Abstract
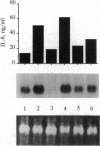
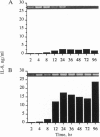
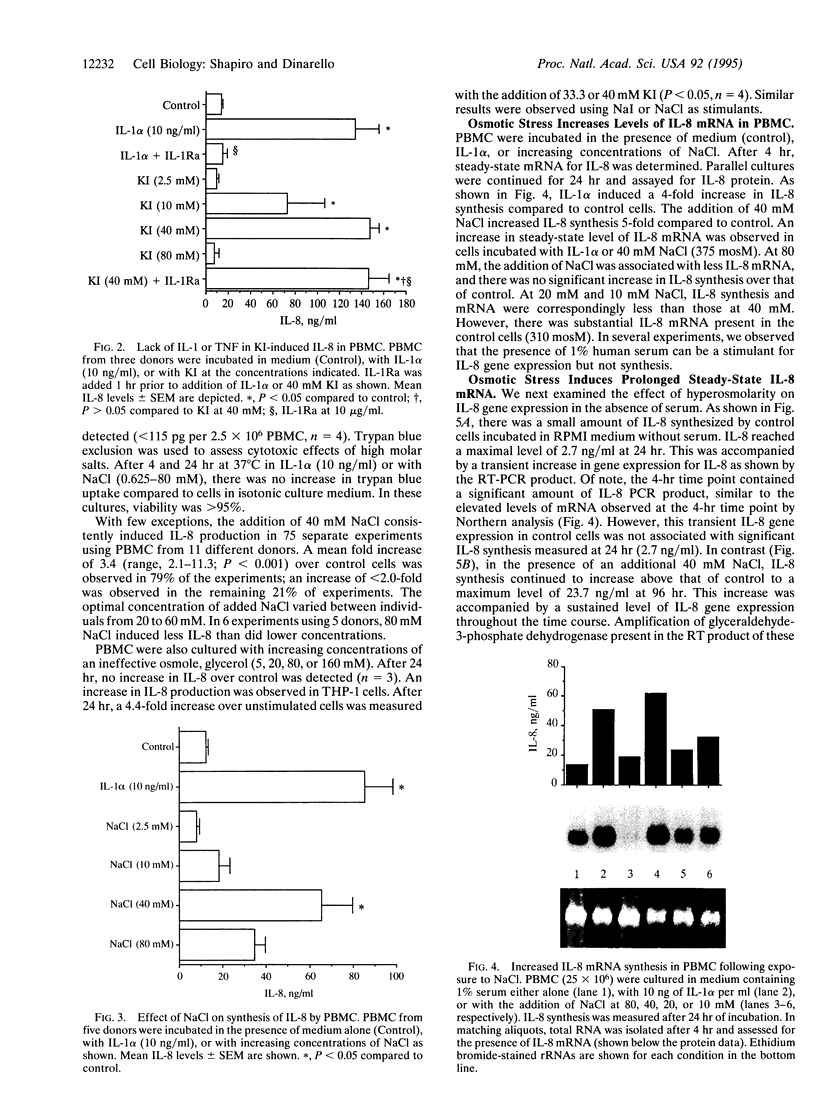
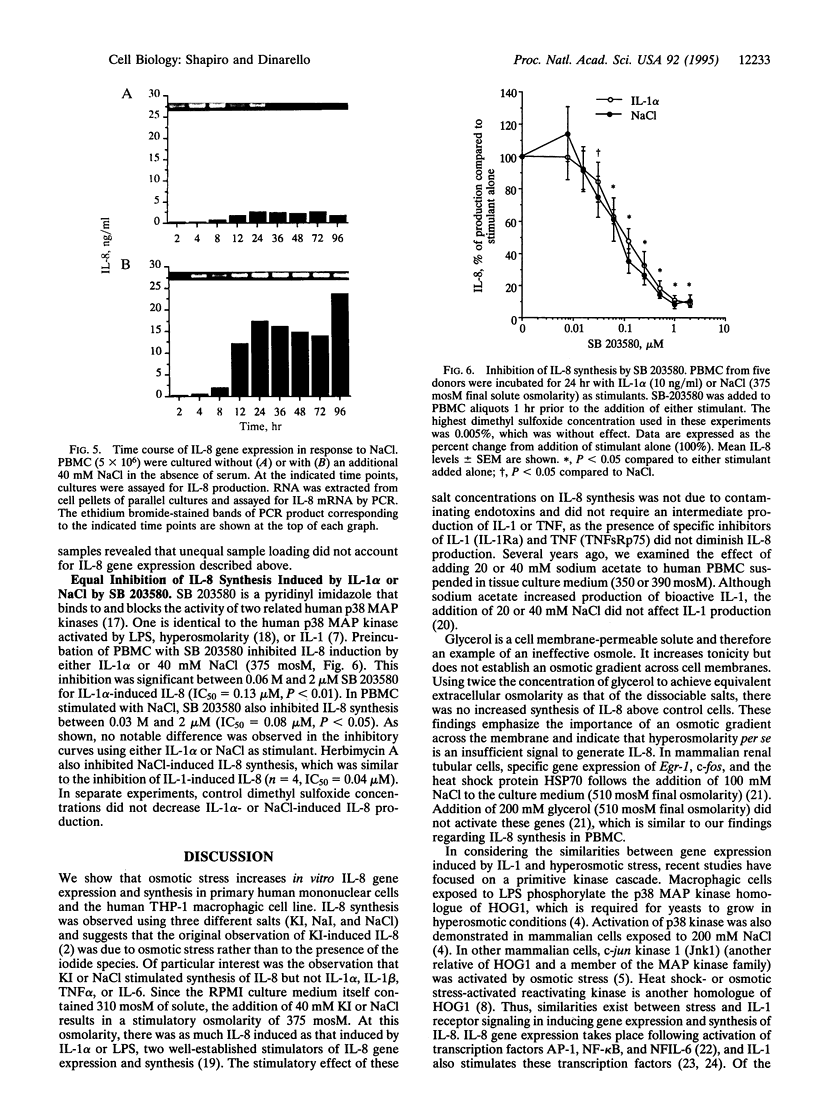
These studies were undertaken to investigate the therapeutic mechanism of saturated solutions of KI, used to treat infectious and inflammatory diseases. The addition of 12-50 mM KI to cultured human peripheral blood mononuclear cells resulted in 319-395 mosM final solute concentration and induced interleukin (IL)-8 synthesis. Maximal IL-8 production was seen when 40 mM salt was added (375 mosM) and was equal to IL-8 induced by endotoxin or IL-1 alpha. However, there was no induction of IL-1 alpha, IL-1 beta, or tumor necrosis factor to account for the synthesis of IL-8; the effect of KI was not due to contaminating endotoxins. Hyperosmolar NaCl also induced IL-8 and increased steady-state levels of IL-8 mRNA similar to those induced by IL-1 alpha. IL-8 gene expression was elevated for 96 hr in peripheral blood mononuclear cells incubated with hyperosmolar NaCl. In human THP-1 macrophagic cells, osmotic stimulation with KI, NaI, or NaCl also induced IL-8 production. IL-1 signal transduction includes the phosphorylation of the p38 mitogen-activated protein kinase that is observed following osmotic stress. Using specific blockade of this kinase, a dose-response inhibition of hyperosmolar NaCl-induced IL-8 synthesis was observed, similar to that in cells stimulated with IL-1. Thus, these studies suggest that IL-1 and osmotic shock utilize the same mitogen-activated protein kinase for signal transduction and IL-8 synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M., Dewald B., Moser B. Interleukin-8 and related chemotactic cytokines--CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Beeson P. B. Effects of iodides on inflammatory processes. Perspect Biol Med. 1994 Winter;37(2):173–181. doi: 10.1353/pbm.1994.0012. [DOI] [PubMed] [Google Scholar]
- Bingel M., Lonnemann G., Koch K. M., Dinarello C. A., Shaldon S. Enhancement of in-vitro human interleukin-1 production by sodium acetate. Lancet. 1987 Jan 3;1(8523):14–16. doi: 10.1016/s0140-6736(87)90703-3. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., van der Meer J. W., Kwiatkowski D., Endres S., Lonnemann G., Burke J. F., Dinarello C. A. Interleukin-1 beta in human plasma: optimization of blood collection, plasma extraction, and radioimmunoassay methods. Lymphokine Res. 1988 Winter;7(4):457–467. [PubMed] [Google Scholar]
- Cohen D. M., Wasserman J. C., Gullans S. R. Immediate early gene and HSP70 expression in hyperosmotic stress in MDCK cells. Am J Physiol. 1991 Oct;261(4 Pt 1):C594–C601. doi: 10.1152/ajpcell.1991.261.4.C594. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Ikejima T., Warner S. J., Orencole S. F., Lonnemann G., Cannon J. G., Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987 Sep 15;139(6):1902–1910. [PubMed] [Google Scholar]
- Freshney N. W., Rawlinson L., Guesdon F., Jones E., Cowley S., Hsuan J., Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994 Sep 23;78(6):1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Galcheva-Gargova Z., Dérijard B., Wu I. H., Davis R. J. An osmosensing signal transduction pathway in mammalian cells. Science. 1994 Aug 5;265(5173):806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- Han J., Lee J. D., Bibbs L., Ulevitch R. J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994 Aug 5;265(5173):808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Han J., Richter B., Li Z., Kravchenko V., Ulevitch R. J. Molecular cloning of human p38 MAP kinase. Biochim Biophys Acta. 1995 Mar 16;1265(2-3):224–227. doi: 10.1016/0167-4889(95)00002-a. [DOI] [PubMed] [Google Scholar]
- Junger W. G., Liu F. C., Loomis W. H., Hoyt D. B. Hypertonic saline enhances cellular immune function. Circ Shock. 1994 Apr;42(4):190–196. [PubMed] [Google Scholar]
- Lee J. C., Laydon J. T., McDonnell P. C., Gallagher T. F., Kumar S., Green D., McNulty D., Blumenthal M. J., Heys J. R., Landvatter S. W. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994 Dec 22;372(6508):739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Lonnemann G., Endres S., van der Meer J. W., Cannon J. G., Dinarello C. A. A radioimmunoassay for human interleukin-1 alpha: measurement of IL-1 alpha produced in vitro by human blood mononuclear cells stimulated with endotoxin. Lymphokine Res. 1988 Summer;7(2):75–84. [PubMed] [Google Scholar]
- Mattox K. L., Maningas P. A., Moore E. E., Mateer J. R., Marx J. A., Aprahamian C., Burch J. M., Pepe P. E. Prehospital hypertonic saline/dextran infusion for post-traumatic hypotension. The U.S.A. Multicenter Trial. Ann Surg. 1991 May;213(5):482–491. doi: 10.1097/00000658-199105000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming Y. Z., Di X., Gomez-Sanchez E. P., Gomez-Sanchez C. E. Improved downward capillary transfer for blotting of DNA and RNA. Biotechniques. 1994 Jan;16(1):58–59. [PubMed] [Google Scholar]
- Muegge K., Vila M., Gusella G. L., Musso T., Herrlich P., Stein B., Durum S. K. Interleukin 1 induction of the c-jun promoter. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7054–7058. doi: 10.1073/pnas.90.15.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaida N., Shiroo M., Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989 Aug 15;143(4):1366–1371. [PubMed] [Google Scholar]
- Porat R., Poutsiaka D. D., Miller L. C., Granowitz E. V., Dinarello C. A. Interleukin-1 (IL-1) receptor blockade reduces endotoxin and Borrelia burgdorferi-stimulated IL-8 synthesis in human mononuclear cells. FASEB J. 1992 Apr;6(7):2482–2486. doi: 10.1096/fasebj.6.7.1532945. [DOI] [PubMed] [Google Scholar]
- Reed L. L., Manglano R., Martin M., Hochman M., Kocka F., Barrett J. The effect of hypertonic saline resuscitation on bacterial translocation after hemorrhagic shock in rats. Surgery. 1991 Oct;110(4):685–690. [PubMed] [Google Scholar]
- Rouse J., Cohen P., Trigon S., Morange M., Alonso-Llamazares A., Zamanillo D., Hunt T., Nebreda A. R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994 Sep 23;78(6):1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Schindler R., Dinarello C. A. A method for removing interleukin-1- and tumor necrosis factor-inducing substances from bacterial cultures by ultrafiltration with polysulfone. J Immunol Methods. 1989 Jan 17;116(2):159–165. doi: 10.1016/0022-1759(89)90199-3. [DOI] [PubMed] [Google Scholar]
- Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S. C., Dinarello C. A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990 Jan 1;75(1):40–47. [PubMed] [Google Scholar]
- Shirakawa F., Mizel S. B. In vitro activation and nuclear translocation of NF-kappa B catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol Cell Biol. 1989 Jun;9(6):2424–2430. doi: 10.1128/mcb.9.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. W., Endres S., Lonnemann G., Cannon J. G., Ikejima T., Okusawa S., Gelfand J. A., Dinarello C. A. Concentrations of immunoreactive human tumor necrosis factor alpha produced by human mononuclear cells in vitro. J Leukoc Biol. 1988 Mar;43(3):216–223. doi: 10.1002/jlb.43.3.216. [DOI] [PubMed] [Google Scholar]