml), anthracycline-resistant HL-60 (HL-60AR; IC50 = 11.22 μg/ml), human breast carcinoma (MDA-MB-231; IC50 = 4.17 μg/ml), MDA-MB-231BCRP (IC50 = 19.45 μg/ml), human malignant glioblastoma (U87MG; IC50 = 13.48 μg/ml), EGFRvIII expressing glioma cells (U87MGΔEGFR; IC50 = 7.44 μg/ml), etc.[19,20] Piperine [6 in Figure 2 and Table 3] might be an active constituent.[21,22]
A recent ethnopharmacological study in Morocco calculated the percent importance of 14 plants selected by 100 herbalists for significance against cancer. Piper cubeba (蓽澄茄 Bì Chéng Qié) was one of the most important plants against cancer.[23] Lignans, such as (-)-cubebin, are the major constituents of P. cubeba.[24] Research results show that the P. cubeba extract (P9605) and the synthetic lignan cubebin might have potential therapeutic use against prostate cancer growth by targeting multiple aspects of the androgen-signaling pathway.[25,26]
In Papua New Guinea, a patient with suspected cancer or other internal sores drinks the juice squeezed from heated bark of Piper gibbilimbum C.DC. with traditional ash salt.[27] Gibbilimbols D (IC50 = 2.1 μg/ml) [19 in Table 3] and B (IC50 = 3.9 μg/ml) [20 in Table 3] from this plant are cytotoxic toward KB cells.[28]
The seed of the Nigerian plant Piper guineense Schum and Thonn reportedly possesses anticancer properties.[29] The plant is also reported to treat cancer in Cameroon.[20] A methanolic extract of its seed was cytotoxic against leukemia CEM/ADR5000 cells (IC50 = 8.20 μg/ml).[20] However, the active constituents remain unclear.
Piper longum L. (syn. Piper latifolium Forst.) is a well-known tropical food and medicinal plant. In traditional medical practice in the Cook Islands, 12 leaves of this plant and a similar number of those of Thespesia populnea (L.) Soland (Malvaceae) are pounded in a wooden bowl with little water and the solution is washed on the chest of a person with suspected breast cancer.[30] P. longum is also used to treat tumors in Indian Ayurvedic medicine. Piplartine,[18] cepharadione A (15), and piperolactams A (16) and B (17) are the active principles.[31,32]
In Thailand, the root of Piper nigrum L. (black pepper plant), in the form of ghee, powders, enemas, and balms, is applied to abdominal tumors. The plant can also be used to treat abdominal fullness, adenitis, cancer, cholera, cold, colic, kidney stone, and headache.[33] Black pepper (黑胡椒 Hēi Hú Jiāo) is used in some formulae to treat respiratory or gastric cancers in China.[13,14] Piperine might be the major active principle from P. nigrum.[22,34]
In Bolivia, a reported Piper species known as Tudhar is used to treat uterine cancer.[35] However, the scientific name of the plant was not confirmed.
EXTRACTS FROM PIPER PLANTS WITH CYTOTOXIC ACTIVITY IN VITRO
Many crude extracts from Piper (胡椒 Hú Jiāo) plants have been evaluated for in vitro cytotoxicity based on ethnomedical knowledge, chemotaxonomic information, or random screening. Among them, 35 extracts of 24 Piper species have shown inhibitory activity (IC50 < 30 μg/ml) against at least one tumor cell line [Table 2]. Extracts from P. aduncum L., Piper barbatum Kunth, Piper fragile Benth., Piper jacquemontianum Kunth, and Piper pellucidum L. showed the highest potential against at least one tumor cell line, with an IC50 value less than 4 μg/ml.[16,36]
COMPOUNDS FROM PIPER PLANTS WITH CYTOTOXIC ACTIVITY IN VITRO
Chemical constituents of Piper (胡椒 Hú Jiāo) plants mainly include amide alkaloids, phenylpropanoids, lignans, neolignans, terpenes, steroids, kawapyrones, piperolides, flavonoids, and alkenylphenols.[9,45,46,47] Thirty-two compounds (1-32) have been reported to exhibit cytotoxic activity toward at least one tumor cell line with an IC50 value less than 4 μg/ml [Table 3]. These compounds include amide alkaloids (1-17), a lignan (18), alkenylphenols (19-22), chalcones and dihydrochalcones (25-27), piperolides (28-31), and other chemical classifications. The amide alkaloids account for 53% of the total active principles. Chabamide G [13 in Figure 2 and Table 3] and sintenpyridone [5 in Figure 2 and Table 3] exhibited the most potent activity against human colon adenocarcinoma (COLO-205; IC50 = 0.018 μg/ml) and HT-29 (IC50 = 0.025 μg/ml) cell lines, respectively.[48] Piplartine is the most promising compound showing toxicity against dozens of cell lines along with significant in vivo activity [Tables 3 and 4].[18,49,50,51,52,53]
COMPOUNDS FROM PIPER PLANTS WITH ANTITUMOR ACTIVITY IN VIVO
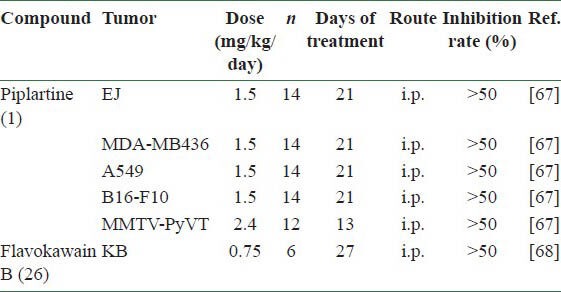
A few Piper (胡椒 Hú Jiāo) compounds have been studied for in vivo antitumor activity. Piplartine (1) and flavokawain B [26 in Figure 1] exhibited significant inhibitory effects on the growth of at least one tumor cell line in vivo at concentrations less than 15 mg/kg body weight [Table 4]. Piplartine was tested against xenograft models of human bladder carcinoma (EJ), human breast carcinoma (MDA-MB436), human alveolar carcinoma epithelial (A549), murine melanoma (B16-F10), and MMTV-polyomavirus middle T antigen transgenic mice model (MMTV-PyVT). Animals were treated for 13 or 21 days at a dose of 1.5 or 2.4 mg/kg/day. Marked antitumor effects were observed in the treated tumor-bearing mice with inhibition rates near those with positive controls, paclitaxel (10 mg/kg/day) and cisplatin (1 mg/kg/day).[67] In addition, flavokawain B treatment (0.75 mg/kg/day) significantly inhibited in vivo growth of human KB cell-derived tumor xenografts in nude mice.[68]
ANTICANCER MECHANISMS OF ACTION OF PIPER COMPOUNDS
Piplartine
Piplartine, also known as piperlongumine, comprises approximately 0.11% content in the fruit of P. longum.[18,69] The compound kills cancer cells by targeting the stress response to reactive oxygen species (ROS). Piplartine induces apoptosis selectively in cells that have a cancer genotype by targeting a non-oncogene co-dependency acquired through expression of the cancer genotype in response to transformation-induced oxidative stress.[67] Structure-activity relationships suggest that the electrophilicity of the C2-C3 olefin is critical for the observed effects on cancer cells.[70] The latest studies suggest that cancer cell lines are more resilient to chemically induced increases in ROS levels than previously thought and highlight that electrophilicity may be more closely associated with cancer-selective cell death than ROS elevation.[71]
Piplartine may target p38 signaling to cause selective killing of cancer cells and autophagy.[72,73] Research results suggest that the anticancer activity of piplartine involves inhibition of the ubiquitin-proteasome system at a pre-proteasomal step, prior to de-ubiquitination of malfolded protein substrates at the proteasome, and that the previously reported induction of ROS is a consequence of this inhibition.[74]
Piplartine can down-regulate Epstein–Barr virus encoded latent membrane protein 1 (EBV-encoded LMP1), cellular myelocytomatosis oncogene (Myc), constitutive nuclear factor kappa B (NF-κB) activity, and a host of LMP1-Myc-NF-κB-regulated target genes, while the LMP1-NF-κB-Myc axis plays the central role in B-lineage neoplasia.[51] Piplartine-dependent cytotoxicity is affected in part by reduced NF-κB and Myc activity.[50]
Piplartine induces rapid depletion of the androgen receptor in prostate cancer cells. Consequently, piplartine may afford novel opportunities for both prevention and treatment of prostatic malignancy.[75] Piplartine may act, at least in part, on the mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway to cause colon cancer cell death.[76]
Piperine
Piperine (6) is a major component of black (P. nigrum) and long (P. longum) pepper. The content of piperine in black pepper varies between 5% and 9%.[77] Piperine can inhibit human fibrosarcoma (HT-1080) cell expression of matrix metalloproteinase (MMP)-9, thereby interfering with tumor cell migration and invasion.[78] Piperine inhibits HER2 gene expression at the transcriptional level implying that it may be a potential agent for the prevention and treatment of human breast cancer with HER2 overexpression.[79] Piperine-induced cytotoxicity against human rectal tumor (HRT)-18 cells may be mediated at least in part by ROS.[80] Piperine also exhibits an antiproliferative effect on human prostate cancer cells by inducing cell cycle arrest and autophagy.[81]
Flavokawain B
Kava (Piper methysticum Forst.) is a perennial plant indigenous to the Pacific Islands. Some data indicate that the more kava consumed by a population, the lower the cancer incidence in that population.[82] Flavokawain B, constituting about 0.015% of kava extracts, appears to be a potent antiproliferative agent against a wide variety of cancer cells.[83,84] Flavokawain B–induced apoptosis, at least in part, requires Bim expression.[66] In KB cells, the induction of apoptosis by flavokawain B may involve both the death receptor and mitochondrial pathway.[68] Flavokawain B also has a pro-apoptotic effect on synovial sarcomas cell lines.[85] Flavokawain B induces apoptosis of non-small cell lung cancer H-460 cells via Bax-initiated mitochondrial and c-Jun N-terminal kinase (JNK) pathways.[86] In osteosarcoma cell lines, apoptotic induction by flavokawain B involves both extrinsic and intrinsic pathways. Flavokawain B also causes G2/M phase cell cycle arrest.[64]
CONCLUSION
Piper (胡椒 Hú Jiāo) plants are important sources for research on and development of new anticancer agents. Ten Piper plants have been used as traditional medicines to treat cancer or cancer-like symptoms. In various studies, 35 extracts from 24 Piper species and 32 compounds from Piper plants were found to possess in vitro cytotoxic activity. Among them, the amide alkaloid piplartine (1) represents the most promising candidate showing cytotoxicity against dozens of cell lines, together with excellent in vivo activity. Piper plants comprise about 2000 species, most of which have not been studied for their chemical constituents and anticancer effects. Thus, further in vitro and in vivo anticancer research studies on Piper plants and their isolates are worthwhile.
ABBREVIATIONS USED
143B, human osteosarcoma; 4T1, murine mammary carcinoma; A549, human alveolar carcinoma epithelial; A2780, human ovarian carcinoma; B-16, murine melanoma; B16-F10, murine melanoma; BCRP, breast cancer resistance protein; C4-2B, human prostate cancer; CCRF-CEM, human leukemic lymphoblast; CEM, human lymphoblastic leukemia; CEM/ADR5000, human acute T-lymphoblastic leukemia; CHCl3, chloroform; CH2Cl2, dichloromethane; COLO-205, human colon adenocarcinoma; DU-145, human prostate carcinoma; EAT, Ehrlich ascites tumor; EBV, Epstein Barr virus; EJ, human bladder carcinoma; ERK, extracellular signal-regulated kinase; EtOH, ethanol; MCF-7, human breast carcinoma; H-460, human large cell lung carcinoma; HCT116 p53+/+, p53-expressing human colon cancer cell; HCT116 p53−/−, p53-knockout human colon cancer cell; HeLa, human cervix adenocarcinoma; HEp-2, human laryngeal carcinoma; Hep-G2, human hepatocarcinoma; IC50, concentration giving 50% inhibition; HL-60, human myeloid leukemia; human promyelocytic leukemia; HL-60AR, anthracycline-resistant HL-60; HRT-18, human rectal tumor; HT-1080, human fibrosarcoma; K562, human erythromyeloblastoid leukemia; KB, human nasopharynx carcinoma; LNCaP, human prostate carcinoma; MCF-7, human breast carcinoma; MDA-MB-231, human breast carcinoma; MDA-MB436, human breast carcinoma; MEK, mitogen-activated protein kinase; MeOH, methanol; Mia PaCa2, human pancreatic adenocarcinoma; MMTV-PyVT, MMTV-polyomavirus middle T antigen transgenic mice model; Molt-4, human lymphocytic leukemia; NCI-H460, human non-small cell lung cancer; NF-κB, nuclear factor kappa B; P-388, mouse lymphocytic leukemia; PC-3, human prostate adenocarcinoma; PE, petroleum ether; ROS, reactive oxygen species; SF-268, glioma; SK-LMS-1, human leiomyosarcoma; SK-MEL-2, human skin melanoma; U87MG, human malignant glioblastoma; U87MGΔEGFR, EGFRvIII expressing glioma cells; WPMY-1, human prostatic stromal myofibroblast.
ACKNOWLEDGMENTS
This work was funded by the Natural Science Foundation of Yunnan Province, China (No. 2011FZ205), the National Natural Science Foundation of China (Nos 31070288 and 31161140345), and the Ministry of Science and Technology of China (No. 2012FY110300-7). Thanks are also due to partial support from NIH grant CA177584-01 from the National Cancer Institute awarded to K. H. Lee.
REFERENCES
- 1.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–35. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng YC, Xia NH, Gilbert MG. Vol. 4. Beijing/St. Louis: Science Press/Missouri Botanical Garden Press; 1999. Flora of China; pp. 110–29. [Google Scholar]
- 3.Zhong Hua Ben Cao. Vol. 3. Shanghai: Shanghai Scientific and Technical Publishers; 1999. Editorial Board of ‘Zhong Hua Ben Cao’, State Administration of Traditional Chinese Medicine of the People's Republic of China; pp. 424–49. [Google Scholar]
- 4.Li SM, Long CL, Liu FY, Lee S, Guo Q, Li R, et al. Herbs for medicinal baths among the traditional Yao communities of China. J Ethnopharmacol. 2006;108:59–67. doi: 10.1016/j.jep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Zheng XL, Xing FW. Ethnobotanical study on medicinal plants around Mt. Yinggeling, Hainan Island, China. J Ethnopharmacol. 2009;124:197–210. doi: 10.1016/j.jep.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 6.Peng CZ, Qi JJ, Li XE. Medicinal plants traditionally used by Hani people in Yuanyang, Yunnan, China. Lishizhen Med Mater Med Res. 2010;21:428–31. [Google Scholar]
- 7.Long CL, Li R. Ethnobotanical studies on medicinal plants used by the Red-headed Yao People in Jinping, Yunnan Province, China. J Ethnopharmacol. 2004;90:389–95. doi: 10.1016/j.jep.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Liu HX, Chen K, Sun QY, Yang FM, Hu GW, Wang YH, et al. Nudibaccatumone, a trimer comprising a phenylpropanoid and two sesquiterpene moieties from Piper nudibaccatum. J Nat Prod. 2013;76:732–6. doi: 10.1021/np300703u. [DOI] [PubMed] [Google Scholar]
- 9.Yang SX, Sun QY, Yang FM, Hu GW, Luo JF, Wang YH, et al. Sarmentosumols A to F, new mono-and dimeric alkenylphenols from Piper sarmentosum. Planta Med. 2013;79:693–6. doi: 10.1055/s-0032-1328400. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Su Y, Luo JF, Gu W, Niu HM, Li Y, et al. New amide alkaloids from Piper longum fruits. Nat Prod Bioprospect. 2013;3:277–81. [Google Scholar]
- 11.Tang GH, Chen DM, Qiu BY, Sheng L, Wang YH, Hu GW, et al. Cytotoxic amide alkaloids from Piper boehmeriaefolium. J Nat Prod. 2011;74:45–9. doi: 10.1021/np100606u. [DOI] [PubMed] [Google Scholar]
- 12.Mahanta P, Ghanim A, Gopinath K. Chemical constituents of Piper sylvaticum (Roxb) and Piper boehmerifolium (Wall) J Pharm Sci. 1974;63:1160–1. doi: 10.1002/jps.2600630733. [DOI] [PubMed] [Google Scholar]
- 13.Xin YW, Qi WD, Han CY. Traditional Chinese medicine for treating respiratory cancer. CN Patent: 101455834 A. 2009 [Google Scholar]
- 14.Chen TC. Observation of the medicine made by oneself in treating with 97 cases with gastric diseases. J Pract Med Technol. 2008;15:593–4. [Google Scholar]
- 15.Alonso-Castro AJ, Villarreal ML, Salazar-Olivo LA, Gomez-Sanchez M, Dominguez F, Garcia-Carranca A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J Ethnopharmacol. 2011;133:945–72. doi: 10.1016/j.jep.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 16.Calderón ÁI, Vázquez Y, Solís PN, Caballero-George C, Zacchino S, Gimenez A, et al. Screening of Latin American plants for cytotoxic activity. Pharm Biol. 2006;44:130–40. [Google Scholar]
- 17.Orjala J, Wright AD, Behrends H, Folkers G, Sticher O, Rüegger H, et al. Cytotoxic and antibacterial dihydrochalcones from Piper aduncum. J Nat Prod. 1994;57:18–26. doi: 10.1021/np50103a003. [DOI] [PubMed] [Google Scholar]
- 18.Bezerra DP, Pessoa C, de Moraes MO, Saker-Neto N, Silveira ER, Costa-Lotufo LV. Overview of the therapeutic potential of piplartine (piperlongumine) Eur J Pharm Sci. 2013;48:453–63. doi: 10.1016/j.ejps.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Kuete V, Sandjo LP, Wiench B, Efferth T. Cytotoxicity and modes of action of four Cameroonian dietary spices ethno-medically used to treat cancers: Echinops giganteus, Xylopia aethiopica, Imperata cylindrica and Piper capense. J Ethnopharmacol. 2013;149:245–53. doi: 10.1016/j.jep.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Kuete V, Krusche B, Youns M, Voukeng I, Fankam AG, Tankeo S, et al. Cytotoxicity of some Cameroonian spices and selected medicinal plant extracts. J Ethnopharmacol. 2011;134:803–12. doi: 10.1016/j.jep.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen ME, Metzler B, Stafford GI, Van Staden J, Jäger AK, Rasmussen HB. Amides from Piper capense with CNS activity: A preliminary SAR analysis. Molecules. 2009;14:3833–43. doi: 10.3390/molecules14093833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umadevi P, Deepti K, Venugopal DV. Synthesis, anticancer and antibacterial activities of piperine analogs. Med Chem Res. 2013;22:5466–71. [Google Scholar]
- 23.Daoudi A, El Youbl AE, Bagrel D, Aarab L. In vitro anticancer activity of some plants used in Moroccan traditional medicine. J Med Plants Res. 2013;7:1182–9. [Google Scholar]
- 24.Usia T, Watabe T, Kadota S, Tezuka Y. Potent CYP3A4 inhibitory constituents of Piper cubeba. J Nat Prod. 2005;68:64–8. doi: 10.1021/np0401765. [DOI] [PubMed] [Google Scholar]
- 25.Kreuter MH, Yam J, Berger-Büter K. The use of extracts or materials extracted from Piper cubeba L. as an effective component in a drug for the treatment of cancer diseases. WO Patent: 2009021347. 2009 [Google Scholar]
- 26.Yam J, Kreuter M, Drewe J. Piper cubeba targets multiple aspects of the androgen-signalling pathway. A potential phytotherapy against prostate cancer growth? Planta Med. 2008;74:33–8. doi: 10.1055/s-2007-993758. [DOI] [PubMed] [Google Scholar]
- 27.Holdsworth D, Kerenga K. A survey of medicinal plants in the Simbu Province, Papua New Guinea. Pharm Biol. 1987;25:183–7. [Google Scholar]
- 28.Orjala J, Mian P, Rali T, Sticher O. Gibbilimbols A-D, cytotoxic and antibacterial alkenylphenols from Piper gibbilimbum. J Nat Prod. 1998;61:939–41. doi: 10.1021/np970529i. [DOI] [PubMed] [Google Scholar]
- 29.Soladoye MO, Amusa N, Raji-Esan S, Chukwuma E, Taiwo A. Ethnobotanical survey of anti-cancer plants in Ogun State, Nigeria. Ann Biol Res. 2010;1:261–73. [Google Scholar]
- 30.Holdsworth D. Traditional medicinal plants of Rarotonga, Cook Islands. Part II. Pharm Biol. 1991;29:71–9. [Google Scholar]
- 31.Kim KH, Choi JW, Choi SU, Ha SK, Kim SY, Park HJ, et al. The chemical constituents of Piper kadsura and their cytotoxic and anti-neuroinflammtaory activities. J Enzyme Inhib Med Chem. 2011;26:254–60. doi: 10.3109/14756366.2010.496363. [DOI] [PubMed] [Google Scholar]
- 32.Desai SJ, Prabhu BR, Mulchandani NB. Aristolactams and 4,5-dioxoaporphines from Piper longum. Phytochemistry. 1988;27:1511–5. [Google Scholar]
- 33.Chaveerach A, Mokkamul P, Sudmoon R, Tanee T. Ethnobotany of the genus Piper (Piperaceae) in Thailand. Ethnobot Res Appl. 2006;4:223–31. [Google Scholar]
- 34.Li S, Lei Y, Jia Y, Li N, Wink M, Ma Y. Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine. 2011;19:83–7. doi: 10.1016/j.phymed.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 35.Bourdy G, DeWalt SJ, de Michel LR, Roca A, Deharo E, Munoz V, et al. Medicinal plants uses of the Tacana, an Amazonian Bolivian ethnic group. J Ethnopharmacol. 2000;70:87–109. doi: 10.1016/s0378-8741(99)00158-0. [DOI] [PubMed] [Google Scholar]
- 36.Widowati W, Wijaya L, Wargasetia TL, Bachtiar I, Yelliantty Y, Laksmitawati DR. Antioxidant, anticancer, and apoptosis-inducing effects of Piper extracts in HeLa cells. J Exp Integr Med. 2013;3:225–30. [Google Scholar]
- 37.Mahavorasirikul W, Viyanant V, Chaijaroenkul W, Itharat A, Na-Bangchang K. Cytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitro. BMC Complement Altern Med. 2010;10:55. doi: 10.1186/1472-6882-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz LE, Munoz DR, Prieto RE, Cuervo SA, Gonzalez DL, Guzman JD, et al. Antioxidant, antitubercular and cytotoxic activities of Piper imperiale. Molecules. 2012;17:4142–57. doi: 10.3390/molecules17044142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chavez P, Sanchez I, González F, Rodríguez J, Axelrod F. Cytotoxicity correlations of Puerto Rican plants using a simplified brine shrimp lethality screening procedure. Pharm Biol. 1997;35:222–6. [Google Scholar]
- 40.Li X, Liu Z, Xu X, Blair CA, Sun Z, Xie J, et al. Kava components down-regulate expression of AR and AR splice variants and reduce growth in patient-derived prostate cancer xenografts in mice. PloS One. 2012;7:e31213. doi: 10.1371/journal.pone.0031213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ee GC, Lim CM, Lim CK, Rahmani M, Shaari K, Bong CF. Alkaloids from Piper sarmentosum and Piper nigrum. Nat Prod Res. 2009;23:1416–23. doi: 10.1080/14786410902757998. [DOI] [PubMed] [Google Scholar]
- 42.Roy UB, Vijayalaxmi KK. Evaluation of in vitro antitumor property of ethanolic extract of Piper Nigrum seeds. Int J Innov Res Stud. 2013;2:282–302. [Google Scholar]
- 43.Taylor P, Arsenak M, Abad MJ, Fernandez A, Milano B, Gonto R, et al. Screening of Venezuelan medicinal plant extracts for cytostatic and cytotoxic activity against tumor cell lines. Phytother Res. 2013;27:530–9. doi: 10.1002/ptr.4752. [DOI] [PubMed] [Google Scholar]
- 44.Zainal Ariffin SH, Wan Omar WH, Zainal Ariffin Z, Safian MF, Senafi S, Megat Abdul Wahab R. Intrinsic anticarcinogenic effects of Piper sarmentosum ethanolic extract on a human hepatoma cell line. Cancer Cell Int. 2009;9:6. doi: 10.1186/1475-2867-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott IM, Jensen HR, Philogene BJ, Arnason JT. A review of Piper spp. (Piperaceae) phytochemistry, insecticidal activity and mode of action. Phytochem Rev. 2008;7:65–75. [Google Scholar]
- 46.Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jha A, et al. Phytochemistry of the genus Piper. Phytochemistry. 1997;46:597–673. [Google Scholar]
- 47.Kato MJ, Furlan M. Chemistry and evolution of the Piperaceae. Pure Appl Chem. 2007;79:529–38. [Google Scholar]
- 48.Rao VR, Suresh G, Babu KS, Raju SS, Vishnu vardhan MV, Ramakrishna S, et al. Novel dimeric amide alkaloids from Piper chaba Hunter: Isolation, cytotoxic activity, and their biomimetic synthesis. Tetrahedron. 2011;67:1885–92. [Google Scholar]
- 49.Bezerra DP, Militão GC, De Castro FO, Pessoa C, de Moraes MO, Silveira ER, et al. Piplartine induces inhibition of leukemia cell proliferation triggering both apoptosis and necrosis pathways. Toxicol In Vitro. 2007;21:1–8. doi: 10.1016/j.tiv.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Han SS, Son DJ, Yun H, Kamberos NL, Janz S. Piperlongumine inhibits proliferation and survival of Burkitt lymphoma in vitro. Leukemia Res. 2013;37:146–54. doi: 10.1016/j.leukres.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han SS, Tompkins VS, Son DJ, Kamberos NL, Stunz LL, Halwani A, et al. Piperlongumine inhibits LMP1/MYC-dependent mouse B-lymphoma cells. Biochem Biophys Res Commun. 2013;436:660–5. doi: 10.1016/j.bbrc.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duh CY, Wu YC, Wang SK. Cytotoxic pyridone alkaloids from the leaves of Piper aborescens. J Nat Prod. 1990;53:1575–7. doi: 10.1021/np50072a030. [DOI] [PubMed] [Google Scholar]
- 53.Bezerra DP, Pessoa C, de Moraes MO, Silveira ER, Lima MS, Elmiro FM, et al. Antiproliferative effects of two amides, piperine and piplartine, from Piper species. Z Naturforsch C. 2005;60:539–43. doi: 10.1515/znc-2005-7-805. [DOI] [PubMed] [Google Scholar]
- 54.Duh CY, Wu YC, Wang SK. Cytotoxic pyridone alkaloids from Piper aborescens. Phytochemistry. 1990;29:2689–91. doi: 10.1021/np50072a030. [DOI] [PubMed] [Google Scholar]
- 55.Chen JJ, Duh CY, Huang HY, Chen IS. Cytotoxic constituents of Piper sintenense. HeIv Chim Acta. 2003;86:2058–64. [Google Scholar]
- 56.Chen JJ, Huang YC, Chen YC, Huang YT, Want SW, Peng CY, et al. Cytotoxic amides from Piper sintenense. Planta Med. 2002;68:980–5. doi: 10.1055/s-2002-35660. [DOI] [PubMed] [Google Scholar]
- 57.Ee GCL, Lim CM, Rahmani M, Shaari K, Bong CF. Pellitorine, a potential anti-cancer lead compound against HL60 and MCF-7 cell lines and microbial transformation of piperine from Piper Nigrum. Molecules. 2010;15:2398–404. doi: 10.3390/molecules15042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma J, Jones SH, Marshall R, Johnson RK, Hecht SM. A DNA-damaging oxoaporphine alkaloid from Piper caninum. J Nat Prod. 2004;67:1162–4. doi: 10.1021/np040056x. [DOI] [PubMed] [Google Scholar]
- 59.Elban MA, Chapuis JC, Li M, Hecht SM. Synthesis and biological evaluation of cepharadiones A and B and related dioxoaporphines. Bioorg Med Chem. 2007;15:6119–25. doi: 10.1016/j.bmc.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 60.Valadares MC, de Carvalho IC, de Oliveira L, Junior, Vieira Mde S, Benfica PL, de Carvalho FS, et al. Cytotoxicity and antiangiogenic activity of grandisin. J Pharm Pharmacol. 2009;61:1709–14. doi: 10.1211/jpp/61.12.0017. [DOI] [PubMed] [Google Scholar]
- 61.Lopes AP, Bagatela BS, Rosa PC, Nanayakkara DN, Tavares Carvalho JC, Maistro EL, et al. Antioxidant and cytotoxic effects of cude extract, fractions and 4-nerolidylcathecol from aerial parts of Pothomorphe umbellata L. (Piperaceae) Biomed Res Int 2013. 2013 doi: 10.1155/2013/206581. 206581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mongelli E, Romano A, Desmarchelier C, Coussio J, Ciccia G. Cytotoxic 4-nerolidylcatechol from Pothomorphe peltata inhibits topoisomerase I activity. Planta Med. 1999;65:376–8. doi: 10.1055/s-2006-960793. [DOI] [PubMed] [Google Scholar]
- 63.Tabudravu JN, Jaspars M. Anticancer activities of constituents of kava (Piper methysticum) S Pac J Nat Appl Sci. 2005;23:26–9. [Google Scholar]
- 64.Ji T, Lin C, Krill LS, Eskander R, Guo Y, Zi XL, et al. Flavokawain B, a kava chalcone, inhibits growth of human osteosarcoma cells through G2/M cell cycle arrest and apoptosis. Mol Cancer. 2013;12:55. doi: 10.1186/1476-4598-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eskander RN, Randall LM, Sakai T, Guo Y, Hoang B, Zi X. Flavokawain B, a novel, naturally occurring chalcone, exhibits robust apoptotic effects and induces G2/M arrest of a uterine leiomyosarcoma cell line. J Orthop Res. 2012;38:1086–94. doi: 10.1111/j.1447-0756.2011.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang YX, Li XB, Liu ZB, Simoneau AR, Xie J, Zi XL. Flavokawain B, a kava chalcone, induces apoptosis via up-regulation of death-receptor 5 and Bim expression in androgen receptor negative, hormonal refractory prostate cancer cell lines and reduces tumor growth. Int J Cancer. 2010;127:1758–68. doi: 10.1002/ijc.25210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li XY, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–4. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Lin E, Lin WH, Wang SY, Chen CS, Liao JW, Chang HW, et al. Flavokawain B inhibits growth of human squamous carcinoma cells: Involvement of apoptosis and cell cycle dysregulation in vitro and in vivo. J Nutr Biochem. 2012;23:368–78. doi: 10.1016/j.jnutbio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 69.Bi Y, Liu JH, Luo R, Wang QS, Wu X. Simultaneous determination of piperine and piperlongumine in Piper longum by HPLC. Chin J Exper Tradit Med Formulae. 2012;18:47–50. [Google Scholar]
- 70.Adams DJ, Dai MJ, Pellegrino G, Wagner BK, Stern AM, Shamji AF, et al. Synthesis, cellular evaluation, and mechanism of action of piperlongumine analogs. Proc Natl Acad Sci USA. 2012;109:15115–20. doi: 10.1073/pnas.1212802109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adams DJ, Boskovic ZV, Theriault JR, Wang AJ, Stern AM, Wagner BK, et al. Discovery of small-molecule enhancers of reactive oxygen species that are nontoxic or cause genotype-selective cell death. ACS Chem Biol. 2013;8:923–9. doi: 10.1021/cb300653v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu JM, Pan F, Li L, Liu QR, Chen Y, Xiong XX, et al. Piperlongumine selectively kills glioblastoma multiforme cells via reactive oxygen species accumulation dependent JNK and p38 activation. Biochem Biophys Res Commun. 2013;437:87–93. doi: 10.1016/j.bbrc.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Wang J, Xiao X, Shan Y, Xue B, Jiang G, et al. Piperlongumine induces autophagy by targeting p38 signaling. Cell Death Dis. 2013;4:e824. doi: 10.1038/cddis.2013.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jarvius M, Fryknas M, D’Arcy P, Sun C, Rickardson L, Gullbo J, et al. Piperlongumine induces inhibition of the ubiquitin-proteasome system in cancer cells. Biochem Biophys Res Commun. 2013;431:117–23. doi: 10.1016/j.bbrc.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 75.Golovine KV, Makhov PB, Teper E, Kutikov A, Canter D, Uzzo RG, Kolenko VM. Piperlongumine induces rapid depletion of the androgen receptor in human prostate cancer cells. The Prostate. 2013;73:23–30. doi: 10.1002/pros.22535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Randhawa H, Kibble K, Zeng H, Moyer M, Reindl K. Activation of ERK signaling and induction of colon cancer cell death by piperlongumine. Toxicol in Vitro. 2013;17:1626–33. doi: 10.1016/j.tiv.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;302:645–50. doi: 10.1124/jpet.102.034728. [DOI] [PubMed] [Google Scholar]
- 78.Hwang YP, Yun HJ, Kim HG, Han EH, Choi JH, Chung YC, et al. Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by piperine via the inhibition of PKC alpha/ERK1/2-dependent matrix metalloproteinase-9 expression. Toxicol Lett. 2011;203:9–19. doi: 10.1016/j.toxlet.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 79.Do MT, Kim HG, Choi JH, Khanal T, Park BH, Tran TP, et al. Antitumor efficacy of piperine in the treatment of human HER2-overexpressing breast cancer cells. Food Chem. 2013;141:2591–9. doi: 10.1016/j.foodchem.2013.04.125. [DOI] [PubMed] [Google Scholar]
- 80.Yaffe PB, Doucette CD, Walsh M, Hoskin DW. Piperine impairs cell cycle progression and causes reactive oxygen species-dependent apoptosis in rectal cancer cells. Exp Mol Pathol. 2013;94:109–14. doi: 10.1016/j.yexmp.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 81.Ouyang DY, Zeng LH, Pan H, Xu LH, Wang Y, Liu KP, et al. Piperine inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and autophagy. Food Chem Toxicol. 2013;60:424–30. doi: 10.1016/j.fct.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 82.Steiner G. The correlation between cancer incidence and kava consumption. Hawaii Med J. 2000;59:420–2. [PubMed] [Google Scholar]
- 83.Chen GG, Lai PB. Berlin: Springer; 2012. Novel apoptotic regulators in carcinogenesis; pp. 189–204. [Google Scholar]
- 84.Dharmaratne RW, Nanayakkara NP, Khan IA. Kavalactones from Piper methysticum, and their 13 C NMR spectroscopic analyses. Phytochemistry. 2002;59:429–33. doi: 10.1016/s0031-9422(01)00443-5. [DOI] [PubMed] [Google Scholar]
- 85.Sakai T, Eskander RN, Guo Y, Kim KJ, Mefford J, Hopkins J, et al. Flavokawain B, a kava chalcone, induces apoptosis in synovial sarcoma cell lines. J Orthop Res. 2012;30:1045–50. doi: 10.1002/jor.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.An J, Gao Y, Wang J, Zhu Q, Ma Y, Wu J, et al. Flavokawain B induces apoptosis of non-small cell lung cancer H460 cells via Bax-initiated mitochondrial and JNK pathway. Biotechnol Lett. 2012;34:1781–8. doi: 10.1007/s10529-012-0976-6. [DOI] [PubMed] [Google Scholar]


