Abstract
More than 75% of the cases of non-small cell lung cancer (NSCLC) are diagnosed in advanced stages (IIIA-IV). Although in these patients the role of surgery is unclear, complete tumor resection can be achieved in selected cases, with good long-term survival. In this review, current indications for surgery in advanced NSCLC are discussed. In stage IIIA (N2), surgery after induction chemotherapy seems to be the best option. The indication of induction chemotherapy plus radiotherapy is debatable due to potential postoperative complications but recently reported experiences have not shown a higher postoperative risk in patients after chemo and radiotherapy induction even if pneumonectomy is performed. In cases of unexpected N2 found during thoracotomy, lobectomy plus systematic nodal dissection is recommended mostly for patients with single station disease. In stage IIIB, surgery is only the choice for resectable T4N0-1 cases and should not be indicated in cases of N2 disease. Favorable outcomes are reported after extended resections to the spine and mediastinal structures. Thorough and individualized discussion of each stage IIIB case is encouraged in the context of a multidisciplinary team. For stage IV oligometastatic cases, surgery can still be included when planning multimodality treatment. Brain and adrenal gland are the two most common sites of oligometastases considered for local ablative therapy.
Keywords: Non-small cell lung cancer (NSCLC), surgical therapy, extended pulmonary resection, multimodality treatment
Introduction
Lung cancer is still the leading cause of cancer death amongst men in most European countries. Due to the rapid course of the disease after diagnosis, standardised mortality rates are quite similar to those of incidence for both sexes (1) and in more than 75% of the cases the disease is diagnosed in advanced stages (2). Both for patients with locally advanced or distant spread of the disease, radio and chemotherapy alone or in different combination regimens have demonstrated considerable improvements in terms of long-term survival and control of symptoms (3,4) but surgery still has a paramount role in the therapy of advanced non-small cell lung cancer (NSCLC). In this review, we are focusing on the role of therapeutic surgical interventions in cases of NSCLC patients with clinical stage IIIA, IIIB or IV (5). Without the intention of performing a systematic review of the literature, we have conducted a Pubmed-based search including manuscripts published in English from 2000 to 2014 combining the following key words: “non-small cell lung cancer (NSCLC)”, “advanced clinical stage”, “clinical stage IIIA or IIIB or IV”, “oligometastatic disease” and “extended lung resection”. Whenever existing, we have reviewed first systematic reviews and meta-analysis; then randomized clinical trials and well-designed studies of cases and controls. Case series have been reviewed only for the infrequent situations of extended operations including the spine or large thoracic vessels.
Surgery alone or as a component of multimodality therapy regimens in clinical stage IIIA (N2) NSCLC
The appropriate therapy for stage IIIA (N2) NSCLC (Figure 1) is not clearly established. Accurate preoperative staging of mediastinal lymph nodes in patients with potentially resectable NSCLC is of paramount importance. Currently, invasive mediastinal staging either by fine needle aspiration using EBUS-EUS or by tissue sampling by video mediastinoscopy classifies correctly almost 90% of cases (6) but still some patients are found to have macro or microscopically evidence of mediastinal spread at thoracotomy if mediastinal staging is performed according to internationally admitted criteria (7). Surgical treatment could have a different role depending of the time when the case if classified as pathological N2 and on the number of affected lymph node stations.
Figure 1.
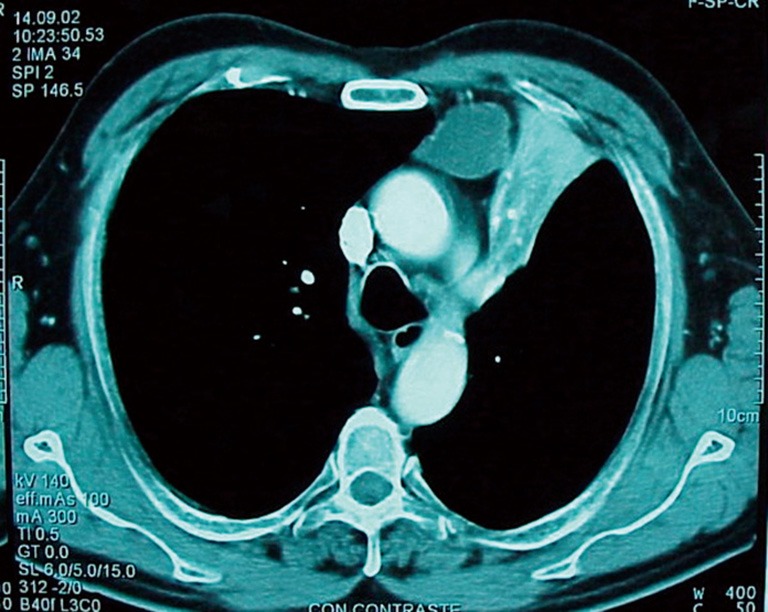
Squamous cell carcinoma of the left upper lobe with large lymph node metastasis in the prevascular area. This case could be considered for induction treatment and further re-assessment for surgery.
Unexpected N2 disease found at thoracotomy
When unsuspected N2 disease is found during scheduled lung resection for clinical stage I or II NSCLC, complete resection can be attempted by lobectomy plus systematic mediastinal lymph node resection; an alternative is aborting the procedure and referring the patient for induction chemotherapy. If complete resection is performed, some authors have reported 35-75% survival rates (8-10) in cases with single-node station N2 and adjuvant chemotherapy. In spite of the number of nodal stations involved, routine platinum-based adjuvant chemotherapy is highly recommended (11). Pneumonectomy in unexpected N2 disease is not recommended since the chances of long-term survival are comparable if the lung is left in place and the patient scheduled for chemo and radiotherapy (12). In experienced hands, VATS lobectomy seems to be comparable with thoracotomy to achieve complete resection is selected cases without the necessity of conversion to open surgery (13,14).
Combined protocols of surgery, chemo and radiotherapy
The efficacy of chemotherapy induction followed by complete resection has been studied in several meta-analysis of published randomized clinical trials. As published by Manser et al. (15), long-term and disease-free survival achieved by surgery alone is lower compared to preoperative chemoradiotherapy followed by resection. On the contrary, Nakamura et al. (16) conclude that the benefit of induction therapy for stage IIIA patients is unclear since none of the hazard ratios at any time after surgery resulted significant. These differences could be a consequence of heterogeneity in selected chemotherapy protocols (17). Standardization of the chemotherapy schemes used for induction in clinical stage IIIA cases would be welcome.
The role of induction using concurrent or sequential chemoradiotherapy is also debatable. In a recently published meta-analysis (18) the authors conclude that given the lack of survival benefit and the potential disadvantages of induction chemoradiation, these regimes should be considered only in the context of randomized clinical trials. In fact, in a randomized trial by the German Lung Cancer Cooperative Group (19) the authors found that preoperative chemoradiation in addition to chemotherapy increased mediastinal downstaging, but did not improve survival. In addition, a caveat against pneumonectomy was stated since treatment-related mortality reached 14% in patients treated by pneumonectomy.
More recently, some Japanese studies (20,21) have reported a high rate of down staging in stage IIIA patients treated with induction chemo radiotherapy—including S-1 and cisplatin-followed by surgery. These series included also pneumonectomies without increased mortality. Although the reported rates of 5-year overall survival are good (around 60%), probably the series are biased by very strict case selection criteria.
Extended resection in cases of IIIB tumours
Despite subsequent revisions, the current UICC staging system defined as IIIB tumors lesions classified T4N2 and all TxN3 diseases (5). Surgery in this context seems to be anecdotal and debated considering such categories as unresectable diseases with a dismal prognosis of about 7% (22). However, there may be a different biologic behavior in patients with local T4 disease and those with N3 nodal diseases within the stage classification. Although there are very few data reporting long-term survivors from extended resections of lung cancers involving the superior vena cava, aorta, left atrium, main pulmonary artery, or esophagus, it has been well accepted that very selected patients with mediastinal invasion along with SVC invasion but without mediastinal node involvement can be viewed as candidates for surgery. The factors that are found to possibly affect survival are the completeness of resection, the lymph node status, and the subclavian artery involvement (23). Of course, variations exist in the surgical results for patients with tumor invading the adjacent organs. Each case should be discussed at the tumor board to plan the best treatment strategy. If T4N0-N1 (IIIA) patients should be treated with aggressive multidisciplinary therapy in a manner that maximizes the chance for long-term cure, it is not the case for T4N2 disease (IIIB). This subgroup should be considered as a potential contraindication to resection of T4 tumors (Figure 2). The 5-year survival rates are 43% vs. 17% for T4N0-1 and for T4N2-3 respectively (24). These results have been also confirmed by other reports (25-27). Multiple nodal station and especially with a N2 disease is a worsen prognosis factor for T4 tumor (28). Advances in the perioperative management and postoperative care, along with a careful patient selection, will likely make the operative mortality and morbidity less prohibitive and yield a more favorable prognosis. Sleeve lobectomy has to be considered whenever possible because pneumonectomy is still a contraindication in this setting, particularly when it is a right resection (29).
Figure 2.

T4N2 adenocarcinoma invading the spine. Due to mediastinal spread to the 4R region, salvage surgery is not the choice in this case.
Another aspect of surgery for IIIB disease stands on the concept of “salvage resection”. In fact, for stage IIIB, many patients are treated with definitive CRT. The current protocol includes concomitant CRT with radiation exceeding 59 Gy. In this context, 24% to 35% of patients with locally advanced NSCLC experience isolated local relapse (30-32). The results of salvage lung resection have been reported by Bauman et al. (33), reporting on 24 patients with stage IIIB in more 35% of patients. The median duration of surgery was 5.5 hours (2 to 9 hours). Median estimated blood loss was 250 mL (0 to 4,400 mL). The median hospital length of stay was 8 days (4 to 46 days). In-hospital mortality was 4% with a 58% morbidity rate. Median overall survival was 30 months and the estimated 3-year survival was 47%. Salvage lung resection after definitive radiation for NSCLC seems to be technically feasible, with acceptable toxicity even when performed at a delayed interval (34). Although oncologic outcomes are encouraging, with a subset of long-term survivors, determination of efficacy requires prospective validation in a rigorously defined population.
Role of surgery in stage IV disease
Despite recent progress in oncologic therapy, a vast number of patients with NSCLC will develop distant metastasis. The standard therapy for metastatic cancer is systemic therapy. As reviewed recently (35), the use of emerging therapies such pemetrexed or monoclonal antibodies for patients with nonsquamous histology and good performance status and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors for patients having an EGFR mutation, is slowly improving the rate of medium-term survivors in stage IV.
The current UICC classification defined as stage IV tumors all M1a disease with existence of a controlateral lung nodulesand M1b disease with distant metastases (5). If there is an indubitably place of surgery for bilateral synchronous lesions (M1a), there is also a place for stage M1b when distant metastases are limited (or oligometastases) and seem accessible to a curative resection. Patients with solitary foci of metastatic disease represent a subgroup with a better prognosis instead of others stage IV patients. Studies have indicated that surgical resection may enhance the survival rate of patients in this setting (34,36,37). Patients who have resectable primary tumors and a solitary site of metastasis, based on a thorough metastatic work-up, benefit from surgical resection (primary tumor and solitary metastasis). The role of adjuvant chemotherapy and radiation depends on the individual and patient setting. There have been several case series indicating an improvement in the long-term (5-year) survival rates of patients after surgical resection of solitary metastases of the brain, adrenal gland, liver and other sites (38-44). Local ablative therapies include surgical resection, stereotactic body radiation therapy, and radiofrequency ablation. Brain and adrenal glands are the two most common sites of oligometastases considered for local ablative therapy.
The concept of oligometastases was proposed first by Hellman and Wechselbaum in 1995 (45) and revised in 2006 as synchronous and metachronous oligometastases (46). Synchronous oligometastases (sync-oligometastases) are defined as 1 to 5 distant metastases that can be treated by local therapy to achieve long-term survival and indicate a state of oligometastases with active but controllable primary lesions. The most important prognostic factor for sync-oligometastases is the status of the primary lesion. Metachronous oligometastases has been proposed by Niibe et al. (47) for NSCLC (oligo-recurrence). Oligo-recurrence is thus defined as 1 to 5 distant metachronous metastases that can be treated by local therapy, under conditions of a controlled primary lesion. More favorable subgroups of oligometastases have subsequently been classified (47) (Table 1).
Table 1. Prognosis of oligometastases. Adapted from Niibe et al. (47).
| Favorable | Relatively favorable | Relatively unfavorable | Unfavorable |
|---|---|---|---|
| Oligorecurrence 1 or 2 (brain and adrenal gland) | Oligorecurrence 3 to 5 (brain and adrenal gland) | 3 to 5 (brain, adrenal gland, others) | >5 or polymetastases |
| Sync-oligometastases 1 or 2 (brain and adrenal gland) | Sync-oligometastases 3 to 5 (brain and adrenal gland) | >5 or polymetastases |
For solitary brain metastases, the standard of care should include consideration of surgical resection and ablation. Favorable criteria include control of the primary tumor, a negative metastatic survey, good performance status, and a significant metachronous interval (38). Patients who have symptomatic synchronous brain lesions should undergo brain local ablative therapy (surgery, stereotactic or whole brain radiation) first to prevent neurologic complications. Subsequent platinum-based chemotherapy followed by resection of the primary tumor should be performed only if there is no evidence of mediastinal lymph node involvement and absence of progression of the disease. Complete resection of all disease should be performed. Operative mortality and morbidity for this combined approach is low. An adjuvant whole brain radiation could be discussed according to the number of brain lesions. Such multimodal treatment results in acceptable long-term outcome. Median, 1-year, and 2-year survival are 22.5 months, 69%, and 42% respectively (38). Response to preoperative chemotherapy before focal treatment was the main favorable prognostic factor (38,39).
According to the current classification, stage IV disease includes also M1a defined as contralateral lung nodules (5). Most of the time, the lesions are synchronous at diagnosis. Distinguishing synchronous primary lung cancers from advanced disease is important because prognosis and treatments are very different and a surgical approach may result in survival similar to solitary cancers. Determining this distinction with certainty, however, is challenging. Studies have suggested that bilateral surgical resection may enhance the survival rate of patients in this setting (48-50). A study from Marseille (51) reports their experience after resection of 125 patients with synchronous lung cancer. Resection for bilateral synchronous lung cancer was performed in 31 patients. The overall 5-year survival for the whole group was 34%. Survival after resection of bilateral lung cancer was near 35%. De Leyn et al. published the result of 57 patients who were planned for bilateral resection for bilateral synchronous lesions proven or suspicious for lung cancer. The median survival after bilateral resection of synchronous bilateral pathological proven lung cancer was 49 months, with a 5-year survival rate of 38% (52). These results suggested that selected patients with bilateral lung cancer may benefit from an aggressive approach, with acceptable morbidity and mortality, and rewarding long-term survival. Mun et al. (53) described their experiences with single-stage surgical treatment in 19 patients with synchronous bilateral lung cancer. They reported an excellent 5-year survival rate of 76%. Finley et al. reported that the gender female could affect positively the overall survival (54).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43-66 [DOI] [PubMed] [Google Scholar]
- 3.Laine AM, Westover KD, Choy H. Radiation therapy as a backbone of treatment of locally advanced non-small cell lung cancer. Semin Oncol 2014;41:57-68 [DOI] [PubMed] [Google Scholar]
- 4.Non-small Cell Lung Cancer Collaborative Group Chemotherapy for non-small cell lung cancer. Cochrane Database Syst Rev 2000;(2):CD002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobin LH, Gospodarowicz MK, Wittekind C. eds. TNM Classification of Malignant Tumours, 7th Edition. New York: Wiley-Blackwell, 2009. [Google Scholar]
- 6.De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98 [DOI] [PubMed] [Google Scholar]
- 7.Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92 [DOI] [PubMed] [Google Scholar]
- 8.Cerfolio RJ, Bryant AS. Survival of patients with unsuspected N2 (stage IIIA) nonsmall-cell lung cancer. Ann Thorac Surg 2008;86:362-6; discussion 366-7 [DOI] [PubMed] [Google Scholar]
- 9.Funakoshi Y, Takeuchi Y, Kusumoto H, et al. Which subgroup of patients with pathologic N2 non-small cell lung cancer benefit from surgery? J Cancer Res Clin Oncol 2012;138:1027-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue M, Sawabata N, Takeda S, et al. Results of surgical intervention for p-stage IIIA (N2) non-small cell lung cancer: acceptable prognosis predicted by complete resection in patients with single N2 disease with primary tumor in the upper lobe. J Thorac Cardiovasc Surg 2004;127:1100-6 [DOI] [PubMed] [Google Scholar]
- 11.Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e314S-40S. [DOI] [PubMed] [Google Scholar]
- 12.Jiménez MF, Varela G, Novoa NM, et al. Results of surgery for non-small cell lung cancer with N2 involvement unsuspected before thoracotomy. Arch Bronconeumol 2008;44:65-9 [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Wang J.Comparison of clinical outcomes for patients with clinical N0 and pathologic N2 non-small cell lung cancer after thoracoscopic lobectomy and open lobectomy: a retrospective analysis of 76 patients. J Surg Oncol 2012;106:431-5 [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Zhou W, Zhang H, et al. Feasibility and long-term efficacy of video-assisted thoracic surgery for unexpected pathologic N2 disease in non-small cell lung cancer. Ann Thorac Med 2013;8:170-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manser R, Wright G, Hart D, et al. Surgery for early stage non-small cell lung cancer. Cochrane Database Syst Rev 2005;(1):CD004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura H, Kawasaki N, Taguchi M, et al. Role of preoperative chemotherapy for non-small-cell lung cancer: a meta-analysis. Lung Cancer 2006;54:325-9 [DOI] [PubMed] [Google Scholar]
- 17.Bozcuk H, Abali H, Coskun S, et al. The correlates of benefit from neoadjuvant chemotherapy before surgery in non-small-cell lung cancer: a metaregression analysis. World J Surg Oncol 2012;10:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah AA, Berry MF, Tzao C, et al. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg 2012;93:1807-12 [DOI] [PubMed] [Google Scholar]
- 19.Thomas M, Rübe C, Hoffknecht P, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol 2008;9:636-48 [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi M, Toyokawa G, Ohba T, et al. Preoperative concurrent chemoradiotherapy of S-1/cisplatin for stage III non-small cell lung cancer. Ann Thorac Surg 2013;96:1783-9 [DOI] [PubMed] [Google Scholar]
- 21.Kato M, Onishi H, Matsumoto K, et al. Preoperative chemoradiotherapy using cisplatin plus S-1 can induce downstaging in patients with locally advanced (stage III) non-small-cell lung cancer. Anticancer Res 2012;32:5099-104 [PubMed] [Google Scholar]
- 22.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7 [DOI] [PubMed] [Google Scholar]
- 23.Yildizeli B, Dartevelle PG, Fadel E, et al. Results of primary surgery with T4 non-small cell lung cancer during a 25-year period in a single center: the benefit is worth the risk. Ann Thorac Surg 2008;86:1065-75; discussion 1074-5 [DOI] [PubMed] [Google Scholar]
- 24.Kang CH, Ra YJ, Kim YT, et al. The impact of multiple metastatic nodal stations on survival in patients with resectable N1 and N2 nonsmall-cell lung cancer. Ann Thorac Surg 2008;86:1092-7 [DOI] [PubMed] [Google Scholar]
- 25.Shargall Y, de Perrot M, Keshavjee S, et al. 15 years single center experience with surgical resection of the superior vena cava for non-small cell lung cancer. Lung Cancer 2004;45:357-63 [DOI] [PubMed] [Google Scholar]
- 26.Osaki T, Sugio K, Hanagiri T, et al. Survival and prognostic factors of surgically resected T4 non-small cell lung cancer. Ann Thorac Surg 2003;75:1745-51; discussion 1751. [DOI] [PubMed]
- 27.Yang HX, Hou X, Lin P, et al. Survival and risk factors of surgically treated mediastinal invasion T4 non-small cell lung cancer. Ann Thorac Surg 2009;88:372-8 [DOI] [PubMed] [Google Scholar]
- 28.Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-99S. [DOI] [PubMed] [Google Scholar]
- 29.Yildizeli B, Fadel E, Mussot S, et al. Morbidity, mortality, and long-term survival after sleeve lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;31:95-102 [DOI] [PubMed] [Google Scholar]
- 30.Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J Clin Oncol 2005;23:5910-7 [DOI] [PubMed] [Google Scholar]
- 31.Roth JA, Atkinson EN, Fossella F, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer 1998;21:1-6 [DOI] [PubMed] [Google Scholar]
- 32.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999;17:2692-9 [DOI] [PubMed] [Google Scholar]
- 33.Bauman JE, Mulligan MS, Martins RG, et al. Salvage lung resection after definitive radiation (>59 Gy) for non-small cell lung cancer: surgical and oncologic outcomes. Ann Thorac Surg 2008;86:1632-8; discussion 1638-9 [DOI] [PubMed] [Google Scholar]
- 34.Sonett JR, Suntharalingam M, Edelman MJ, et al. Pulmonary resection after curative intent radiotherapy (>59 Gy) and concurrent chemotherapy in non-small-cell lung cancer. Ann Thorac Surg 2004;78:1200-5; discussion 1206 [DOI] [PubMed] [Google Scholar]
- 35.Socinski MA, Evans T, Gettinger S, et al. Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e341S-68S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu C, Chang EL, Hassenbusch SJ, 3rd, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer 2006;106:1998-2004 [DOI] [PubMed] [Google Scholar]
- 37.Yamanaka R.Medical management of brain metastases from lung cancer (Review) Oncol Rep 2009;22:1269-76 [DOI] [PubMed] [Google Scholar]
- 38.Schuchert MJ, Luketich JD. Solitary sites of metastatic disease in non-small cell lung cancer. Curr Treat Options Oncol 2003;4:65-79 [DOI] [PubMed] [Google Scholar]
- 39.Girard N, Cottin V, Tronc F, et al. Chemotherapy is the cornerstone of the combined surgical treatment of lung cancer with synchronous brain metastases. Lung Cancer 2006;53:51-8 [DOI] [PubMed] [Google Scholar]
- 40.Getman V, Devyatko E, Dunkler D, et al. Prognosis of patients with non-small cell lung cancer with isolated brain metastases undergoing combined surgical treatment. Eur J Cardiothorac Surg 2004;25:1107-13 [DOI] [PubMed] [Google Scholar]
- 41.Bonnette P, Puyo P, Gabriel C, et al. Surgical management of non-small cell lung cancer with synchronous brain metastases. Chest 2001;119:1469-75 [DOI] [PubMed] [Google Scholar]
- 42.Granone P, Margaritora S, D’Andrilli A, et al. Non-small cell lung cancer with single brain metastasis: the role of surgical treatment. Eur J Cardiothorac Surg 2001;20:361-6 [DOI] [PubMed] [Google Scholar]
- 43.Billing PS, Miller DL, Allen MS, et al. Surgical treatment of primary lung cancer with synchronous brain metastases. J Thorac Cardiovasc Surg 2001;122:548-53 [DOI] [PubMed] [Google Scholar]
- 44.Gaspar LE. Brain metastases in lung cancer. Expert Rev Anticancer Ther 2004;4:259-70 [DOI] [PubMed] [Google Scholar]
- 45.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10 [DOI] [PubMed] [Google Scholar]
- 46.Niibe Y, Kenjo M, Kazumoto T, et al. Multi-institutional study of radiation therapy for isolated para-aortic lymph node recurrence in uterine cervical carcinoma: 84 subjects of a population of more than 5,000. Int J Radiat Oncol Biol Phys 2006;66:1366-9 [DOI] [PubMed] [Google Scholar]
- 47.Niibe Y, Chang JY, Onishi H, et al. Oligometastases/Oligo-recurrence of lung cancer. Pulm Med 2013;2013:438236. [DOI] [PMC free article] [PubMed]
- 48.Nakata M, Sawada S, Yamashita M, et al. Surgical treatments for multiple primary adenocarcinoma of the lung. Ann Thorac Surg 2004;78:1194-9 [DOI] [PubMed] [Google Scholar]
- 49.Rea F, Zuin A, Callegaro D, et al. Surgical results for multiple primary lung cancers. Eur J Cardiothorac Surg 2001;20:489-95 [DOI] [PubMed] [Google Scholar]
- 50.Tsunezuka Y, Matsumoto I, Tamura M, et al. The results of therapy for bilateral multiple primary lung cancers: 30 years experience in a single centre. Eur J Surg Oncol 2004;30:781-5 [DOI] [PubMed] [Google Scholar]
- 51.Trousse D, Barlesi F, Loundou A, et al. Synchronous multiple primary lung cancer: an increasing clinical occurrence requiring multidisciplinary management. J Thorac Cardiovasc Surg 2007;133:1193-200 [DOI] [PubMed] [Google Scholar]
- 52.De Leyn P, Moons J, Vansteenkiste J, et al. Survival after resection of synchronous bilateral lung cancer. Eur J Cardiothorac Surg 2008;34:1215-22 [DOI] [PubMed] [Google Scholar]
- 53.Mun M, Kohno T.Single-stage surgical treatment of synchronous bilateral multiple lung cancers. Ann Thorac Surg 2007;83:1146-51 [DOI] [PubMed] [Google Scholar]
- 54.Finley DJ, Yoshizawa A, Travis W, et al. Predictors of outcomes after surgical treatment of synchronous primary lung cancers. J Thorac Oncol 2010;5:197-205 [DOI] [PubMed] [Google Scholar]


