Abstract
Objective
To examine use of CT colonography (CTC) in the English Bowel Cancer Screening Programme (BCSP) and investigate detection rates.
Design
Retrospective analysis of routinely coded BCSP data. Guaiac faecal occult blood test (gFOBt)-positive screenees undergoing CTC from June 2006 to July 2012 as their first-line colonic investigation were included. Abnormalities found at CTC, subsequent polyp, adenoma and cancer detection and positive predictive value (PPV) were calculated. Detection rates were compared with those observed in gFOBt-positive screenees investigated by colonoscopy. Multilevel logistic regression was used to examine factors associated with variable detection.
Results
2731 screenees underwent CTC. Colorectal cancer (CRC) or polyps were suspected in 1027 individuals (37.6%; 95% CI 33.8% to 41.4%); 911 of these underwent confirmatory testing. 124 screenees had CRC (4.5%) and 533 had polyps (19.5%), 468 adenomatous (17.1%). Overall detection was 24.1% (95% CI 21.5% to 26.6%) for CRC or polyps and 21.7% (95% CI 19.2% to 24.1%) for CRC or adenoma. Advanced neoplasia was detected in 504 screenees (18.5%; 95% CI 16.1% to 20.8%). PPV for CRC or polyp was 72.1% (95% CI 66.6% to 77.6%). By comparison, 9.0% of 72 817 screenees undergoing colonoscopy had cancer and 50.6% had polyps; advanced neoplasia was detected in 32.7%. CTC detection rates and PPV were higher at centres with experienced radiologists (>1000 examinations) and at high-volume centres (>175 cases/radiologist/annum). Centres using three-dimensional interpretation detected more neoplasia.
Conclusions
In the BCSP, detection rates after positive gFOBt are lower for CTC than colonoscopy, although populations undergoing the two tests are different. Centres with more experienced radiologists have higher detection and accuracy. Rigorous quality assurance of BCSP radiology is needed.
Keywords: Computer Tomography, Colorectal Neoplasia, Screening
Significance of this study.
What is already known about this subject?
Meta-analysis and randomised trials suggest CT colonography (CTC) is not significantly different to colonoscopy for detection of colorectal cancer.
CTC is used in the Bowel Cancer Screening Programme (BCSP) when colonoscopy is contraindicated or incomplete.
BCSP endoscopists are rigorously quality assured: CTC quality assurance is in development.
What are the new findings?
In the BCSP, 18.5% of guaiac faecal occult blood test-positive screenees investigated with CTC had advanced neoplasia detected, compared with 32.7% for colonoscopy.
Screenees investigated with CTC and colonoscopy are fundamentally different populations and reasons for differences in detection rates are speculative at this stage.
Centres with radiologists of higher average CTC experience, greater CTC workload and using three-dimensional interpretation had significantly higher detection rates.
How might it impact on clinical practice in the foreseeable future?
Urgent quality assurance, encompassing technical and interpretive quality, is needed in the BCSP in case these data are due to suboptimal CTC practice.
Minimum standards for CTC radiologists should be applied.
Service reconfiguration may be necessary to ensure BCSP CTC examinations are interpreted by experienced radiologists.
Introduction
UK colorectal cancer (CRC) mortality is relatively poor compared with Europe1 and the USA.2 Prognosis is related to stage at diagnosis, with 5-year survival ranging from 94% for cancers confined to the bowel wall to 5% for those with distant metastases.3 Detecting early-stage disease in asymptomatic individuals via screening with guaiac-based faecal occult blood testing (gFOBt) reduces disease-specific mortality by 16% in those offered screening and 25% in those who accept it.4 gFOBt screening is also cost-effective, at £3000 per quality-adjusted life-year gained.5 Consequently, since April 2006, the Department of Health in England has funded a national programme of biennial gFOBt screening at age 60–69 years (subsequently extended to age 70–74; those 75 or over may ‘opt-in’).
Fifty percent of subjects with a positive gFOBt result have underlying neoplasia, and a positive result must be investigated further, usually via colonoscopy. However, total colonoscopy is occasionally not possible or is considered too hazardous (eg, due to comorbidity). In these screenees, CT colonography (CTC) has recently replaced barium enema as the alternative test recommended by the English Bowel Cancer Screening Programme (BCSP).6 This paper describes usage and outcomes for CTC for the first 6 years of the English BCSP.
Methods
Programme structure
The BCSP has been described previously.7 In summary, eligible English residents are sent an invitation letter and information leaflet, and subsequently a gFOBt kit (Hemascreen; Immunostics, New Jersey, USA) with a prepaid return envelope. Kits are returned to one of five screening hub laboratories. Subjects testing positive are informed by post and invited to meet a specialist screening practitioner (most often a nurse) at one of 58 screening centres. The nature of colonoscopy and its risks and benefits are explained, and a health assessment performed. Consenting screenees undergo colonoscopy, performed by a screening-accredited practitioner. The Programme was progressively introduced across England, beginning in July 2006 and completing ‘roll-out’ in 2011.
Although colonoscopy is preferred for screenees testing positive for gFOBt, CTC may substitute if the subject is unable to safely complete colonoscopic bowel preparation, is too frail or immobile to undergo colonoscopy although potentially fit for necessary treatment, or colonoscopy is deemed otherwise contraindicated.6 CTC is also performed following incomplete colonoscopy. Barium enema was used until randomised trial data made available to the BCSP in 2010 suggested its detection rates for neoplasia were significantly inferior to CTC.8
Data identification
The Bowel Cancer Screening System (BCSS) is the BCSP clinical database, populated with screenee demographics from the English National Health Application and Infrastructure Services systems. As episode results become available, they are manually entered at the screening centres. We used these routinely coded data to perform a service evaluation approved by the BCSP Evaluation Group and the BCSP Radiology Quality Assurance Group, primarily focusing on CTC abnormality rates, subsequent confirmatory testing, detection of cancers and polyps and adenomas. A waiver to publish anonymised data gathered retrospectively was obtained from the Joint Research Office of the Chief Investigator.
We identified screenees having CTC within the BCSP as the first-line whole-colon investigation following positive gFOBt between June 2006 and July 2012. We excluded screenees undergoing completion CTC after incomplete colonoscopy since many of these will be due to an obstructing tumour (and hence would inflate the CTC cancer detection rate via inclusion bias). We recorded patient details (age, sex, American Society of Anesthesiologists (ASA) grade, screening centre attended and number of times screened previously), abnormalities found at CTC (suspected polyps and cancers, their diameter and segmental location), further tests performed after CTC, results from subsequent endoscopy (including polyp diameter and locations) and histology for resected polyps and cancers. The same BCSP database was used to extract polyp and cancer size, morphology and histology for all colonoscopies performed as the first whole-colon test for evaluation of positive gFOBt in 2011 and 2012. The largest cancer/polyp diagnosis made in each individual was recorded, allowing comparison of per-screenee detection rates.
Statistical analysis
Data were analysed using R V.2.15.1.9 The CTC abnormality rate was defined as the proportion of screenees undergoing CTC at which a polyp or cancer was recorded on BCSS. Abnormality rates were calculated for suspected CRC or large (10 mm+) polyp, suspected 6–9 mm polyps or suspected diminutive (≤5 mm) polyps. CRC and 10 mm+ polyps were grouped together since large polyps were described variably by different radiologists; that is, as a cancer or large polyp. Detection rate was defined as the proportion of individuals undergoing CTC who ultimately had a histologically confirmed CRC or adenoma matching the CTC abnormality. Matching was permitted if the CTC and endoscopic sizes were within 50% and lesions were in the same or adjacent colonic segment.10 Advanced neoplasia was defined as CRC, adenoma of 10 mm or greater, a >20% villous component, or high-grade dysplasia. Positive predictive value (PPV) was defined as the number of screenees believed to have CRC or a polyp on CTC and in whom at least one matched lesion was subsequently confirmed, divided by the total number of screenees undergoing such additional diagnostic testing following abnormal CTC. Analysis was performed on a per-screenee basis. Sensitivity cannot be calculated because no reference result is available for all participants, particularly those testing negative initially; 95% CIs for proportions were calculated from 2000 random bootstrap samples to account for clustering by centre.
To determine screenee and centre factors associated with detection, multilevel (random effects) binary logistic regression was performed, with a matched polyp or cancer as the outcome variable. screenee-level covariates included screenee age, gender, screening round for the individual (ie, number of times that individual had previously been screened) and centre-level covariates were average radiologist CTC experience, preferred reading strategy (two-dimensional (2D) or 3D), centre CTC workload and bowel preparation technique. Screenee-level covariates were taken directly from the central database, whereas centre-level covariates were taken from a prior national survey of CTC practice within the BCSP performed by the authors.11 Since data were grouped by screening centre, a hierarchical model incorporated centre as a random effects term using the package hglm.12 Funnel plots were used to compare centre variations in CTC abnormality rate, detection rate and PPV. We identified centres with a detection rate more than 2 and 3 SDs (corresponding to calculated 95% and 99.9% control limits) from the overall average. Plots were drawn in R using ggplot2,13 according to Spiegelhalter,14 using exact binomial control limits. Risk-adjusted detection rates were calculated by analogy with risk-adjusted mortality used for monitoring surgical performance.15 16 The number of observed neoplasms diagnosed by each centre was divided by the expected number of positives predicted by the logistic regression model. This observed:expected ratio was then multiplied by the national average neoplasia detection rate to give a risk-adjusted detection rate for each centre.
Results
Screenee characteristics and overall usage of CTC
From June 2006 to July 2012, 2753 FOBt-positive screenees were investigated with CTC as their first whole-colon test in the BCSP; 1521 men and 1232 women, spread over three screening rounds; 22 subjects withdrew prior to investigation, leaving 2731 for subsequent analysis. Screenee demographics are summarised in table 1. The reduction in numbers in each successive screening round is due to the gradual Programme roll-out, which did not complete national coverage until 2010.
Table 1.
Sex and age of screenees investigated by CTC, split by the individual's round of screening
| Characteristic | Screening round | |||
|---|---|---|---|---|
| 1 | 2 | 3 | Total | |
| Men | ||||
| 60–64 years | 370 | 184 | 6 | 560 |
| 65–69 years | 299 | 284 | 42 | 625 |
| 70–74 years | 123 | 122 | 34 | 279 |
| 75+ years | 36 | 5 | 0 | 41 |
| Total | 828 | 595 | 82 | 1505 |
| Women | ||||
| 60–64 years | 307 | 146 | 2 | 455 |
| 65–69 years | 218 | 236 | 36 | 490 |
| 70–74 years | 100 | 115 | 30 | 245 |
| 75+ years | 33 | 2 | 1 | 36 |
| Total | 658 | 499 | 69 | 1226 |
| Grand total (M+W) | 1486 | 1094 | 151 | 2731 |
CTC, CT colonography.
Median age was 66.5 years (IQR 63.3–69.5 years); males median 66.4 years (IQR 63.2–69.5 years), females median 66.6 years (IQR 63.3–69.5 years). CTC was used as the initial colonic investigation following positive gFOBt in only 2.3% of screenees overall, although this figure varied substantially across the 58 centres (0.039%–9.7%, IQR 0.80–3.1%).
CTC findings, use of confirmatory tests and detection rates
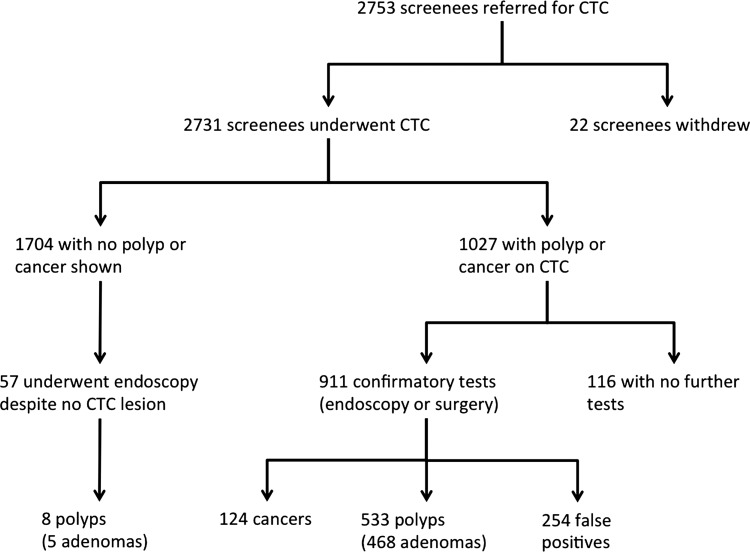
Overall, 1027 individuals were suspected to have CRC or polyps at CTC, an abnormality rate of 37.6% (95% CI 33.8% to 41.4%). 462 screenees were diagnosed with CRC or a 10 mm+ polyp at CTC (16.9%), 176 screenees were suspected to have 6–9 mm polyps (6.4%) and 115 individuals were felt to have diminutive polyps (4.2%). In 274 screenees (10.0%), the size and location of the CTC abnormality provoking endoscopy was absent from the database, although endoscopic size was available; 427 of the 462 screenees with suspected CRC or 10 mm+ polyp underwent endoscopy or surgery (92.4%), 371 of whom were found to have at least one matched abnormality (PPV=86.9%; 95% CI 82.7% to 90.9%). A smaller proportion of those with suspected 6–9 mm and ≤5 mm polyps underwent confirmatory tests, with PPV also declining as lesion size reduced (table 2). Ultimately, of the 1027 screenees with a suspected CRC or polyp on CTC, 911 underwent confirmatory testing (88.8%; 95% CI 86.2% to 91.4%), and 657 had at least one matched abnormality (24.1% of the total; 95% CI 21.5% to 26.6%), giving a per-patient PPV of 72.1%; 95% CI 66.5% to 77.8% (table 2).
Table 2.
CTC abnormalities, confirmatory tests and detection of matched abnormalities stratified by size of abnormality shown on CTC
| Largest abnormality suspected at CTC in 2731 gFOBt-positive individuals | |||||
|---|---|---|---|---|---|
| CRC or 10 mm+ polyp | 6–9 mm polyp | ≤5 mm polyp | Size not recorded | Any suspected polyp/cancer | |
| Number (% of total, (95% CI)) | 462 (16.9% (14.9% to 18.9%)) | 176 (6.4% (5.1% to 7.7%)) | 115 (4.2% (3.2% to 5.2%)) | 274 (10.0% (7.2% to 13.1%)) | 1027 (37.6% (33.8% to 41.4%)) |
| Number (% of those with CTC abnormality) undergoing confirmatory test (95% CI) | 427 of 462 (92.4% (89.6% to 95.1%)) | 136 of 176 (77.3% (70.8% to 83.7%)) | 76 of 115 (66.1% (53.2% to 79.7%)) | 272 of 274 (99.3% (98.3% to 100%)) | 911 of 1027 (88.7% (86.2% to 91.4%)) |
| Number (% of total) with matched abnormality (95% CI) | 371 (13.6% (11.4% to 15.7%)) | 99 (3.6% (2.6% to 4.6%)) | 40 (1.5% 0.9 to 2.0%)) | 147 (5.4% (3.6% to 7.2%)) | 657 (24.1% (41.5% to 26.7%)) |
| Histological diagnoses | 106 CRC 247 adenomas 18 non-adenomatous polyps |
87 adenomas 12 non-adenomatous polyps |
22 adenomas 18 non-adenomatous polyps |
18 CRC 112 adenomas 17 non-adenomatous polyps |
124 CRC 468 adenomas 65 non-adenomatous polyps |
| Positive predictive value for matched CRC or polyp (95% CI) | 86.9% (82.7% to 90.9%) | 72.8% (62.8% to 82.7%) | 52.6% (43.5% to 61.4%) | 54.0% (46.1% to 62.2%) | 72.1% (66.6% to 77.6%) |
| Positive predictive value for matched CRC or adenoma (95% CI) | 82.7% (78.6% to 86.5%) | 64.0% (52.3% to 74.9%) | 28.9% (18.3% to 39.5%) | 47.8% (38.8% to 53.6%) | 64.9% (59.3% to 70.5%) |
CRC, Colorectal cancer; CTC, CT colonography; gFOBt, guaiac faecal occult blood test.
The 116 screenees with abnormal CTC but not undergoing confirmatory testing included 10 with suspected CRC (1 with metastases), 25 individuals with 10 mm+ polyps, 40 screenees with 6–9 mm polyps, 39 with suspected diminutive polyps and two screenees in whom the CTC polyp diameter was missing. The reasons for no further testing included death, loss to follow-up, patient refusal and clinical decisions to not pursue small polyps in frail screenees.
Additionally, 57 endoscopies were performed without a prior CTC diagnosis of cancer or polyp. Twelve of these were for suspected benign submucosal abnormalities, three for suspected colitis and no reason was recorded for the remaining 42. These 42 endoscopies detected eight additional polyps, five of which were adenomas (including a 11 mm adenoma and a 7 mm adenoma; figure 1).
Figure 1.
Flowchart of screenees included.
Polyp location and morphology was available from CTC for 1062 polyps in 629 screenees. Most polyps (740; 69.7%) were distal to the splenic flexure; 150 (14.4%) rectal, 447 (42.1%) sigmoid, 107 (10.1%) descending colon and 36 (3.4%) at the splenic flexure. The remaining 322 (30.3%) were split as follows: 119 in the transverse (11.2%), 47 at the hepatic flexure (4.4%), 87 in the ascending (8.2%) and 69 in the caecum (6.5%). Polyps were generally either sessile (688; 64.8%) or pedunculated (328; 30.9%), with relatively few flat lesions (46; 4.3%). Cancer location was available for 110 of the confirmed 124 tumours and showed a similar distal predominance, with 30 (24.2%) rectal, 48 (38.7%) sigmoid, 6 (4.8%) descending colon, 2 (1.6%) splenic flexure, 7 (5.6%) transverse, 4 (3.2%) hepatic flexure, 7 (5.6%) ascending colon, 6 (4.8%) caecal and 14 (11.3%) missing data.
Comparison with colonoscopy detection rates
Colonoscopy data were available for 72 817 screenees; 6577 were found to have CRC (9.0%), 14 992 had a 10 mm+ polyp as the largest colonic abnormality (20.6%), 5773 had 6–9 mm polyps (7.9%) and 16 085 had only diminutive polyps (22.1%). Advanced neoplasia was detected in 23 830 (32.7%). Table 3 shows colonoscopic and CTC detection rates at various size thresholds. For screenees with no polyp diameter recorded at CTC, the endoscopic size was used.
Table 3.
Per-screenee detection rates at CTC and colonoscopy by the largest lesion demonstrated
| CTC (n=2731) | Colonoscopy (n=72 817) | p Value* | |
|---|---|---|---|
| Colorectal cancer (%) | 124† (4.5) | 6577 (9.0) | <0.0001 |
| 10 mm+ polyp (%) | 340 (12.4) | 14 992 (20.6) | <0.0001 |
| 10 mm+ adenoma (%) | 320 (11.7) | 13 571 (18.6) | <0.0001 |
| 6–9 mm polyp (%) | 119 (4.4) | 5773 (7.9) | <0.0001 |
| 6–9 mm adenoma (%) | 103 (3.8) | 5243 (7.2) | <0.0001 |
| ≤5 mm polyp (%) | 75 (2.7) | 16 085 (22.1) | <0.0001 |
| ≤5 mm adenoma (%) | 45 (1.6) | 10 870 (14.9) | <0.0001 |
| 6 mm+ adenoma or CRC (%) | 547 (20.0) | 25 391 (34.9) | <0.0001 |
| 10 mm+ adenoma or CRC (%) | 444 (16.3) | 20 148 (27.7) | <0.0001 |
| Advanced neoplasia (%) | 504 (18.5) | 23 830 (32.7) | <0.0001 |
For CTC, at each size threshold the number of screenees with a matched lesion at confirmatory testing (endoscopy or surgery) is reported. For colonoscopy, the size categories are the endoscopic size for polyps and histologic size for adenomas.
Probability value for χ2 test.
†10 additional screenees had no confirmatory testing but suspected CRC on CT.
CRC, colorectal cancer; CTC, CT colonography.
Factors associated with variable CTC abnormality rates, detection rates and PPV
Outcome after CTC stratified by sex and screening round is shown in table 4. CTC abnormality was commoner in men than in women (632 of 1505 men, 42.0% vs 395 of 1226 women, 32.2%, χ2=27.0, p<0.001). Detection rates were also greater in men: 395 of 1505 (26.2%) men had adenoma/CRC confirmed compared with 197 of 1226 (16.1%) women (χ2=40.6, p<0.001). PPV was greater for men, with 436 matched abnormalities detected at 564 endoscopies or resections (77.3%) compared with 221 from 347 for women (63.7%, χ2=19.1, p<0.001).
Table 4.
Total screenees imaged by CTC, subsequent abnormalities found at CTC, and ultimate detection rates for cancer, large polyp (10 mm+), medium-sized polyp (6–9 mm), any polyp or cancer, any adenoma or cancer and advanced neoplasia, each split by sex and individual's screening round
| First round | Second round | Third round | Total | |
|---|---|---|---|---|
| Total imaged by CTC | ||||
| Men | 828 | 595 | 82 | 1505 |
| Women | 658 | 499 | 69 | 1226 |
| Total | 1486 | 1094 | 151 | 2731 |
| Number (%) with polyp or cancer suspected on CTC | ||||
| Men | 373 (45.0) | 235 (39.5) | 24 (29.3) | 632 (42.0) |
| Women | 231 (35.1) | 148 (29.7) | 16 (23.2) | 395 (32.2) |
| Total | 604 (40.6) | 383 (35.0) | 40 (26.5) | 1027 (37.6) |
| Number (%) with proven cancer | ||||
| Men | 55 (6.6) | 32 (5.4) | 2 (2.4) | 89 (5.9) |
| Women | 21 (3.2) | 14 (2.8) | 0 (0.0) | 35 (2.9) |
| Total | 76 (5.1) | 46 (4.2) | 2 (1.3) | 124 (4.5) |
| Number (%) with matched 10 mm+ polyp | ||||
| Men | 142 (17.1) | 79 (13.3) | 6 (7.3) | 227 (15.1) |
| Women | 78 (11.9) | 31 (6.2) | 4 (5.8) | 113 (9.2) |
| Total | 220 (14.8) | 110 (10.1) | 10 (6.6) | 340 (12.4) |
| Number (%) with matched 6–9 mm polyp | ||||
| Men | 36 (4.3) | 31 (5.2) | 4 (4.9) | 71 (4.7) |
| Women | 24 (3.6) | 21 (4.2) | 3 (4.3) | 48 (3.9) |
| Total | 60 (4.0) | 52 (4.8) | 7 (4.6) | 119 (4.4) |
| Number (%) with any matched polyp or cancer | ||||
| Men | 263 (31.8) | 159 (26.7) | 14 (17.1) | 436 (29.0) |
| Women | 142 (21.6) | 72 (14.4) | 7 (10.1) | 221 (18.0) |
| Total | 405 (27.3) | 231 (21.1) | 21 (13.9) | 657 (24.1) |
| Number (%) with any matched adenoma or cancer | ||||
| Men | 237 (28.6) | 144 (24.2) | 14 (17.1) | 395 (26.2) |
| Women | 126 (19.1) | 64 (12.8) | 7 (10.1) | 197 (16.1) |
| Total | 363 (24.4) | 208 (19.0) | 21 (13.9) | 592 (21.7) |
| Number (%) with advanced neoplasia | ||||
| Men | 209 (25.2) | 119 (20.0) | 12 (14.6) | 340 (22.6) |
| Women | 106 (16.1) | 50 (10.0) | 8 (11.6) | 164 (13.4) |
| Total | 315 (21.2) | 169 (15.4) | 20 (13.2) | 504 (18.5) |
To calculate percentages for each cell of the table, the total number of screenees of the corresponding gender and screening round was used as the denominator.
CTC, CT colonography.
Both reported abnormality at CTC, and detection rate diminished in successive screening rounds, with detection of adenomas and cancers falling from 24.4% in round 1 to 19.0% in round 2, and 13.9% in round 3 (test for equality of proportions, p<0.001), a finding consistent across all age groups. Conversely, combined adenoma/CRC detection rate increased with age (table 5), ranging from 20.6% in the youngest group to 37.7% in those aged 75 years or more (who, since they exceeded the upper boundary for invitation, had ‘opted-in’). PPV for CTC was unaffected by screening round or age, although there was a non-significant trend for higher PPV in the oldest age category when compared with younger screenees (p=0.08).
Table 5.
Number of screenees imaged by CTC, subsequent referrals for further testing, and ultimate detection for cancer, large polyp (10 mm+), medium-sized polyp (6–9 mm), any polyp or cancer, any adenoma or cancer and advanced neoplasia, split by age and sex
| 60–64 years | 65–69 years | 70–74 years | 75+ years | |
|---|---|---|---|---|
| Number imaged by CTC | ||||
| Men | 560 | 625 | 279 | 41 |
| Women | 455 | 489 | 246 | 36 |
| Total | 1015 | 1114 | 525 | 77 |
| Number (%) with suspected polyp or cancer on CTC | ||||
| Men | 227 (40.5) | 252 (40.3) | 131 (47.0) | 22 (53.7) |
| Women | 134 (29.5) | 164 (33.5) | 77 (31.3) | 20 (55.6) |
| Total | 361 (35.6) | 416 (37.3) | 208 (39.6) | 42 (54.5) |
| Number (%) with proven cancer | ||||
| Men | 25 (4.5) | 38 (6.1) | 19 (6.8) | 7 (17.1) |
| Women | 14 (3.1) | 12 (2.5) | 8 (3.3) | 1 (2.8) |
| Total | 39 (3.8) | 50 (4.5) | 27 (5.1) | 8 (10.4) |
| Number (%) with matched 10 mm+ polyp | ||||
| Men | 86 (15.4) | 91 (14.6) | 46 (16.5) | 4 (9.8) |
| Women | 45 (9.9) | 43 (8.8) | 19 (7.7) | 6 (16.7) |
| Total | 131 (12.9) | 134 (12.0) | 65 (12.4) | 10 (13.0) |
| Number (%) with matched 6–9 mm polyp | ||||
| Men | 27 (4.8) | 28 (4.5) | 14 (5.0) | 2 (4.9) |
| Women | 14 (3.1) | 20 (4.1) | 9 (3.7) | 5 (13.9) |
| Total | 41 (4.0) | 48 (4.3) | 23 (4.4) | 7 (9.1) |
| Number (%) with any matched polyp or cancer | ||||
| Men | 158 (28.2) | 180 (28.8) | 83 (29.7) | 15 (36.6) |
| Women | 78 (17.1) | 89 (18.2) | 41 (16.7) | 13 (36.1) |
| Total | 236 (23.3) | 269 (24.1) | 124 (23.6) | 28 (36.4) |
| Number (%) with any matched adenoma or cancer | ||||
| Men | 141 (25.2) | 159 (25.4) | 80 (28.7) | 15 (36.6) |
| Women | 68 (14.9) | 78 (16.0) | 37 (15.0) | 14 (38.9) |
| Total | 209 (20.6) | 237 (21.3) | 117 (22.3) | 29 (37.7) |
| Number (%) with advanced neoplasia | ||||
| Men | 115 (20.5) | 145 (23.2) | 69 (24.7) | 11 (26.8) |
| Women | 58 (12.7) | 63 (12.9) | 32 (13.0) | 11 (30.6) |
| Total | 173 (17.0) | 208 (18.7) | 101 (19.2) | 22 (28.6) |
Percentages use the total number of screenees imaged by CTC of that sex and age category as the denominator.
Age, sex and individual's screening round were highly significant predictors of CTC detection rates in both univariate and multivariate analysis (table 6).
CTC, CT colonography.
Between-centre variation for CTC abnormality, detection of adenomas and cancers and PPV
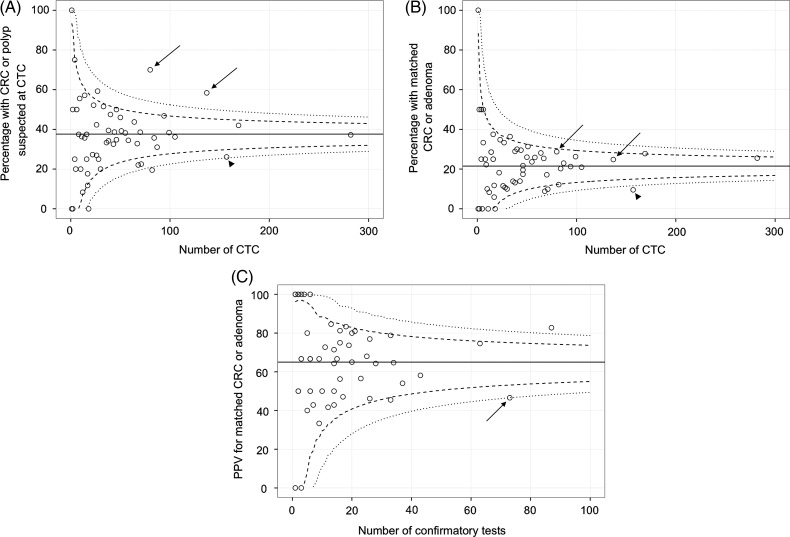
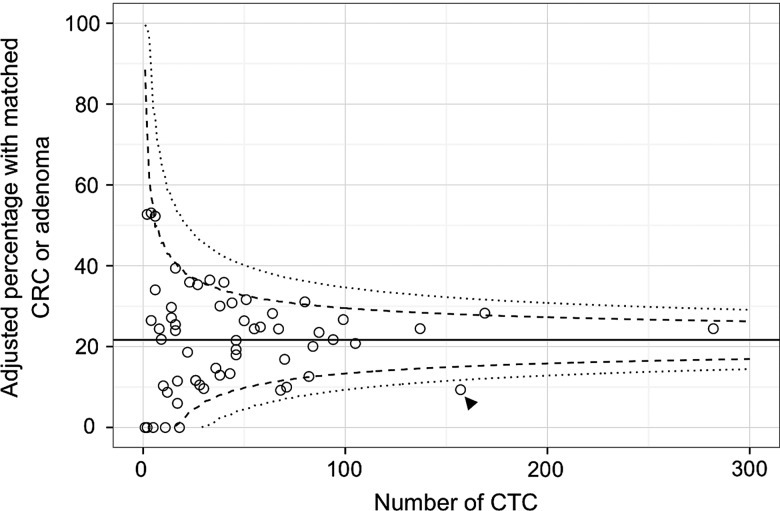
Rates of CTC abnormality (figure 2A), subsequent detection of adenomas or cancers (figure 2B), and PPV for CTC (figure 2C) were relatively homogeneous across screening centres: only one centre showed a significantly lower detection rate than the average. Overall, PPV at each centre showed moderate positive correlation with that centre's detection rate (Spearman r=0.63). Because it is possible that different categories of screenee were referred for CTC at different centres (eg, a greater proportion of women or young screenees, which would reduce prevalence of abnormality: table 6) thereby influencing subsequent detection rates, we calculated a risk-adjusted detection rate for each centre by correcting for these covariates with logistic regression. The risk-adjusted funnel plot is shown in figure 3 and is similar to the unadjusted data: The low detection centre retains a lower detection rate when adjusting for the population being imaged. Supporting this, the regression model estimate for this centre gave an OR of 0.55 (95% CI 0.20 to 0.96) for detection of adenomas or cancer, implying that the lower detection rate was not simply due to case-mix.
Figure 2.
(A) Unadjusted CT colonography (CTC) abnormality rate, (B) unadjusted detection rates of matched adenomas and cancer and (C) positive predictive value (PPV) for CTC by screening centre. The dashed lines correspond to upper and lower 95% control limits, and the dotted lines represent 99.9% limits. Two centres had a high abnormality rate at CTC but both lay within the control limits when considering proven adenomas and cancers (arrows in (A) and (B)). One of these centres had a PPV that was at the lower control limit (arrow in (C)). A different centre had a borderline CTC abnormality rate and low adenoma/cancer detection (arrowheads in (A) and (B)).
Table 6.
Screenee factors associated with detection of adenomas or CRC, derived by logistic regression and expressed as ORs
| Screenee factor | Univariate OR (95% CI) | Multivariate OR (95% CI) | p Value* |
|---|---|---|---|
| Men versus women | 1.83 (1.52 to 2.22) | 1.84 (1.52 to 2.23) | <0.001 |
| Screened for second time versus first time | 0.73 (0.60 to 0.89) | 0.72 (0.59 to 0.87) | <0.001 |
| Screened for third time versus first time | 0.49 (0.30 to 0.76) | 0.44 (0.26 to 0.69) | <0.001 |
| Age (per year increase) | 1.03 (1.01 to 1.05) | 1.04 (1.02 to 1.06) | <0.001 |
*Probability value for the Wald z-score for both univariate and multivariate analysis.
CRC, Colorectal cancer;
Figure 3.
Adjusted detection rate at CT colonography by screening centre with 95% (dashed) and 99.9% (dotted) control limits. The low-detection outlier shown in figure 2B is unchanged (arrowhead).
Centre factors associated with referral rates, detection rates and PPV
Although not available from BCSS, a prior survey of the 58 screening centres gathered information on CTC technique, estimated annual centre workload per radiologist (including symptomatic as well as screening practice), and average experience of the interpreting radiologists at that centre (both symptomatic and screening cases).11 Two of the 58 centres provided incomplete responses, accounting for small differences from the data presented above.
Average radiologist experience strongly influenced the proportion of CTC reported as abnormal, combined adenoma/CRC detection rate, and PPV: all increased in line with experience (table 7). Similarly, centres performing more CTC (including symptomatic practice) reported more abnormalities, detected more adenomas/CRC and had a higher PPV than lower-volume centres; 3D interpretation (either a primary 3D strategy or equal use of 3D and 2D) was also beneficial, with significantly higher rates of CTC abnormality and adenoma/CRC detection, at equivalent PPV when compared with centres using a 2D approach. Bowel preparation had a variable effect on diagnostic performance, with centres combining bowel purgation with faecal tagging detecting slightly more adenomas and cancers than centres using either purgation or tagging alone although the difference was not statistically significant. PPV was significantly lower for the combined preparation (table 7).
Table 7.
Institutional factors associated with varying CTC abnormality rates, detection of adenomas and cancers, and PPV
| Variable | Abnormality rate at CTC (%) | Detection of adenomas and cancers (%) | PPV (%) |
|---|---|---|---|
| Average radiologist experience | |||
| <300 cases | 154/474 (32.5) | 82/474 (17.3) | 82/134 (61.2) |
| 300–999 cases | 384/1006 (38.2) | 205/1006 (20.4) | 205/340 (60.3) |
| 1000+ cases | 450/1119 (40.2)* | 282/1119 (25.2)* | 382/400 (70.5)* |
| Average radiologist workload | |||
| <175 cases/radiologist/annum | 291/851 (34.2) | 145/851 (17.0) | 145/247 (58.7) |
| ≥175 cases/radiologist/annum | 697/1748 (39.9)† | 424/1748 (24.3)† | 424/627 (67.6)† |
| Interpretation strategy | |||
| Primary two-dimensional interpretation | 316/973 (32.5) | 183/973 (18.8) | 183/286 (64.0) |
| Three-dimensional interpretation | 711/1758 (40.4)† | 409/1758 (23.3)† | 409/625 (57.5) |
| Bowel preparation | |||
| Either purgation or tagging | 493/1456 (33.9) | 301/1456 (20.7) | 301/439 (68.6)† |
| Both purgation and tagging combined | 495/1143 (43.3)† | 268/1143 (23.4) | 268/435 (61.6) |
*χ2 Test for trend in proportions, p<0.05.
†χ2 Test, p<0.05 when compared with the other category of the same variable.
CTC, CT colonography; PPV, positive predictive value
The logistic regression model used screenee age, gender and screening with additional covariates of average radiologist experience (<300 total CTC experience, 300–999, 1000+), centre workload (<175 CTC/radiologist/annum vs 175+), bowel preparation (cleansing and tagging combined vs either alone) and reading strategy (2D vs 3D). All except the method of bowel preparation remained significant in a random effects model (table 8). The interaction between experience and reading strategy was also modelled, but was not significant.
Table 8.
Screenee and institutional factors associated with detection rates following CTC, derived via a multilevel logistic regression model and expressed as ORs
| Variable | Crude OR | p Value* | Random effects model OR | p Value* | |
|---|---|---|---|---|---|
| Screenee factors | Men (vs women) | 1.86 (1.56 to 2.23) | <0.001 | 1.83 (1.50 to 2.23) | <0.001 |
| Screened for second time (vs first time) | 0.73 (0.60 to 0.87) | <0.001 | 0.75 (0.61 to 0.93) | <0.001 | |
| Screened for third time (vs first time) | 0.46 (0.29 to 0.69) | <0.001 | 0.43 (0.26 to 0.70) | <0.001 | |
| Age (per year increase) | 1.04 (1.02 to 1.06) | <0.001 | 1.04 (1.02 to 1.06) | <0.001 | |
| Institutional factors | Three-dimensional review of images (vs primary two-dimensional) | 1.31 (1.07 to 1.60) | 0.010 | 1.38 (1.02 to 1.95) | 0.023 |
| Experience of radiologist (vs <300 cases) | |||||
| High (1000+ cases) | 1.49 (1.16 to 1.94) | 0.020 | 1.44 (1.02 to 2.04) | 0.038 | |
| Medium (300–999 cases) | 1.18 (0.90 to 1.54) | 0.23 | 1.22 (0.87 to 1.72) | 0.26 | |
| High workload (>175 cases per radiologist per annum, vs less than this) | 1.52 (1.22 to 1.89) | 0.017 | 1.41 (1.02 to 1.93) | 0.032 | |
| Use of purgation combined with tagging (vs either alone) | 1.14 (0.95 to 1.38) | 0.16 | 1.12 (0.85 to 1.48) | 0.40 |
*Probablility value for the Wald z-score. Italic values are significant at the 5% level.
CTC, CT colonography.
Stage of cancers detected
Dukes’ stage was available for 116 of the 124 patients with histologically proven cancer. Seven of the eight cancers with a missing Dukes’ stage were T1 lesions but had no N stage, possibly due to local resection (precluding a pathological N stage). Stage compared with distribution in the whole BCSP (reported in reference17) is shown in table 9. Overall stage distribution was similar, suggesting that CTC-diagnosed neoplasms are equally likely to be found at an early, curable stage.
Table 9.
Stage distribution of cancers confirmed by endoscopy following CTC alone compared with cancer stage across the whole BCSP
| Stage | CTC total* | CTC percentage | BCSP total† | BCSP percentage |
|---|---|---|---|---|
| Dukes A | 37 | 29.8 | 614 | 28.9 |
| Dukes B | 38 | 30.6 | 517 | 24.4 |
| Dukes C | 34 | 27.4 | 497 | 23.4 |
| Dukes D | 7 | 5.6 | 121 | 5.7 |
| Missing | 8 | 6.5 | 374 | 17.6 |
*10 further cancers were suspected radiologically but not confirmed histologically.
†This total will include a small number of cancers diagnosed by CTC, albeit likely fewer than 15.
BCSP, Bowel Cancer Screening Programme; CTC, CT colonography.
Discussion
The success of the BCSP depends on thorough colonic investigation after positive gFOBT. Colonoscopy is the reference standard and has been instituted nationally with excellent safety and quality.18 Nevertheless, around 2% of screenees cannot undergo colonoscopy7 (rising to 9% at some centres19), so CTC is recommended.6 Meta-analysis suggests CTC has equivalent sensitivity to colonoscopy for cancer,20 and around 90% sensitivity for adenomas of 1 cm or greater.21 22 Detection rates of advanced neoplasia at screening CTC and colonoscopy were equal in two large cohort studies,10 23 and randomised data suggest that CTC is not significantly different from colonoscopy for detection of cancers and large polyps in symptomatic patients.24 CTC has also been evaluated specifically in gFOBt-positive screenees, achieving a sensitivity of 95% for cancer and 1 cm+ polyps combined25 and 86.5% for 6 mm+ advanced adenomas.26 These data suggest CTC can substitute for colonoscopy when the latter is undesirable.
We found substantially lower detection rates for CTC than for colonoscopy. Cancer detection rates were 4.5% for BCSP screenees undergoing CTC and 9.0% for those having colonoscopy. Similarly, whereas 20.6% of screenees undergoing colonoscopy had a 10 mm+ polyp as the largest colonic abnormality, only 12.4% of screenees undergoing CTC had a large polyp confirmed. Detection of advanced neoplasia was 32.7% and 18.5%, respectively.
There are several possible explanations for this. Most obviously, populations undergoing CTC or colonoscopy are different since CTC is performed when colonoscopy is contraindicated. Because referral criteria for CTC are subject to interpretation, selection bias might reduce the prevalence of neoplasia in those having CTC. For example, CTC might be used preferentially in those deemed low-risk because of recent normal colonoscopy or a strong alternative explanation for a positive gFOBt. Atlhough we have no direct evidence of this, such biases are possible. Additionally, 11% of screenees with pathology suspected at CTC did not undergo further testing, thereby reducing detection rates of confirmed neoplasia. Differences between polyp diameters at CTC and colonoscopy may also alter detection rates at various size thresholds, since neither technique measures diameter perfectly.27 We found there was a tendency for polyp diameters to cluster at 5 mm intervals (data not shown), implying ‘rounding’ to the nearest 5 mm, which might skew detection rates based on size thresholds. Furthermore, many subjects investigated with CTC take anticoagulants such as warfarin or antiplatelets, including clopidogrel. Whether these affect gFOBt is controversial,28–30 but clopidogrel, in particular, is implicated as a cause of false-positive gFOBt.31 Women are also relatively over-represented at CTC when compared with colonoscopy (1.2 : 1 M:F ratio in our study vs 1.5 : 1 in the report of the first million individuals screened).7 Since women also have lower prevalence of neoplasia, this will diminish overall CTC detection rates.
However, lower detection rates may be because CTC, as currently practiced in the BCSP, has unexpectedly low sensitivity. Interval cancer rates may help determine if CTC misses significantly more cancers than colonoscopy; these data are currently being collected. Although the available literature suggests CTC has similar sensitivity to colonoscopy for cancers and large polyps, these data may not generalise to the BCSP. BCSP endoscopists have undergone extensive quality assurance. Conversely, CTC in the BCSP is relatively novel and has not been subject to similar stipulations. Multicentre studies of CTC have used radiologists meeting specific training requirements,32 highly experienced readers33 or motivated subspecialists with additional training.8 24 Radiologists in the BCSP, while almost universally trained in basic CTC interpretation and, frequently, subspecialists in gastrointestinal imaging,11 may not have attained a similar degree of experience or training. Like colonoscopy prior to the BCSP,34 it is known that radiologist performance may be highly variable, with sensitivity for 1cm+ lesions ranging between 67 and 100% in one multicentre study.32 Although a recent analysis of detection rates from a single US centre showed relatively little variation in neoplasia detection between radiologists,35 replicating this across multiple centres may be more difficult.
A quality assurance programme for CTC, as for colonoscopy, should help minimise differences in diagnostic accuracy. Despite CTC having lower detection rates than colonoscopy, it remains the preferred radiological investigation in the BCSP when colonoscopy is not desirable.6 Reinforcing this, cancer detection rates for barium enema in the BCSP were even lower than for CTC (3.6%, full data not shown). It is important to note that this is a difficult population to image. These screenees are frail and have already been judged unsuitable for colonoscopy. Therefore, achieving optimal CTC image quality and diagnostic performance is challenging. Even if the lower detection rates we describe are partly due to missed lesions at CTC, there is no guarantee that an alternative test (including colonoscopy) would fare better—these individuals will likely be difficult to endoscope safely, and neoplasia detection rates might be no different. We would therefore caution against interpreting our data to argue for or against CTC in frailer screenees, since the outcomes of the alternative (colonoscopy) are unknown.
We found that centres with increasing average radiologist experience and higher CTC workload had higher detection rates, supporting the hypothesis that CTC requires considerable experience to maximise diagnostic performance. Our data raise the possibility that less experienced BCSP radiologists are missing lesions, although this has not been directly proven because data are unrandomised. An alternative explanation is further selection bias. Less experienced radiologists are likely to work at centres whose CTC service is nascent. The presumed selection biases contributing to lower CTC detection rates (vs colonoscopy) nationally might be particularly severe at these centres because referrers have less confidence in CTC. Notably, average radiologist experience did not become significant in our study until the number of cases interpreted exceeded 1000, substantially greater than previous training recommendations.36 Adenoma detection rates by endoscopists continue to increase over several hundred examinations.37 A higher total CTC caseload per radiologist per annum was also associated with superior detection. Interestingly, it did not appear that radiologists with higher detection rates were simply over-reporting equivocal findings at CTC. In fact, the converse was true: radiologist experience was associated with significantly higher PPV, suggesting that not only were more experienced radiologists more likely to report an abnormality at CTC, they were also more likely to be correct when doing so.
Interpretation using 3D images (ie, ‘virtual colonoscopy’) was associated with higher detection rates. While this may be because 3D visualisation helps detect polyps, it may also be a proxy for other measures that influence diagnostic performance; for example, a high-quality workstation, training, or sufficient reporting time to incorporate 3D search. Existing literature is unclear if 3D interpretation is superior38 or not.39 Nevertheless, whether directly associated or not, we found a primary 2D strategy was associated with lower diagnosis and detection rates. The precise method used for bowel preparation did not seem to be a significant factor after adjusting for age, sex and the individual's prior screening history.
PPV in our study was lower at 72.1% than prior studies of both FOBt-positives (87%40) and average-risk individuals (92–96%41–43), although we found PPV for CRC and 1 cm+ polyps was good (87%). The difference may partly be due to different patient populations—our study describes screenees with relative contraindications to colonoscopy, whereas, existing literature does not preselect in this manner. As noted above, these individuals are difficult to image, and therefore, equivocal findings may have been reported as positive, lowering PPV.
Our study is limited by the fact we used routinely coded data, the accuracy of which is unknown currently. Of note, analysis of other large databases (including the National Bowel Cancer Audit Project, NBOCAP) suggested inadequate performance that was ultimately due to upload of inaccurate data.44 Our own local audit at two of the largest centres in terms of CTC volume suggests the database accuracy exceeds 90% (data not shown). However, this may not apply to all centres. The planned introduction of a minimum dataset for CTC reporting in the BCSP should improve accuracy of centrally held data. A further limitation is that centre-level data regarding specific CTC factors (eg, average radiologist experience and reading strategy) were inferred from a separate survey.11
In summary, screenees undergoing CTC as the primary investigation following positive gFOBt in the English BCSP have a 18.5% rate of advanced neoplasia, compared with 32.7% for colonoscopy. The reasons for this difference are likely to be multifactorial, but selection bias and/or suboptimal diagnostic performance of CTC are possible. Radiologist inexperience was associated with lower detection rates and PPV. A primary 2D reading strategy was also associated with lower detection rates. A rigorous quality assurance process for radiology in the BCSP is being instituted and will devise methods to ensure high-quality imaging is available universally.
Footnotes
Correction notice: This article has been corrected since it was published Online First. ‘Two-dimensional’ in table 7 has been amended to read ‘Three-dimensional’.
Contributors: AP, SH, AG, SAT, JP and DB conceived and designed the study. CN extracted the data and designed the study. PB designed the statistical analysis plan. AP, PB and SH analysed the data. AP drafted the paper. All authors revised the paper and approved it. SH is guarantor.
Funding: This research was funded partly by the UK Department of Health via a National Institute for Health Research Programme Grant (RP-PG-0407-10338) and by the Royal College of Radiologists’ Kodak Fund Scholarship. This work was undertaken at UCLH and UCL, which receives a proportion of funding from the NIHR Biomedical Research Centre funding scheme. The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health.
Competing interests: None.
Ethics approval: Joint Research Office, University College London Hospitals.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sant M, Allemani C, Santaquilani M, et al. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer 2009;45:931–91 [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Surveillance Epidemiology and End Results Cancer Statistics Review: Cancer of the Colon (Invasive). http://seer.cancer.gov/csr/1975_2008/browse_csr.php?section=6&page=sect_06_table.13.html (accessed Sep 2012).
- 3.National Cancer Intelligence Network. Colorectal cancer survival by stage—NCIN data briefing. http://www.ncin.org.uk/publications/data_briefings/colorectal_cancer_survival_by_stage.aspx (accessed Sep 2012).
- 4.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348:1472–7 [DOI] [PubMed] [Google Scholar]
- 5.Tappenden P, Chilcott J, Eggington S, et al. Option appraisal of population-based colorectal cancer screening programmes in England. Gut 2007;56:677–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor SA, Burling DN, Patnick J. Guidelines for the use of imaging in the NHS Bowel Cancer Screening Programme: Second Edition. 2012. http://www.cancerscreening.nhs.uk/bowel/publications/nhsbcsp05.pdf (accessed Jan 2013).
- 7.Logan RFA, Patnick J, Nickerson C, et al. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012;61:1439–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halligan S, Wooldrage K, Dadswell E, et al. Computed tomographic colonography versus barium enema for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): a multicentre randomised trial. Lancet 2013;381:1185–93 [DOI] [PubMed] [Google Scholar]
- 9.R Core Team. R: A language and enviroment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org (accessed Jan 2013).
- 10.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med 2003;349:2191–200 [DOI] [PubMed] [Google Scholar]
- 11.Plumb AA, Halligan S, Taylor SA, et al. CT colonography in the English Bowel Cancer Screening Programme: national survey of current practice. Clin Radiol 2013;68:479–87 [DOI] [PubMed] [Google Scholar]
- 12.Ronnegard L, Shen X, Alam M. hglm: a package for fitting hierarchical generalized linear models. 2010. http://journal.r-project.org/archive/2010–2/RJournal_2010–2_Roennegaard~et~al.pdf (accessed Jan 2013).
- 13.Wickham H. ggplot2: elegant graphics for data analysis. New York, NY: Springer, 2009 [Google Scholar]
- 14.Spiegelhalter DJ. Funnel plots for comparing institutional performance. Stat Med 2005;24:1185–202 [DOI] [PubMed] [Google Scholar]
- 15.Morris EJA, Taylor EF, Thomas JD, et al. Thirty-day postoperative mortality after colorectal cancer surgery in England. Gut 2011;60:806–13 [DOI] [PubMed] [Google Scholar]
- 16.Mayer EK, Bottle A, Aylin P, et al. What is the role of risk-adjusted funnel plots in the analysis of radical cystectomy volume-outcome relationships?. BJU Int 2011;108:844–50 [DOI] [PubMed] [Google Scholar]
- 17.Morris EJA, Whitehouse LE, Farrell T, et al. A retrospective observational study examining the characteristics and outcomes of tumours diagnosed within and without of the English NHS Bowel Cancer Screening Programme. Br J Cancer 2012;107:757–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee TJW, Rutter MD, Blanks RG, et al. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut 2012;61:1050–7 [DOI] [PubMed] [Google Scholar]
- 19.Goddard AF, Nickerson C, Blanks RG, et al. Current role of radiology as the first investigation in the English Bowel Cancer Screening Programme (BCSP). Gut 2012;61(Suppl2):A326–7 [Google Scholar]
- 20.Pickhardt PJ, Hassan C, Halligan S, et al. Colorectal cancer: CT colonography and colonoscopy for detection—systematic review and meta-analysis. Radiology 2011;259:393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halligan S, Altman DG, Taylor SA, et al. CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology 2005;237:893–904 [DOI] [PubMed] [Google Scholar]
- 22.De Haan MC, van Gelder RE, Graser A, et al. Diagnostic value of CT-colonography as compared to colonoscopy in an asymptomatic screening population: a meta-analysis. Eur Radiol 2011;21:1747–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim DH, Pickhardt PJ, Leung WK, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med 2007;357:1403–12 [DOI] [PubMed] [Google Scholar]
- 24.Atkin W, Dadswell E, Wooldrage K, et al. Computed tomographic colonography versus colonoscopy for investigation of patients with symptoms suggestive of colorectal cancer (SIGGAR): a multicentre randomised trial. Lancet 2013;381:1194–202 [DOI] [PubMed] [Google Scholar]
- 25.Liedenbaum MH, de Vries AH, van Rijn AF, et al. CT colonography with limited bowel preparation for the detection of colorectal neoplasia in an FOBT positive screening population. Abdom Imaging 2010;35:661–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regge D, Laudi C, Galatola G, et al. Diagnostic accuracy of computed tomographic colonography for the detection of advanced neoplasia in individuals at increased risk of colorectal cancer. JAMA 2009;301:2453–61 [DOI] [PubMed] [Google Scholar]
- 27.Summers RM. Polyp size measurement at CT colonography: what do we know and what do we need to know? Radiology 2010;255:707–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarke P, Jack F, Carey FA, et al. Medications with anticoagulant properties increase the likelihood of a negative colonoscopy in faecal occult blood test population screening. Colorectal Dis 2006;8:389–92 [DOI] [PubMed] [Google Scholar]
- 29.Iles-Shih L, Collins JF, Holub JL, et al. Prevalence of significant neoplasia in FOBT-positive patients on warfarin compared with those not on warfarin. Am J Gastroenterol 2010;105:2030–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahi CJ, Imperiale TF. Do aspirin and nonsteroidal anti-inflammatory drugs cause false-positive fecal occult blood test results? A prospective study in a cohort of veterans. Am J Med 2004;117:837–41 [DOI] [PubMed] [Google Scholar]
- 31.Sawhney MS, McDougall H, Nelson DB, et al. Fecal occult blood test in patients on low-dose aspirin, warfarin, clopidogrel, or non-steroidal anti-inflammatory drugs. Dig Dis Sci 2010;55:1637–42 [DOI] [PubMed] [Google Scholar]
- 32.Johnson CD, Chen M-H, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med 2008;359:1207–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoop EM, de Haan MC, de Wijkerslooth TR, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol 2012;13:55–64 [DOI] [PubMed] [Google Scholar]
- 34.Bowles CJA, Leicester R, Romaya C, et al. A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow? Gut 2004;53:277–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pooler BD, Kim DH, Hassan C, et al. Variation in Diagnostic Performance among Radiologists at Screening CT Colonography. Radiology 2013;268:127–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor SA, Laghi A, Lefere P, et al. European Society of Gastrointestinal and Abdominal Radiology (ESGAR): consensus statement on CT colonography. Eur Radiol 2007;17:575–9 [DOI] [PubMed] [Google Scholar]
- 37.Atkin W, Rogers P, Cardwell C, et al. Wide variation in adenoma detection rates at screening flexible sigmoidoscopy. Gastroenterology 2004;126: 1247–56 [DOI] [PubMed] [Google Scholar]
- 38.Pickhardt PJ, Lee AD, Taylor AJ, et al. Primary 2D versus primary 3D polyp detection at screening CT colonography. AJR Am J Roentgenol 2007;189:1451–6 [DOI] [PubMed] [Google Scholar]
- 39.Hara AK, Blevins M, Chen M-H, et al. ACRIN CT colonography trial: does reader's preference for primary two-dimensional versus primary three-dimensional interpretation affect performance? Radiology 2011;259:435–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liedenbaum MH, van Rijn AF, de Vries AH, et al. Using CT colonography as a triage technique after a positive faecal occult blood test in colorectal cancer screening. Gut 2009;58:1242–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickhardt PJ, Wise SM, Kim DH. Positive predictive value for polyps detected at screening CT colonography. Eur Radiol 2010;20:1651–6 [DOI] [PubMed] [Google Scholar]
- 42.Zueco Zueco C, Sobrido Sampedro C, Corroto JD, et al. CT colonography without cathartic preparation: positive predictive value and patient experience in clinical practice. Eur Radiol 2012;22:1195–204 [DOI] [PubMed] [Google Scholar]
- 43.Iafrate F, Hassan C, Ciolina M, et al. High positive predictive value of CT colonography in a referral centre. Eur J Radiol 2011;80:e289–92 [DOI] [PubMed] [Google Scholar]
- 44.Lewis RS, Graham DG, Watson JD, et al. Learning the hard way: the importance of accurate data. Colorectal Dis 2012;14:1015–18 [DOI] [PubMed] [Google Scholar]