Abstract
The European Society of Thoracic Surgeons (ESTS) Database is a free registry created by ESTS in 2001. The current online version was launched in 2007. It runs currently on a Dendrite platform with extensive data security and frequent backups. The main features are a specialty-specific, procedure-specific, prospectively maintained, periodically audited and web-based electronic database, designed for quality control and performance monitoring, which allows for the collection of all general thoracic procedures. Data collection is the “backbone” of the ESTS database. It includes many risk factors, processes of care and outcomes, which are specially designed for quality control and performance audit. The user can download and export their own data and use them for internal analyses and quality control audits. The ESTS database represents the gold standard of clinical data collection for European General Thoracic Surgery. Over the past years, the ESTS database has achieved many accomplishments. In particular, the database hit two major milestones: it now includes more than 235 participating centers and 70,000 surgical procedures. The ESTS database is a snapshot of surgical practice that aims at improving patient care. In other words, data capture should become integral to routine patient care, with the final objective of improving quality of care within Europe.
Keywords: General thoracic surgery, database, education
Introduction
The European Society of Thoracic Surgeons (ESTS) Database was established by Richard Berrisford in 2001 as an initiative for quality improvement and patient safety among European Thoracic Surgeons. Since then, it has grown considerably over the years to include now a total of more than 70,000 procedures providing clinical information on more than 50,000 lung resections. Data input on other thoracic procedures is also increasing. Indeed, the ESTS database was recently updated to include more fields on surgical treatment for Thymic tumors. Further expansion of data fields to capture outcome after Esophageal, Mesothelioma, Neuro Endocrine Tumors or Chest wall and trauma surgery is to be expected in the coming years based on a proposal of the ESTS database committee.
Current functioning
The ESTS Database was founded by the ESTS Database Committee with the aim to develop risk-adjusted instruments for assessing the performance of thoracic surgery units across Europe. The first version of the Database lead to the publication of the first risk- adjusted multinational risk-score for mortality (1), which has been then applied to compare the performance of different units (2).
The second version of the Database was launched online in July 2007 and has so far accrued approximately 235 general thoracic surgical units throughout Europe, that send data on their patients on a voluntary basis. It is a specialty-specific, procedure-specific, prospectively maintained, periodically audited and web-based electronic database, designed for quality control and performance monitoring, which allows for the collection of all general thoracic procedures (3,4). Importantly, data is anonymously reported, independently accessed and encrypted to other users. Participation to the Database project is totally free and voluntary, but strongly recommended by the ESTS. It is possible to access the Database from the ESTS website or by using the following address: https://ests.dendrite.it/csp/ests/intellect/login.csp. To join the Database, your own personal login account is needed. You can request it by downloading and completing an application form from the ESTS homepage (http://www.ests.org).
Importantly, units with their own institutional database can submit their anonymous data as an excel spreadsheet to the ESTS Database without duplicating their imputing work. Units without an internal database can use the free online ESTS Database as an institutional registry for their own purposes at the same time contributing to the European data collection.
As the ESTS Database approached a more mature stage at the end of the 2000’, and more demanding aspects of data management were required, it has been decided to make use of professional expertise in running and managing contents, data flow, data merge and so on of the ESTS Registry; in Nov 2009 the ESTS Council awarded this task to Dendrite Clinical System Italia srl. Since 1993 Dendrite has established a highly respected track record in setting up and running a variety of International Registries, with an underlying philosophy of long term partnership with numerous clinical associations within and outside Europe. The main reasons for Dendrite’s widespread activity (and consequently the choice by ESTS) include the following items: (I) bottom-up approach to data management: the range of products and services starts from database and electronic patient records and serves Clinicians daily needs; it escalates to hospital-wide systems, to regional, national and finally to international registries; (II) user-friendly inclusion of all who wish to participate: Import Data Module allows any Contributor to use his chosen type of tool to collect data, and Dendrite will perform the correspondence and data merge required to add their data to the main ESTS Database, if there is conformity with the required dataset; (III) fool-proof suite of clinical statistical analysis integrated in the central data collection installation (server); (IV) contributors can retain, download and use own data, from the ESTS site, in MS Excel format, which lends itself to be analyzed by any clinical software product; (V) unblemished track record of data handling integrity: not ever lost, leaked or misplaced third Party data to date.
The ESTS database is managed by a Database Committee, which is responsible for its periodical revisions and updates. The database allows for the annual publication of a European report (“the Silver Book”), which is distributed to all ESTS members as a benchmark of the thoracic surgery practice in Europe. This annually published report presents not only a global analysis of the practice but also provide data in a more analytical fashion in rolling 3-year review. This ESTS annual database report is another prove that our society is fulfilling its mission statement “to improve quality in all aspects to the benefit of your specialty, your practice, and your patients: from clinical and surgical management of patients to education, training, and credentialing of thoracic surgeons in Europe and worldwide”.
To assess surgical performance on an international level is one of the main objectives of the database. The ESTS has developed a composite performance score (5) incorporating process and outcome measures available in the database. Those Units that are above the 50th percentile of the composite score are invited to submit their application to the ESTS Institutional Accreditation Program. An audit by an independent professional company (Adamas) is done on site for those Units that apply for Accreditation by ESTS. Participation to the ESTS Database is a pre-requisite to participate in the European Institutional Quality certification program. Participation will be acknowledged and, if requested, local institutional administrations made aware that your unit is enrolled in a European Thoracic Database aimed at implementing quality of care monitoring and improvement programs endorsed by ESTS and pre-requisite for future clinical Institutional European Accreditation.
The ESTS database represents a source of information for the advancement of knowledge of our specialty. The ESTS database also offers solid grounds for clinical research. Participating units are encouraged to submit their own proposals of clinical research based on the total data present in the ESTS Database. Projects should be submitted to the ESTS database Committee for peer review and, if accepted, the requested and anonymized data will be provided to the proponent of the project. ESTS will retain the responsibility for the final analysis and interpretation of results: the final product needs to be reviewed once again by the Database Committee, who will be responsible for the accuracy and integrity of the results. The proponent of the project will be the first Author of the final manuscript and he/she will be allowed to include, if requested, additional two colleagues, who helped in the elaboration of the manuscript. The members of the Database Committee who contributed to the review process and assisted in the development of the manuscript will be also included in the list of Authors. To date a dozen of publications has arisen from the ESTS database. The ESTS database offers a unique scientific platform for other working groups interested to study specific variables on various topics in the field of thoracic surgery to guide our current practice based on recent knowledge.
Benefits of participations
The ESTS general thoracic surgery database project aimed at providing benefit not only to patients, but also to practice and specialty. The opportunity to participate in a European quality improvement effort for general thoracic surgery has a positive impact at the local, national and international level. Indeed, data incorporating in the ESTS database will contribute to the followings: (I) development of European benchmarks of performance through the analysis of outcomes and processes of care indicators. Participation in a national outcome registry as well as in quality assessment program (either individually or through your institution) is important (6); (II) performance assessment by risk-adjusted outcome and/or process indicators, which will allow to compare a given institutional performance against European benchmarks; (III) analysis and development of new potential outcomes and processes of care indicators that may complement/substitute current quality of care measures. A continuous monitoring of surgical results can be achieved thanks to the CUSUM analysis (7), the creation of a quality score (as done in France with the Epithor program), and/or a mortality analysis derived from the TRISS method (8); (IV) implement a provider-led quality monitoring and improvement program with the aim to improve individual practice; (V) data for research projects, which can be used to assess new technologies/pathways of care that can ultimately lead to improved patient care and outcomes. Those data, collected in a standardized ESTS-endorsed Dataset, can be downloaded at local level and used for your internal quality analyses or institutional research purposes; (VI) use for local hospital administration resource allocation, for individual negotiations, public relations and expert witness (maintain your own data if data is requested or mandated by third parties).
Future and evolution: where do we go next in the burgeoning field of data collection in Europe?
The ESTS database committee main goal is to achieve a progressive evolution from morbidity and mortality to total quality management. In other words, within the ESTS database, data should be analyzed over a long-term period with trending, allowing to modulate short-term variation and provide the ability to assess change over time (4). For this purpose, the database committee will promote in the coming year the following step by step actions:
As aforementioned, the ESTS database was recently updated to include data on surgical treatment for Thymic tumors. Further expansion of the core dataset (“satellite” dataset) to capture outcome after Esophageal, Mesothelioma, Neuro Endocrine Tumors or Chest wall and trauma surgery is to be expected in the coming months, based on a proposal of the ESTS database committee. For each of these satellite databases the database committee will appoint a clinical leading physician.
Creation of a decision-making tool: refinement of the currently existing risk scores (i.e., ESOS or Thoracoscore). For example, the variables of the dataset will be revising to be able to provide a risk assessment for in-hospital mortality based on the Thoracoscore (9) for each ESTS database patients.
As data collection is the backbone of the ESTS database, it is essential to promote the integration of data in the ESTS database. Maintaining and improving data quality remains essential (example of the composite performance score) (5,10,11). Until now, units without an internal database can use the free online ESTS Database as an institutional registry for their own purposes at the same time contributing to the European data collection. In addition, units with their own institutional database can submit their anonymous data as an excel spreadsheet to the ESTS Database without duplicating their imputing work. Following the fruitful example of France with the Epithor® Registry—that send data on lung cancer and thymoma on an annual basis since 2012—data input will also be encouraged via national registries. A close collaboration not only between ESTS and the Dutch Lung Surgery Audit database but also between ESTS and Polish or Hungarian national registries is expected in the coming months.
Enhancing recording process measures thanks to standardized pathways of care: in the future, the surgical audit will be comprised of an accurate analysis of the process of care, including the creation of process indicators. The key idea is that the monitoring of the process can be a surrogate for measuring the outcome itself (12).
Continue the promotion of clinical research within the ESTS database.
Favor collaboration between ESTS and sisters’ Society. Recently, close cooperation was established with the Society of Thoracic Surgeons to create standardizations in nomenclature and variables between both the STS and the ESTS databases so that outcome after thoracic surgery can be compared between the USA and Europe.
Educate database participants and managers. The results of the audit should be incorporated into educational and quality improvement process. Indeed, as a future project, participants will receive a periodic confidential feedback on the quality of their data and their performance against International benchmarks.
Welcome international (outside of European Union) participants.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
Appendix
Database format and submission of data
The first step is to request and obtain a login account through the relevant link found in the ESTS homepage (http//www.ests.org) or by directly sending an email to one of the members of the Database Committee. Once you have a valid login account you can proceed through the following data entry interface (accessible through https://ests.dendrite.it/csp/ests/intellect/login.csp).
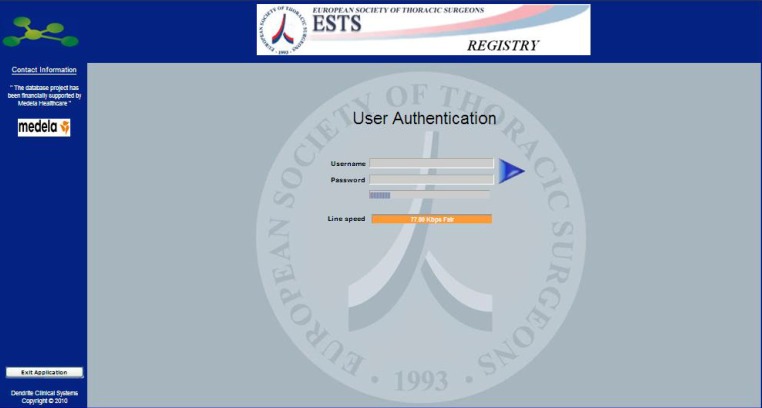
The intellect Web logon screen shown below has been engineered to provide enhanced security facilities:
□ Limiting users to three logon attempts before locking the user-account;
□ Giving information on previously successful and unsuccessful logon attempts;
□ Requiring users to have an eight-character password that contains at least one uppercase character, one lowercase character and one digit.
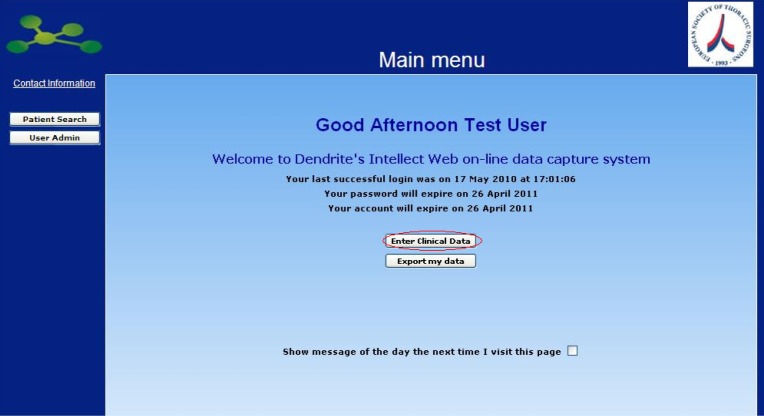
Once you have logged in you are presented with the Database main menu, from which you can add new data, view or edit a procedure, modify your account details, and export your data in Excel for your own purposes.
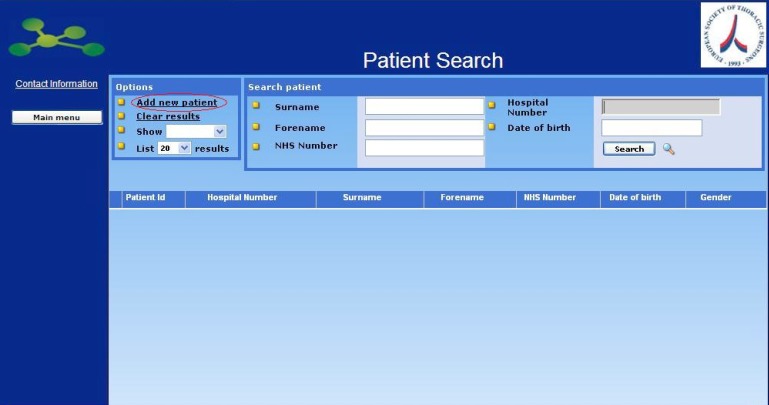
Clicking on the Enter Clinical Data button opens the next screen “Patient Search”, where it is possible to search for patients already in the database or add new patients.
Clicking on the link Add New Patient, that can be found at the left of the screen in the section Options, you will be required to fill in the minimum data required to register a New Patient.
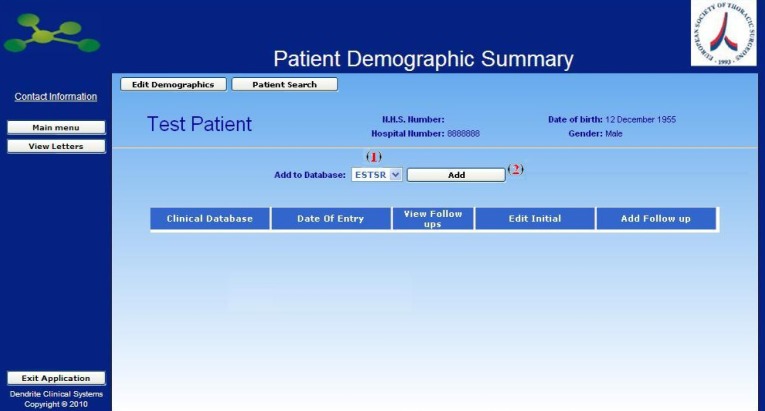
The newly created patient is ready to be entered into the database.
Now it is possible to select the available Database [1] (in our case there is only the one named ESTSR) and add the patient to the chosen Database by clicking on the button [2].
Once you have clicked the Add Button, the first page of the selected Registry will appear. Now you can start inserting clinical data as showed in next page.
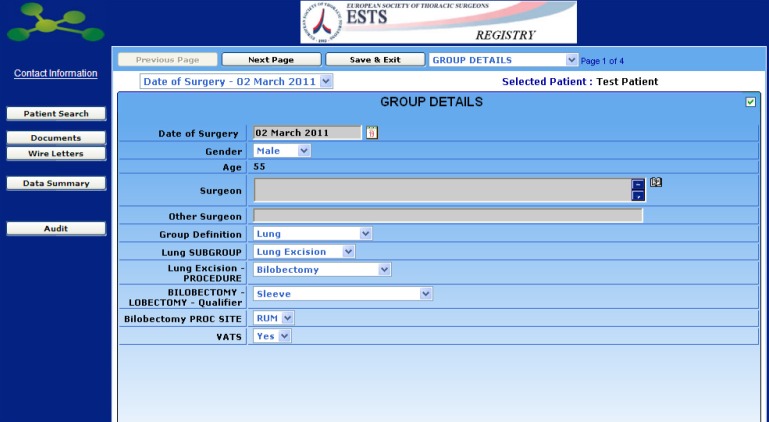
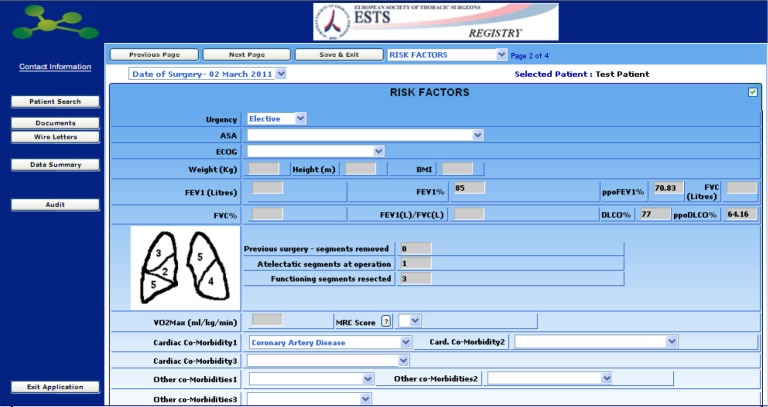
The Database is an all-purpose database designed for all general thoracic surgery procedures, but specifically focused on lung resections for which a number of additional items can be selected, including risk-scores, cardiopulmonary function data and calculation of predicted postoperative pulmonary function through a standardized calculator.
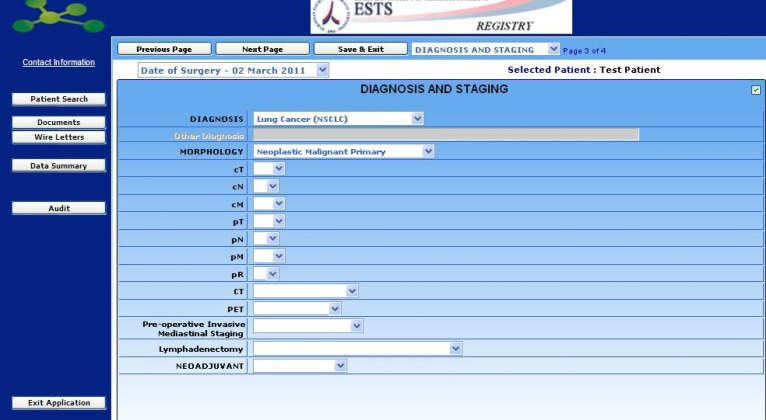
In addition to risk factors, diagnosis and staging details can be added in a following section.
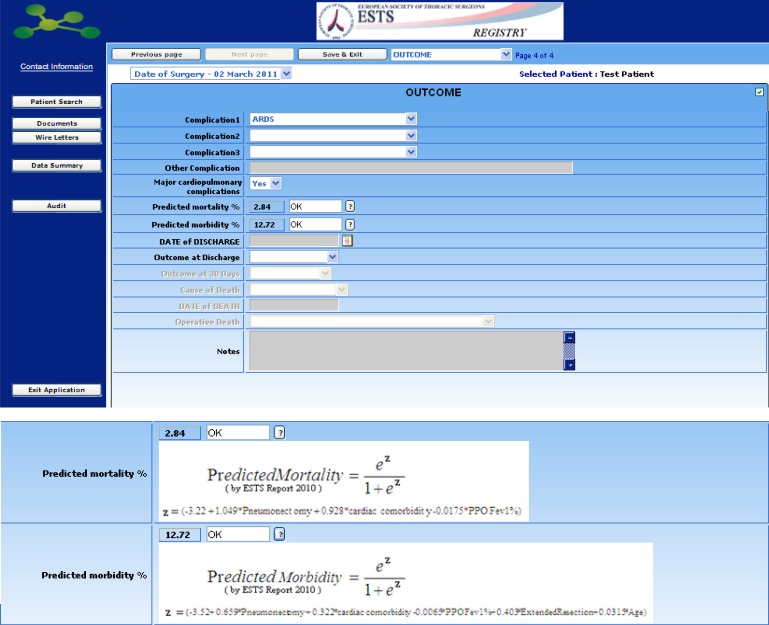
The system auto-calculates for Lung Excision Procedure the Predicted Mortality (%) and Predicted Morbidity (%).
Early outcomes, including in-hospital morbidity, in-hospital and 30-days mortality should be specified in the final section, before submitting the data.
References
- 1.Berrisford R, Brunelli A, Rocco G, et al. The European Thoracic Surgery Database project: modelling the risk of in-hospital death following lung resection. Eur J Cardiothorac Surg 2005;28:306-11 [DOI] [PubMed] [Google Scholar]
- 2.Brunelli A, Varela G, Van Schil P, et al. Multicentric analysis of performance after major lung resections by using the European Society Objective Score (ESOS). Eur J Cardiothorac Surg 2008;33:284-8 [DOI] [PubMed] [Google Scholar]
- 3.Corrall CJ, Wyer PC, Zick LS, et al. Evidence-based emergency medicine. How to find evidence when you need it, part 1: databases, search programs, and strategies. Ann Emerg Med 2002;39:302-6 [DOI] [PubMed] [Google Scholar]
- 4.Brunelli A, Varela G, Berrisford R, et al. Audit, quality control, and performance in thoracic surgery--a European perspective. Thorac Surg Clin 2007;17:387-93, vii. [DOI] [PubMed] [Google Scholar]
- 5.Brunelli A, Berrisford RG, Rocco G, et al. The European Thoracic Database project: composite performance score to measure quality of care after major lung resection. Eur J Cardiothorac Surg 2009;35:769-74 [DOI] [PubMed] [Google Scholar]
- 6.Boyer P, Boutron I, Ravaud P.Scientific production and impact of national registers: the example of orthopaedic national registers. Osteoarthritis Cartilage 2011;19:858-63 [DOI] [PubMed] [Google Scholar]
- 7.Noyez L.Control charts, Cusum techniques and funnel plots. A review of methods for monitoring performance in healthcare. Interact Cardiovasc Thorac Surg 2009;9:494-9 [DOI] [PubMed] [Google Scholar]
- 8.Fallon WF, Jr, Barnoski AL, Mancuso CL, et al. Benchmarking the quality-monitoring process: a comparison of outcomes analysis by trauma and injury severity score (TRISS) methodology with the peer-review process. J Trauma 1997;42:810-5; discussion 815-7 [DOI] [PubMed] [Google Scholar]
- 9.Falcoz PE, Conti M, Brouchet L, et al. The Thoracic Surgery Scoring System (Thoracoscore): risk model for in-hospital death in 15,183 patients requiring thoracic surgery. J Thorac Cardiovasc Surg 2007;133:325-32 [DOI] [PubMed] [Google Scholar]
- 10.Pass HI. Medical registries: continued attempts for robust quality data. J Thorac Oncol 2010;5:S198-9 [DOI] [PubMed] [Google Scholar]
- 11.Salati M, Brunelli A, Dahan M, et al. Task-independent metrics to assess the data quality of medical registries using the European Society of Thoracic Surgeons (ESTS) Database. Eur J Cardiothorac Surg 2011;40:91-8 [DOI] [PubMed] [Google Scholar]
- 12.Rostami R, Nahm M, Pieper CF. What can we learn from a decade of database audits? The Duke Clinical Research Institute experience, 1997--2006. Clin Trials 2009;6:141-50 [DOI] [PMC free article] [PubMed] [Google Scholar]