Introduction
Most patients with multiple sclerosis (MS) experience walking impairment during the course of their disease. From an MS patient's perspective, walking ability is the most important bodily function.1 Currently, sustained-release oral fampridine (fampridine-SR) is the only drug approved for the symptomatic treatment of walking disability due to MS. Fampridine is a potassium channel blocker that improves the impaired axonal conduction associated with CNS demyelination.2 However, only a subpopulation of MS patients with walking disability respond to fampridine.3 Herein, we prospectively evaluated whether pre-therapy motor-evoked potentials (MEP), reflecting pyramidal demyelination by an increased central motor conduction time (CMCT), are suited as a predictive therapeutic response marker.
Methods
This prospective observational study was approved by the local ethics committee. Twenty-five patients with definite MS who were undergoing planned therapy with fampridine-SR for clinical reasons were recruited consecutively from our MS outpatient clinic from July 2012 to August 2013. Written informed consent was obtained from all participants. Five patients dropped out of the study due to adverse effects (n=3) or irregular drug intake (n=2); 20 patients were included for further analysis (see online supplementary table 1 for clinical characteristics).
Fampridine-SR 10 mg was administered to the patients twice daily. A timed 25-foot walk (T25FW, two times per measurement) and a timed 50 m walk were assessed before and 14 (range: 13–14) days after treatment initiation. Fampridine responders were prospectively defined by an improvement of >20% in both timed walks.4 However, as only three (15%) of the patients fulfilled this criterion, a posthoc analysis with an arbitrary response definition lowered to an improvement of >10% in both timed walks was added as to verify the results. Though unable to complete the 50 m walk, patient number 5 was considered as a responder in this posthoc assessment, as his T25FW improved by 13%, and his measured maximum walking distance increased from 28 m to 40 m (43%). All timed walks were assessed in the morning hours (8:00 to 11:00).
Tibialis anterior MEPs were elicited before treatment exactly according to a standard protocol that we previously described in detail.5 Briefly, the motor cortex and L5 lumbar roots were stimulated using a circular high-performance coil attached to a Magstim 200 stimulator (Micromed, Freiburg, Germany). For preactivation, patients were asked to perform a weak voluntary contraction of the foot dorsiflexors (about 20% of maximum force). For cortical stimulation, stimulus intensity was adjusted to 1.5 times the motor threshold or to the maximal output if the threshold exceeded 66% of the stimulator output. Lumbar roots were stimulated at 100% of the maximal stimulator output. CMCT was calculated as the difference between the cortical MEP latency and the peripheral motor conduction time. The data were evaluated in a blinded manner. The means of the individual right and left CMCTs were determined as a global measure of pyramidal affection. To account for the relationship between CMCT and body height (H, in cm), the upper normal limit of CMCT (ULCMCT) was calculated using the following formula: ULCMCT=(0.08 ms/cm*H) + 3.7 ms.6 To compare CMCT prolongations, the difference (ΔCMCT) between individual CMCTs and body height-related ULCMCT was calculated.
All statistical analyses were performed using GraphPad PRISM 5 (La Jolla, California, USA). As most data did not show a normal distribution according to the Shapiro–Wilk test, non-parametric analyses were performed as indicated (Mann–Whitney U test for unpaired or Wilcoxon signed-rank test for paired data; Spearman correlation coefficients).
Results
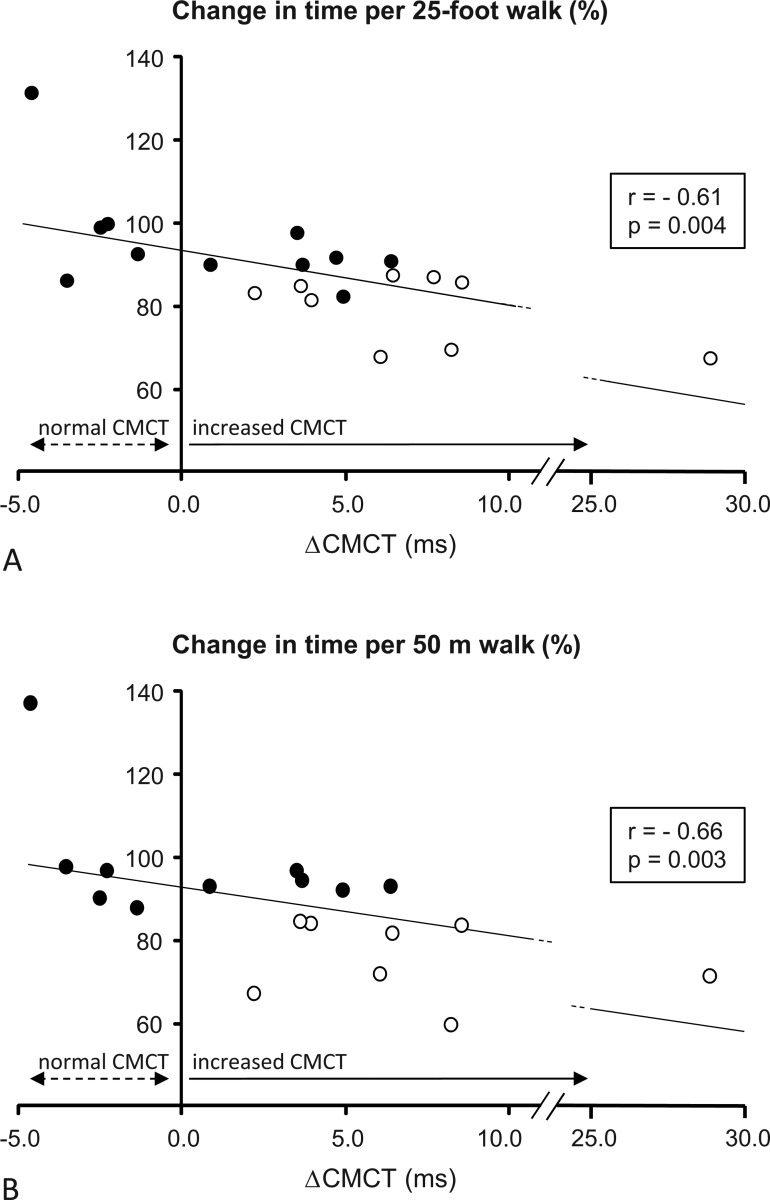
A significant overall improvement was observed for both timed walks in fampridine-SR-treated patients. For the T25FW, the median difference was −1.0 s (range −8.1 to +1.7, Wilcoxon p=0.001), and for the 50 m walk, the median difference was −5.0 s (range: −47.0 to +13.0 s, Wilcoxon p=0.002). At baseline, ΔCMCT was negatively correlated with a change in the speed of the T25FW (Spearman r=−0.61, p=0.004, figure 1A) and the 50 m walk (Spearman r=−0.66, p=0.003, figure 1B) in fampridine-SR-treated patients. Baseline ΔCMCT differed significantly between responders, as prospectively defined by >20% improvement in both timed walks (n=3, ΔCMCT 6.1, 28.8, and 8.2 ms), and non-responders (n=17, median baseline ΔCMCT 3.6, range −4.6 to 8.5 ms; Mann–Whitney p=0.034). Posthoc analysis with an arbitrary, less conservative response definition (improvement of >10% in both timed walks) confirmed a significant difference of ΔCMCT between responders (n=9, median baseline ΔCMCT 6.4, range 2.2 to 28.8 ms) and non-responders (n=11, median baseline ΔCMCT 0.9, range −4.6 to 6.4 ms; Mann–Whitney p=0.007). Importantly, and independent from the response definition, all patients with normal CMCT at baseline (n=5) were non-responders, whereas all responders and a subpopulation of the non-responders demonstrated a pathological increase in CMCT before therapy. Of note, the Expanded Disability Status Scale (EDSS) scores and timed walks at baseline did not differ between responders and non-responders.
Figure 1.
Correlations of baseline ΔCMCT (difference between individual CMCT and body height-related upper normal limit of CMCT) with changes in time of the (A) timed 25-foot walk and (B) 50 m walk in patients treated with fampridine. Black dots: fampridine non-responders (n=11), circles: fampridine responders (n=9; two patients were unable to complete the 50 m walk). Notably, all patients with normal CMCT (ie, negative ΔCMCT) were non-responders.
Discussion
In our study, all fampridine responders had a prolonged baseline CMCT, whereas none of the patients with a normal baseline CMCT responded. This finding is consistent with the hypothesis that a fampridine-mediated blockade of potassium currents in demyelinated CNS axons, reducing the probability of conduction blocks, is responsible for the improvement of walking ability observed in some patients with MS.2 However, only a subpopulation of patients who demonstrated electrophysiological evidence for pyramidal demyelination responded to fampridine. Thus, walking disabilities of non-responders with prolonged CMCT may primarily result from other causes inside (eg, axonal loss) or outside of the pyramidal tract. Although the observed correlations between pretreatment CMCT and clinical treatment response pointed towards a therapeutically relevant effect of fampridine on demyelinated axons, additional effects, such as a potentiation of synaptic transmission or an increase in skeletal muscle twitch tension as previously described,7 still appear to be of potential relevance.
This study was limited by the fact that only three out of 20 patients fulfilled the prospective response definition of an improvement of more than 20% in both timed walks. The 20% threshold is accepted as clinically meaningful by a majority of authors.4 For posthoc analysis, we used an arbitrarily liberalised response criterion, defining responders by an improvement of at least 10% in both timed walks. This revealed a similar relative distribution of responders and non-responders as observed in the pivotal trials.3 The results confirmed our primary analysis. It appears conceivable that day-to-day variations in walking ability, as common in MS patients, somehow limited the correct identification of responders and non-responders in our setting, where walking ability was assessed only once before and once while on treatment. Accordingly, a repeated measurements approach, as used for the pivotal trials, might have revealed even stronger correlations.
In conclusion, our study suggests that patients with MS with a normal pretherapy CMCT are very unlikely to benefit from fampridine, whereas patients with a prolonged CMCT have a higher chance to respond to treatment than non-stratified patients. Upon independent confirmation, measuring CMCT may help neurologists and MS patients determine whether fampridine-SR is worth trying or not.
Supplementary Material
Footnotes
Contributors: DZ: study concept and design, data acquisition, statistical data analysis and interpretation, manuscript preparation; had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. KR: data acquisition, manuscript preparation. SB: clinical examination of the patients, data acquisition. MB: study concept and design, clinical examination of patients, statistical data analysis and interpretation, manuscript preparation.
Competing interests: None.
Ethics approval: Ethics Committee at the Faculty of Medicine, University of Würzburg.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Heesen C, Bohm J, Reich C, et al. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler 2008;14:988–91 [DOI] [PubMed] [Google Scholar]
- 2.Bostock H, Sears TA, Sherratt RM. The effects of 4-aminopyridine and tetraethylammonium ions on normal and demyelinated mammalian nerve fibres. J Physiol 1981;313:301–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pikoulas TE, Fuller MA. Dalfampridine: a medication to improve walking in patients with multiple sclerosis. Ann Pharmacother 2012;46:1010–5 [DOI] [PubMed] [Google Scholar]
- 4.Kragt JJ, van der Linden FA, Nielsen JM, et al. Clinical impact of 20% worsening on timed 25-foot walk and 9-hole peg test in multiple sclerosis. Mult Scler 2006;12:594–8 [DOI] [PubMed] [Google Scholar]
- 5.Kallmann BA, Fackelmann S, Toyka KV, et al. Early abnormalities of evoked potentials and future disability in patients with multiple sclerosis. Mult Scler 2006;12:58–65 [DOI] [PubMed] [Google Scholar]
- 6.Claus D. Central motor conduction: method and normal results. Muscle Nerve 1990;13:1125–32 [DOI] [PubMed] [Google Scholar]
- 7.Smith KJ, Felts PA, John GR. Effects of 4-aminopyridine on demyelinated axons, synapses and muscle tension. Brain 2000;123:171–84 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.