Abstract
Alzheimer disease (AD) is the most common form of age-related dementia. The etiology of AD is considered to be multifactorial as only a negligible percentage of cases have a familial or genetic origin. Glycogen synthase kinase-3 (GSK-3) is regarded as a critical molecular link between the two histopathological hallmarks of the disease, namely senile plaques and neurofibrillary tangles. In this review, we summarize current data regarding the involvement of this kinase in several aspects of AD development and progression, as well as key observations highlighting GSK-3 as one of the most relevant targets for AD treatment.
Keywords: GSK-3β, Alzheimer disease, kinase, neurodegeneration, tau proteins
Alzheimer disease (AD) is a neurodegenerative disorder, first described by the German psychiatrist Alois Alzheimer in 1906. AD is the most common form of age-related dementia. The estimated annual incidence of this disease appears to increase exponentially with age, from approximately 53 new cases per 1,000 people between the ages of 65 and 74 to 231 new cases per 1,000 people over 85 (Hebert et al., 2001; Alzheimer’s Association, 2012). Although mostly unknown, the etiology of AD is considered to be multifactorial. Only a negligible percentage of cases have a familial origin, while most are linked to environmental, non-genetic risk factors of diverse nature (Blennow et al., 2006). AD is characterized by a progressive loss of episodic memory and by cognitive and behavioral impairments. The most relevant histopathological hallmarks of the disease are extracellular senile plaques composed by amyloid-β (Aβ) protein and neurofibrillary tangles (NFTs), the latter formed mainly by hyperphosphorylated tau protein.
The anatomical changes in AD are highly selective for certain brain areas, although alterations can be widespread at advanced stages of the disease. Nevertheless, as one of the most affected brain structures, the entorhinal cortex (EC) is considered an invariant focus of pathology in all cases (Van Hoesen et al., 1991). Anatomical studies have revealed that the EC gives rise to axons that bi-directionally interconnect the hippocampus and the rest of the cortex. Accordingly, it is widely accepted that the EC functions as a gateway to the hippocampus, a brain structure that plays a key role in memory acquisition and consolidation. The EC–hippocampus disconnection that occurs in AD is believed to play a prominent role in the aggravation of the memory impairments that characterize this neurodegenerative disease.
Glycogen synthase kinase-3 (GSK-3) is a highly conserved protein-serine/threonine kinase that was first isolated from skeletal muscle in 1980 as one of five enzymes capable of phosphorylating glycogen synthase (Embi et al., 1980). It was subsequently demonstrated that insulin triggers the inactivation of this kinase. In mammals, GSK-3 is encoded by two highly related genes encoding GSK-3α and GSK-3β, respectively. In the brain, GSK-3β regulates many crucial cellular processes, acting as a key switch that controls numerous signaling pathways (Doble and Woodgett, 2007; Forde and Dale, 2007). The dysregulation of this kinase occurs in the development of cancer, diabetes, AD, schizophrenia, and bipolar disorder, among others. Thus, given its relevance in pathophysiological processes, GSK-3 β is widely considered a therapeutic target of interest.
GSK-3β AS A MOLECULAR LINK BETWEEN Aβ AND TAU
The Aβ peptide has been widely considered the cornerstone of AD pathogenesis, and its precursor protein APP is one of the most studied molecules in the field of AD research. The APP is a glycosylated surface membrane protein (Kang et al., 1987). Aβ is a cleavage product derived from the transmembrane domain of this large precursor protein. APP undergoes post-translational processing, involving cleavage by various secretases and proteases, via two major pathways. Firstly, in the non-amyloidogenic pathway, APP is sequentially cleaved by α- and γ-secretases, thus giving rise to easily degradable fragments. Three members of the α-disintegrin and metalloproteinase (ADAM) family (ADAM-10, ADAM-17, and ADAM-9) have been proposed to form the α-secretase complex (Buxbaum et al., 1998; Koike et al., 1999). GSK-3β may down-regulate the activity of this complex by inhibiting ADAM activity (Zhang et al., 2012). In addition to another three proteins (APH1, PEN2, and nicastrin), presenilin (PS) 1 and 2 function as the catalytic core of the γ-secretase complex. GSK-3β also regulates Aβ production by interfering with APP cleavage at the γ-secretase complex step, since both APP and PS1 are also substrates of this kinase (Cai et al., 2012). In vitro studies suggest that GSK-3β affects PS1 function, which is required for the generation of the toxic Aβ (Uemura et al., 2007).
In contrast, in the amyloidogenic pathway, APP is cleaved by β-secretase, generating a membrane-associated fragment (Zhang et al., 2012). Subsequently, γ-secretase releases Aβ, which tends to aggregate, giving rise to senile plaques and other insoluble oligomeric forms of the protein. The putative β-secretase, also known as β-site APP cleaving enzyme 1 (BACE1), is a type I transmembrane aspartyl protease whose active site is located on the luminal side of the membrane. The knock-down of bace1 prevents Aβ generation and abolishes amyloid pathology in mice expressing the Swedish mutation of APP (Cai et al., 2001; Luo et al., 2001). The expression level and activity of BACE1 have been found to be elevated in AD patients (Holsinger et al., 2002). Accordingly, GSK-3β inhibition reduces BACE1-mediated cleavage of APP through a NF-κB signaling-mediated mechanism. This observation thus suggests that the inhibition of GSK-3β reduces Aβ pathology (Ly et al., 2013).
In vitro studies (Takashima et al., 1996b) and transgenic animal models of AD (Terwel et al., 2008) indicate that Aβ activates GSK-3β signaling (Takashima et al., 1996a, b) by preventing inhibitory phosphorylation of this enzyme in the case of in vitro studies and by an independent mechanism in the case of animal studies. A similar increase in GSK-3β activity has been observed in the brains of AD patients (Leroy et al., 2007). A feed-forward loop is established after GSK-3β pathological activation by Aβ, which subsequently contributes to abnormal APP processing and to synaptic failure (Deng et al., 2014). Consistent with this, GSK-3β inhibition has been shown to reduce Aβ production in AD murine models (Phiel et al., 2003; Rockenstein et al., 2007b) and to decrease Aβ-induced neurotoxicity in cultured neurons (Koh et al., 2008).
In post-mitotic neurons, the microtubule network is of particular significance in supporting axon function. Microtubule-associated proteins (MAPs) facilitate and regulate microtubule formation and stability. Tau is a MAP that is found mainly in the axonal compartment under physiological conditions. Tau associates with microtubules and stabilizes their polymerization. It has been suggested that the presence of tau is required for Aβ-induced toxicity (Rapoport et al., 2002; Santacruz et al., 2005; Roberson et al., 2007). NFTs comprise mainly hyperphosphorylated forms of tau protein. In contrast to normal tau, the hyperphosphorylated form of the protein acquires the shape of paired helical filaments (PHF-tau). Accumulating evidence indicates that the phosphorylated state of tau is closely associated with AD pathology (Augustinack et al., 2002). Accordingly, Aβ induces the formation of tau fibrils in culture (Ferrari et al., 2003). PHF-tau has been described to be an aggregated and insoluble deposit in the somatodendritic compartment (Gotz et al., 1995). In addition, this form of tau is often truncated at the C-terminal domain and is highly resistant to the action of phosphatases and proteases. While non-phosphorylated tau is a flexible protein, PHF-tau is an insoluble misfolded protein. During the course of NFT formation, tau progressively acquires a rigid conformation.
The distinct phosphorylation states of tau correspond to its physiological roles (Bretteville and Planel, 2008; Sergeant et al., 2008), and phosphorylation of some of its serine/threonine residues elicits a biological effect (Fuster-Matanzo et al., 2012). The three tau kinases, GSK-3β, CDK-5, and PKA, associate with both tau and microtubules. Although they show a wide spectrum of phosphorylation, the major phosphorylatable sites of tau for each kinase are limited in preference (Hashiguchi and Hashiguchi, 2013). Multisite phosphorylation occurs in PHF-tau and is explained by the catalytic activities of the different kinases, although the functional significance of this phenomenon is not completely understood. Indeed, a direct association of tau with GSK-3β takes place as a functional unit (Sun et al., 2002; Chun et al., 2004). Although GSK-3β phosphorylates at least 36 residues in tau (Hanger et al., 2007), the main phosphorylation sites identified for this kinase are Ser199, Thr231, Ser396, and Ser413 (Billingsley and Kincaid, 1997). A moderate phosphorylation of Ser46, Thr50, and Ser202/Thr205 has also been reported (Illenberger et al., 1998), and minor phosphorylation of other residues has been described (Hanger et al., 2007). A complete description of these phosphorylation sites is provided in an extensive review by Hashiguchi and Hashiguchi (2013).
In the pre-tangle stage of AD, scattered deposits of phospho-Thr231-tau are detected in the brains of patients (Luna-Munoz et al., 2007). Interestingly, similar to many other residues of tau, the phosphorylation of Thr231 demands the combined action of CDK-5 and GSK-3β (Li and Paudel, 2006; Li et al., 2006). GSK-3β requires a priming phosphorylation of this residue by other tau kinases. This phosphorylation reduces tau binding to microtubules (Sengupta et al., 1998). A similar mechanism has been described for Ser404 and other residues. Thus, the combined action of CDK-5 and GSK-3β seems to be required for the development of the epitope characteristics of PHF-tau (Plattner et al., 2006; Sengupta et al., 2006). Interestingly, the protein phosphatases PP-1 and PP-2 effectively dephosphorylate these sites, in such a way that the overall tau phosphorylation state is determined by the balance between kinase and phosphatase action. Subsequently, cleavage and conformational changes of tau occur after its phosphorylation. After neuronal cell death, intracellular NFTs are released into the extracellular space (Dickson et al., 1992). Interestingly, growing evidence indicates that hyperphosphorylated tau activates GSK-3β through an increase in oxidative stress, neuroinflammation, and apoptosis (Saeki et al., 2011). In addition, GSK-3β impairs lysosomal acidification, a process that entails an inadequate clearance of non-functional proteins (Avrahami et al., 2013).
In summary, increased GSK-3β activity has been used to model events occurring in AD, interventions that exacerbate cognitive impairments, and neuropathology in rodent models of AD (Gomez-Sintes et al., 2011). Conditional overexpression of GSK-3β in mouse hippocampal neurons results in impaired performance in the Morris water maze, hyperphosphorylation of tau, reactive astrogliosis and microgliosis, and neuronal death (Lucas et al., 2001; Hernandez et al., 2002). Restoring normal levels of GSK-3β activity reverses spatial memory deficits, reduces tau hyperphosphorylation, and decreases reactive gliosis and neuronal death (Engel et al., 2006). The deletion of tau in GSK-3β-overexpressing mice significantly ameliorates memory impairments, thus indicating that tau phosphorylation contributes to this cognitive impairment (Gomez de Barreda et al., 2010).
PHYSIOLOGICAL AND PATHOLOGICAL REGULATION OF GSK-3β ACTIVITY
GSK-3β is constitutively active in most tissues and most commonly regulated by inhibitory phosphorylation on Ser9. GSK-3β can be phosphorylated on this serine by several kinases. This observation allows for an effective mechanism for several intracellular signaling pathways to control the activity of this kinase. However, the dysregulation of these signal transduction pathways results in failure to adequately repress GSK-3β, thus allowing GSK-3β to remain abnormally active. Such a status contributes to various pathologies, including neurodegenerative and mood disorders, diabetes, and cancer.
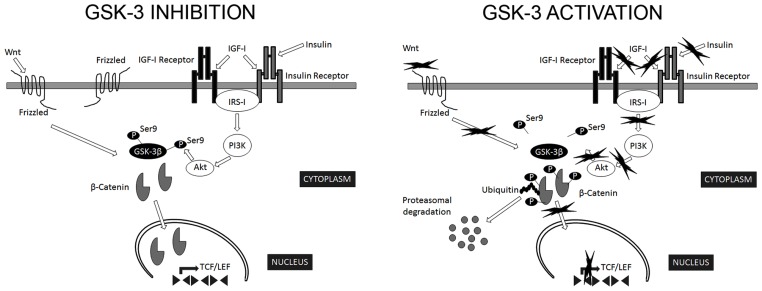
The most relevant extracellular signaling pathway known to regulate GSK-3β activity is that of insulin/insulin-like growth factor I (Figure 1). In addition, a number of kinases phosphorylate Ser9 of GSK-3β in the context of specific signaling pathways: PKB targets GSK-3β in response to insulin (Sutherland et al., 1993); PKA phosphorylates GSK-3β in Ser9 in response to cAMP (Cross et al., 1995); p90RSK/MAPKAP kinase-1 phosphorylates GSK-3β following activation by EGF or PDGF (Sutherland and Cohen, 1994; Fang et al., 2000); and p70S6K targets GSK-3β in response to stimulation by insulin and other growth factors (Godemann et al., 1999).
FIGURE 1.
Regulation of GSK-3β activity. GSK-3β is constitutively active in most tissues and most commonly regulated by inhibitory phosphorylation on Ser9. The most relevant extracellular signaling pathways known to negatively regulate GSK-3β activity are those of insulin/insulin-like growth factor I and Wnt. In the canonical Wnt signaling pathway, Wnt stabilizes levels of β-catenin. Subsequently, stabilized β-catenin initiates the transcription of target genes. GSK-3β phosphorylates several components of this transduction pathway, β-catenin being the most widely characterized. Phosphorylated β-catenin is recognized by ubiquitin and targeted for proteasomal degradation (Wu and He, 2006). In addition, Akt phosphorylate Ser9 of GSK-3β in the context of insulin signaling pathway (Sutherland et al., 1993). Consequently, signals that modify GSK-3β activity are expected to alter β-catenin levels (Forde and Dale, 2007).
Interestingly, Aβ interferes not only with insulin but also with Wnt signaling pathways (Townsend et al., 2007; Magdesian et al., 2008). GSK-3β is a key transducer of the Wnt pathway (Figure 1), the components of which are involved in AD onset (Christian et al., 2002). It has been suggested that auto-inhibitory phosphorylation on Ser9 participates in the regulation of GSK-3β activity in response to Wnt (Saito et al., 1994; Fukumoto et al., 2001). In the canonical Wnt signaling pathway, Wnt stabilizes levels of β-catenin. Subsequently, stabilized β-catenin initiates the transcription of target genes. GSK-3β phosphorylates several components of this transduction pathway, β-catenin being the most widely characterized. Phosphorylated β-catenin is recognized by ubiquitin and targeted for proteasomal degradation (Wu and He, 2006). Consequently, signals that modify GSK-3β activity are expected to alter β-catenin levels (Forde and Dale, 2007). In addition, other components of Wnt signaling pathway, such as DKK1, negatively regulate these pathways, thus activating GSK-3β and contributing to the pathological events triggered by Aβ (Alvarez et al., 2004).
It has been proposed that GSK-3β activity also depends on the phosphorylation of Tyr216 (Kannan and Neuwald, 2004). The underlying mechanisms responsible for regulating tyrosine phosphorylation of GSK-3β remain controversial. In addition, it is still unclear whether GSK-3β autophosphorylation is an intramolecular or intermolecular event.
An interesting alternative regulatory mechanism of GSK-3β activity involves the action of the calcium-dependent protease calpain. GSK-3β is a calpain substrate (Goni-Oliver et al., 2007) and its cleavage by calpain produces the release of the inhibitory domain containing Ser9. Only in the GSK-3α isoform (but not in GSK-3β) is this region surrounded by glycine stretches, a feature that has been proposed to differentially regulate inhibitory phosphorylation and cleavage by calpain in both isoforms (Goni-Oliver et al., 2009).
NEURAL CONSEQUENCES OF THE DYSREGULATION OF GSK-3β ACTIVITY
CHOLINERGIC SYSTEM ALTERATION
A marked loss of cholinergic neurons in certain cortical areas is a well-known feature of AD brain (Whitehouse et al., 1981; Plotkin and Jarvik, 1986). It has been proposed that GSK-3β plays a key role in choline metabolism, which involves the regulation of choline acetyltransferase (ChAT) and acetylcholinesterase (Yates et al., 1983; Samadi et al., 2011). In fact, the loss of cholinergic neurons in the basal forebrain and hippocampus correlates with a transient decrease in Ser9 phosphorylation of GSK-3β and a concomitant increase in tau phosphorylation (Hoshi et al., 1996; Wang et al., 2009). In addition, cholinergic stimulation in the hippocampus, striatum, and cortex causes a rapid increase in Ser9 phosphorylation of GSK-3β (Wang et al., 2009).
AXONAL TRANSPORT AND MICROTUBULE DYNAMICS IMPAIRMENT
Axonopathy and cytoskeletal disruption play a crucial role in AD (Kokubo et al., 2005; Robert and Mathuranath, 2007). GSK-3β has the capacity to phosphorylate several MAPs, thus regulating axonal stability through direct interaction with microtubules. GSK-3β-phosphorylated forms of tau and MAP-2 exhibit decreased affinity toward microtubules and are less stable (Lovestone et al., 1996; Sanchez et al., 2000; Zumbrunn et al., 2001). This microtubule destabilization is detrimental for the maintenance of axonal structure and appropriate synapse function (Sergeant et al., 2008). Importantly, Aβ plaques can lead to axonal dystrophy, causing profound impairment of axonal transport, great detriment to cognitive function, extensive synapse loss, and cell death (Rauk, 2008). Growing evidence indicates that axonal transport failure makes a significant contribution to AD pathology (Pope et al., 1993).
During neural development, GSK-3β is involved in axon formation and elongation (Bartzokis et al., 2003). In this regard, it impairs mitochondrial anterograde and retrograde axonal transport in vitro, a process that involves tau and MAP-1B, respectively (Jimenez-Mateos et al., 2006; Montenegro-Venegas et al., 2010; Llorens-Martin et al., 2011), and these alterations can have severe consequences on synapse function as a result of energy depletion. Accordingly, tau overexpression disrupts axonal transport, causing vesicular aggregation, a phenomenon reversed by GSK-3β inhibitors (Soutar et al., 2010).
In addition, PS1 regulates kinesin-related axonal transport by a mechanism involving GSK-3β activity (Ryan and Pimplikar, 2005) and the modulation of its role in controlling kinesin binding to microtubules at sites of vesicle release (Pigino et al., 2003).
APOPTOSIS
Interestingly, GSK-3β promotes both pro-and anti-apoptotic effects. In this regard, it regulates the two major apoptotic pathways: intrinsic and extrinsic. GSK-3β triggers cell death through the activation of the mitochondrial intrinsic pro-apoptotic pathway while it inhibits the death receptor-mediated extrinsic apoptotic pathway (Beurel and Jope, 2006). After activation of the former, this kinase induces apoptosis in response to a wide range of detrimental stimuli, such as DNA damage (Watcharasit et al., 2003), hypoxia (Loberg et al., 2002), growth factor deprivation (Pap and Cooper, 1998; Johnson-Farley et al., 2006), and heat shock (Bijur et al., 2000). As a part of this pro-apoptotic cascade, GSK-3β phosphorylates and inhibits eIF2B (Welsh and Proud, 1993; Pap and Cooper, 2002). A murine model of neuronal GSK-3β overexpression developed by our group shows enhanced apoptosis in certain sensitive areas of the brain such as the hippocampal formation, which is crucial for memory and learning and strongly affected in AD (Fuster-Matanzo et al., 2011; Llorens-Martin et al., 2013). Although the exact mechanism by which GSK-3β overexpression induces apoptosis in these cells is unclear, it has been proposed that the combination of cell-autonomous effects and other effects indirectly mediated by inflammatory changes act in a coordinated manner to induce hippocampal neuron death (Llorens-Martin et al., 2013). However, given the regulation of the extrinsic apoptotic pathway by GSK-3β, it should be considered that this kinase modulates crucial steps in each of the two major pathways of apoptosis, but in opposing directions. Consequently, inhibitors of GSK-3β provide protection from intrinsic apoptotic signaling but potentiate that of extrinsic apoptosis (Gomez-Sintes et al., 2007). These observations should be taken into account when designing new therapeutic approaches and novel GSK-3β inhibitors.
SYNAPTIC EFFECTS
Synaptic loss is currently the best neurobiological correlate of cognitive deficits in AD. In addition to the synapse loss caused by neuronal cell death, living neurons lose synapses in AD (Coleman and Yao, 2003). It has been proposed that the mechanism allowing information storage in the brain involves changes in synaptic connection weights, including long-term potentiation (LTP) and long-term depression (LTD). The finding that LTP inhibits GSK-3β activity and that this kinase is required for LTD suggests that LTP regulates LTD (Peineau et al., 2007). Although the exact mechanism underlying this regulation remains unclear, it has been demonstrated that constitutive GSK-3β activity enhances basal α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) endocytosis (Wei et al., 2010). This phenomenon, leads to the dissociation of AMPAR-containing vesicles from kinesin (Du et al., 2010). In addition, tau and PS1 may be additional targets for GSK-3β regulation of synaptic plasticity, or, alternatively, different transcription factors or miRNAs may be involved in the protein synthesis-dependent phase of LTD (Manahan-Vaughan, 2010).
Of particular interest is whether the balance between LTP and LTD leads to functional impairments in memory storage similar to those described in AD (Bradley et al., 2012). LTD induces the removal of AMPARs from individual synapses (Luthi et al., 1999) in a process known as synapse silencing. These silent synapses are either reactivated through new AMPAR insertion (unsilencing; Isaac et al., 1995; Liao et al., 1995) or eliminated (Bastrikova et al., 2008; Egashira et al., 2010). Synapse elimination is particularly important during development and crucial for pruning unnecessary synaptic connections (Rabacchi et al., 1992; Bhatt et al., 2009). In fact, NMDAR-triggered apoptosis requires AMPAR endocytosis (Wang et al., 2004), a process known as synaptosis. In adulthood, synaptosis is down-regulated, and it is assumed that NMDAR-related LTD is used for adjusting synaptic weights rather than for eliminating synapses. Collingridge and colleagues suggested that neurodegeneration is often triggered by the reactivation of synaptosis, which leads to apoptosis of vulnerable neuronal populations (Bradley et al., 2012). The imbalance between these mechanisms may lead to the pathological elimination of synapses, which in turn leads to neuronal death. In this regard, we have demonstrated that neuronal GSK-3β overexpression causes a drastic decrease in postsynaptic density number and volume in hippocampal granule neurons (Llorens-Martin et al., 2013), a phenomenon that may be related to cognitive impairment and altered LTP generation previously observed in these mice (Hernandez et al., 2002; Hooper et al., 2007). In agreement, Aβ causes synaptic toxicity (Cullen et al., 1997; Witton et al., 2010). The use of GSK-3β inhibitors protects synapses from the deleterious effects of Aβ (Shipton et al., 2011), thus suggesting that GSK-3β activation is required for the pathological effect of Aβ on synaptic plasticity.
INFLAMMATION
Among the functions regulated by GSK-3β, inflammation has recently emerged as one of the most relevant for neurodegenerative disorders (Sudduth et al., 2013). GSK-3β itself is an important positive regulator of the inflammatory process (Martin et al., 2005; Jope et al., 2007). Within the brain, microglial cells are considered to be equivalent to macrophages in the periphery and key guardian immune cells. Numerous stressors activate microglia, including neurodegenerative diseases, leading to a chronic inflammatory response and migration of responsive cells from the periphery. During long-term inflammatory responses, chronically activated (primed) glia appear to be detrimental to neuronal function and survival. Therefore, it is relevant that GSK-3β has been identified as a prominent regulator of inflammation. GSK-3β promotes the production of various pro-inflammatory cytokines, such as interleukin-6 (IL-6), IL-1β, and tumor necrosis factor (TNF; Martin et al., 2005). In addition, this kinase decreases the production of the anti-inflammatory cytokine IL-10. Remarkably, in vivo administration of GSK-3β inhibitors confers protection from endotoxin shock (Martin et al., 2005). Data from our group showed that GSK-3β overexpression in neurons leads to the appearance of a unique pattern of cytokines in the brain in vivo (Llorens-Martin et al., 2013). In addition, we have demonstrated that this pro-inflammatory environment is detrimental for immature neurons as it inhibits their appropriate maturation (Fuster-Matanzo et al., 2013) and leads them to acquire an aberrant morphology (named “V” shape) markedly similar to that found in AD patient granule neurons (Llorens-Martin et al., 2013).
CELL CYCLE DYSREGULATION
The formation of dynamically re-arranged synaptic connections during continuous structural remodeling entails that neurons must permanently withdraw from the cell cycle (Arendt, 2003). As elegantly exposed in the “Dr. Jekyll and Mr. Hyde concept,” formulated by Arendt, after leaving the cell cycle, differentiated neurons modulate synaptic plasticity through molecular mechanisms primarily developed to control proliferation (Arendt, 2003, 2009). The up-regulation of a various molecular effectors involved in the activation and progression of the cell cycle occurs at early stages of neurodegeneration in AD (Arendt et al., 1996; Nagy et al., 1997a, b). Although the cause of this failure remains to be elucidated, recent evidence indicates that molecular mechanisms controlling synaptic plasticity and cell cycle are shared in the same cells, and, consequently, attempts to increase plasticity during initial stages of AD are sometimes disastrous for hippocampal function. At the molecular level, the Sonic hedgehog (Shh) and Wnt signaling pathways cooperate to orchestrate cellular proliferation, differentiation, and pattern formation during both development and adult neurogenesis. As previously discussed, GSK-3β plays a crucial role in modulating both pathways. Although the underlying mechanism regulating GSK-3β activity in response to Shh remains to be determined, Zhang et al. (2005) proposed the formation of a multi-protein complex similar to that required for efficient phosphorylation of β-catenin in the Wnt pathway. However, the physiological relevance of this interaction has yet to be revealed.
ADULT HIPPOCAMPAL NEUROGENESIS
New neurons are continuously added to the hippocampal dentate gyrus (DG) throughout lifetime (Kempermann et al., 1998; Knoth et al., 2010). During differentiation stages, newborn neurons sequentially increase their dendritic tree complexity and send axons toward the CA3 region (Zhao et al., 2006). Growing evidence indicates that newborn neurons are crucial for hippocampal function and hippocampal-dependent memory (Bischofberger, 2007). One of the most important regulators of adult hippocampal neurogenesis (AHN) is GSK-3β. In this regard, it has been demonstrated that overexpression of this kinase impairs adult neurogenesis (Sirerol-Piquer et al., 2011; Fuster-Matanzo et al., 2013) and causes a depletion in the number of proliferative clusters within the hippocampal DG. In addition, we have recently reported that GSK-3β overexpression has dual effects on newborn neurons, blocking the differentiation of newborn neurons, thus supporting the notion that their maturation is impaired. We have observed that GSK-3β overexpression leads to alterations in the rate of death and survival of newborn neurons, as well as in the expression pattern of the immature neuron marker doublecortin (Fuster-Matanzo et al., 2013). In accordance, Spittaels et al. (2002) demonstrated that GSK-3β influences the post-natal maturation of neurons in vivo in a transgenic model overexpressing a constitutively active form of the enzyme. In addition, overexpression of this kinase causes morphological and connectivity alterations similar to those observed in the granule neurons of AD patients (Llorens-Martin et al., 2013). Given the relevance of newborn neurons in hippocampal-dependent learning, it is reasonable to assume that the alterations in AHN lead to cognitive impairments. In fact, murine model overexpressing GSK-3β in the hippocampus shows impaired hippocampal-dependent learning (Hernandez et al., 2002).
AD THERAPIES INVOLVING GSK-3β INHIBITION
Growing evidence indicates that GSK-3β contributes to the pathology of several neurodegenerative diseases. Thus, there is increasing interest in applying GSK-3β inhibitors to treat these disorders. Lithium is a GSK-3 inhibitor that binds directly to GSK-3β (Klein and Melton, 1996) and increases the inhibitory phosphorylation in Ser9 of GSK-3β (Jope, 2003). Lithium is used as a mood stabilizer in patients suffering from mood disorders. Various effects of lithium are caused by GSK-3β inhibition (Jope, 2011), and lithium administration reduces the neuropathology and cognitive deficits in rats that have received intra-hippocampal injections of Aβ (De Ferrari et al., 2003), rats overexpressing GSK-3β (Liu et al., 2010), and several murine models overexpressing human APP (Rockenstein et al., 2007b; Ghosal et al., 2009; Toledo and Inestrosa, 2010). However, some studies report poor effects of lithium on behavior in other murine models of AD (Caccamo et al., 2007; Fiorentini et al., 2010; Sudduth et al., 2012). It is interesting to note that although certain cognitive tasks are improved by lithium treatment in healthy rodents, this metal does not significantly affect cognitive performance.
Recent years have witnessed the development of an increasing number of novel GSK-3β inhibitors, many of which are ATP-competitive. However, particularly promising are the non-ATP-competitive GSK-3β inhibitors, since they tend to be more selective and less toxic (King et al., 2014). The classical ATP-competitive GSK-3 inhibitors include Indirubin (Leclerc et al., 2001), Paullone compounds (Leost et al., 2000), SB415286 and SB216763 (Coghlan et al., 2000), and AR-A014418 (Bhat et al., 2003). Several well-known non-competitive ATP binding site inhibitors of GSK-3 are L803-mts (Plotkin et al., 2003; Kaidanovich-Beilin et al., 2004), TDZD-8 (Martinez et al., 2002), and VP0.7 (Palomo et al., 2011). The treatment of healthy rodents with GSK-3β inhibitors produces no remarkable effects on behavioral cognitive scores (Thotala et al., 2008). Conversely, genetic reduction of GSK-3β activity appears to be detrimental for hippocampal memory acquisition (Kimura et al., 2008). In contrast, GSK-3β overexpression (both the native and constitutively active forms of the enzyme) leads to cognitive impairment (Hernandez et al., 2002; Dewachter et al., 2009). In this regard, inhibitors of GSK-3β have been reported to rescue cognitive deficits in several murine models of AD. Treatment with NP12, AR-A014418, and Indirubin decreases memory deficits in the Morris water maze and reduces tau phosphorylation and amyloid deposition in various models of transgenic mice overexpressing human APP (Sereno et al., 2009; Ding et al., 2010; Ly et al., 2013). In addition, 5XFAD mice treated with L803-mts exhibit improved hippocampal-dependent learning capacity (Avrahami et al., 2013). Genetic approaches aimed to knock down either GSK-3α or GSK-3β have also been shown to improve cognitive impairments in several murine models of AD (Rockenstein et al., 2007a, b; Hurtado et al., 2012).
The promising ability of GSK-3 inhibitors to alleviate the AD-like phenotype of various murine models of AD has brought about several clinical studies in patients with this neurodegenerative disease, although contradictory data regarding the success of these treatments have been reported by different clinical trials (del Ser et al., 2013). It should be taken into account that GSK-3 is essential for cell life, and there is a concern that its inhibition could prevent cells from operating normally (Martinez et al., 2011).
Lithium has been shown to exert certain protection against the development of cognitive impairments in bipolar disorder patients (Nunes et al., 2007; Kessing et al., 2010). Importantly, patients in early-stage AD receiving lithium treatment showed improved cognitive function (Leyhe et al., 2009; Forlenza et al., 2011, 2012), although other studies showing no such enhancement have also been reported (Macdonald et al., 2008; Pomara, 2009).
CONCLUSIONS AND FURTHER DIRECTIONS
GSK-3β is not a conventional kinase. It plays critical roles in neurodevelopment and in both physiological and pathological aging. In AD, a functional link between Aβ and tau unequivocally implicates the dysregulation of GSK-3β activity. In recent decades, Aβ was considered the cornerstone of AD etiology. However, the present consensus is that the disease has a multifactorial origin. Growing evidence supports inflammation as one of the most deleterious inputs to the aging brain. Given the relevance of GSK-3β in regulating crucial steps of the inflammatory cascade, efforts should be channeled into the development of novel and selective inhibitors that safely regulate the activity of this kinase, and, in parallel, block the inflammatory and self-propagating cascade that it triggers in previously damaged brain areas. Although the involvement of GSK-3β in multiple pathways controlling most of the crucial aspects of cell physiology complicates the design of specific inhibitors, it is of paramount importance to address the whole spectrum of GSK-3β actions on cell biology under both physiological and pathological conditions. A promising avenue are also regenerative strategies focused on the capacity of certain neural populations to be continuously generated and integrated into pre-existing neural circuits (adult neurogenesis). Given the pivotal role played by GSK-3β in the regulation of these processes, it is imperative to perform exhaustive research into the therapeutic potential of GSK-3β inhibitors. Such drugs would allow the normal development and functional integration of newborn neurons in the hippocampal formation previously damaged by the progression of the disease.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- Alvarez A. R., Godoy J. A., Mullendorff K., Olivares G. H., Bronfman M., Inestrosa N. C. (2004). Wnt-3a overcomes beta-amyloid toxicity in rat hippocampal neurons. Exp. Cell Res. 297 186–196 10.1016/j.yexcr.2004.02.028 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2012). 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 8 131–168 10.1016/j.jalz.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Arendt T. (2003). Synaptic plasticity and cell cycle activation in neurons are alternative effector pathways: the ‘Dr. Jekyll and Mr. Hyde concept’ of Alzheimer’s disease or the yin and yang of neuroplasticity. Prog. Neurobiol. 71 83–248 10.1016/j.pneurobio.2003.09.007 [DOI] [PubMed] [Google Scholar]
- Arendt T. (2009). Synaptic degeneration in Alzheimer’s disease. Acta Neuropathol. 118 167–179 10.1007/s00401-009-0536-x [DOI] [PubMed] [Google Scholar]
- Arendt T., Rodel L., Gartner U., Holzer M. (1996). Expression of the cyclin-dependent kinase inhibitor p16 in Alzheimer’s disease. Neuroreport 7 3047–3049 10.1097/00001756-199611250-00050 [DOI] [PubMed] [Google Scholar]
- Augustinack J. C., Schneider A., Mandelkow E. M., Hyman B. T. (2002). Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 103 26–35 10.1007/s004010100423 [DOI] [PubMed] [Google Scholar]
- Avrahami L., Farfara D., Shaham-Kol M., Vassar R., Frenkel D., Eldar-Finkelman H. (2013). Inhibition of glycogen synthase kinase-3 ameliorates beta-amyloid pathology and restores lysosomal acidification and mammalian target of rapamycin activity in the Alzheimer disease mouse model: in vivo and in vitro studies. J. Biol. Chem. 288 1295–1306 10.1074/jbc.M112.409250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G., Cummings J. L., Sultzer D., Henderson V. W., Nuechterlein K. H., Mintz J. (2003). White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch. Neurol. 60 393–398 10.1001/archneur.60.3.393 [DOI] [PubMed] [Google Scholar]
- Bastrikova N., Gardner G. A., Reece J. M., Jeromin A., Dudek S. M. (2008). Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc. Natl. Acad. Sci. U.S.A. 105 3123–3127 10.1073/pnas.0800027105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E., Jope R. S. (2006). The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog. Neurobiol. 79 173–189 10.1016/j.pneurobio.2006.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R., Xue Y., Berg S., Hellberg S., Ormo M., Nilsson Y., et al. (2003). Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J. Biol. Chem. 278 45937–45945 10.1074/jbc.M306268200 [DOI] [PubMed] [Google Scholar]
- Bhatt D. H., Zhang S., Gan W. B. (2009). Dendritic spine dynamics. Annu. Rev. Physiol. 71 261–282 10.1146/annurev.physiol.010908.163140 [DOI] [PubMed] [Google Scholar]
- Bijur G. N., De Sarno P., Jope R. S. (2000). Glycogen synthase kinase-3beta facilitates staurosporine- and heat shock-induced apoptosis. Protection by lithium. J. Biol. Chem. 275 7583–7590 10.1074/jbc.275.11.7583 [DOI] [PubMed] [Google Scholar]
- Billingsley M. L., Kincaid R. L. (1997). Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem. J. 323(Pt 3) 577–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger J. (2007). Young and excitable: new neurons in memory networks. Nat. Neurosci. 10 273–275 10.1038/nn0307-273 [DOI] [PubMed] [Google Scholar]
- Blennow K., de Leon M. J., Zetterberg H. (2006). Alzheimer’s disease. Lancet 368 387–403 10.1016/S0140-6736(06)69113-7 [DOI] [PubMed] [Google Scholar]
- Bradley C. A., Peineau S., Taghibiglou C., Nicolas C. S., Whitcomb D. J., Bortolotto Z. A., et al. (2012). A pivotal role of GSK-3 in synaptic plasticity. Front. Mol. Neurosci. 5:13 10.3389/fnmol.2012.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretteville A., Planel E. (2008). Tau aggregates: toxic, inert, or protective species? J. Alzheimers Dis. 14 431–436 [DOI] [PubMed] [Google Scholar]
- Buxbaum J. D., Thinakaran G., Koliatsos V., O’Callahan J., Slunt H. H., Price D. L., et al. (1998). Alzheimer amyloid protein precursor in the rat hippocampus: transport and processing through the perforant path. J. Neurosci. 18 9629–9637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A., Oddo S., Tran L. X., LaFerla F. M. (2007). Lithium reduces tau phosphorylation but not A beta or working memory deficits in a transgenic model with both plaques and tangles. Am. J. Pathol. 170 1669–1675 10.2353/ajpath.2007.061178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Wang Y., McCarthy D., Wen H., Borchelt D. R., Price D. L., et al. (2001). BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat. Neurosci. 4 233–234 10.1038/85064 [DOI] [PubMed] [Google Scholar]
- Cai Z., Zhao Y., Zhao B. (2012). Roles of glycogen synthase kinase 3 in Alzheimer’s disease. Curr. Alzheimer Res. 9 864–879 10.2174/156720512802455386 [DOI] [PubMed] [Google Scholar]
- Coghlan M. P., Culbert A. A., Cross D. A., Corcoran S. L., Yates J. W., Pearce N. J., et al. (2000). Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 7 793–803 10.1016/S1074-5521(00)00025-9 [DOI] [PubMed] [Google Scholar]
- Coleman P. D., Yao P. J. (2003). Synaptic slaughter in Alzheimer’s disease. Neurobiol. Aging 24 1023–1027 10.1016/j.neurobiolaging.2003.09.001 [DOI] [PubMed] [Google Scholar]
- Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995). Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378 785–789 10.1038/378785a0 [DOI] [PubMed] [Google Scholar]
- Christian S. L., Sims P. V., Gold M. R. (2002). The B cell antigen receptor regulates the transcriptional activator beta-catenin via protein kinase C-mediated inhibition of glycogen synthase kinase-3. J. Immunol. 169 758–769 10.4049/jimmunol.169.2.758 [DOI] [PubMed] [Google Scholar]
- Chun J., Kwon T., Lee E. J., Kim C. H., Han Y. S., Hong S. K., et al. (2004). 14-3-3 Protein mediates phosphorylation of microtubule-associated protein tau by serum- and glucocorticoid-induced protein kinase 1. Mol. Cells 18 360–368 [PubMed] [Google Scholar]
- Cullen W. K., Suh Y. H., Anwyl R., Rowan M. J. (1997). Block of LTP in rat hippocampus in vivo by beta-amyloid precursor protein fragments. Neuroreport 8 3213–3217 10.1097/00001756-199710200-00006 [DOI] [PubMed] [Google Scholar]
- De Ferrari G. V., Chacon M. A., Barria M. I., Garrido J. L., Godoy J. A., Olivares G., et al. (2003). Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils. Mol. Psychiatry 8 195–208 10.1038/sj.mp.4001208 [DOI] [PubMed] [Google Scholar]
- del Ser T., Steinwachs K. C., Gertz H. J., Andres M. V., Gomez-Carrillo B., Medina M., et al. (2013). Treatment of Alzheimer’s disease with the GSK-3 inhibitor tideglusib: a pilot study. J. Alzheimers Dis. 33 205–215 10.3233/JAD-2012-120805 [DOI] [PubMed] [Google Scholar]
- Deng Y., Xiong Z., Chen P., Wei J., Chen S. S., Yan Z. (2014). beta-amyloid impairs the regulation of N-methyl-D-aspartate receptors by glycogen synthase kinase 3. Neurobiol. Aging 35 449–459 10.1016/j.neurobiolaging.2013.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewachter I., Ris L., Jaworski T., Seymour C. M., Kremer A., Borghgraef P., et al. (2009). GSK3beta, a centre-staged kinase in neuropsychiatric disorders, modulates long term memory by inhibitory phosphorylation at serine-9. Neurobiol. Dis. 35 193–200 10.1016/j.nbd.2009.04.003 [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Ksiezak-Reding H., Liu W. K., Davies P., Crowe A., Yen S. H. (1992). Immunocytochemistry of neurofibrillary tangles with antibodies to subregions of tau protein: identification of hidden and cleaved tau epitopes and a new phosphorylation site. Acta Neuropathol. 84 596–605 10.1007/BF00227736 [DOI] [PubMed] [Google Scholar]
- Ding Y., Qiao A., Fan G. H. (2010). Indirubin-3′-monoxime rescues spatial memory deficits and attenuates beta-amyloid-associated neuropathology in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 39 156–168 10.1016/j.nbd.2010.03.022 [DOI] [PubMed] [Google Scholar]
- Doble B. W., Woodgett J. R. (2007). Role of glycogen synthase kinase-3 in cell fate and epithelial–mesenchymal transitions. Cells Tissues Organs 185 73–84 10.1159/000101306 [DOI] [PubMed] [Google Scholar]
- Du J., Wei Y., Liu L., Wang Y., Khairova R., Blumenthal R., et al. (2010). A kinesin signaling complex mediates the ability of GSK-3beta to affect mood-associated behaviors. Proc. Natl. Acad. Sci. U.S.A. 107 11573–11578 10.1073/pnas.0913138107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira Y., Tanaka T., Soni P., Sakuragi S., Tominaga-Yoshino K., Ogura A. (2010). Involvement of the p75(NTR) signaling pathway in persistent synaptic suppression coupled with synapse elimination following repeated long-term depression induction. J. Neurosci. Res. 88 3433–3446 10.1002/jnr.22505 [DOI] [PubMed] [Google Scholar]
- Embi N., Rylatt D. B., Cohen P. (1980). Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur. J. Biochem. 107 519–527 10.1111/j.1432-1033.1980.tb06059.x [DOI] [PubMed] [Google Scholar]
- Engel T., Hernandez F., Avila J., Lucas J. J. (2006). Full reversal of Alzheimer’s disease-like phenotype in a mouse model with conditional overexpression of glycogen synthase kinase-3. J. Neurosci. 26 5083–5090 10.1523/JNEUROSCI.0604-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X., Yu S. X., Lu Y., Bast R. C., Woodgett J. R., Jr., Mills G. B. (2000). Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc. Natl. Acad. Sci. U.S.A. 97 11960–11965 10.1073/pnas.220413597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A., Hoerndli F., Baechi T., Nitsch R. M., Gotz J. (2003). beta-Amyloid induces paired helical filament-like tau filaments in tissue culture. J. Biol. Chem. 278 40162–40168 10.1074/jbc.M308243200 [DOI] [PubMed] [Google Scholar]
- Fiorentini A., Rosi M. C., Grossi C., Luccarini I., Casamenti F. (2010). Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice. PLoS ONE 5:e14382 10.1371/journal.pone.0014382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde J. E., Dale T. C. (2007). Glycogen synthase kinase 3: a key regulator of cellular fate. Cell. Mol. Life Sci. 64 1930–1944 10.1007/s00018-007-7045-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlenza O. V., de Paula V. J., Machado-Vieira R., Diniz B. S., Gattaz W. F. (2012). Does lithium prevent Alzheimer’s disease? Drugs Aging 29 335–342 10.2165/11599180-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Forlenza O. V., Diniz B. S., Radanovic M., Santos F. S., Talib L. L., Gattaz W. F. (2011). Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br. J. Psychiatry 198 351–356 10.1192/bjp.bp.110.080044 [DOI] [PubMed] [Google Scholar]
- Fukumoto S., Hsieh C. M., Maemura K., Layne M. D., Yet S. F., Lee K. H., et al. (2001). Akt participation in the Wnt signaling pathway through Dishevelled. J. Biol. Chem. 276 17479–17483 10.1074/jbc.C000880200 [DOI] [PubMed] [Google Scholar]
- Fuster-Matanzo A., Llorens-Martin M., de Barreda E. G., Avila J., Hernandez F. (2011). Different susceptibility to neurodegeneration of dorsal and ventral hippocampal dentate gyrus: a study with transgenic mice overexpressing GSK3beta. PLoS ONE 6:e27262 10.1371/journal.pone.0027262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster-Matanzo A., Llorens-Martin M., Jurado-Arjona J., Avila J., Hernandez F. (2012). Tau protein and adult hippocampal neurogenesis. Front. Neurosci. 6: 104 10.3389/fnins.2012.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster-Matanzo A., Llorens-Martin M., Sirerol-Piquer M. S., Garcia-Verdugo J. M., Avila J., Hernandez F. (2013). Dual effects of increased glycogen synthase kinase-3beta activity on adult neurogenesis. Hum. Mol. Genet. 22 1300–1315 10.1093/hmg/dds533 [DOI] [PubMed] [Google Scholar]
- Ghosal K., Vogt D. L., Liang M., Shen Y., Lamb B. T., Pimplikar S. W. (2009). Alzheimer’s disease-like pathological features in transgenic mice expressing the APP intracellular domain. Proc. Natl. Acad. Sci. U.S.A. 106 18367–18372 10.1073/pnas.0907652106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godemann R., Biernat J., Mandelkow E., Mandelkow E. M. (1999). Phosphorylation of tau protein by recombinant GSK-3beta: pronounced phosphorylation at select Ser/Thr-Pro motifs but no phosphorylation at Ser262 in the repeat domain. FEBS Lett. 454 157–164 10.1016/S0014-5793(99)00741-3 [DOI] [PubMed] [Google Scholar]
- Gomez de Barreda E., Perez M., Gomez Ramos P., de Cristobal J., Martin-Maestro P., Moran A., et al. (2010). Tau-knockout mice show reduced GSK3-induced hippocampal degeneration and learning deficits. Neurobiol. Dis. 37 622–629 10.1016/j.nbd.2009.11.017 [DOI] [PubMed] [Google Scholar]
- Gomez-Sintes R., Hernandez F., Bortolozzi A., Artigas F., Avila J. J., Zaratin P., et al. (2007). Neuronal apoptosis and reversible motor deficit in dominant-negative GSK-3 conditional transgenic mice. EMBO J. 26 2743–2754 10.1038/sj.emboj.7601725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sintes R., Hernandez F., Lucas J. J., Avila J. (2011). GSK-3 mouse models to study neuronal apoptosis and neurodegeneration. Front. Mol. Neurosci. 4:45 10.3389/fnmol.2011.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni-Oliver P., Avila J., Hernandez F. (2009). Calpain-mediated truncation of GSK-3 in post-mortem brain samples. J. Neurosci. Res. 87 1156–1161 10.1002/jnr.21932 [DOI] [PubMed] [Google Scholar]
- Goni-Oliver P., Lucas J. J., Avila J., Hernandez F. (2007). N-terminal cleavage of GSK-3 by calpain: a new form of GSK-3 regulation. J. Biol. Chem. 282 22406–22413 10.1074/jbc.M702793200 [DOI] [PubMed] [Google Scholar]
- Gotz J., Probst A., Spillantini M. G., Schafer T., Jakes R., Burki K., et al. (1995). Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J. 14 1304–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanger D. P., Byers H. L., Wray S., Leung K. Y., Saxton M. J., Seereeram A., et al. (2007). Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J. Biol. Chem. 282 23645–23654 10.1074/jbc.M703269200 [DOI] [PubMed] [Google Scholar]
- Hashiguchi M., Hashiguchi T. (2013). Kinase-kinase interaction and modulation of tau phosphorylation. Int. Rev. Cell Mol. Biol. 300 121–160 10.1016/B978-0-12-405210-9.00004-7 [DOI] [PubMed] [Google Scholar]
- Hebert L. E., Beckett L. A., Scherr P. A., Evans D. A. (2001). Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis. Assoc. Disord. 15 169–173 10.1097/00002093-200110000-00002 [DOI] [PubMed] [Google Scholar]
- Hernandez F., Borrell J., Guaza C., Avila J., Lucas J. J. (2002). Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments. J. Neurochem. 83 1529–1533 10.1046/j.1471-4159.2002.01269.x [DOI] [PubMed] [Google Scholar]
- Holsinger R. M., McLean C. A., Beyreuther K., Masters C. L., Evin G. (2002). Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann. Neurol. 51 783–786 10.1002/ana.10208 [DOI] [PubMed] [Google Scholar]
- Hooper C., Markevich V., Plattner F., Killick R., Schofield E., Engel T., et al. (2007). Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur. J. Neurosci. 25 81–86 10.1111/j.1460-9568.2006.05245.x [DOI] [PubMed] [Google Scholar]
- Hoshi M., Takashima A., Noguchi K., Murayama M., Sato M., Kondo S., et al. (1996). Regulation of mitochondrial pyruvate dehydrogenase activity by tau protein kinase I/glycogen synthase kinase 3beta in brain. Proc. Natl. Acad. Sci. U.S.A. 93 2719–2723 10.1073/pnas.93.7.2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado D. E., Molina-Porcel L., Carroll J. C., Macdonald C., Aboagye A. K., Trojanowski J. Q., et al. (2012). Selectively silencing GSK-3 isoforms reduces plaques and tangles in mouse models of Alzheimer’s disease. J. Neurosci. 32 7392–7402 10.1523/JNEUROSCI.0889-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illenberger S., Zheng-Fischhofer Q., Preuss U., Stamer K., Baumann K., Trinczek B., et al. (1998). The endogenous and cell cycle-dependent phosphorylation of tau protein in living cells: implications for Alzheimer’s disease. Mol. Biol. Cell 9 1495–1512 10.1091/mbc.9.6.1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac J. T., Nicoll R. A., Malenka R. C. (1995). Evidence for silent synapses: implications for the expression of LTP. Neuron 15 427–434 10.1016/0896-6273(95)90046-2 [DOI] [PubMed] [Google Scholar]
- Jimenez-Mateos E. M., Gonzalez-Billault C., Dawson H. N., Vitek M. P., Avila J. (2006). Role of MAP1B in axonal retrograde transport of mitochondria. Biochem. J. 397 53–59 10.1042/BJ20060205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Farley N. N., Travkina T., Cowen D. S. (2006). Cumulative activation of Akt and consequent inhibition of glycogen synthase kinase-3 by brain-derived neurotrophic factor and insulin-like growth factor-1 in cultured hippocampal neurons. J. Pharmacol. Exp. Ther. 316 1062–1069 10.1124/jpet.105.094433 [DOI] [PubMed] [Google Scholar]
- Jope R. S. (2003). Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol. Sci. 24 441–443 10.1016/S0165-6147(03)00206-2 [DOI] [PubMed] [Google Scholar]
- Jope R. S. (2011). Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front. Mol. Neurosci. 4:16 10.3389/fnmol.2011.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope R. S., Yuskaitis C. J., Beurel E. (2007). Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem. Res. 32 577–595 10.1007/s11064-006-9128-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O., Milman A., Weizman A., Pick C. G., Eldar-Finkelman H. (2004). Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol. Psychiatry 55 781–784 10.1016/j.biopsych.2004.01.008 [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., et al. (1987). The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325 733–736 10.1038/325733a0 [DOI] [PubMed] [Google Scholar]
- Kannan N., Neuwald A. F. (2004). Evolutionary constraints associated with functional specificity of the CMGC protein kinases MAPK, CDK, GSK, SRPK, DYRK, and CK2alpha. Protein Sci. 13 2059–2077 10.1110/ps.04637904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Kuhn H. G., Gage F. H. (1998). Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 18 3206–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessing L. V., Forman J. L., Andersen P. K. (2010). Does lithium protect against dementia? Bipolar Disord. 12 87–94 10.1111/j.1399-5618.2009.00788.x [DOI] [PubMed] [Google Scholar]
- Kimura T., Yamashita S., Nakao S., Park J. M., Murayama M., Mizoroki T., et al. (2008). GSK-3beta is required for memory reconsolidation in adult brain. PLoS ONE 3:e3540 10.1371/journal.pone.0003540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. K., Pardo M., Cheng Y., Downey K., Jope R. S., Beurel E. (2014). Glycogen synthase kinase-3 inhibitors: rescuers of cognitive impairments. Pharmacol. Ther. 141 1–12 10.1016/j.pharmthera.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P. S., Melton D. A. (1996). A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. U.S.A. 93 8455–8459 10.1073/pnas.93.16.8455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth R., Singec I., Ditter M., Pantazis G., Capetian P., Meyer R. P., et al. (2010). Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 5:e8809 10.1371/journal.pone.0008809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S. H., Noh M. Y., Kim S. H. (2008). Amyloid-beta-induced neurotoxicity is reduced by inhibition of glycogen synthase kinase-3. Brain Res. 1188 254–262 10.1016/j.brainres.2007.10.064 [DOI] [PubMed] [Google Scholar]
- Koike H., Tomioka S., Sorimachi H., Saido T. C., Maruyama K., Okuyama A., et al. (1999). Membrane-anchored metalloprotease MDC9 has an alpha-secretase activity responsible for processing the amyloid precursor protein. Biochem. J. 343(Pt 2) 371–375 10.1042/0264-6021:3430371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubo H., Kayed R., Glabe C. G., Yamaguchi H. (2005). Soluble Abeta oligomers ultrastructurally localize to cell processes and might be related to synaptic dysfunction in Alzheimer’s disease brain. Brain Res. 1031 222–228 10.1016/j.brainres.2004.10.041 [DOI] [PubMed] [Google Scholar]
- Leclerc S., Garnier M., Hoessel R., Marko D., Bibb J. A., Snyder G. L., et al. (2001). Indirubins inhibit glycogen synthase kinase-3 beta and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors? J. Biol. Chem. 276 251–260 10.1074/jbc.M002466200 [DOI] [PubMed] [Google Scholar]
- Leost M., Schultz C., Link A., Wu Y. Z., Biernat J., Mandelkow E. M., et al. (2000). Paullones are potent inhibitors of glycogen synthase kinase-3beta and cyclin-dependent kinase 5/p25. Eur. J. Biochem. 267 5983–5994 10.1046/j.1432-1327.2000.01673.x [DOI] [PubMed] [Google Scholar]
- Leroy K., Yilmaz Z., Brion J. P. (2007). Increased level of active GSK-3beta in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol. Appl. Neurobiol. 33 43–55 10.1111/j.1365-2990.2006.00795.x [DOI] [PubMed] [Google Scholar]
- Leyhe T., Eschweiler G. W., Stransky E., Gasser T., Annas P., Basun H., et al. (2009). Increase of BDNF serum concentration in lithium treated patients with early Alzheimer’s disease. J. Alzheimers Dis. 16 649–656 10.3233/JAD-2009-1004 [DOI] [PubMed] [Google Scholar]
- Li T., Hawkes C., Qureshi H. Y., Kar S., Paudel H. K. (2006). Cyclin-dependent protein kinase 5 primes microtubule-associated protein tau site-specifically for glycogen synthase kinase 3beta. Biochemistry 45 3134–3145 10.1021/bi051635j [DOI] [PubMed] [Google Scholar]
- Li T., Paudel H. K. (2006). Glycogen synthase kinase 3beta phosphorylates Alzheimer’s disease-specific Ser396 of microtubule-associated protein tau by a sequential mechanism. Biochemistry 45 3125–3133 10.1021/bi051634r [DOI] [PubMed] [Google Scholar]
- Liao D., Hessler N. A., Malinow R. (1995). Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375 400–404 10.1038/375400a0 [DOI] [PubMed] [Google Scholar]
- Liu X. S., Xue Q. S., Zeng Q. W., Li Q., Liu J., Feng X. M., et al. (2010). Sevoflurane impairs memory consolidation in rats, possibly through inhibiting phosphorylation of glycogen synthase kinase-3beta in the hippocampus. Neurobiol. Learn. Mem. 94 461–467 10.1016/j.nlm.2010.08.011 [DOI] [PubMed] [Google Scholar]
- Llorens-Martin M., Fuster-Matanzo A., Teixeira C. M., Jurado-Arjona J., Ulloa F., et al. (2013). GSK-3beta overexpression causes reversible alterations on postsynaptic densities and dendritic morphology of hippocampal granule neurons in vivo. Mol. Psychiatry 18 451–460 10.1038/mp.2013.4 [DOI] [PubMed] [Google Scholar]
- Llorens-Martin M., Lopez-Domenech G., Soriano E., Avila J. (2011). GSK3beta is involved in the relief of mitochondria pausing in a tau-dependent manner. PLoS ONE 6:e27686 10.1371/journal.pone.0027686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loberg R. D., Vesely E, Brosius F. C., III. (2002). Enhanced glycogen synthase kinase-3beta activity mediates hypoxia-induced apoptosis of vascular smooth muscle cells and is prevented by glucose transport and metabolism. J. Biol. Chem. 277 41667–41673 10.1074/jbc.M206405200 [DOI] [PubMed] [Google Scholar]
- Lovestone S., Hartley C. L., Pearce J., Anderton B. H. (1996). Phosphorylation of tau by glycogen synthase kinase-3 beta in intact mammalian cells: the effects on the organization and stability of microtubules. Neuroscience 73 1145–1157 10.1016/0306-4522(96)00126-1 [DOI] [PubMed] [Google Scholar]
- Lucas J. J., Hernandez F., Gomez-Ramos P., Moran M. A., Hen R., Avila J. (2001). Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 20 27–39 10.1093/emboj/20.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Munoz J., Chavez-Macias L., Garcia-Sierra F., Mena R. (2007). Earliest stages of tau conformational changes are related to the appearance of a sequence of specific phospho-dependent tau epitopes in Alzheimer’s disease. J. Alzheimers Dis. 12 365–375 [DOI] [PubMed] [Google Scholar]
- Luo Y., Bolon B., Kahn S., Bennett B. D., Babu-Khan S., Denis P., et al. (2001). Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat. Neurosci. 4 231–232 10.1038/85059 [DOI] [PubMed] [Google Scholar]
- Luthi A., Chittajallu R., Duprat F., Palmer M. J., Benke T. A., Kidd F. L., et al. (1999). Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF–GluR2 interaction. Neuron 24 389–399 10.1016/S0896-6273(00)80852-1 [DOI] [PubMed] [Google Scholar]
- Ly P. T., Wu Y., Zou H., Wang R., Zhou W., Kinoshita A., et al. (2013). Inhibition of GSK3beta-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J. Clin. Invest. 123 224–235 10.1172/JCI64516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald A., Briggs K., Poppe M., Higgins A., Velayudhan L., Lovestone S. (2008). A feasibility and tolerability study of lithium in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 23 704–711 10.1002/gps.1964 [DOI] [PubMed] [Google Scholar]
- Magdesian M. H., Carvalho M. M., Mendes F. A., Saraiva L. M., Juliano M. A., Juliano L. J., et al. (2008). Amyloid-beta binds to the extracellular cysteine-rich domain of Frizzled and inhibits Wnt/beta-catenin signaling. J. Biol. Chem. 283 9359–9368 10.1074/jbc.M707108200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D. (2010). MicroRNAs contribute to LTP in the hippocampus in vivo (Commentary on Wibrand et al.). Eur. J. Neurosci. 31 634–635 10.1111/j.1460-9568.2010.07140.x [DOI] [PubMed] [Google Scholar]
- Martin M., Rehani K., Jope R. S., Michalek S. M. (2005). Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 6 777–784 10.1038/ni1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A., Alonso M., Castro A., Perez C., Moreno F. J. (2002). First non-ATP competitive glycogen synthase kinase 3 beta (GSK-3beta) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J. Med. Chem. 45 1292–1299 10.1021/jm011020u [DOI] [PubMed] [Google Scholar]
- Martinez A., Gil C., Perez D. I. (2011). Glycogen synthase kinase 3 inhibitors in the next horizon for Alzheimer’s disease treatment. Int. J. Alzheimers Dis. 2011 280502 10.4061/2011/280502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro-Venegas C., Tortosa E., Rosso S., Peretti D., Bollati F., Bisbal M., et al. (2010). MAP1B regulates axonal development by modulating Rho-GTPase Rac1 activity. Mol. Biol. Cell 21 3518–3528 10.1091/mbc.E09-08-0709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z., Esiri M. M., Cato A. M., Smith A. D. (1997a). Cell cycle markers in the hippocampus in Alzheimer’s disease. Acta Neuropathol. 94 6–15 10.1007/s004010050665 [DOI] [PubMed] [Google Scholar]
- Nagy Z., Esiri M. M., Smith A. D. (1997b). Expression of cell division markers in the hippocampus in Alzheimer’s disease and other neurodegenerative conditions. Acta Neuropathol. 93 294–300 10.1007/s004010050617 [DOI] [PubMed] [Google Scholar]
- Nunes P. V., Forlenza O. V., Gattaz W. F. (2007). Lithium and risk for Alzheimer’s disease in elderly patients with bipolar disorder. Br. J. Psychiatry 190 359–360 10.1192/bjp.bp.106.029868 [DOI] [PubMed] [Google Scholar]
- Palomo V., Soteras I., Perez D. I., Perez C., Gil C., Campillo N. E., et al. (2011). Exploring the binding sites of glycogen synthase kinase 3. Identification and characterization of allosteric modulation cavities. J. Med. Chem. 54 8461–8470 10.1021/jm200996g [DOI] [PubMed] [Google Scholar]
- Pap M., Cooper G. M. (1998). Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akti cell survival pathway. J. Biol. Chem. 273 19929–19932 10.1074/jbc.273.32.19929 [DOI] [PubMed] [Google Scholar]
- Pap M., Cooper G. M. (2002). Role of translation initiation factor 2B in control of cell survival by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta signaling pathway. Mol. Cell. Biol. 22 578–586 10.1128/MCB.22.2.578-586.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau S., Taghibiglou C., Bradley C., Wong T. P., Liu L., Lu J., et al. (2007). LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron 53 703–717 10.1016/j.neuron.2007.01.029 [DOI] [PubMed] [Google Scholar]
- Phiel C. J., Wilson C. A., Lee V. M., Klein P. S. (2003). GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature 423 435–439 10.1038/nature01640 [DOI] [PubMed] [Google Scholar]
- Pigino G. G., Morfini, Pelsman A., Mattson M. P., Brady S. T., Busciglio J. (2003). Alzheimer’s presenilin 1 mutations impair kinesin-based axonal transport. J. Neurosci. 23 4499–4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner F., Angelo M., Giese K. P. (2006). The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J. Biol. Chem. 281 25457–25465 10.1074/jbc.M603469200 [DOI] [PubMed] [Google Scholar]
- Plotkin B., Kaidanovich O., Talior I., Eldar-Finkelman H. (2003). Insulin mimetic action of synthetic phosphorylated peptide inhibitors of glycogen synthase kinase-3. J. Pharmacol. Exp. Ther. 305 974–980 10.1124/jpet.102.047381 [DOI] [PubMed] [Google Scholar]
- Plotkin D. A., Jarvik L. F. (1986). Cholinergic dysfunction in Alzheimer disease: cause or effect? Prog. Brain Res. 65 91–103 10.1016/S0079-6123(08)60644-5 [DOI] [PubMed] [Google Scholar]
- Pomara N. (2009). Lithium treatment in Alzheimer’s disease does not promote cognitive enhancement, but may exert long-term neuroprotective effects. Psychopharmacology (Berl.) 205 169–170 10.1007/s00213-009-1510-y [DOI] [PubMed] [Google Scholar]
- Pope W., Enam S. A., Bawa N., Miller B. E., Ghanbari H. A., Klein W. L. (1993). Phosphorylated tau epitope of Alzheimer’s disease is coupled to axon development in the avian central nervous system. Exp. Neurol. 120 106–113 10.1006/exnr.1993.1044 [DOI] [PubMed] [Google Scholar]
- Rabacchi S. A., Bailly Y., Delhaye-Bouchaud N., Herrup K., Mariani J. (1992). Role of the target in synapse elimination: studies in cerebellum of developing lurcher mutants and adult chimeric mice. J. Neurosci. 12 4712–4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M., Dawson H. N., Binder L. I., Vitek M. P., Ferreira A. (2002). Tau is essential to beta -amyloid-induced neurotoxicity. Proc. Natl. Acad. Sci. U.S.A. 99 6364–6369 10.1073/pnas.092136199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauk A. (2008). Why is the amyloid beta peptide of Alzheimer’s disease neurotoxic? Dalton Trans. 1273–1282 10.1039/b718601k [DOI] [PubMed] [Google Scholar]
- Roberson E. D., Scearce-Levie K., Palop J. J., Yan F., Cheng I. H., Wu T., et al. (2007). Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science 316 750–754 10.1126/science.1141736 [DOI] [PubMed] [Google Scholar]
- Robert M., Mathuranath P. S. (2007). Tau and tauopathies. Neurol. India 55 11–16 10.4103/0028-3886.30420 [DOI] [PubMed] [Google Scholar]
- Rockenstein E., Crews L., Masliah E. (2007a). Transgenic animal models of neurodegenerative diseases and their application to treatment development. Adv. Drug Deliv. Rev. 59 1093–1102 10.1016/j.addr.2007.08.013 [DOI] [PubMed] [Google Scholar]
- Rockenstein E., Torrance M., Adame A., Mante M., Bar-On P., Rose J. B., et al. (2007b). Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylation. J. Neurosci. 27 1981–1991 10.1523/JNEUROSCI.4321-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K. A., Pimplikar S. W. (2005). Activation of GSK-3 and phosphorylation of CRMP2 in transgenic mice expressing APP intracellular domain. J. Cell Biol. 171 327–335 10.1083/jcb.200505078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki K., Machida M., Kinoshita Y., Takasawa R., Tanuma S. (2011). Glycogen synthase kinase-3beta2 has lower phosphorylation activity to tau than glycogen synthase kinase-3beta1. Biol. Pharm. Bull. 34 146–149 10.1248/bpb.34.146 [DOI] [PubMed] [Google Scholar]
- Saito Y., Vandenheede J. R., Cohen P. (1994). The mechanism by which epidermal growth factor inhibits glycogen synthase kinase 3 in A431 cells. Biochem. J. 303(Pt 1) 27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi A., Valderas C., de los Rios C., Bastida A., Chioua M., Gonzalez-Lafuente L., et al. (2011). Cholinergic and neuroprotective drugs for the treatment of Alzheimer and neuronal vascular diseases. II. Synthesis, biological assessment, and molecular modelling of new tacrine analogues from highly substituted 2-aminopyridine-3-carbonitriles. Bioorg. Med. Chem. 19 122–133 10.1016/j.bmc.2010.11.040 [DOI] [PubMed] [Google Scholar]
- Sanchez C., Perez M., Avila J. (2000). GSK3beta-mediated phosphorylation of the microtubule-associated protein 2C (MAP2C) prevents microtubule bundling. Eur. J. Cell Biol. 79 252–260 10.1078/S0171-9335(04)70028-X [DOI] [PubMed] [Google Scholar]
- Santacruz K., Lewis J., Spires T., Paulson J., Kotilinek L., Ingelsson M., et al. (2005). Tau suppression in a neurodegenerative mouse model improves memory function. Science 309 476–481 10.1126/science.1113694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A., Kabat J., Novak M., Wu Q., Grundke-Iqbal I., Iqbal K. (1998). Phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Arch. Biochem. Biophys. 357 299–309 10.1006/abbi.1998.0813 [DOI] [PubMed] [Google Scholar]
- Sengupta A., Novak M., Grundke-Iqbal I., Iqbal K. (2006). Regulation of phosphorylation of tau by cyclin-dependent kinase 5 and glycogen synthase kinase-3 at substrate level. FEBS Lett. 580 5925–5933 10.1016/j.febslet.2006.09.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno L., Coma M., Rodriguez M., Sanchez-Ferrer P., Sanchez M. B., Gich I., et al. (2009). A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol. Dis. 35 359–367 10.1016/j.nbd.2009.05.025 [DOI] [PubMed] [Google Scholar]
- Sergeant N., Bretteville A., Hamdane M., Caillet-Boudin M. L., Grognet P., Bombois S., et al. (2008). Biochemistry of tau in Alzheimer’s disease and related neurological disorders. Expert Rev. Proteomics 5 207–224 10.1586/14789450.5.2.207 [DOI] [PubMed] [Google Scholar]
- Shipton O. A., Leitz J. R., Dworzak J., Acton C. E., Tunbridge E. M., Denk F., et al. (2011). Tau protein is required for amyloid {beta}-induced impairment of hippocampal long-term potentiation. J. Neurosci. 31 1688–1692 10.1523/JNEUROSCI.2610-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirerol-Piquer M., Gomez-Ramos P., Hernandez F., Perez M., Moran M. A., Fuster-Matanzo A., et al. (2011). GSK3beta overexpression induces neuronal death and a depletion of the neurogenic niches in the dentate gyrus. Hippocampus 21 910–922 [DOI] [PubMed] [Google Scholar]
- Soutar M. P., Kim W. Y., Williamson R., Peggie M., Hastie C. J., McLauchlan H., et al. (2010). Evidence that glycogen synthase kinase-3 isoforms have distinct substrate preference in the brain. J. Neurochem. 115 974–983 10.1111/j.1471-4159.2010.06988.x [DOI] [PubMed] [Google Scholar]
- Spittaels K., Van den Haute C., Van Dorpe J., Terwel D., Vandezande K., Lasrado R., et al. (2002). Neonatal neuronal overexpression of glycogen synthase kinase-3 beta reduces brain size in transgenic mice. Neuroscience 113 797–808 10.1016/S0306-4522(02)00236-1 [DOI] [PubMed] [Google Scholar]
- Sudduth T. L., Schmitt F. A., Nelson P. T., Wilcock D. M. (2013). Neuroinflammatory phenotype in early Alzheimer’s disease. Neurobiol. Aging 34 1051–1059 10.1016/j.neurobiolaging.2012.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudduth T. L., Wilson J. G., Everhart A., Colton C. A., Wilcock D. M. (2012). Lithium treatment of APPSwDI/NOS2-/- mice leads to reduced hyperphosphorylated tau, increased amyloid deposition and altered inflammatory phenotype. PLoS ONE 7:e31993 10.1371/journal.pone.0031993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Qureshi H. Y., Cafferty P. W., Sobue K., Agarwal-Mawal A., et al. (2002). Glycogen synthase kinase-3beta is complexed with tau protein in brain microtubules. J. Biol. Chem. 277 11933–11940 10.1074/jbc.M107182200 [DOI] [PubMed] [Google Scholar]
- Sutherland C., Cohen P. (1994). The alpha-isoform of glycogen synthase kinase-3 from rabbit skeletal muscle is inactivated by p70 S6 kinase or MAP kinase-activated protein kinase-1 in vitro. FEBS Lett. 338 37–42 10.1016/0014-5793(94)80112-6 [DOI] [PubMed] [Google Scholar]
- Sutherland C., Leighton I. A., Cohen P. (1993). Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem. J. 296(Pt 1) 15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A., Noguchi K., Michel G., Mercken M., Hoshi M., Ishiguro K., et al. (1996a). Exposure of rat hippocampal neurons to amyloid beta peptide (25–35) induces the inactivation of phosphatidyl inositol-3 kinase and the activation of tau protein kinase I/glycogen synthase kinase-3 beta. Neurosci. Lett. 203 33–36 10.1016/0304-3940(95)12257-5 [DOI] [PubMed] [Google Scholar]
- Takashima A., Sato M., Mercken M., Tanaka S., Kondo S., Honda T., et al. (1996b). Localization of Alzheimer-associated presenilin 1 in transfected COS-7 cells. Biochem. Biophys. Res. Commun. 227 423–426 10.1006/bbrc.1996.1523 [DOI] [PubMed] [Google Scholar]
- Terwel D., Muyllaert D., Dewachter I., Borghgraef P., Croes S., Devijver H., et al. (2008). Amyloid activates GSK-3beta to aggravate neuronal tauopathy in bigenic mice. Am. J. Pathol. 172 786–798 10.2353/ajpath.2008.070904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thotala D. K., Hallahan D. E., Yazlovitskaya E. M. (2008). Inhibition of glycogen synthase kinase 3 beta attenuates neurocognitive dysfunction resulting from cranial irradiation. Cancer Res. 68 5859–5868 10.1158/0008-5472.CAN-07-6327 [DOI] [PubMed] [Google Scholar]
- Toledo E. M., Inestrosa N. C. (2010). Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer’s disease. Mol. Psychiatry 15 272–285 228 10.1038/mp.2009.72 [DOI] [PubMed] [Google Scholar]
- Townsend M., Mehta T., Selkoe D. J. (2007). Soluble Abeta inhibits specific signal transduction cascades common to the insulin receptor pathway. J. Biol. Chem. 282 33305–33312 10.1074/jbc.M610390200 [DOI] [PubMed] [Google Scholar]
- Uemura K., Kuzuya A., Shimozono Y., Aoyagi N., Ando K., Shimohama S., et al. (2007). GSK3beta activity modifies the localization and function of presenilin 1. J. Biol. Chem. 282 15823–15832 10.1074/jbc.M610708200 [DOI] [PubMed] [Google Scholar]
- Van Hoesen G. W., Hyman B. T., Damasio A. R. (1991). Entorhinal cortex pathology in Alzheimer’s disease. Hippocampus 1 1–8 10.1002/hipo.450010102 [DOI] [PubMed] [Google Scholar]
- Wang H., Wang R., Zhao Z., Ji Z., Xu S., Holscher C., et al. (2009). Coexistences of insulin signaling-related proteins and choline acetyltransferase in neurons. Brain Res. 1249 237–243 10.1016/j.brainres.2008.10.046 [DOI] [PubMed] [Google Scholar]
- Wang Y., Ju W., Liu L., Fam S., D’Souza S., Taghibiglou C., et al. (2004). alpha-Amino-3-hydroxy-5-methylisoxazole-4-propionic acid subtype glutamate receptor (AMPAR) endocytosis is essential for N-methyl-D-aspartate-induced neuronal apoptosis. J. Biol. Chem. 279 41267–41270 10.1074/jbc.C400199200 [DOI] [PubMed] [Google Scholar]
- Watcharasit P., Bijur G. N., Song L., Zhu J., Chen X., Jope R. S. (2003). Glycogen synthase kinase-3beta (GSK3beta) binds to and promotes the actions of p53. J. Biol. Chem. 278 48872–48879 10.1074/jbc.M305870200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Liu W., Yan Z. (2010). Regulation of AMPA receptor trafficking and function by glycogen synthase kinase 3. J. Biol. Chem. 285 26369–26376 10.1074/jbc.M110.121376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh G. I., Proud C. G. (1993). Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem. J. 294(Pt 3) 625–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse P. J., Price D. L., Clark A. W., Coyle J. T., DeLong M. R. (1981). Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 10 122–126 10.1002/ana.410100203 [DOI] [PubMed] [Google Scholar]
- Witton J., Brown J. T., Jones M. W., Randall A. D. (2010). Altered synaptic plasticity in the mossy fibre pathway of transgenic mice expressing mutant amyloid precursor protein. Mol. Brain 3 32 10.1186/1756-6606-3-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., He X. (2006). Threonine 41 in beta-catenin serves as a key phosphorylation relay residue in beta-catenin degradation. Biochemistry 45 5319–5323 10.1021/bi0601149 [DOI] [PubMed] [Google Scholar]
- Yates C. M., Simpson J., Gordon A., Maloney A. F., Allison Y., Ritchie I. M., et al. (1983). Catecholamines and cholinergic enzymes in pre-senile and senile Alzheimer-type dementia and Down’s syndrome. Brain Res. 280 119–126 10.1016/0006-8993(83)91179-4 [DOI] [PubMed] [Google Scholar]
- Zhang H., Ma Q., Zhang Y. W., Xu H. (2012). Proteolytic processing of Alzheimer’s beta-amyloid precursor protein. J. Neurochem. 120(Suppl.1) 9–21 10.1111/j.1471-4159.2011.07519.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhao Y., Tong C., Wang G., Wang B., Jia J., et al. (2005). Hedgehog-regulated Costal2-kinase complexes control phosphorylation and proteolytic processing of Cubitus interruptus. Dev. Cell 8 267–278 10.1016/j.devcel.2005.01.001 [DOI] [PubMed] [Google Scholar]
- Zhao C., Teng E. M., Summers R. G., Ming G. L., Jr., Gage F. H. (2006). Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 26 3–11 10.1523/JNEUROSCI.3648-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbrunn J., Kinoshita K., Hyman A. A., Nathke I. S. (2001). Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr. Biol. 11 44–49 10.1016/S0960-9822(01)00002-1 [DOI] [PubMed] [Google Scholar]