Abstract
To compare the diagnostic value of gadolinium (Gd) and ultrasmall superparamagnetic iron oxide (SPIO) contrast media for characterization of focal liver lesions (FLL), we retrospectively evaluated the results of magnetic resonance (MR) imaging in 68 patients (40 M, 28 F, age from 22 to 81 yrs) of which 36 with diagnosis of colo-rectal cancer, 26 with hepatic cirrhosis and 6 with incidental imaging detection of FLL. MR (Gyroscan Intera 1.5 T, Philips Medical Systems) study was performed using T1 and T2 fast-field-echo (FFE) and T2 turbo-spin-echo (TSE) sequences in axial and coronal views. Dynamic multi-phases gadolinium Gd-enhanced T1-FFE-Bh images were obtained in arterial, portal and equilibrium phases, followed by SPIO-enhanced T2-FFE scans. A qualitative analysis of pre- and post-contrast MR images to classify FLL as benign or malignant was performed using a 3-point scoring system: 0= benign; 1= suspicious for malignancy; 2= malignant. A total of 118 lesions were evaluated. In particular, histology (n=18), cytology (n=14) or clinical-imaging follow-up data (n=86) demonstrated 4 adenomas, 29 cysts, 3 focal steatosis, 25 hemangiomas, 1 focal vascular abnormality, 5 fibrotic lesions as well as 13 regenerative nodules, 6 dysplastic, 14 hepatocellular carcinomas (HCC), 17 metastasis and 1 cholangiocarcinoma. For MR imaging, diagnostic accuracy, sensitivity, specificity, positive (PPV) and negative (NPV) predictive values of Gd vs. SPIO images were respectively 83% vs. 92%, 79% vs. 74%, 85% vs. 99% (P=0.002), 68% vs. 96% (P=0.005) and 91% vs. 90%, respectively. The results suggest that SPIO-MR provides a diagnostic incremental value, as specificity and PPV, particularly to characterize FLL compared to Gd-MR; thus, we strongly recommend the use of SPIO when liver lesion characterization is requested and Gd images are uncertain.
Keywords: MR imaging, focal liver lesions (FLL), gadolinium, superparamagnetic iron oxide (SPIO), contrast media
Introduction
The medical innovation regarding the procedures for treatment of focal liver lesions (FLL), both primary and metastatic, showed a significant increase of patient survey in such cases (1-4). Therefore, it is fundamental to early identify the presence of FLL as well as to accurately characterize the nature of such lesions, as benign or malignant, to establish the appropriate treatment planning. For this purpose, diagnostic imaging has a main role both to non-invasively identify and characterize FLL allowing the differentiation between benign and malignant lesions.
The more common imaging techniques used for the identification and characterization of FLL are represented by ultrasound (US), computed tomography (CT) and magnetic resonance (MR) scans (5-9). In particular, the technological innovation and the availability of new contrast media determined a significant improvement of the diagnostic potential of such modalities. However, US is operator-dependent as well as this modality shows technical limits as high patient body weight and intestinal meteorism, while CT requires radiation exposure and intravenous iodine contrast medium injection. Conversely, MR represents the current technique of choice in this setting since it is free of ionizing radiation as well as it shows high contrast resolution using several sequences and different types of contrast media. In this regard, the more common used MR contrast media are represented by gadolinium-chelates which show an extra-cellular hepatic distribution, while the intra-cellular contrast media consist of compounds with biliary excretion or those with distribution in the reticuloendothelial system cells (RES) (10-12). In particular, although there is general agreement about the superiority of MR with extra-cellular contrast medium compared to the baseline study without contrast, many clinical investigations have been performed comparing imaging results obtained with extra- and intra-cellular contrast media in order to define the more appropriate MR protocol to identify and characterize FLL (13-29).
In this study, we compared the diagnostic value of gadolinium-chelate and superparamagnetic iron oxide (SPIO) MR imaging for the characterization of FLL in patients with colo-rectal cancer, with hepatic cirrhosis or with incidental occurrence of FLL in order to establish the diagnostic role of SPIO in such patients to differentiate benign and malignant FLL.
Materials and methods
Population
Informed consent, a requirement of the protocol approved by the Institutional Clinical Research Subpanel on Human Studies at our Institute, was obtained in all patients. Sixty-eight patients with the suspicion or the US/CT evidence of FLL were retrospectively evaluated (40 males and 28 females ranging, age range: 22-81 years, mean age: 59 years): 36 patients presented colo-rectal cancer, 26 patients liver cirrhosis and 6 patients incidental FLL on US or CT scans performed for different clinical reasons, for a total of 118 FLL analyzed. All patients underwent abdominal MR study with both extra-cellular (gadolinium-chelate) and intra-cellular (SPIO) contrast media using a protocol in a single day. The standard of reference to compare MR findings in FLL were histology (n=18), biopsy (n=14) or clinical-imaging (US/CT) follow-up (n=86) data. In particular, in patients with colo-rectal cancer, at laparotomy liver lesions were identified by means of bimanual palpation integrated with intraoperative-US and the intraoperative biopsy of the more representative lesion was performed as well as the absence of liver lesions was confirmed by CT follow-up study. Conversely, in patients with liver cirrhosis or with incidental FLL the presence of lesions was confirmed by histology, biopsy or US/CT follow-up.
Magnetic resonance imaging
A 1.5 T MR system (Gyroscan Intera 1.5 T, Philips Medical Systems, Best, and Holland) with a phased-array body coil was used. The following scan protocol was used: transverse breath-hold dual-echo (in and opposed phase) T1-weighted fast-field-echo (FFE) (repetition time, 217 msec; opposed-phase echo time, 2.3 msec; in-phase echo time, msec 4.6; flip angle, 80°; section thickness 5 mm, intersection gap 1 mm; end-expiration breath hold); transverse breath-hold T2-weighted turbo-spin-echo (TSE) with (repetition time msec/echo time msec, 417/80; TSE factor, 68; section thickness, 4 mm, intersection gap, 0 mm) and without fat saturation (repetition time msec/echo time msec, 417/80; TSE factor, 55; section thickness, 4 mm; intersection gap, 0 mm); coronal breath-hold T2-weighted TSE (repetition time msec/echo time msec, 587/80; TSE factor 96; section thickness 6 mm, intersection gap 1 mm); transverse T2-weighted FFE (repetition time msec/echo time msec, 150/14; flip angle, 90°; section thickness, 6 mm, intersection gap, 1 mm). Successively gadolinium-chelate scan protocol was performed; the images were obtained with transverse breath-hold T1-weighted turbo field echo (TFE) with fat suppression (repetition time msec/echo time msec, 3.6/1.7; flip angle, 10°; TFE factor, 60; section thickness, 6 mm; intersection gap, –3 mm) before and after bolus injection of gadopentetate dimeglumine (Magnevist; Berlex Laboratories; dose: 0.1 mmol/kg; rate of injection: 3 mL/sec followed by injection of 20 mL of normal saline flush); images were acquired during the arterial (25 seconds), portal (60 seconds) and equilibrium (180 seconds) phases. As last step, SPIO scan protocol was performed: after iv injection of Ferucarbotran (Resovist; Schering, Berlin, Germany; dose: 0.7-0.12 mmol/Kg), the images were obtained 15 minutes from the end of injection repeating transverse breath-hold T2-weighted TSE with and without fat saturation and transverse T2-weighted FFE.
Imaging analysis
MR images were separately evaluated by two radiologists without knowledge of histology, biopsy or follow-up data. MR images performed after contrast media administration were independently evaluated and compared with pre-contrast images; in case of disagreement between the first two radiologists, a third expert radiologist was consulted for further assessment. For each patient, the number, the size and the location of FLL, according to Couinaud segmental division, were analyzed on MR imaging; in particular, the size of FLL was measured in cm. For lesion characterization as benign or malignant, a scoring analysis was performed using the following scores: 0= benign, 1= suspicious for malignancy and 2= malignant. For MR images after gadolinium, the following criteria for benign (score 0) FLL have been used: no contrast enhancement in simple cystic lesions, progressive contrast filling in hemangiomas, homogeneous contrast enhancement in arterial phase with isointensity to normal liver tissue on delayed images in case of adenoma or focal nodular hyperplasia; conversely, the following criteria for malignant (score 2) FLL have been used: early contrast enhancement in arterial phase with progressive wash-out and “ring” image on equilibrium phase in case of hepatocellular carcinoma (HCC), non-homogeneous contrast enhancement on delayed phase in case of cholangiocarcinoma or only capsular contrast enhancement on the subsequent phases in case of metastases; the score 1 (suspicious for malignancy) was assigned to FLL that showed faint contrast enhancement only in arterial phase. For MR images after SPIO, no contrast enhancement by FLL was considered criterion of malignancy (score 2); however, for benign FLL such as simple cysts, hemangiomas and fibrotic nodules, although no contrast enhancement was found for the presence of substitutive tissue (liquid, blood, fibrotic) a benign score was considered (score =0); in particular, in these FLL the results of both T1 and T2 weighted pre-contrast images were also considered for tissue characterization; the score 1 (suspicious for malignancy) was used when in FLL were not observed the characteristics of scores 0 and 2. The results of MR images were compared to the standard of reference data to assign true or false finding, positive or negative; the concordance and the discordance between the two groups of post-contrast MR images were evaluated. Furthermore, for each group of post-contrast MR images diagnostic accuracy, sensibility, specificity, positive and negative predictive values (PPV and NPV) were calculated; in particular, for this analysis FLL assigned to score 1 were considered as malignant. The statistic significance of the discordant findings between the two groups of post-contrast MR images as well as of the different results in diagnostic accuracy, sensibility, specificity, PPV and NPV was assessed using Mc Nemar, chi-square and proportions tests, as appropriate, considering significant a P value <0.05.
Results
In 49 out of 68 patients (72%) a total of 118 FLL was observed, while no FLL were detected in the remaining 19 patients; in particular, these latter patients were evaluated during the staging for colo-rectal carcinoma. Of the 49 patients with FLL, 17 patients were part of the group studied during the staging for colo-rectal carcinoma, 26 patients had liver cirrhosis and 6 patients showed incidental FLL.
Table 1 illustrates the features in terms of histology and size of the 118 FLL; a total of 86 benign FLL was observed represented by 29 simple cysts, 25 hemangiomas, 4 adenomas, 5 fibrotic nodules, 3 focal steatosis, 1 vascular abnormality, 13 regenerative nodules and 6 dysplastic nodules. Conversely, the remaining 32 FLL were malignant represented by 14 HCC, 17 colo-rectal metastases and 1 cholangiocarcinoma. In particular, of the 36 patients with colo-rectal carcinoma 17 showed FLL of which 7 patients with metastases (n=17) and 10 with benign lesions such as hemangiomas (n=11), cysts (n=29), focal steatosis (n=1) and fibrotic nodules (n=3). Furthermore, FLL were detected in all patients with liver cirrhosis (n=26) of which 9 patients with hepatocellular carcinoma (n=14), 2 patients with dysplastic nodules (n=6), 6 patients with regenerative nodules (n=13) and 9 patients with benign lesions (5 hemangiomas, 2 focal steatosis, 2 adenomas, 2 fibrotic nodules and 1 vascular abnormality). Finally, in the 6 patients with incidental FLL a total of 12 lesions was found of which 2 adenomas, 9 hemangiomas and 1 cholangiocarcinoma.
Table 1. Size features on MR imaging of focal liver lesions.
| Lesion type | Size (range in cm) | Number |
|---|---|---|
| Simple cyst | <0.5 | 18 |
| Simple cyst | 1.0-2.0 | 11 |
| Hemangioma | 0.5-3 | 25 |
| Focale steatosis | 1.5-2 | 3 |
| Focale fibrosis | 0.5-1.5 | 5 |
| Adenoma | 1.0-4.0 | 4 |
| Vascular lesion | 1.5 | 1 |
| Regenerative nodule | 1.0-2.0 | 13 |
| Dysplastic nodule | 0.5-3.0 | 6 |
| Hepatocellular carcinoma | 1.0-3.0 | 14 |
| Cholangiocarcinoma | 5.0 | 1 |
| Metastasis | 0.3-8.8 | 17 |
MR, magnetic resonance.
Table 2 illustrates the comparative results between the two groups of post-contrast MR images in terms of diagnostic accuracy, sensibility, specificity, PPV and NPV. In particular, the difference statistically significant between the two groups of post-contrast MR images was observed only in diagnostic specificity and PPV that were significantly (P<0.01) higher for SPIO MR images compared to those of gadolinium MR scans.
Table 2. MR imaging results after contrast media.
| Gadolinium (%) | SPIO (%) | |
|---|---|---|
| Accuracy | 83 | 92 |
| Sensibility | 79 | 74 |
| Specificity | 85 | 99* |
| Positive predictive value | 68 | 96* |
| Negative predictive value | 91 | 90 |
*, P<0.01; MR, magnetic resonance; SPIO, superparamagnetic iron oxide.
In the majority of FLL (n=98, 83%) the results of MR images obtained with the two contrast media were concordant. In particular, 74 cases of concordance in terms of benign lesions (score =0) were observed, while the remaining 24 cases were represented by malignant lesions (score =2); in this group of FLL, no cases assigned to score 1 were found for both post-contrast MR images. Conversely, in the remaining FLL (n=20, 17%) the results of MR images obtained with the two contrast media were discordant (P<0.01). Table 3 illustrates the comparative findings between the two groups of post-contrast MR images in discordant cases. In particular, in 13 FLL (8 hemangiomas, 1 adenoma and 4 dysplastic nodules) the result of MR imaging after gadolinium (score 1) was classified as false positive for malignancy, while that of MR scan after SPIO (score 0) was classified as true negative (Figures 1,2). In a case of regenerative nodule the result of MR imaging after gadolinium (score 0) was classified as true negative for malignancy, while that of MR scan after SPIO (score 2) was classified as false positive. In 4 cases of FLL by hepatocellular carcinoma the result of MR imaging after gadolinium (score 2) was classified as true positive for malignancy, while that of MR scan after SPIO (score 0) was classified as false negative. Finally, in the last two FLL of malignant nature (1 hepatocellular carcinoma and 1 cholangiocarcinoma) the result of MR imaging after gadolinium (score 0) was classified as false negative for malignancy, while that of MR scan after SPIO (score 2) was classified as true positive (Figure 3).
Table 3. Discordant MR results (17%) on images after contrast media in FLL (n=20).
| Lesion | Size (cm) | Gadolinium [score] | SPIO [score] |
|---|---|---|---|
| Adenoma | 2.0 | FP [1] | TN [0] |
| Hemangioma | 1.0 | FP [1] | TN [0] |
| Hemangioma | 0.5 | FP [1] | TN [0] |
| Hemangioma | 0.5 | FP [1] | TN [0] |
| Hemangioma | 0.5 | FP [1] | TN [0] |
| Hemangioma | 0.5 | FP [1] | TN [0] |
| Hemangioma | 1.0 | FP [1] | TN [0] |
| Hemangioma | 1.0 | FP [1] | TN [0] |
| Hemangioma | 0.5 | FP [1] | TN [0] |
| Rigenerative nodule | 1.0 | TN [0] | FP [2] |
| Dysplastic nodule | 1.0 | FP [1] | TN [0] |
| Dysplastic nodule | 0.5 | FP [1] | TN [0] |
| Dysplastic nodule | 1.0 | FP [1] | TN [0] |
| Dysplastic nodule | 0.5 | FP [1] | TN [0] |
| Hepatocellular Ca | 1.0 | TP [2] | FN [0] |
| Hepatocellular Ca | 1.5 | TP [2] | FN [0] |
| Hepatocellular Ca | 1.0 | TP [2] | FN [0] |
| Hepatocellular Ca | 1.0 | TP [2] | FN [0] |
| Hepatocellular Ca | 1.0 | FN [0] | TP [2] |
| Metastasis | 1.3 | FN [0] | TP [2] |
MR, magnetic resonance; FLL, focal liver lesions; FP, false positive; TN, true negative; TP, true positive; FN, false negative; Ca, carcinoma.
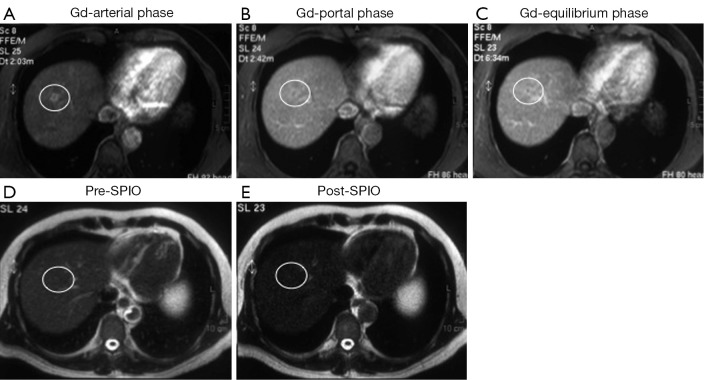
Figure 1.
A small (1 cm) capillary hemangioma, proven by biopsy, was located in the segment VIII; dynamic magnetic resonance (MR) images after gadolinium (Gd) administration show focal faint enhancement by the lesion (white circle) only in the arterial phase (A) with no focal abnormality in portal (B) and equilibrium (C) phases (score =1; false positive finding); T2 weighted MR images before (D) and after (E) superparamagnetic iron oxide (SPIO) administration show homogeneous contrast distribution in the liver with diffuse hypointensity and no focal abnormalities (score =0; true negative finding).
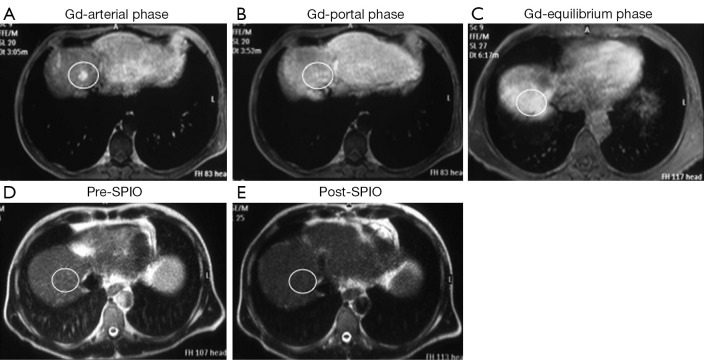
Figure 2.
A small (1 cm) dysplastic nodule, proven by biopsy, was located in the segment VIII; dynamic magnetic resonance (MR) images after gadolinium (Gd) administration show focal faint enhancement by the lesion (white circle) only in the arterial phase (A) with no focal abnormality in portal (B) and equilibrium (C) phases (score =1; false positive finding); T2 weighted MR images before (D) and after (E) superparamagnetic iron oxide (SPIO) administration show homogeneous contrast distribution in the liver with diffuse hypointensity and no focal abnormalities (score =0; true negative finding).
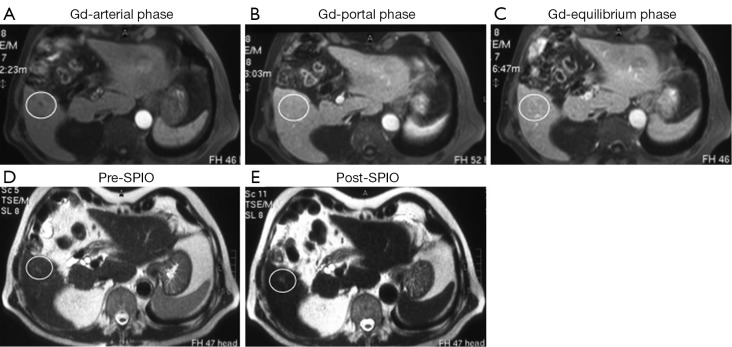
Figure 3.
A small (5 mm) hepatocellular carcinoma, proven by biopsy, was located in the segment VI; dynamic magnetic resonance (MR) images after gadolinium (Gd) administration show no focal abnormalities in the arterial (A), portal (B) and equilibrium (C) phases (score =0; false negative finding); T2 weighted MR images before (D) and after (E) superparamagnetic iron oxide (SPIO) administration show diffuse liver hypointensity and focal faint hyperintensity (white circle) in the VI hepatic segment in the post-contrast MR image (E) (score =2; true positive finding).
Discussion
MR imaging represents the diagnostic modality of choice to identify and characterize FLL (9-12). For this purpose, different contrast media have been proposed as gadolinium chelates with extra-cellular distribution or agents with intra-cellular liver concentration and biliary excretion as well as compounds with distribution in the cells of the RES. However, although the availability of different contrast media, a standard MR imaging protocol for the identification and characterization of FLL has not been clearly defined.
In this study, we report our experience regarding the characterization of FLL using MR imaging and two different contrast media. In particular, we directly compared in patients with clinical suspicion or imaging evidence by US and/or CT scans of FLL the results of MR images obtained after gadolinium chelate administration with those of MR images acquired after SPIO injection in order to define the diagnostic role of SPIO to characterize FLL. The results of our study demonstrated a statistically significant difference only of the diagnostic specificity and PPV in the comparative analysis between gadolinium and SPIO MR images showing higher values of SPIO. In particular, this difference was observed since in 20 small (0.5-2.0 cm) FLL discordant results between MR contrast media images were found. Of note, in such FLL MR imaging has been previously suggested as an effective method for tissue characterization (13). In particular, a major number (n=13) of false positive findings of gadolinium images was observed compared to that (n=1) of SPIO scans; these lesions were represented by 8 hemangiomas, 1 adenoma and 4 dysplastic nodules of small size (0.5-2.0 cm) in which the enhancement of gadolinium was suspicious for malignancy (score 1); conversely, in these lesions the distribution of SPIO was homogeneous and it ruled out the suspicion of malignancy. Particularly, in the eight cases of small capillary hemangiomas, confirmed by biopsy, the small lesion size (0.5-1.0) as well as the vascular lesion features might justify the early and faint gadolinium enhancement only during the arterial phase as also the corresponding homogeneous concentration of SPIO in the liver tissue where the small lesions were located. Of note, it is necessary to underline that these capillary hemangiomas were not detectable both on T1 and T2 pre-contrast images which, thus, were not helpful for lesion identification and characterization. In the individual FLL represented by a small (2 cm) hepatic adenoma the homogeneous distribution of SPIO allowed to rule out malignancy. In this regard, although in the majority of hepatic adenomas no RES cells have been described and thus no SPIO concentration is detectable, it is described in the literature that in some cases hepatic adenomas are able to concentrate SPIO with significant reduction of MR signal intensity on T2-weighted images, as occurred in our case (14). In the four FLL represented by dysplastic nodules, the discordant results between the two contrast media are not amazing since neoangiogenesis and the presence of RES cells have been demonstrated in such lesions. Furthermore, in terms of diagnostic sensitivity and negative predictive results the values were higher for gadolinium MR images compared to those of SPIO scans since a lower number of false negative cases for malignancy was observed (n=2 vs. 4). In particular, the four FLL in which the results of MR SPIO images were false negative for malignancy were represented by small (1.0-1.5 cm) well-differentiated HCC in which the high grade of lesion differentiation might justify SPIO concentration. Conversely, the two FLL in which the results of MR gadolinium images were false negative for malignancy were represented by a small (1.0 cm) hepatocellular carcinoma in which the small size and/or the hypovascular features of the lesion might justify the absence of contrast enhancement in arterial phase, and by a small (1.3 cm) colo-rectal metastasis in which the better diagnostic accuracy of MR SPIO imaging has been previously demonstrated (15). However, the observed difference in diagnostic sensitivity and NPV was not statistically significant since a limited number of discordant cases of our series. Therefore, the results of our study suggest a diagnostic role of MR SPIO imaging for the characterization of FLL in terms of high diagnostic specificity and PPV.
The comparative studies available in the literature regarding the direct comparison between gadolinium and SPIO contrast media to identify and characterize FLL report heterogeneous results (16-32). In particular, these discordant findings were observed since different patient population were investigated; in fact, it is appropriate to distinguish comparative studies performed in patients with chronic liver disease (cirrhosis), with suspicion of liver metastases or with incidental FLL. In patients with liver chronic disease (cirrhosis), the majority of comparative studies (16-22) showed a higher diagnostic accuracy of gadolinium MR imaging to identify hepatocellular carcinoma. However, in such patients an investigation (23) demonstrated a higher diagnostic accuracy of SPIO MR imaging as well as another study (24) reported similar results of the two contrast media in terms of diagnostic sensitivity, but a higher diagnostic specificity of gadolinium MR imaging. Conversely, in patients with suspicion of liver metastases comparative studies showed a higher diagnostic accuracy of SPIO MR imaging in two series (25,26) and a similar diagnostic accuracy between the two contrast media in an individual study (27). Finally, in patients with incidental FLL the comparative results suggest a higher diagnostic accuracy of SPIO MR imaging in hypovascular or small (<1 cm) FLL (28-30) as well as a higher diagnostic accuracy of gadolinium MR imaging to identify hypervascular FLL (29). This heterogeneity of MR imaging experiences determinated that in other studies (31,32) a combined MR protocol with double contrast medium for the characterization of FLL has been proposed. Furthermore, in two recent comparative studies between MR SPIO imaging and integrated positron emission tomography with computed tomography (PET-CT) using F-18 FDG in patients evaluated during the follow-up for colo-rectal carcinoma, it has been demonstrated a higher diagnostic accuracy of MR SPIO imaging both to identify and characterize liver metastases (15,33). Therefore, there is a wide evidence that MR liver imaging with SPIO represents a valid alternative to gadolinium to identify and characterize FLL showing a complementary role. In addition, the morpho-functional features of MR liver imaging are also reflected by other different MR techniques to evaluate FLL such as diffusion-sequence, spectroscopy and elastography (34,35). Furthermore, in MR imaging the characterization of FLL may be performed using contrast media with hepato-biliary excretion (36-38) as gadolinium-BOPTA (Multihance) and gadolinium-EOB-DTPA (Primovist) of which the former shows minimal hepato-biliary excretion (3-5%) of the administered dose, while the latter has a major (50%) biliary distribution. In particular, this difference determinates a delayed (1 hour) imaging in case of gadolinium-BOPTA administration, while in case of gadolinium-EOB-DTPA early (20 min) imaging is allowed with significant advantages for patient management as well as for machine time scheduling. Finally, it is appropriate to underline the further advantage of these two contrast media that both, with a single intravenous injection, allow the early extra-cellular liver imaging as well as the delayed intra-cellular hepato-biliary imaging phase. Thus, MR imaging provides specific diagnostic information to identify and characterize FLL on the basis of several liver functions, cellular or extra-cellular, of which the presence of RES cells is reflected by the distribution of SPIO. However, it is necessary to underline that the commercial availability of this contrast agent is currently limited to the market in Japan, although it is specific in its functional intra-cellular features. This commercial aspect may explain the limited number of published scientific studies using SPIO in last years; however, a clinical study (39) has been recently performed in Japan describing the use of MR SPIO imaging in patients with hepatocellular carcinoma treated with radiofrequency, hence demonstrating the persistent scientific interest of clinical applications of this contrast agent.
However, some comments are necessary about the limitations of our study. First, the patient population was heterogeneous since three sub-groups of patients were studied represented by patients with liver cirrhosis, patients in staging for colo-rectal cancer and patients with incidental FLL. Second, each sub-group of patients consisted of a small number of patients. Third, the correlation between MR imaging results and pathology data was limited since it was obtained only in the 27% of FLL in which histology or biopsy data were available; conversely, the majority (73%) of standard reference data to compare MR findings was represented by clinical-imaging follow criteria.
In conclusion, our results suggest a complementary role between SPIO and gadolinium MR imaging to characterize FLL; in particular, MR imaging with SPIO provides a diagnostic incremental value in terms of specificity and PPV to characterize FLL compared to gadolinium. Thus, we strongly recommend the use of SPIO when liver lesion characterization is requested and gadolinium images are not diagnostic; therefore, as also suggested by others, a combined MR imaging protocol using both contrast media might be considered and widely investigated.
Acknowledgements
Paper presented as EPOS (10.1594/ecr2013/C-1305) during the European Congress of Radiology (ECR), Vienna, 7-11 March 2013.
Disclosure: The authors declare no conflict of interest.
References
- 1.Gervais DA, Goldberg SN, Brown DB, et al. Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol 2009;20:3-8 [DOI] [PubMed] [Google Scholar]
- 2.Helmberger T.Interventional procedures for hepatic metastases. Chirurg 2010;81:542-50 [DOI] [PubMed] [Google Scholar]
- 3.Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: patient selection and postoperative outcome. Liver Transpl 2004;10:S39-45 [DOI] [PubMed] [Google Scholar]
- 4.Schwartz M.Liver transplantation in patients with hepatocellular carcinoma. Liver Transpl 2004;10:S81-5 [DOI] [PubMed] [Google Scholar]
- 5.Dai Y, Chen MH, Yin SS, et al. Focal liver lesions: can SonoVue-enhanced ultrasound be used to differentiate malignant from benign lesions? Invest Radiol 2007;42:596-603 [DOI] [PubMed] [Google Scholar]
- 6.Soye JA, Mullan CP, Porter S, et al. The use of contrast-enhanced ultrasound in the characterisation of focal liver lesions. Ulster Med J 2007;76:22-5 [PMC free article] [PubMed] [Google Scholar]
- 7.Winterer JT, Kotter E, Ghanem N, et al. Detection and characterization of benign focal liver lesions with multislice CT. Eur Radiol 2006;16:2427-43 [DOI] [PubMed] [Google Scholar]
- 8.Catala V, Nicolau C, Vilana R, et al. Characterization of focal liver lesions: comparative study of contrast-enhanced ultrasound versus spiral computed tomography. Eur Radiol 2007;17:1066-73 [DOI] [PubMed] [Google Scholar]
- 9.Coenegrachts K.Magnetic resonance imaging of the liver: new imaging strategies for the evaluating focal liver lesions. World J Radiol 2009;1:72-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semelka RC, Helmberger TK. Contrast agents for MR imaging of the liver. Radiology 2001;218:27-38 [DOI] [PubMed] [Google Scholar]
- 11.Harisinghani MG, Jhaveri KS, Weissleder R, et al. MRI contrast agents for evaluating focal hepatic lesions. Clin Radiol 2001;56:714-25 [DOI] [PubMed] [Google Scholar]
- 12.Hammerstingl R, Huppertz A, Breuer J, et al. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral TC for the therapeutic strategy: comparison with intraoperative end histopathologic findings in focal liver lesions. Eur Radiol 2008;18:457-67 [DOI] [PubMed] [Google Scholar]
- 13.Mueller GC, Hussain HK, Carlos RC, et al. Effectiveness of MR imaging in characterizing small hepatic lesions: routine versus expert interpretation. AJR Am J Roentgenol 2003;180:673-80 [DOI] [PubMed] [Google Scholar]
- 14.Schneider G, Grazioli L, Saini S. eds. Imaging of benign focal liver lesions. In: MRI of the liver. Milano, Italy: Springer-Verlag, 2003;133. [Google Scholar]
- 15.Mainenti PP, Mancini M, Mainolfi C, et al. Detection of colo-rectal liver metastases: prospective comparison of contrast enhanced US, multidetector CT, PET/CT, and 1.5 Tesla MR with extracellular and reticulo-endothelial cell specific contrast agents. Abdom Imaging 2010;35:511-21 [DOI] [PubMed] [Google Scholar]
- 16.Tang Y, Yamashita Y, Arakawa A, et al. Detection of hepatocellular carcinoma arising in cirrhotic livers: comparison of gadolinium-and ferumoxides-enhanced MR imaging. AJR Am J Roentgenol 1999;172:1547-54 [DOI] [PubMed] [Google Scholar]
- 17.Ward J, Guthrie JA, Scott DJ, et al. Hepatocellular carcinoma in the cirrhotic liver: double-contrast MR imaging for diagnosis. Radiology 2000;216:154-62 [DOI] [PubMed] [Google Scholar]
- 18.Pauleit D, Textor J, Bachmann R, et al. Hepatocellular carcinoma: detection with gadolinium-and ferumoxides-enhanced MR imaging of the liver. Radiology 2002;222:73-80 [DOI] [PubMed] [Google Scholar]
- 19.Kim YK, Kim CS, Lee YH, et al. Comparison of superparamagnetic iron oxide–enhanced and gadobenate dimeglumine–enhanced dynamic MRI for detection of small hepatocellular carcinomas. AJR Am J Roentgenol 2004;182:1217-23 [DOI] [PubMed] [Google Scholar]
- 20.Kim YK, Kim CS, Han YM. Detection of small hepatocellular carcinoma: comparison of conventional gadolinium-enhanced MRI with gadoliniumenhanced MRI after the administration of ferucarbotran. Br J Radiol 2009;82:468-84 [DOI] [PubMed] [Google Scholar]
- 21.Kim YK, Kim CS, Han YM, et al. Comparison of gadoxetic acid-enhanced MRI and superparamagnetic iron oxide-enhanced MRI for the detection of hepatocellular carcinoma. Clin Radiol 2010;65:358-65 [DOI] [PubMed] [Google Scholar]
- 22.Matsuo M, Kanematsu M, Itoh K, et al. Detection of malignant hepatic tumors: comparison of gadolinium- and ferumoxide-enhanced MR imaging. AJR Am J Roentgenol 2001;177:637-43 [DOI] [PubMed] [Google Scholar]
- 23.Castoldi MC, Fauda V, Scaramuzza D, et al. Hepatic and hepatocarcinoma magnetic resonance: comparison of the results obtained with paramagnetic (gadolinium) and superparamagnetic (iron oxide particles) contrast media. Radiol Med 2000;100:160-7 [PubMed] [Google Scholar]
- 24.Simon G, Link TM, Wörtler K, et al. Detection of hepatocellular carcinoma: comparison of Gd-DTPA- and ferumoxides-enhanced MR imaging. Eur Radiol 2005;15:895-903 [DOI] [PubMed] [Google Scholar]
- 25.del Frate C, Bazzocchi M, Mortele KJ, et al. Detection of liver metastases: comparison of gadobenate dimeglumine-enhanced and ferumoxides-enhanced MR imaging examinations. Radiology 2002;225:766-72 [DOI] [PubMed] [Google Scholar]
- 26.Ward J, Robinson PJ, Guthrie JA, et al. Liver metastases in candidates for hepatic resection: comparison of helical CT and gadolinium- and SPIO-enhanced MR imaging. Radiology 2005;237:170-80 [DOI] [PubMed] [Google Scholar]
- 27.Kim YK, Lee YH, Kwak HS, et al. Detection of liver metastases: gadoxetic acid-enhanced three-dimensional MR imaging versus ferucarbotran-enhanced MR imaging. Eur J Radiol 2010;73:131-6 [DOI] [PubMed] [Google Scholar]
- 28.Di Cesare E, Cariello G, Barile A, et al. Magnetic resonance imaging of hepatic focal lesions: dynamic contrastographic evaluation with gadolinium versus reticulo-endothelial hepato-specific contrast media. Radiol Med 2000;100:245-50 [PubMed] [Google Scholar]
- 29.Kim MJ, Kim JH, Chung JJ, et al. Focal hepatic lesions: detection and characterization with combination gadolinium- and superparamagnetic iron oxide–enhanced MR imaging. Radiology 2003;228:719-26 [DOI] [PubMed] [Google Scholar]
- 30.Cheng WZ, Zeng MS, Yan FH, et al. Ferucarbotran versus Gd-DTPA-enhanced MR imaging imaging in the detection of focal hepatic lesions. World J Gastroenterol 2007;13:4891-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heilmaier C, Lutz AM, Bolog N, et al. Focal liver lesions: detection and characterization at double-contrast liver MR Imaging with ferucarbotran and gadobutrol versus single-contrast liver MR imaging. Radiology 2009;253:724-33 [DOI] [PubMed] [Google Scholar]
- 32.Takahama K, Amano Y, Hayashi H, et al. Detection and characterization of focal liver lesions using superparamagnetic iron oxide-enhanced magnetic resonance imaging: comparison between ferumoxides-enhanced T1-weighted imaging and delayed-phase gadolinium-enhanced T1-weighted imaging. Abdom Imaging 2003;28:525-30 [DOI] [PubMed] [Google Scholar]
- 33.Bacigalupo L, Aufort S, Eberlé MC, et al. Assessment of liver metastases from colorectal adenocarcinoma following chemotherapy: SPIO-MRI versus FDG-PET/TC. Radiol Med 2010;115:1087-100 [DOI] [PubMed] [Google Scholar]
- 34.Haradome H, Grazioli L, Morone M, et al. T2-weighted and diffusion-weighted MRI for discriminating benign from malignant focal liver lesions: diagnostic abilities of single versus combined interpretations. J Magn Reson Imaging 2012;35:1388-96 [DOI] [PubMed] [Google Scholar]
- 35.Lee JM, Choi BI. Hepatocellular nodules in liver cirrhosis: MR evaluation. Abdom Imaging 2011;36:282-9 [DOI] [PubMed] [Google Scholar]
- 36.Seale MK, Catalano OA, Saini S, et al. Hepatobiliary–specific MR contrast agents: role in imaging the liver anb biliary tree. Radiographics 2009;29:1725-48 [DOI] [PubMed] [Google Scholar]
- 37.Golfieri R, Grazioli L, Orlando E, et al. Which is the best MRI marker of malignancy for atypical cirrhotic nodules: hypointensity in hepatobiliary phase alone or combined with other features? Classification after Gd-EOB-DTPA administration. J Magn Reson Imaging 2012;36:648-57 [DOI] [PubMed] [Google Scholar]
- 38.Grazioli L, Bondioni MP, Haradome H, et al. Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology 2012;262:520-9 [DOI] [PubMed] [Google Scholar]
- 39.Koda M, Tokamaga S, Miyoshi K, et al. Ablative margin states by magnetic resonance imaging with ferucarbotran in radiofrequency ablation for hepatocellular carcinoma can predict local tumor progression. J Gastroenterol 2013;48:1283-92 [DOI] [PubMed] [Google Scholar]