Abstract
In this study we assessed the influence of the three different recovery interventions massage (MSG), electrical muscle stimulation (EMS), and passive rest (PR) on lactate disappearance and muscle recovery after exhausting exercise bouts. Twelve healthy male sport students participated in the study. They attended the laboratory on five test days. After measurement of O2max and a baseline Wingate test (WGb), the three recovery interventions were tested in random counterbalanced order. High intensity exercise, which consisted of six exhausting exercise bouts (interspersed with active recovery), was followed by MSG, EMS or PR application (24 minutes); then the final Wingate test (WGf) was performed. Lactate, heart rate, peak and mean power, rating of perceived exertion (RPE), and total quality of recovery (TQR) were recorded. In WGf mean power was significantly higher than in WGb for all three recovery modalities (MSG 6.29%, EMS 5.33%, PR 4.84% increase, p < 0.05), but no significant differences in mean and peak power were observed between the three recovery modes (p > 0.05). The heart rate response and the changes in blood lactate concentration were identical in all three interventions during the entire protocol (p = 0.817, p = 0.493, respectively). RPE and TQR scores were also not different among the three interventions (p > 0.05). These results provide further evidence that MSG and EMS are not more effective than PR in the process of recovery from high intensity exercise.
Keywords: electromyostimulation, massage, recovery, muscle performance
INTRODUCTION
Many sport events are characterized by intermittent activity patterns and short inter-bout rest periods. Fast muscle recovery after fatiguing exercise is a crucial factor for better performance in these sports [28]. In this context muscle fatigue can be defined as any exercise-induced reduction in voluntary muscle force or power [11]. Muscle fatigue may arise from peripheral changes at the level of the muscle, such as exercise-induced alterations in muscle homeostasis (e.g. depletion of creatine phosphate or accumulation of inorganic phosphate), a relative mismatch of oxygen supply by capillaries to cellular energy demand or alterations in excitability due to K+ efflux from the cell [1]. Another possibility is the development of ‘central’ fatigue, which denotes that the central ner vous system fails to drive the motoneurons adequately [11].
It is evident that in the sport setting there is need for suitable methods to enhance the rate of recovery after fatiguing exercise. Thus, many research groups have tried to define the most appropriate recovery interventions [5, 7, 24, 28, 29, 32, 40]. One efficient mechanism of recovery enhancement at muscle level may be the augmented removal of exercise metabolites by improving microcirculation. Massage (MSG) has been assumed to be an efficient technique in this regard. But scientific studies could not substantiate the effects of massage on physiological variables affecting muscle recovery from exercise. Also, the efficacy of manual massage after exercise as a means to improve performance or to shorten the time needed for muscle recovery could not be validated [5, 16, 28, 35, 37, 38]. Light exercise after strenuous exercise may be even more effective than manual massage in improving blood flow [13, 37].
In this context, electrical muscle stimulation (EMS) has been introduced as an additional method to facilitate recovery by enhancing removal of metabolites [5, 9]. EMS employs transcutaneous electrical currents to peripherally stimulate motor neurons and induce muscle contraction and relaxation cycles which may increase blood flow via the ‘muscle pump effect’. But concrete evidence about the positive effects of EMS intervention on fatigued muscle after exhausting exercise is still lacking [20, 24, 25, 30, 31].
With these considerations in mind, the aim of this study was to examine the effects of three recovery modalities (EMS, Massage and Passive Rest) after exhausting exercise on blood lactate, heart rate, rate of perceived exertion (RTE), total quality of recovery (TQR), and subsequent power output in the Wingate test. We hypothesized that EMS and massage would allow a better recovery than passive resting modality and hence would improve the subsequent high intensity exercise performance.
MATERIALS AND METHODS
Subjects
This study follows the Helsinki Declaration [14] to fulfil the ethical standard requirements. Twelve healthy male game players (mean (SD) age: 20.92 (2.47) years, body mass: 68.42 (7.25) kg, height: 174.25 (6.11) cm, O2 max: 50.67 (4.37) ml · kg−1 · min−1) volunteered to participate in the study. All participants regularly took part in sports involving bouts of high intensity effort such as soccer and basketball. After being informed about the nature and protocol of the experiment, the volunteers provided their informed consent. Obligatory requirements for participation in the study were not to have received electromyostimulation in the last 6 months, and not to present medical contraindications related to application of electrical muscle stimulation (back problems, heart rhythm disorders, and recent surgical operations).
Experimental procedures
We adapted and modified the experimental procedures from Robertson et al. [32]. Subjects entered the laboratory on five separate occasions at least 48 hours apart and at the same time of day. Familiarization was completed on the first and second visit to ensure that they all knew the protocol. During these two visits baseline performance parameters were obtained: a baseline Wingate test (WGb) was performed to acquire peak power (Pp) and mean power (Pm). The peak power was measured in the first 5-second interval of the Wingate test. A ramp test on a cycle ergometer (Ergoline® S100) yielded maximal oxygen uptake (25 W · min−1 increments). The following three visits constituted the experimental phase. The study had a counterbalanced crossover design. Each of the subjects was subjected to the following recovery protocols in a randomized counterbalanced order: (a) massage, (b) electrical muscle stimulation, and (c) passive rest.
Dietary intake, and exercise intensity and duration were recorded for two days before the familiarization visit. Subjects replicated this dietary intake habit for two days before each subsequent laboratory visit. Subjects were instructed not to exercise heavily in the 24 hours before the visits. They were also asked not to consume food 2 hours before testing. They were only tested when they had complied with their individual dietary intake and exercise pattern.
On arrival for each test session subjects were seated, and after a 10-minute rest period, a baseline blood sample for lactate was drawn. They performed a standardized light warm up of five minutes and a short stretching period (three minutes of static stretches of hamstrings, calf, and quadriceps muscle groups). Then they performed six standardized 30-second high intensity bouts of exercise on the cycle ergometer (load was 85% of load in WGb, 60 rpm), each interspersed with 30 seconds of active recovery (unloaded cycling with 60 rpm). Power output was monitored during these high intensity bouts of exercise through a PC interface. On completion of the six high intensity bouts, subjects were delegated to the three recovery interventions, all lasting 24 minutes.
After the recovery intervention period, the subject completed the same standardized five-minute warm up and three minutes of static stretching as previously described. Then the subjects tried to reach maximal power output in the subsequent final Wingate test (WGf). Heart rate was recorded continuously by a heart rate monitor (Polar® RS800). Perceived exertion (RPE) was assessed by the Borg scale (scale ranging from “no exertion at all” (6 points) to “maximal effort” (20 points)) and the quality of recovery was evaluated by the Total Quality of Recovery (TQR) scale (scale ranging from “very very poor recovery” (6 points) to “very very good recovery” (20 points)) [6, 23].
Capillary blood samples were drawn for lactate analysis at rest, 5 minutes after the six high intensity bouts, at completion of the recovery intervention, and 5 minutes after the Wingate test. Blood lactate was measured using the Scout lactate analyser (SensLab GmbH, Leipzig, Germany). Wingate test variables were recorded through a PC interface and included peak power (Pp) and mean power (Pm).
Massage intervention
The massage was applied for a total of 24 minutes by two certified specialists. The massage techniques were standardized (Table 1) and applied synchronously to both legs. During the first application subjects were lying in a prone position on a standard treatment couch for 12 minutes. Then they assumed a supine position for 12 minutes, and the massage routine was repeated. Most strokes were grade 1 or 2, but three grade 3 effleurage strokes, using a clenched fist, were applied in a centripetal direction to the left and right iliotibial band midway through the supine massage. All massage was administered using a conventional bland mineral oil (40 mL)—that is, 10 mL per massage area.
TABLE 1.
MASSAGE PROTOCOL
| Massage technique | Description | Grade |
|---|---|---|
| Stroking | Whole hand two handed in a centripetal direction | Four strokes grade 1, two strokes grade 2 range: 1/2 |
| Effleurage | Whole hand two handed, centripetal and multidirectional | Grade 1 up to grade 2 |
| Kneading | Whole hand two handed, centripetal and centrifugal | Grade 1 up to grade 2 |
| Picking up | Whole hand two handed v-shaped, centripetal | Grade 1 up to grade 2 |
| Wringing | Whole hand two handed, centripetal, centrifugal, multidirectional | Grade 1 |
| Rolling | Muscle rolling, centripetal | Grade 2 |
| Effleurage | Whole hand two handed, centripetal | Grade 2 |
Electrical muscle stimulation
EMS recovery consisted in simultaneous electrical stimulation of both lower limbs (quadriceps and hamstring muscles) in a supine position. Electrical stimulation was elicited using bipolar electrodes and two portable battery-powered 4-channel electrical stimulators (CompexMI-Sport, Medicompex SA, Ecublens, Switzerland). For the quadriceps femoris, a cathode (10x5 cm rectangular electrode) was positioned on the upper part of the thigh, and two anodes (5x5 cm) were placed over the motor point of the vastus lateralis and medialis. For the hamstring muscles, the cathode was positioned proximally below the gluteal fold, and the anode (10x5 cm) was placed over the belly of the hamstring muscles. Stimulation frequency starting at a frequency of 9 Hz decreased during the intervention period until reaching 1 Hz (rise time = 1.5 second; pulse width= 400 µseconds; fall time= 0.5 seconds). The subjects selected the most comfortable intensity (i.e., level 10-20 mA). Recovery interventions lasted 24 minutes, according to the duration of the EMS recovery programme [36].
Passive rest
Subjects quietly lay in a supine position during the passive rest period.
Statistical analysis
A one-way analysis of variance with repeated measures was used to calculate the statistical significance of the difference between initial measurement and the end of recovery (for mean and peak power), and a two-way analysis of variance (recovery intervention x time) with repeated measures was used to detect significant differences across the three different recovery interventions (for blood lactate concentration, heart rate response, rating of perceived exertion, and total quality of recovery) with checks for sphericity completed (within subjects design). Post-hoc comparisons were performed with a Bonferroni adjustment of the alpha level (0.05). Descriptive statistics (means, standard deviation, standard error, and percentile change) were used for dependent variables. Pearson correlations between variables were calculated. The level of significance was determined at p < 0.05 and analyses were performed using SPSS 14 for Windows.
RESULTS
All subjects coped well with the test procedures and all showed compliance with pre-test diet and exercise controls.
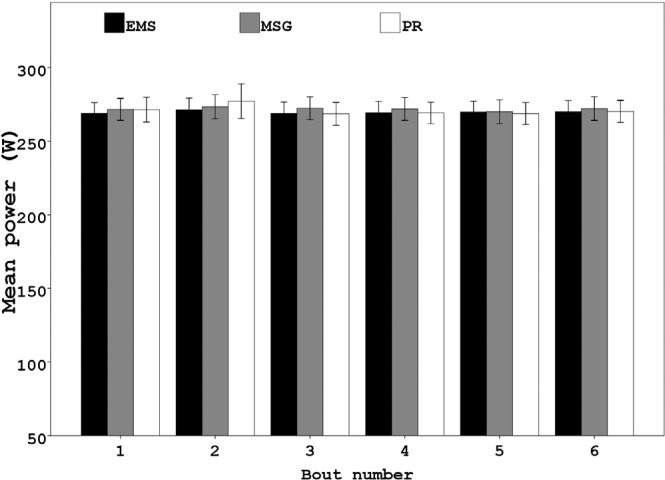
There was no significant difference between interventions in the power output sustained during the standardized high intensity exercise bouts (F2, 22= 0.698, p= 0.51, η 2= 0.06) (Figure 1).
FIG. 1.
MEAN (SEM) POWER OUTPUT IN EACH OF THE SIX EXHAUSTING EXERCISE BOUTS
Note: No significant difference was observed between trials at any time (p > 0.05).
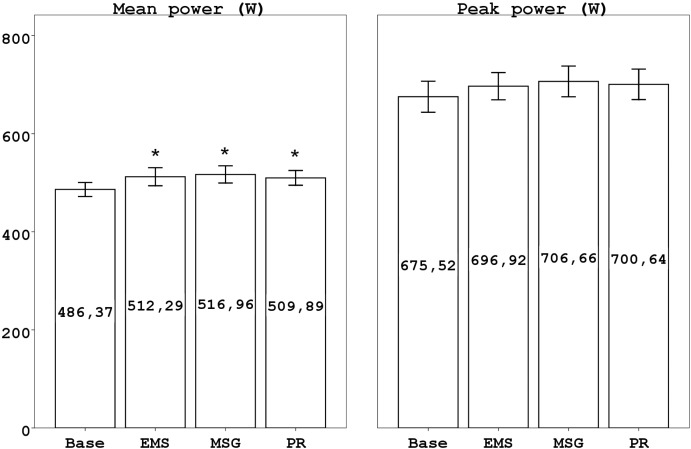
The one-way analysis of variance with repeated measures revealed that Pm values in WGf were higher than those in WGb for all three intervention modalities (F3= 7.83, p< 0.001, η 2=0.42). Between the three different recovery interventions there was no significant difference in mean power output of WGf (p > 0.05) (Figure 2).
FIG. 2.
MEAN (SEM) PM AND PP VALUES AT BASELINE AND AFTER EACH OF THE RECOVERY INTERVENTIONS
Note: Mean power values in EMS, Massage, and Passive recovery interventions were significantly different from baseline (*p<.05). Peak power values were not significantly different from baseline (p > 0.05).
For Pp values of WGf, there were neither significant differences between the three intervention modalities nor was there a difference to WGb Pp values (F1.79, 19.64= 0.848, p= 0.432, η 2=0.07) (Figure 2).
O2 max as a covariate had no effect on these parameters (F3,30=1.650, p = 0.199, η2=0.14 for mean power, F3,30=0.453, p = 0.717, η2=0.04 for peak power).
According to baseline values mean changes after the three interventions were 5.33% for Pm, 3.17% for Pp in EMS intervention; 6.29% for Pm, 4.61% for Pp in MSG intervention; and 4.84% for Pm, 3.71% for Pp in PR.
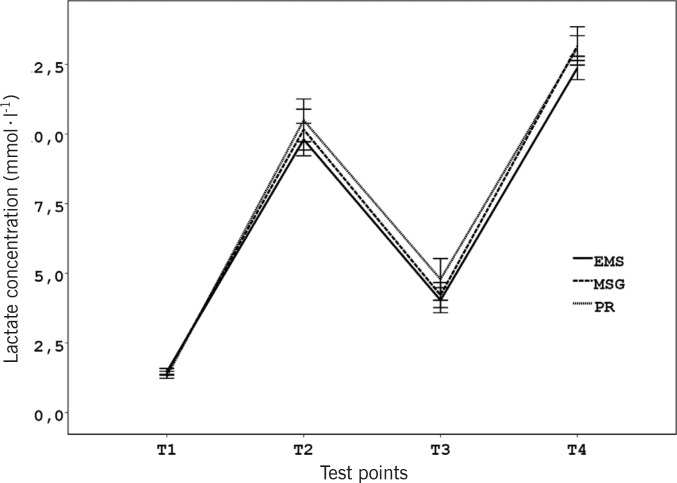
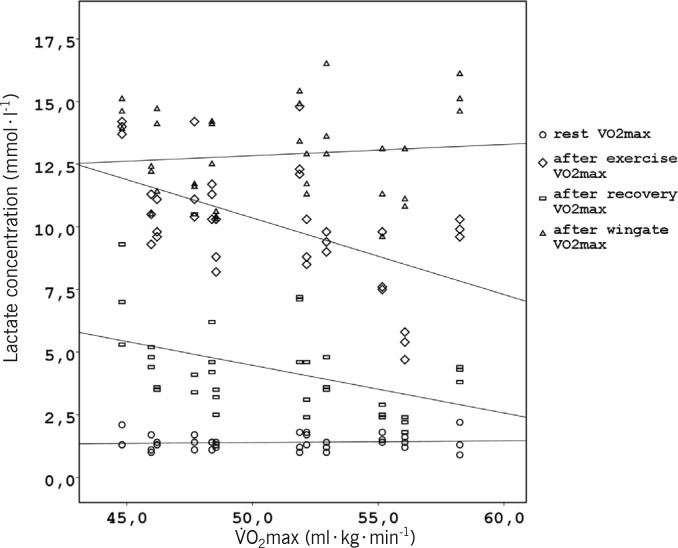
Regarding blood lactate concentrations, there was no significant main effect of the type of recovery (F2, 22= 1.81, p = 0.186, η2=0.14), and no significant interaction between the type of recovery and test points (F6, 66= 0.91, p= 0.493, η2=0.08) (Figure 4). However, there was a significant main effect difference among the four test points (F1.87, 20.57= 226.51, p< 0.001, η2=0.95), and only in this case was a significant interaction between O2max and test points observed (F = 5.60, p = 0.004, η2=0.36). O2max was responsible for 36% of variation of blood lactate concentrations regardless of the recovery interventions. Blood lactate concentrations were negatively correlated with O2max after the high intensity exercise bouts (r= -0.55, p= 0.001) and after the recovery interventions (r= -0.42, p= 0.011), but not during rest or after WGf (p > 0.05). This also indicated the appropriate standards of the test protocol (Figure 3).
FIG. 4.
MEAN (SEM) BLOOD LACTATE CONCENTRATION AT REST (T1), AFTER THE EXHAUSTING EXERCISE BOUTS (T2), 24 MINUTES OF INTERVENTION (T3), AND FIVE MINUTES AFTER THE WINGATE TEST (T4)
Note: No significant difference was observed between the three recovery interventions at any time (p > 0.05).
FIG. 3.
CORRELATION BETWEEN VO2MAX, BLOOD LACTATE AND TEST POINTS AFTER THE EXHAUSTING EXERCISE BOUTS (r = -0.55, p = 0.001) AND AFTER THE RECOVERY INTERVENTION (r = -0.42, p = 0.011).
Regarding the heart rate response, there was no significant main effect of the type of recovery intervention (F2, 22= 0.466, p= 0.634, η2=0.041), and no significant interaction between the type of recovery and test points (F16, 176= 0.674, p= 0.817, η2=0.058) (Table 2).
TABLE 2.
MEAN AND MAXIMUM (SEM) HEART RATE RESPONSES (BEATS/MIN) AT INITIAL WINGATE TEST, AT REST, AT STANDARDIZED EXHAUSTING EXERCISE BOUTS, AT INTERVENTION FOR 24 MINUTES, AND AT THE FINAL WINGATE TEST IN THE EMS, MSG AND PR TRIALS
| WGb (mean-30s) | WGb (max-30s) | Resting (mean- 10m in) | Exh. Exer bouts (mean-6min) | Exh. exer bouts (max-6min) | Intervention (mean-24min) | WGf (mean-30s). | WGf (max-30s) | After WGf (mean-5min) | |
|---|---|---|---|---|---|---|---|---|---|
| EMS | 65.67 (2.01) | 162.50 (2.70) | 185.75 (2.65) | 89.08 (2.44) | 173.42 (2.52) | 188.75 (2.40) | 125.00 (2.55) | ||
| MSG | 172.92 (3.09) | 187.17 (2.32) | 64.67(2.37) | 161.00 (2.71) | 183.33 (3.52) | 89.50 (2.02) | 174.00 (2.77) | 188.67 (3.17) | 124.50 (2.60) |
| PR | 65.42 (2.62) | 163.08 (3.48) | 185.33 (2.83) | 92.75 (3.24) | 173.08 (2.58) | 188.42 (2.55) | 126.00 (3.84) |
There was no significant main effect of the type of recovery intervention on TQR (F2, 22=2.126, p= 0.143, η2=0.16) and RPE (F2, 22= 0.076, p= 0.927, η2=0.007). There was no significant interaction between the type of recovery and TQR (F2, 22= 3.028, p= 0.069, η2=0.22), and this was also true for RPE (F2, 22= 0.090, p= 0.915, η2=0.008) (Table 3). A significant negative correlation between blood lactate concentration and TQR scores was observed only after the EMS intervention (r= -0.74, p < 0.01).
TABLE 3.
MEAN (SEM) RPE AND TQR AT REST, AFTER THE STANDARDIZED EXHAUSTING EXERCISE BOUTS, AFTER 24 MINUTES OF INTERVENTION, AND AFTER WINGATE TEST IN THE EMS, MSG AND PR TRIALS
| TQR at rest | RPE after exhausting exercise bouts | TQR after recovery | RPE after Wingate test | |
|---|---|---|---|---|
| EMS | 190.33 (0.23) | 150.83 (0.87) | 180.08 (0.51) | 150.83 (1.06) |
| very very good recovery | 80% effort-hard | very very good recovery | 80% effort-hard | |
| MSG | 190.42 (0.23) | 150.58 (0.80) | 190.25 (0.35) | 160.00 (0.73) |
| very very good recovery | 80% effort-hard | very very good recovery | 85% effort-hard | |
| PR | 190.50 (0.15) | 150.67 (0.96) | 170.83 (0.53) | 150.58 (0.82) |
| very very good recovery | 80% effort-hard | very good recovery | 80% effort-hard |
DISCUSSION
This investigation was carried out to evaluate the effects of three different recovery interventions after exhausting exercise on performance and physiological parameters in the subsequent anaerobic Wingate test. No significant differences were demonstrated between the effects of EMS, MSG, and PR on physiological and psychological recovery after the high intensity exercise. Wingate test scores (mean and peak power) were not significantly different between the three different passive recovery interventions after the high intensity exercise. However, in the Wingate test a significant higher mean power output was observed after all three recovery interventions compared to baseline scores. Such increase of power output (roughly 5%) during exhaustive exercise has been reported when preceded by prior heavy (“priming”) exercise [21]. To our knowledge this could be the first study reporting equivocal effects of different recovery interventions after such priming exercise.
In all trials we carefully tried to guarantee an identical exercise and recovery profile; mean power output was nearly identical during the high-intensity cycle exercise for all three conditions. This was also reflected in the blood lactate and heart rate responses; blood lactate concentrations and heart rate were not significantly different between the three recovery protocols at any time point. Although the subject's O2max capacity showed a significant correlation with blood lactate concentrations after the exhausting exercise and all three recovery interventions, the overall effect on individual lactate variations was modest (p = 0.004, η2=0.36).
Studies on recovery interventions were criticized because of lack of standardization of exercise and recovery protocols. Therefore, we kept the duration of the massage intervention identical to that of the EMS intervention, both of which were sufficiently long to be effective [32]. We employed a standardized massage intervention for all subjects with identical type, intensity, and duration of strokes. The EMS protocol of low frequency (1 to 9 Hz) was chosen to resemble massage by increasing blood flow and endorphin release, as well as to reduce spasms and increase relaxation [20, 42]. Further standardization procedures targeted dietary intake and exercise patterns in the days preceding the visits to the laboratory. We tried to ensure similar pre-exercise muscle glycogen content and acid-base status, because both of these factors significantly affect the ability to perform high intensity work as well as the metabolite responses to exercise and recovery interventions [12, 13, 22, 29].
Only a few studies in the literature have focused on the efficiency of different recovery modalities and EMS [8, 17, 24, 26, 36]. To our knowledge, our study is the first comparison of recovery interventions using EMS and MSG. This is surprising, because similar physiological benefits have been proposed for both modalities. It was hypothesized that EMS and MSG may accelerate metabolite clearance and then improve recovery of neuromuscular function following high-intensity exercise [4, 29, 39, 42]. However, the lack of an observed effect on lactate clearance by MSG or EMS compared with PR in our study implies that there were no changes in muscle blood flow and/ or lactate efflux or removal in either recovery modality. Although blood flow was not assessed in this study, our findings are supported by studies which also failed to show any advantageous effect of MSG on lactate clearance and blood flow [10, 13, 27, 33, 37]. Massage could even become counterproductive by increasing skin blood flow without an increase in arterial blood flow, and so potentially diverting blood flow away from recovering muscle [18]. Similarly, studies investigating the effects of EMS on blood flow showed that low frequency stimulation increased skin blood flow, especially when applied above the motor threshold inducing muscle contractions [9]. But it was also possible to increase blood flow in the femoral artery by low frequency EMS at intensities sufficient to produce contractions equivalent to 15% of Maximal Voluntary Contraction (MVC) [39, 42]. In other studies these changes in hemodynamic functions could not be produced by application of EMS [2, 19, 41]. These discrepancies can be attributed to the wide variety of EMS parameters employed and different assessment methods of blood flow. However, it seems obvious that when EMS produces sufficient muscle contraction, the increased metabolic demand should also enhance blood flow [3, 39].
Another possibility of recovery improvement by EMS is the finding that electromyostimulation leads to an increase in the activation rate of motor units; this increase in neural drive seems to originate from spinal as well as supraspinal centres [34]. But on the whole, evidence that EMS improves post recovery performance is scarce, and a significant effect has not been observed in the literature [24]. EMS had no positive impact on post-recovery anaerobic exercise performance and maximal voluntary contraction force [26, 36]. EMS also had no significant effect on physiological parameters of submaximal aerobic performance [8]. In a sport specific rock climbing test, EMS was even detrimental to performance when compared with active recovery [17]. The results of our study confirm these findings, because EMS did not produce any advantageous recovery profile or perception, and had no differential effect on maximal performance in the Wingate test.
In our study we also evaluated psychological load by RPE and psychological recovery by TQR. Both EMS and MSG have been reported to provide psychological regeneration in addition to physiological restoration. Especially for MSG, beneficial effects on release of endorphins, decreased arousal levels and enhanced perception of recovery have been observed [8, 15]. Although non-significant, the higher TQR score we observed after the massage intervention in our study support this line of thought. But the validity of the TQR scale for monitoring perceived recovery after high intensity exercise needs further investigation.
CONCLUSIONS
In conclusion, the results of this study suggest that neither massage nor electrical muscle stimulation as a method of post-exercise recovery intervention represents a performance enhancement modality superior to passive rest only.
Acknowledgements
The funding sources of this study (SAG-A-070808-0209) were provided by the Scientific Research Projects Department of Marmara University.
References
- 1.Allen D.G, Lamb G.D, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol. Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 2.Balogun J.A, Tang S, He Y, Hsieh J.M, Katz J. Effects of high volt galvanic stimulation of ST36 and ST37 acupuncture points on peripheral blood flow and skin temperature. Disabil. Rehabil. 1996;18:523–528. doi: 10.3109/09638289609166039. [DOI] [PubMed] [Google Scholar]
- 3.Balogun J.A, Biasci S, Han L. The effects of acupuncture, electroneedling and transcutaneous electrical stimulation therapies on peripheral haemodynamic functioning. Disabil. Rehabil. 1998;20:41–48. doi: 10.3109/09638289809166052. [DOI] [PubMed] [Google Scholar]
- 4.Bale P, James H. Massage, warm-down and rest as recuperative measures after short-term intense exercise. Physiother. Sport. 1991;13:4–7. [Google Scholar]
- 5.Barnett A. Using recovery modalities between training sessions in elite athletes: does it help? Sports Med. 2006;36:781–796. doi: 10.2165/00007256-200636090-00005. [DOI] [PubMed] [Google Scholar]
- 6.Borg G, Ljunggren G, Ceci R. The increase of perceived exertion, aches and pain in the arms, heart rate and blood lactate during exercise on a bicycle ergometer. Eur. J. Appl. Physiol. 1985;54:343–349. doi: 10.1007/BF02337176. [DOI] [PubMed] [Google Scholar]
- 7.Coffey V, Leveritt M, Gill N. Effect of recovery modality on 4-hour repeated treadmill running performance and changes in physiological variables. J. Sci. Med. Sport. 2004;7:1–10. doi: 10.1016/s1440-2440(04)80038-0. [DOI] [PubMed] [Google Scholar]
- 8.Cortis C, Tessitore A, D'Artibale E, Meeusen R, Capranica L. Effects of post-exercise recovery interventions on physiological, psychological, and performance parameters. Int. J. Sports Med. 2010;31:327–335. doi: 10.1055/s-0030-1248242. [DOI] [PubMed] [Google Scholar]
- 9.Cramp F.L, McCullough G.R, Lowe A.S, Walsh D.M. Transcutaneous electric nerve stimulation: the effect of intensity on local and distal cutaneous blood flow and skin temperature in healthy subjects. Arch. Phys. Med. Rehabil. 2002;83:5–9. doi: 10.1053/apmr.2002.27478. [DOI] [PubMed] [Google Scholar]
- 10.Dolgener F.A, Morien A. The effect of massage on lactate disappearance. J. Strength Condit. Res. 1993;7:159–162. [Google Scholar]
- 11.Gandevia S.C. Spinal and Supraspinal Factors in Human Muscle Fatigue. Physiol. Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 12.Greenhaff P.L, Gleeson M, Maughan R.J. The effects of dietary manipulation on blood acid-base status and the performance of high-intensity exercise. Eur. J. Appl. Physiol. 1987;56:331–337. doi: 10.1007/BF00690901. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Goswami A, Sadhukhan A.K. Comparative study of lactate removal in short term massage of extremities, active recovery and a passive recovery period after supramaximal exercise sessions. Int. J. Sports Med. 1996;17:106–110. doi: 10.1055/s-2007-972816. [DOI] [PubMed] [Google Scholar]
- 14.Harriss D.J, Atkinson G. Ethical standards in sport and exercise science research. Int. J. Sports Med. 2009;30:701–702. doi: 10.1055/s-0029-1237378. [DOI] [PubMed] [Google Scholar]
- 15.Hemmings B, Smith M, Graydon J, Dyson R. Effects of massage on physiological restoration, perceived recovery, and repeated sports performance. Br. J. Sports Med. 2000;34:109–114. doi: 10.1136/bjsm.34.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemmings B.J. Physiological, psychological and performance effects of massage therapy in sport: a review of the literature. Phys. Ther. Sport. 2001;2:165–170. [Google Scholar]
- 17.Heyman E, De Geus B, Mertens I, Meeusen R. Effects of four recovery methods on repeated maximal rock climbing performance. Med. Sci. Sports Exerc. 2009;41:1303–1310. doi: 10.1249/MSS.0b013e318195107d. [DOI] [PubMed] [Google Scholar]
- 18.Hinds T, McEwan I, Perkes J, Dawson E, Ball D, George K. Effects of massage on limband skin blood flow after quadriceps exercise. Med. Sci. Sports Exerc. 2004;36:1308–1313. doi: 10.1249/01.mss.0000135789.47716.db. [DOI] [PubMed] [Google Scholar]
- 19.Indergand H.J, Morgan M.J. Effects of high-frequency transcutaneous electrical nerve stimulation on limb blood flow in healthy humans. Phys. Ther. 1994;74:361–367. doi: 10.1093/ptj/74.4.361. [DOI] [PubMed] [Google Scholar]
- 20.Johnston B.D. ElectroMyoStimulation. Synergy [Internet] 2004. [cited 2010 Feb 20]. Available from: http://www.trimform.no/electromyo.pdf.
- 21.Jones A.M, Koppo K, Burnley M. Effects of prior exercise on metabolic and gas exchange responses to exercise. Sports Med. 2003;33:949–971. doi: 10.2165/00007256-200333130-00002. [DOI] [PubMed] [Google Scholar]
- 22.Jones N.L, Sutton J.R, Taylor R. Effects of pH on cardiorespiratory and metabolic responses to exercise. J. Appl. Physiol. 1977;43:959–964. doi: 10.1152/jappl.1977.43.6.959. [DOI] [PubMed] [Google Scholar]
- 23.Kentta G, Hassmen P. Overtraining and Recovery: A Conceptual Model”. Sports Med. 1998;26:1–16. doi: 10.2165/00007256-199826010-00001. [DOI] [PubMed] [Google Scholar]
- 24.Lattier G, Millet G.Y, Martin A, Martin V. Fatigue and recovery after highintensity exercise. Part II: Recovery interventions. Int. J. Sports Med. 2004;25:509–515. doi: 10.1055/s-2004-820946. [DOI] [PubMed] [Google Scholar]
- 25.Maffiuletti N.A, Cometti G, Amiridis I.G, Martin A, Pousson M, Chatard J.C. The effects of electromyostimulation training and basketball practice on muscle strength and jumping ability. Int. J. Sports Med. 2000;21:437–443. doi: 10.1055/s-2000-3837. [DOI] [PubMed] [Google Scholar]
- 26.Martin V, Millet G.Y, Lattier G, Perrod L. Effect of recovery modes after knee extensor muscles eccentric contractions. Med. Sci. Sports Exerc. 2004;36:1907–1915. doi: 10.1249/01.mss.0000145526.43208.08. [DOI] [PubMed] [Google Scholar]
- 27.Martin N.A, Zoeller R.F, Robertson R.J. The comparative effects of sports massage, active recovery, and rest in promoting blood lactate clearance after supramaximal leg exercise. J. Athl. Train. 1998;33:30–35. [PMC free article] [PubMed] [Google Scholar]
- 28.Mika A, Mika P, Fernhall B. Comparison of recovery strategies on muscle performance after fatiguing exercise. Am. J. Phys. Med. Rehabil. 2007;86:474–481. doi: 10.1097/PHM.0b013e31805b7c79. [DOI] [PubMed] [Google Scholar]
- 29.Monedero J, Donne B. Effect of recovery interventions on lactate removal and subsequent performance. Int. J. Sports Med. 2000;21:593–597. doi: 10.1055/s-2000-8488. [DOI] [PubMed] [Google Scholar]
- 30.Pichon F, Chatard J.C, Martin A, Cometti G. Electrical stimulation and swimming performance. Med. Sci. Sports Exerc. 1995;27:1671–1676. [PubMed] [Google Scholar]
- 31.Pournezam M, Andrews B.J, Baxendale R.H, Phillips G.F, Paul J.P. Reduction of muscle fatigue in man by cyclical stimulation. J. Biomed. Eng. 1988;10:196–200. doi: 10.1016/0141-5425(88)90100-8. [DOI] [PubMed] [Google Scholar]
- 32.Robertson A, Watt J.M, Galloway S.D.R. Effects of leg massage on recovery from high intensity cycling exercise. Br. J. Sports Med. 2004;38:173–176. doi: 10.1136/bjsm.2002.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shoemaker J.K, Tiidus P.M, Mader R. Failure of manual massage to alter limb blood flow: measures by Doppler ultrasound. Med. Sci. Sports Exerc. 1997;29:610–614. doi: 10.1097/00005768-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Smith G.V, Alon G, Roys S.R, Gullapalli R.P. Functional MRI determination of a dose-response relationship to lower extremity neuromuscular electrical stimulation. Exp. Brain Res. 2003;150:33–39. doi: 10.1007/s00221-003-1405-9. [DOI] [PubMed] [Google Scholar]
- 35.Steele D.S, Duke A.M. Metabolic factors contributing to altered Ca2_regulation in skeletal muscle fatigue. Acta Physiol. Scand. 2003;179:39–48. doi: 10.1046/j.1365-201X.2003.01169.x. [DOI] [PubMed] [Google Scholar]
- 36.Tessitore A, Meeusen R, Cortis C, Capranica L. Effects of different recovery interventions on anaerobic performances following preseason soccer training. J. Strength Cond. Res. 2007;21:745–750. doi: 10.1519/R-20386.1. [DOI] [PubMed] [Google Scholar]
- 37.Tiidus P.M, Shoemaker J.K. Effleurage massage, muscle blood flow and long-term post-exercise strength recovery. Int. J. Sports Med. 1995;16:478–483. doi: 10.1055/s-2007-973041. [DOI] [PubMed] [Google Scholar]
- 38.Tiidus P.M. Manual massage and recovery of muscle function following exercise: a literature review. J. Orthop. Sports Phys. Ther. 1997;25:107–112. doi: 10.2519/jospt.1997.25.2.107. [DOI] [PubMed] [Google Scholar]
- 39.Tracy J.E, Currier D.P, Threlkeld A.J. Comparison of selected pulse frequencies from two different electrical stimulators on blood flow in healthy subjects. Phys. Ther. 1988;68:1526–1532. [PubMed] [Google Scholar]
- 40.Vanderthommen M, Soltani K, Maquet D, Crielaard J.M, Croisier J.L. Does neuromuscular electrical stimulation influence muscle recovery after maximal isokinetic exercise? Isokinetics Exerc. Sci. 2007;1:143–149. [Google Scholar]
- 41.Walker D.C, Currier D.P, Threlkeld A.J. Effects of high voltage pulsed electrical stimulation on blood flow. Phys. Ther. 1988;68:481–485. doi: 10.1093/ptj/68.4.481. [DOI] [PubMed] [Google Scholar]
- 42.Zicot M, Rigaux P. Effect of the frequency of neuromuscular electric stimulation of the leg on femoral arterial blood flow. J. Maladies Vasculaires. 1995;20:9–13. [PubMed] [Google Scholar]