Chronic liver disease is a major public health problem worldwide. Liver fibrosis, a common feature of almost all chronic liver diseases, involves the accumulation of collagen, proteoglycans, and other macromolecules in the extracellular matrix. The accumulation of proteins in the extracellular matrix promotes the formation of scars that bridge together adjacent portal triads and central veins. Ultimately, progressive hepatic fibrosis leads to cirrhosis, a characteristic of all end-stage liver disease. Clinically, liver fibrosis usually has an insidious onset and progresses slowly over decades. Patients remain asymptomatic or have only mild, nonspecific symptoms until the development of cirrhosis (1).
To date, the conventional imaging diagnostic tests available in clinical practice are not sensitive or specific enough to function as screening tests for detecting liver fibrosis. In patients with precirrhotic stages of liver fibrosis, as well as patients with early cirrhosis, the liver parenchyma usually has a normal appearance or may exhibit only subtle, nonspecific heterogeneity on conventional MR images. A number of MR imaging techniques have been investigated to identify or to assign a grade to liver cirrhosis. These include double contrast material-enhanced MR imaging (2), MR elastography (3-5), and diffusion weighted imaging (3,5,6). The reproducibility and intersite variability of these techniques have not been well established.
A mechanism for magnetic resonance (MR) tissue contrast, spin-lattice relaxation time in the rotating frame (T1ρ), has been investigated in biomedical applications. In T1ρ imaging, the equilibrium magnetization, M0, established by the static magnetic field, B0, is flipped into the transverse plane first. This magnetization in the transverse plane relaxes like a normal free induction decay but in the presence of an on-resonant continuous wave radiofrequency pulse, which is much weaker than B0 and is called spin-lock radiofrequency pulse. The relaxation rate constant of this transverse magnetization with regard to the duration of spin-lock radiofrequency pulse is T1ρ relaxation time. T1ρ is sensitive to low frequency motional processes comparable to the Larmor frequency of the spin-lock radiofrequency pulse, thus it can be used to investigate macromolecular composition and proton exchange in tissues (7). Because liver fibrosis involves the accumulation of a number of biologic macromolecules, including collagen and proteoglycans, it was hypothesized that T1ρ MR imaging may be sensitive for evaluation of liver fibrosis (8). The first experiment was carried out using a rat biliary duct ligation (BDL) model which results in cholestatic liver injury, fibrosis, and cirrhosis. It was concluded that liver fibrosis is associated with T1ρ value increases, and T1ρ MR imaging can be sensitive in the evaluation of liver fibrosis progression (8) (Figure 1). This concept was further confirmed by another liver fibrosis model in rats induced by carbon tetrachloride intoxication (9). Both these animal experiments were conducted at 3T with a spin-lock frequency of 500 Hz.
Figure 1.
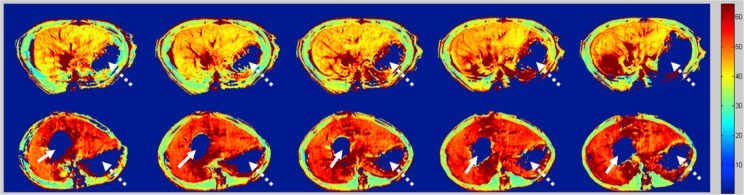
Color-coded T1ρ maps of liver in a sham-operated rat (control, top row) and liver in a BDL rat 24 days after surgery (bottom row). BDL rat liver demonstrates higher T1ρ value (red) than does the control rat liver (yellow orange). Solid arrow, dilated biliary duct; dotted arrow, gas in the stomach [reproduced from reference (8)].
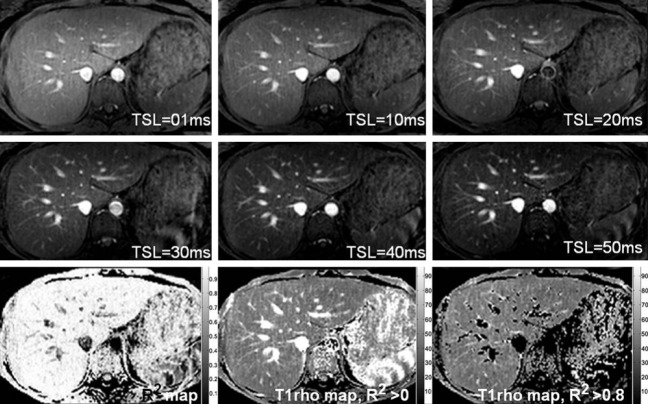
Liver T1ρ value in healthy volunteers has also been obtained at 3T. At clinical 3T, three representative axial slices were selected to cut through the upper, middle and lower liver. A rotary echo spin-lock pulse was implemented in a two-dimensional fast-field echo (FFE) sequence. Spin-lock frequency was 500 Hz, and the spin-lock times (TSLs) of 1, 10, 20, 30, 40 and 50 ms were used for T1ρ mapping. The images were acquired slice by slice during breath-holding. Regions of interest were manually placed on each slice of the liver parenchyma region, excluding artefacts and vessels. The normal liver T1ρ value ranged from 38.6 to 48.3 ms (mean 43.0 ms, median 42.6 ms) (Figure 2) (10). The feasibility of using three or two TSLs to measure liver T1ρ relaxation time was explored (11). Adopting 3 TSLs of 1, 20, and 50 ms can be an acceptable alternative for the liver T1ρ measurement (12-14).
Figure 2.
The upper and middle rows show liver T1ρ-weighted images acquired with spin lock times (TSL) ranged from 1 to 50 ms. Note vessels demonstrate high signal on T1ρ-weighted images. The lower row shows coefficient of determination (R2) map (left), T1ρ map without R2 evaluation (middle), and T1ρ map with R2 >0.8 evaluation [right; reproduced from reference (10)].
The first clinical study of T1ρ liver imaging in liver cirrhosis patients was reported at the International Society of Magnetic Resonance in Medicine annual conference in 2012 (15). Nine patients with established liver cirrhosis were recruited. MRI data acquisition was performed on a 3T clinical scanner with the technique similar to a previous report (12). TSLs of 1, 20, and 50 ms were used for T1ρ mapping. TE and TR for FFE acquisition were 1.16 and 2.3 ms respectively. The voxel size was 1.50×1.50×7.00 mm3. The flip angle was 40 degrees and the number of signal averages (NSA) was 3. A sensitivity-encoding (SENSE) factor of 1.5 was applied for parallel imaging to reduce the acquisition time. To quantify liver T1ρ value, three to five regions-of-interest (ROIs) of approximately 100-200 mm2 were manually placed on the liver right robe parenchyma region on the T1ρ maps for each slice, excluding observable artifacts and blood vessels. With T1ρ value measured from the right liver lobes, the mean value (± SD) was 51.1±8.1 ms (Figure 3), significantly higher than the mean value of 43.0±2.2 ms (P<0.01) in healthy volunteers reported in our previous study (10). It is known that hepatic fibrosis and hepatic cirrhosis may not be homogeneously distributed across liver (16). We measured the T1ρ value of the right lobe instead of both right and left lobes, because it is common that right lobe undergoes more severe cirrhotic changes while the left lobe tends to regenerate and become hypertrophied.
Figure 3.
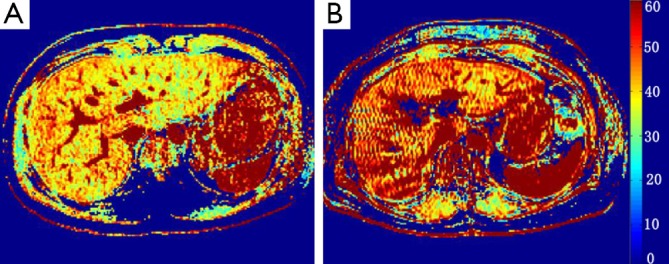
(A) Liver T1ρ map of a healthy volunteer; (B) liver T1ρ map of a patient with liver cirrhosis [reproduced from reference (15)].
Recently, two studies tested at 1.5 T whether MR T1ρ imaging of fibrotic liver disease is feasible, investigated whether liver T1ρ imaging allows assessment of the severity of liver cirrhosis, and assessed the normal liver T1ρ range in healthy patients (17,18). In Allkemper et al.’s study, respiratory triggering and 3-D fast field-echo sequence was used. The spin-lock frequency was also 500 Hz. By taking the advantage of the higher temporal efficiency of 3D sequence, larger liver volume was covered (26 sections). SLT of 10, 20, 40, and 80 ms was applied. At 1.5 T, Allkemper et al. was able to use comparatively long SLT of 80 ms (while in our study at 3.0 T the longest SLT was limited to 50 ms restricted by the scanner). A total of 25 healthy volunteers and 34 patients with liver cirrhosis underwent whole-liver T1ρ MR imaging. The authors concluded that mean T1ρ values of volunteers (mean, 40.9±2.9 ms; range, 33.9-46.3 ms) were significantly lower than those of patients who were Child-Pugh class A (45.4±1.6 ms; P<0.001), B (50.0±3.0 ms; P<0.001), or C (54.0±3.7 ms; P<0.001), and significant differences were found between each Child-Pugh stage (A vs. B, P<0.002; B vs. C, P<0.009; A vs. C, P<0.001).
Allkemper et al.’ demonstrated that mean T1ρ values did not correlate significantly with necroinflammatory activity, the degree of steatosis, or the presence of iron load. In an experimental study in rat liver fibrosis model with carbon tetrachloride intoxication, we also showed that acute necroinflammation did not lead to an increased liver T1ρ (9). However, theoretically, the presence of liver iron load may increase T2* effect and this would influence T1ρ measurement. Further studies are needed to clarify the influence of iron load on T1ρ measurement.
In Rauscher et al.’s study, 10 healthy control subjects and 21 patients with clinically diagnosed liver cirrhosis were examined at 1.5 T. T1ρ weighted images were acquired using a 2D Turbo FLASH sequence with spin-lock preparation. The spin-lock frequency was 400 Hz and the TSLs included 4, 8, 16, 32 and 48 ms. T1ρ measurements were performed on one representative section of the liver. Mean liver T1ρ values in patients with liver cirrhosis (57.4±7.4 ms) were significantly higher than those of healthy subjects (47.8±4.2 ms; range, 41.7-56.1 ms; P=0.0007). In their study, 8 patients had a Child-Pugh score A, 11 patients had a Child-Pugh score B, and 2 patients had a Child-Pugh score C. Both patients with Child-Pugh score A and Child-Pugh score B revealed a significant difference compared to the healthy control group, and the difference for patients with Child-Pugh score B compared to healthy control subjects was more significant (P=0.001 compared to P=0.01). There was no significant difference in the T1ρ values between patients with Child-Pugh score A and patients with Child-Pugh score B. Due to the limited sample size of patients with Child-Pugh score C (n=2) a statistical comparison was not possible.
Allkemper et al.’s study found the positive correlation between mean T1ρ value and Child-Pugh classification. On the other hand, while our animal study suggested MRI T1ρ might detect early stage liver fibrosis, this has not been confirmed in Allkemper et al. and Rauscher et al.’s studies, as no patients with early stages of liver fibrosis were investigated in their studies. Our recent human study data at 3.0 T showed in healthy subjects mean liver T1ρ value was 43.2±2.2 ms (range, 38.6-48.0 ms). In Dr Allkemper et al.’s study, for healthy subjects the mean T1ρ value was 40.9±2.9 msec (range, 33.9-46.3 ms). In our study of nine cirrhotic patients (15), the mean liver T1ρ was 51.1±8.1 ms, similar to the Allkemper et al.’s results in Child-Pugh B and C patients. The lower bound T1ρ value in Child-Pugh A patients seems to overlap with the higher end T1ρ value of healthy subjects. It is our experience that with the technique we used (10,12,13,15), poor breathing holding or respiration induced liver displacement between different SLTs could lead to artificially high T1ρ measurement. Allkemper et al. also suggested that ‘since even minor spatial misregistration between imaging examinations with different SLTs may lead to artificially high T1ρ value of liver parenchyma, … liver vessels displayed high signal intensities because of the use of fast field-echo sequences’ (17). Therefore, while we may conclude that the mean normal value of liver T1ρ is likely to be around 41-43 ms (8-10,12,13,17), technical improvement leading to narrow down the normal range may help to increase the sensitivity for detection of early liver fibrosis. Since the micro-vessels in the liver parenchyma may contribute to high T1ρ measurement due to partial volume effect, techniques which can effectively and homogeneously suppress blood flow may be useful. Eventually, T1ρ imaging may form part of parametric assessment of liver function and be combined with other functional hepatic imaging methods or other serum biomarkers to increase its sensitivity and specificity in the detection and staging of early liver fibrosis. To translate MR T1ρ imaging to clinical application for use as an early imaging biomarker for liver fibrosis detection and staging (19), further technical improvements are warranted.
Acknowledgements
Supported by Hong Kong RGC GRF 475911, SEG_CUHK02 and China NSFC grant 81201076.
Disclosure: The authors declare no conflict of interest.
References
- 1.Wallace K, Burt AD, Wright MC. Liver fibrosis. Biochem J 2008;411:1-18 [DOI] [PubMed] [Google Scholar]
- 2.Aguirre DA, Behling CA, Alpert E, et al. Liver fibrosis: noninvasive diagnosis with double contrast material-enhanced MR imaging. Radiology 2006;239:425-37 [DOI] [PubMed] [Google Scholar]
- 3.Talwalkar JA, Yin M, Fidler JL, et al. Magnetic resonance imaging of hepatic fibrosis: emerging clinical applications. Hepatology 2008;47:332-42 [DOI] [PubMed] [Google Scholar]
- 4.Salameh N, Larrat B, Abarca-Quinones J, et al. Early detection of steatohepatitis in fatty rat liver by using MR elastography. Radiology 2009;253:90-7 [DOI] [PubMed] [Google Scholar]
- 5.Bonekamp S, Kamel I, Solga S, et al. Can imaging modalities diagnose and stage hepatic fibrosis and cirrhosis accurately? J Hepatol 2009;50:17-35 [DOI] [PubMed] [Google Scholar]
- 6.Lu PX, Huang H, Yuan J, et al. Intravoxel Incoherent Motion MR Imaging showed lower pure molecular diffusion in fibrotic livers: a report of preliminary results. Proc Intl Soc Mag Reson Med 2013;21:No.4081 Available online: http://www.ismrm.org/13/ep10.htm [Google Scholar]
- 7.Yuan J, Wang YX. Chapter 20: T1rho MR Imaging: Principle, Technology, and Application, in “Medical Imaging: Technology and Applications”. In: Farncombe T, Iniewski K. eds. CRC Press, 2013:565-92. ISBN 9781466582620. Available online: http://www.crcpress.com/product/isbn/9781466582620
- 8.Wang YX, Yuan J, Chu ES, et al. T1rho MR imaging is sensitive to evaluate liver fibrosis: an experimental study in a rat biliary duct ligation model. Radiology 2011;259:712-9 [DOI] [PubMed] [Google Scholar]
- 9.Zhao F, Wang YX, Yuan J, et al. MR T1ρ as an imaging biomarker for monitoring liver injury progression and regression: an experimental study in rats with carbon tetrachloride intoxication. Eur Radiol 2012;22:1709-16 [DOI] [PubMed] [Google Scholar]
- 10.Deng M, Zhao F, Yuan J, et al. Liver T1ρ MRI measurement in healthy human subjects at 3 T: a preliminary study with a two-dimensional fast-field echo sequence. Br J Radiol 2012;85:e590-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan J, Zhao F, Griffith JF, et al. Optimized efficient liver T 1ρ mapping using limited spin lock times. Phys Med Biol 2012;57:1631-40 [DOI] [PubMed] [Google Scholar]
- 12.Zhao F, Deng M, Yuan J, et al. Experimental evaluation of accelerated T1rho relaxation quantification in human liver using limited spin-lock times. Korean J Radiol 2012;13:736-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao F, Yuan J, Deng M, et al. Further exploration of MRI techniques for liver T1rho quantification. Quant Imaging Med Surg 2013;3:308-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang YX, Zhao F, Yuan J, et al. Accelerated T1rho relaxation quantification in intervertebral disc using limited spin-lock times. Quant Imaging Med Surg 2013;3:54-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YX, Zhao F, Wong VW, et al. Liver MR T1rho measurement in liver cirrhosis patients: a preliminary study with a 2D fast field echo sequence at 3T. Proc Intl Soc Mag Reson Med 2012;20:No.1289 Available online: http://submissions.miracd.com/ISMRM2012/Proceedings/files/1289.pdf [Google Scholar]
- 16.Romero-Gómez M, Gómez-González E, Madrazo A, et al. Optical analysis of computed tomography images of the liver predicts fibrosis stage and distribution in chronic hepatitis C. Hepatology 2008;47:810-6 [DOI] [PubMed] [Google Scholar]
- 17.Allkemper T, Sagmeister F, Cicinnati V, et al. Evaluation of Fibrotic Liver Disease with Whole-Liver T1ρ MR Imaging: A Feasibility Study at 1.5 T. Radiology. Available online: http://dx.doi.org/10.1148/radiol.13130342 [DOI] [PubMed]
- 18.Rauscher I, Eiber M, Ganter C, et al. Evaluation of T1ρ as a potential MR biomarker for liver cirrhosis: Comparison of healthy control subjects and patients with liver cirrhosis. Eur J Radiol 2014;83:900-4 [DOI] [PubMed] [Google Scholar]
- 19.Wang YX. Medical imaging in pharmaceutical clinical trials: what radiologists should know. Clin Radiol 2005;60:1051-7 [DOI] [PubMed] [Google Scholar]