Abstract
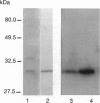
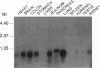
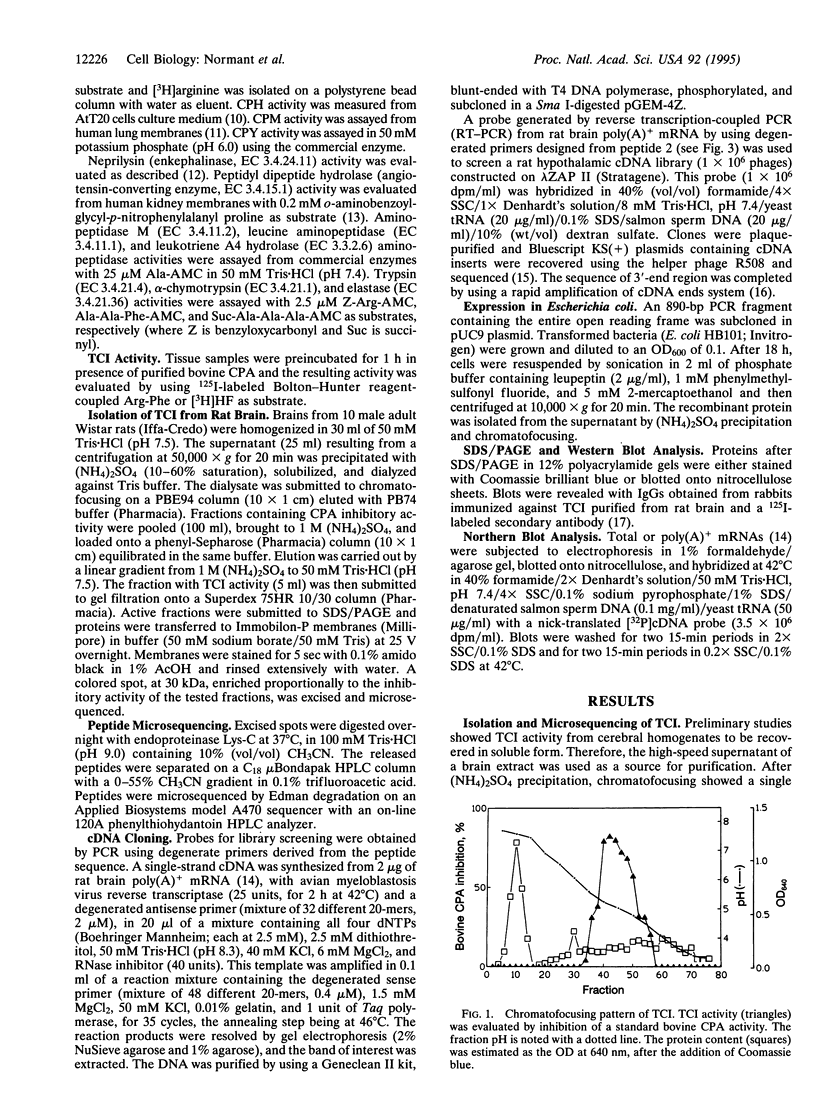
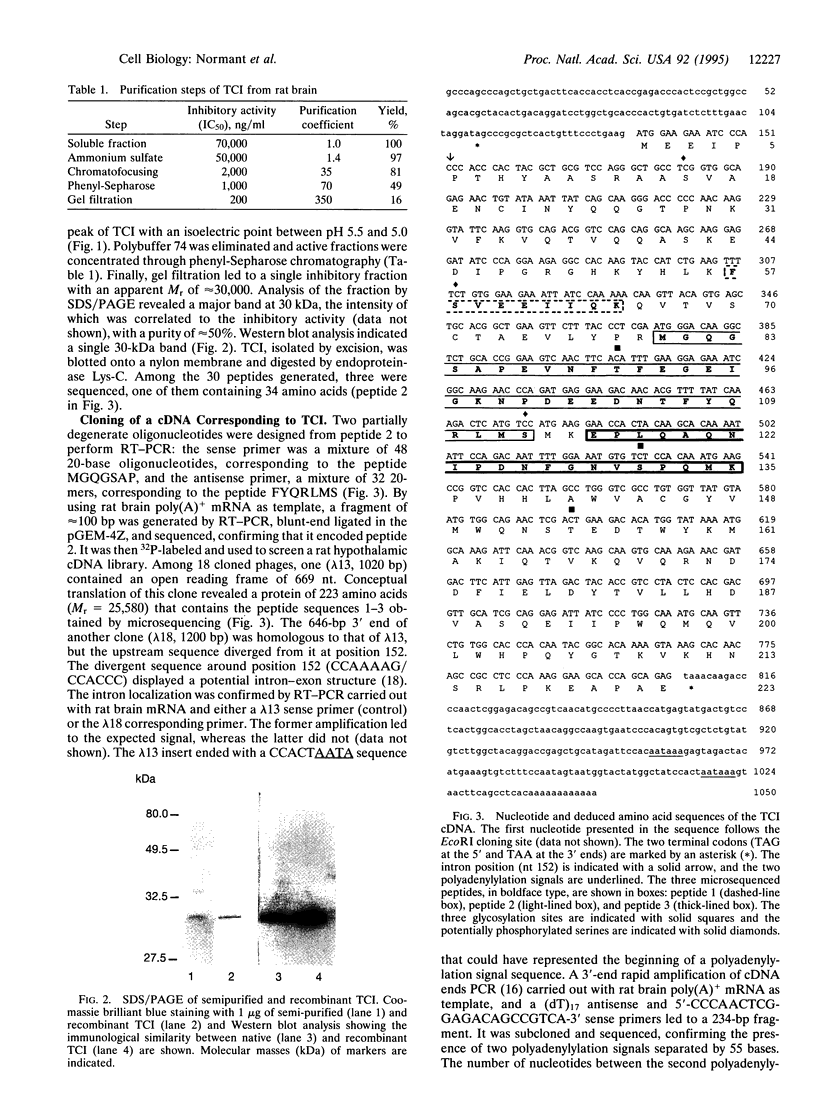
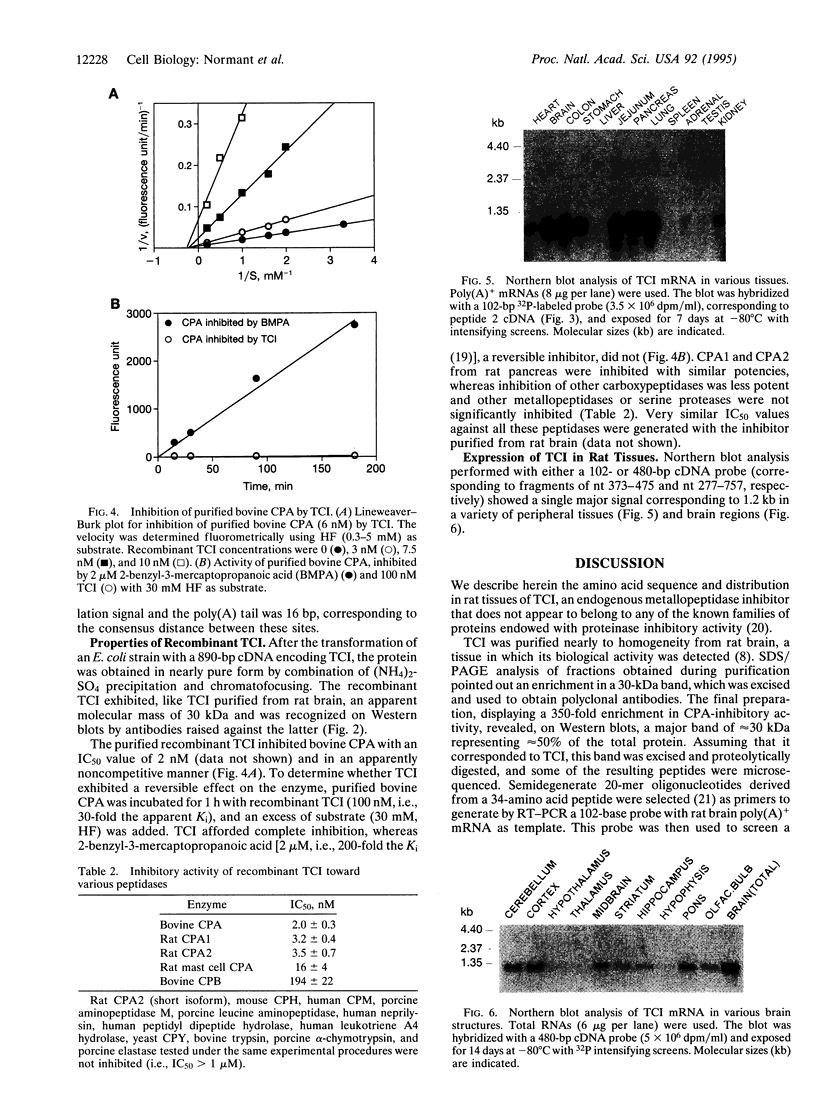
The recent demonstration of the occurrence in rat brain and other nonpancreatic tissues of carboxypeptidase A (CPA) gene transcripts without associated catalytic activity could be ascribed to the presence of a soluble endogenous protein inhibitor. This tissue carboxypeptidase inhibitor (TCI), detected by the inhibition of added bovine pancreatic CPA, was purified from rat brain. Peptides were obtained by partial proteolysis of purified TCI, a protein of approximately 30 kDa, and starting from their sequences, a full-length cDNA encoding a 223-amino acid protein containing three potential phosphorylation sites was cloned from a cDNA library. Its identity with TCI was shown by expression in Escherichia coli of a recombinant protein recognized by antibodies raised against native TCI and display characteristic CPA-inhibiting activity. TCI appears as a hardly reversible, non-competitive, and potent inhibitor of CPA1 and CPA2 (Ki approximately 3 nM) and mast-cell CPA (Ki = 16 nM) and inactive on various other proteases. This pattern of selectivity might be attributable to a limited homology of a 11-amino acid sequence with sequences within the activation segments of CPA and CPB known to interact with residues within their active sites. The widespread expression of TCI in a number of tissues (e.g., brain, lung, or digestive tract) and its apparently cytosolic localization point to a rather general functional role, e.g., in the control of cytosolic protein degradation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avilés F. X., Vendrell J., Guasch A., Coll M., Huber R. Advances in metallo-procarboxypeptidases. Emerging details on the inhibition mechanism and on the activation process. Eur J Biochem. 1993 Feb 1;211(3):381–389. doi: 10.1111/j.1432-1033.1993.tb17561.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode W., Huber R. Natural protein proteinase inhibitors and their interaction with proteinases. Eur J Biochem. 1992 Mar 1;204(2):433–451. doi: 10.1111/j.1432-1033.1992.tb16654.x. [DOI] [PubMed] [Google Scholar]
- Boone T. C., Johnson M. J., De Clerck Y. A., Langley K. E. cDNA cloning and expression of a metalloproteinase inhibitor related to tissue inhibitor of metalloproteinases. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2800–2804. doi: 10.1073/pnas.87.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Bruzdzinski C. J., Riordan-Johnson M., Nordby E. C., Suter S. M., Gelehrter T. D. Isolation and characterization of the rat plasminogen activator inhibitor-1 gene. J Biol Chem. 1990 Feb 5;265(4):2078–2085. [PubMed] [Google Scholar]
- Edwards D. R., Waterhouse P., Holman M. L., Denhardt D. T. A growth-responsive gene (16C8) in normal mouse fibroblasts homologous to a human collagenase inhibitor with erythroid-potentiating activity: evidence for inducible and constitutive transcripts. Nucleic Acids Res. 1986 Nov 25;14(22):8863–8878. doi: 10.1093/nar/14.22.8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker L. D., Snyder S. H. Enkephalin convertase: purification and characterization of a specific enkephalin-synthesizing carboxypeptidase localized to adrenal chromaffin granules. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3886–3890. doi: 10.1073/pnas.79.12.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B., Gros C., Schwartz J. C., Danvy D., Plaquevent J. C., Duhamel L., Duhamel P., Vlaiculescu A., Costentin J., Lecomte J. M. Enantiomers of thiorphan and acetorphan: correlation between enkephalinase inhibition, protection of endogenous enkephalins and behavioral effects. J Pharmacol Exp Ther. 1987 Nov;243(2):666–673. [PubMed] [Google Scholar]
- Gros C., Giros B., Schwartz J. C. Identification of aminopeptidase M as an enkephalin-inactivating enzyme in rat cerebral membranes. Biochemistry. 1985 Apr 23;24(9):2179–2185. doi: 10.1021/bi00330a011. [DOI] [PubMed] [Google Scholar]
- Guasch A., Coll M., Avilés F. X., Huber R. Three-dimensional structure of porcine pancreatic procarboxypeptidase A. A comparison of the A and B zymogens and their determinants for inhibition and activation. J Mol Biol. 1992 Mar 5;224(1):141–157. doi: 10.1016/0022-2836(92)90581-4. [DOI] [PubMed] [Google Scholar]
- Hass G. M., Nau H., Biemann K., Grahn D. T., Ericsson L. H., Neurath H. The amino acid sequence of a carboxypeptidase inhibitor from potatoes. Biochemistry. 1975 Mar 25;14(6):1334–1342. doi: 10.1021/bi00677a036. [DOI] [PubMed] [Google Scholar]
- Homandberg G. A., Litwiller R. D., Peanasky R. J. Carboxypeptidase inhibitors from Ascaris suum: the primary structure. Arch Biochem Biophys. 1989 Apr;270(1):153–161. doi: 10.1016/0003-9861(89)90017-9. [DOI] [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol Cell Biol. 1989 Nov;9(11):5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Molina M. A., Marino C., Oliva B., Avilés F. X., Querol E. C-tail valine is a key residue for stabilization of complex between potato inhibitor and carboxypeptidase A. J Biol Chem. 1994 Aug 26;269(34):21467–21472. [PubMed] [Google Scholar]
- Normant E., Gros C., Schwartz J. C. Carboxypeptidase A isoforms produced by distinct genes or alternative splicing in brain and other extrapancreatic tissues. J Biol Chem. 1995 Sep 1;270(35):20543–20549. doi: 10.1074/jbc.270.35.20543. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Condon M. E., Reid J., Sabo E. F., Cheung H. S., Cushman D. W. Design of potent and specific inhibitors of carboxypeptidases A and B. Biochemistry. 1979 Apr 17;18(8):1427–1430. doi: 10.1021/bi00575a006. [DOI] [PubMed] [Google Scholar]
- Pavloff N., Staskus P. W., Kishnani N. S., Hawkes S. P. A new inhibitor of metalloproteinases from chicken: ChIMP-3. A third member of the TIMP family. J Biol Chem. 1992 Aug 25;267(24):17321–17326. [PubMed] [Google Scholar]
- Rees D. C., Lipscomb W. N. Structure of the potato inhibitor complex of carboxypeptidase A at 2.5-A resolution. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4633–4637. doi: 10.1073/pnas.77.8.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. Fluorescence reaction for amino acids. Anal Chem. 1971 Jun;43(7):880–882. doi: 10.1021/ac60302a020. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidgel R. A., Davis R. M., Tan F. Human carboxypeptidase M. Purification and characterization of a membrane-bound carboxypeptidase that cleaves peptide hormones. J Biol Chem. 1989 Feb 5;264(4):2236–2241. [PubMed] [Google Scholar]