Abstract
Regression of established tumors can be induced by adoptive immunotherapy (AIT) with tumor draining lymph node (DLN) lymphocytes activated with bryostatin and ionomycin (B/I). Tumor antigen-sensitized DLN lymphocytes from BALB/c mice with 10-day 4T1 mammary carcinomas were harvested, activated with B/I, and expanded in culture with either interleukin-2 (IL-2) or IL-7 + IL-15. Cell yields, proliferation, phenotypes, and in vitro responses to tumor antigen were compared for cells grown in different cytokines. These T cells were also tested for antitumor activity against established 4T1 mammary carcinomas after inoculation of tumor cells subcutaneously (s.c.). IL-7/15 resulted in much faster and more prolonged proliferation of B/I-activated T cells than culturing the same cells in IL-2. This resulted in approximately 5–10-fold greater yields of viable cells. Culture in IL-7/15 yielded higher proportions of CD8+ T cells and a higher proportion of cells with a central memory phenotype. T cells grown in IL-2 had higher interferon-gamma (IFN-γ) release responses to tumor antigen than cells grown in IL-7/15. Adoptive transfer of B/I-activated T cells grown in IL-7/15 demonstrated much greater efficacy against 4T1 tumors in vivo. Activation of tumor antigen-sensitized T cells with B/I and culture in IL-7 + IL-15 is a promising modification of standard regimens for production of T cells for use in AIT of cancer.
Keywords: Adoptive immunotherapy, Mammary carcinoma, IL-7, IL-15, IL-2, T lymphocytes
Introduction
Adoptive immunotherapy (AIT) using antigen-sensitized T cells activated and/or expanded in vitro has been extensively investigated as a potential therapy for cancer in humans [1–11]. We have shown that in vitro treatment with bryostatin 1 and ionomycin (B/I) selectively activates antigen-sensitized tumor draining lymph node (tDLN) lymphocytes [7–10, 12]. Bryostatin 1, a macrocyclic lactone derived from Bugula neritina, activates protein kinase C, and ionomycin increases intracellular calcium, mimicking signaling through the CD3/T cell receptor (TcR) complex, and leading to activation and proliferation of T cells [13–16].
In the 4T1 mammary carcinoma model which is very weakly immunogenic and aggressively metastatic, we have previously shown that B/I selectively activates CD62L− or sensitized T cells [12]. Although our previous studies in this model were based on the use of the T cell growth factor interleukin-2 (IL-2) to stimulate the proliferation of antitumor T cells in culture, other cytokines that stimulate T cell growth, via a gamma chain shared with the IL-2 receptor complex, may have a number of important advantages. In particular, IL-7 and IL-15 support the proliferation and survival of T cells more effectively than IL-2 does, and we have reported recently, in the B16 melanoma model, that culturing B/I-activated DLN cells in IL-7 + IL-15 resulted in much greater proliferation and improved survival of T cells than the same type cells grown in IL-2[17]. T cells grown in IL-7/15 were consistently at least as effective as T cells grown in IL-2 at inducing regression of melanoma lung metastases. IL-7/15 are also less likely to stimulate growth of Treg cells, which respond to IL-2 and may suppress antitumor immune responses [18–24]. Moreover, these alternate gamma-chain cytokines have been found to support preferential differentiation of CD8+ T cells toward a central memory (Tcm) phenotype, which, as suggested by some researchers, may be more effective at inducing tumor regression than the effector cells that are more likely to develop when lymphocytes are grown in IL-2 [25–28]. IL-15 also favors greater survival of high versus low avidity cytotoxic T lymphocytes, and higher expression of the CD8 coreceptor [29]. Although we observed a shift in the phenotypes for melanoma-specific T cells grown in IL-7/15 compared with cells grown in IL-2, they were only slightly more effective at inducing regression of melanoma lung metastases at equivalent cell doses. Thus, the major advantage of IL-7/15 over IL-2 in the melanoma model related to the greater yield of viable T cells, and not to any major increase in their in vivo activity.
In this article, however, we show that 4T1 mammary carcinoma-specific T cells activated with B/I not only grow far more rapidly and for a longer time when cultured in IL-7/15 than in IL-2, but also are far more effective at mediating regression of established subcutaneous 4T1 tumors than the same cells grown in IL-2.
Methods
Mice
Virus-free BALB/c mice (National Cancer Institute, Bethesda, MD) and between 8 and 12 weeks of age, caged in groups of six or fewer, were provided with food and water ad libitum. All guidelines of the Virginia Commonwealth University Institutional Animal Care and Use Committee, which conform to the American Association for Accreditation of Laboratory Animal Care and the U.S. Department of Agriculture recommendations for the care and humane experimental use of animals, were followed.
Tumor cell lines
4T1 mammary tumor cell line was kindly provided by Dr. Jane Tsai of the Michigan Cancer Foundation, Detroit, Michigan. Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) with 10% heat-inactivated fetal calf serum (Hyclone, Logan, UT), 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma, St. Louis MO) (modified DMEM). Tumor cells were harvested for inoculation of mice with 0.05% trypsin–EDTA (Fisher, Pittsburgh, PA).
Draining lymph node sensitization
Donor mice were vaccinated in the left hind footpad with 1 × 106 viable 4T1 cells. Ten days after footpad vaccination, when these mice had a growing tumor in the foot, popliteal tDLNs were harvested under sterile conditions.
Lymphocyte activation and in vitro expansion
Draining lymph nodes were harvested and dispersed into a single cell suspension in complete RPMI media at 1 × 106 cells/ml. The cells were activated by incubation with 5 nM bryostatin 1 (provided by the National Cancer Institute, Bethesda, MD) and 10 nM ionomycin (Calbiochem, San Diego, CA) (B/I), and 80U/ml of rIL-2 (Chiron, Emeryville, CA) at 37°C for 18 h. Cells were washed three times with warm, complete RPMI and resuspended at 1–2 × 106 cells/ml with 40 U/ml of rIL-2 or in IL-7 + IL-15 (10 ng/ml each). The cells were allowed to proliferate in culture for an additional 6–14 days and were split every 2–3 days to maintain 1–2 × 106 cells/ml concentration. Fresh cytokines were added at each split to achieve the same final concentration as at culture initiation. Samples were assessed for the number of viable cells, by trypan blue exclusion, at various days of culture (usually at the time of splits), as shown.
Adoptive immunotherapy
Host mice were inoculated in the left flank with 2.5 × 104–5 × 104 4T1 cells. One day prior to AIT, mice were pretreated with cyclophosphamide (CYP, Mead Johnson, Princeton, NJ), 100 mg/kg intraperitoneally (IP). After various time points in culture, (usually 6–10 days), the B/I-activated and expanded DLN lymphocytes were washed twice in phosphate buffered saline, filtered through a 70-μm nylon mesh strainer (Invitrogen, Carlsbad, CA), and injected intravenously (IV) in 0.5 ml into host mice. No systemic cytokines or vaccinations were administered to these tumor-bearing mice.
Flow cytometry
Cells harvested from cultures at various time points were stained with a panel of antibodies and analyzed by dual color flow cytometry for surface marker expression on an ELITE Beckman Coulter flow cytometer. Fluorescently labeled Abs directed against the following markers were obtained from Pharmingen (San Diego, CA): CD4 (GK1.5), CD8 (53-6.7), CD44 (IM7), CD62L (MEL-14), and CD69 (H1.2F3). Intracellular staining for Foxp3 was also performed on permeabilized cells, using PE anti-mouse/rat Foxp3 staining set (eBioscience, San Diego, CA). Appropriate isotype controls were used in all the cases.
Proliferation assays
Lymphocytes activated with B/I were cultured in 96-well plates (at either 12,000 or 25,000 viable cells per well in 0.2 ml) in IL-2 (40U/ml) or IL-7/15 (10 ng/ml each). After 3–9 days of culture, the cells were labeled with 1 μCi per well of [3H]thymidine (PerkinElmer Life and Analytical Sciences, Boston, MA) and were harvested 24 h later with a semiautomated harvester (PHD: Cambridge Technology, Inc., Cambridge, MA). Thymidine uptake was determined by liquid scintillation counting and expressed as the mean cpm ± SD of triplicate wells.
Tumor measurements
In all the AIT experiments, tumor growth was monitored with biweekly measurements of perpendicular diameters. Results are reported as the mean tumor area ± standard error (SE). When the tumor area was greater than 100 mm2 or if a mouse appeared ill, then the animal was euthanized by CO2 inhalation. Complete tumor regression was defined as the absence of a measurable tumor on three consecutive measurements.
Cytokine release assays
Interferon-γ (IFN-γ) release in supernatants from tumor-sensitized and B/I-activated and expanded lymphocytes, in response to stimulation with irradiated 4T1 for 24 h, was assayed using BD OptEIA mouse IFN-γ ELISA sets from BD Biosciences (San Jose, CA).
Statistical analysis
Differences in tumor growth and in vitro assay results (proliferation and IFN-γ release) were assessed by repeated measures analysis of variance (ANOVA) and Tukey–Kramer honestly significant difference test (Tukey’s HSD) using PROC GLIMMIX, version 9.2 (SAS Institute Inc., Cary, N.C.). In vivo experiments included at least six mice per group and were repeated at least twice. A value of P < 0.05 was used throughout to determine significant differences. Descriptive statistics shown are means ± standard errors (SEMs).
Results
Expansion of 4T1 DLN cells in IL-7/15 or alternating cytokines is much greater than in IL-2 alone
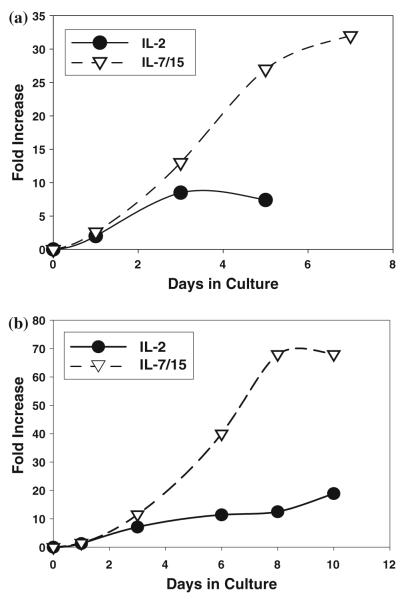
As shown in Fig. 1, 4T1 tumor-DLN cells activated with B/I and cultured in IL-7/15 consistently expanded 20–50-fold in 6 days, compared to only 5–10-fold expansion during the same period of time in IL-2. Moreover, cells cultured in IL-7/15 continued to expand until at least 10–12 days after B/I activation, while the same cells grown in IL-2 started to decline in number after day 5 or day 6 and usually had to be discarded before day 10 because of poor viability. In several experiments (Fig. 1b), IL-7/15-cultured cells expanded 30–70-fold before 2 weeks, without any restimulation with antigen or B/I. The results shown in Fig. 1 are representative of more than a dozen separate experiments. For nine separate experiments in which both IL-2 and IL-7/15 cultured cells were counted on day 7, the median expansions were 9- and 32-fold, respectively.
Fig. 1.
IL-7/15 induce greater expansion of antigen-sensitized, B/I-activated lymphocytes than IL-2. 4T1-sensitized DLN cells were activated with B/I and cultured in IL-2 or IL-7/15. Fold-expansion is based on viable cell counts on each day, compared to the number plated immediately after pulsing with B/I
In order to determine whether the increased yield of cells in IL-7/15 resulted from an increase in proliferation, B/I-activated DLN cells resuspended in either IL-2 or IL-7/15 were pulsed with tritiated thymidine at various days of culture and assessed for thymidine incorporation 24 hours later. As shown in Fig. 2, proliferation was dramatically higher for cells grown in IL-7/15 at both day 6 and day 9. In addition, part of the increased yield of viable cells in alternate cytokines was accounted for by the higher proportion of dead cells (as assessed by trypan blue uptake) in IL-2. For example, at days 3, 6, 9, and 12 in one experiment, the proportions of dead cells in IL-2 were 7.3, 13.5, 30, and 36% versus 1.5, 6.4, 23, and 13% in IL-7/15, respectively (representative of 3 separate experiments).
Fig. 2.
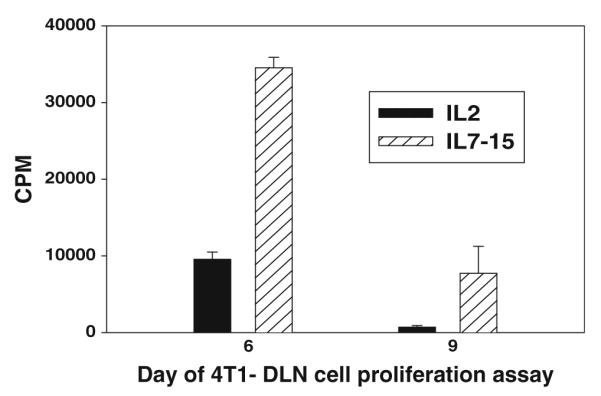
Increased proliferation rate of B/I-activated lymphocytes cultured in IL-7/15 compared to IL-2. DLN cells activated with B/I and cultured in IL-2 or IL-7/15 were pulsed with tritiated thymidine on day 6 or 9 and harvested on glass-fiber filters 24 h. later and counted in a beta scintillation counter. Mean cpm’s ± SEM are shown for triplicate wells [By ANOVA, F(1,8) = 82.45, P < 0.0001 for IL-7/15 cells versus IL-2 cells]. Similar results were obtained on day 3
Phenotypes of cells grown in IL-7/15 versus IL-2
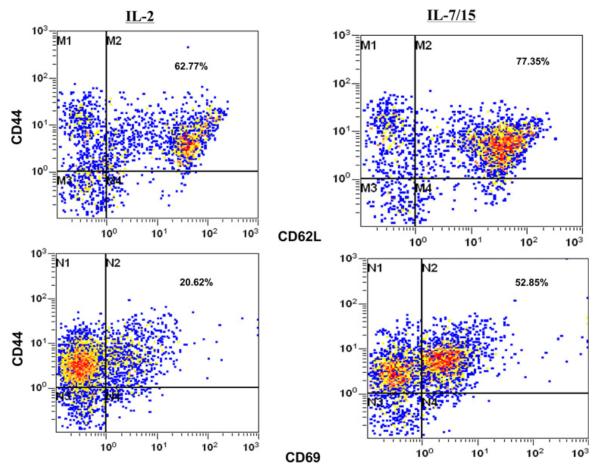
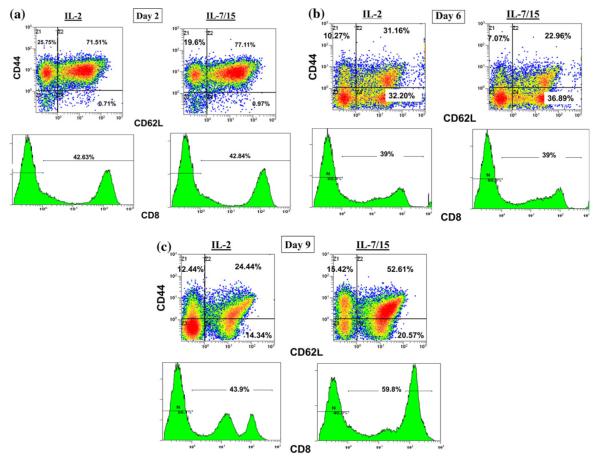
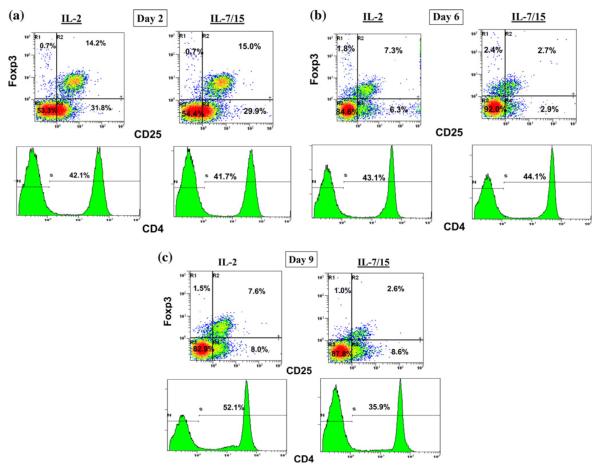
Analysis of the phenotypes grown in different cytokines showed some key differences in the resulting distribution of phenotypes. As shown in Table 1, B/I-activated lymph node cells grown in IL-7/15 had higher proportions of CD8+, CD62L+, and CD69+ cells than cells grown in IL-2. Moreover, with double-staining analysis, IL-7/15 cultured cells had a higher proportion of CD44+CD62L+ double-positive memory cells (Fig. 3). These results are representative of three separate experiments, which are summarized in Table 2. With triple-staining, it was possible to determine that the proportion of CD8+ cells with memory cell phenotypes, as shown in Fig. 4, became higher when cells were grown in IL-7/15. Also, note that on day 9, the CD8+ cells grown in IL-2 were nearly evenly divided between low and high CD8 expressing cells, while nearly all the CD8+ cells grown in IL-7/15 were CD8high. In addition, as the cultures were continued over time, Treg cells, detected as triple-positive CD4+CD25+Foxp3+ cells (Fig. 5), at first declined, but at later time points were more frequent among CD4+ cells grown in IL-2 (14.2, 7.3, and 7.6% on days 2, 6, and 9, respectively) compared to cells grown in IL-7/15 (15, 2.7, and 2.6% on the same days as above). Moreover, as shown in Fig. 5, because CD4+ cells represented lower proportions of viable cells as cultures were maintained in IL-7/15, the proportion of Treg cells in relation to total viable cells was even more disparate between the two conditions by day 9 (4% in IL-2 versus 0.9% in IL-7/15). These results are representative of two separate experiments.
Table 1.
Phenotypes by single staining of DLN cells before (baseline), or on, day 6 or day 7 after activation with B/I and culture in either IL-2 or IL-7/15*
| Phenotype | Baseline |
Grown in IL-2 |
Grown in IL-7/15 |
|||
|---|---|---|---|---|---|---|
| Mean % | SEM | Mean % | SEM | Mean % | SEM | |
| CD4+ | 15 | – | 53 | 3.5 | 38 | 14 |
| CD8+ | 12.5 | 1.5 | 38 | 3.1 | 52 | 6 |
| CD44+ | 25 | 9 | 90 | 4.5 | 93 | 2.5 |
| CD62L+ | 65 | 2.5 | 57.5 | 3.5 | 71 | 7.8 |
| CD69+ | 4 | – | 17 | 2.3 | 37 | 7.2 |
Results shown are means ± SEM for four separate experiments for CD8, CD44, CD62L, 3 for CD69, 2 for CD44 and all but CD4 and CD69 for baseline values; CD4 and CD69 at baseline are from one experiment
Fig. 3.
Phenotypes of B/I-activated cells grown in IL-2 versus IL-7/15. 4T1-sensitized DLN cells were activated with B/I and cultured in IL-2 or IL-7/15 for 8 days. Samples were taken on days 3, 6, and 8 of expansion and incubated with mAb against CD44 and CD62L. Fluorescence of 15,000 cells per sample was analyzed by flow cytometry. Numbers represent percentage of double-positive cells for day 6 samples in this experiment. Results are representative of three separate experiments
Table 2.
Phenotypes of DLN cells double stained for CD44 and CD62L or CD69 after 7 days cultured in IL-2 or IL-7/15*
| Phenotype | Grown in IL-2 |
Grown in IL-7/15 |
||
|---|---|---|---|---|
| Mean % | SEM | Mean % | SEM | |
| CD44+, CD62L+ | 63 | 0.6 | 75 | 3.1 |
| CD44+, CD69+ | 16.7 | 3.4 | 43.3 | 9.7 |
Results shown are means ± SEM for three separate experiments
Fig. 4.
Culture of B/I-activated lymphocytes in IL-7/15 gradually increases proportion of CD44+CD62L+ double-positive CD8+ cells compared with IL-2. 4T1-sensitized DLN cells were activated with B/I and cultured in IL-2 or IL-7/15. Samples were taken on days 2, 6, and 9 of expansion (a, b, and c, respectively) and incubated with mAb against CD8, CD44, and CD62L. Fluorescence of 15,000 cells per sample was analyzed by flow cytometry. Results are representative of three separate experiments
Fig. 5.
Culture of B/I-activated lymphocytes in IL-7/15 decreases proportion of Treg cells compared with IL-2. 4T1-sensitized DLN cells were activated with B/I and cultured in IL-2 or IL-7/15. Samples were taken on days 2, 6, and 9 of expansion (a, b, and c, respectively) and incubated with mAb against CD4, CD25, and Foxp3. Fluorescence of 15,000 cells per sample was analyzed by flow cytometry. Results are representative of two separate experiments
IFN-γ responses of DLN cells grown in alternate cytokines
DLN cells activated with B/I and then cultured in either IL-2 or IL-7/15 were harvested and then cultured for 24 h alone or with irradiated 4T1 stimulator cells. As shown in Fig. 6, cells grown in IL-2 secreted higher unstimulated levels of IFN-γ than did cells grown in IL-7/15. Moreover, IL-2 cultured cells also demonstrated markedly higher levels of IFN-γ secretion in response to tumor cells. Although each repeat experiment was slightly different in timing and IFN-γ levels were quite variable, in three experiments in which cells were tested on day 6 or 7, the mean IFN-γ secretions in response to 4T1 cells were 4,984 pg/ml (±1,755) for IL-2 and 2,683 pg/ml (± 963) for IL-7/15. Thus, cells grown in IL-7/15 had lower levels of effector function by this criterion. As shown previously, the IFN-γ secretion in response to 4T1 cells by DLN cells activated with B/I and expanded in vitro is tumor antigen specific[30, 31].
Fig. 6.
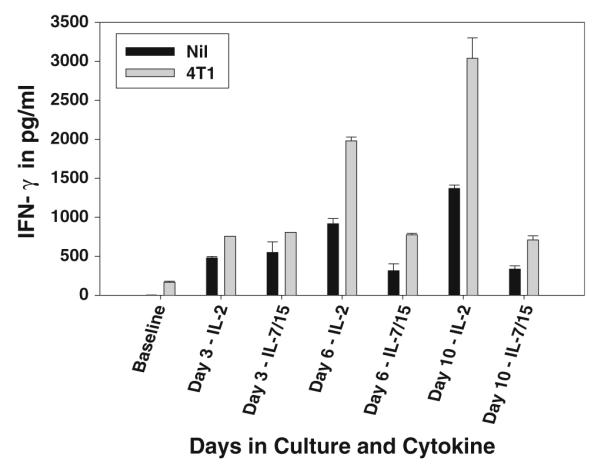
IFN-γ release in response to tumor antigen after expansion in IL-2 or IL-7/15 or alternating cytokines. 4T1-sensitized DLN cells were activated with B/I and cultured in IL-2 or IL-7/15. Cells were harvested from cultures at times shown on × axis. Cells were incubated with stimulants (nil or irradiated 4T1 cells) at a ratio of 10:1 in 24 well plates at a concentration of 1 × 106 lymphocytes/ml. Supernatants were collected 24 h after exposure to stimulants and assayed for IFN-γ using the murine IFN-γ ELISA kit. Baseline refers to the response of cells immediately after harvest of DLN. IFN release from cells cultured in IL-2 was significantly higher than from cells cultured in IL-7/15 on days 6 and 10 [F(1,14) = 92.35 and 344.84, respectively, P < 0.0001]
AIT with T cells grown in IL-7/15 induced tumor regression much more effectively than T cells grown in IL-2
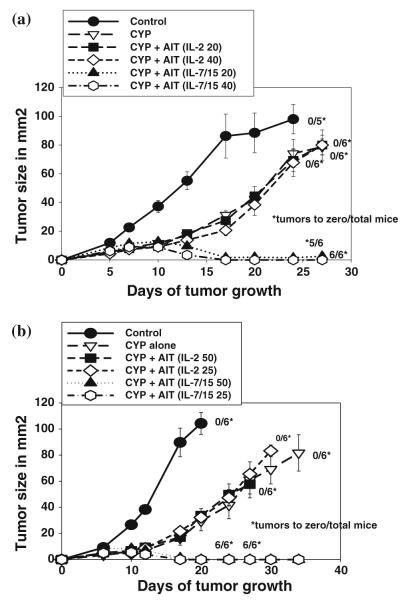
Although we have reported that AIT with 4T1-sensitized tDLN cells activated with B/I and grown in IL-2 could induce regression of established 4T1 tumors, this effect sometimes required infusion of very large numbers of cells, and sometimes was ineffective with T cell doses in the range of 20–50 million cells per mouse. As shown in Fig. 7, with infusion of 75 million T cells per mouse, activated DLN cells grown in IL-2 inhibited tumor growth dramatically compared to control or CYP alone groups, and induced complete regression in four out of six mice [IL-2 AIT vs Control: F(1,164) = 115.99, P < 0.0001; vs CYP: F(1,164) = 64.32, P 7lt; 0.0001]. When cells grown in IL-7/15 were infused at the same cell dose, tumors regressed very rapidly in all the seven mice. [IL-7/15 AIT vs Control F(1,164) = 141.37, P < 0.0001; vs CYP: F(1,164) = 99.8, P < 0.0001].
Fig. 7.
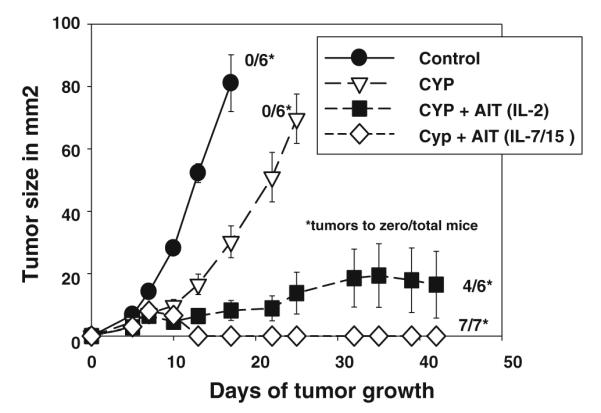
Adoptive immunotherapy versus 4T1 subcutaneous tumors with T cells activated with B/I and cultured in IL-2 or IL-7/15. Recipient BALB/c mice were injected s.c. in the flank with 25,000 4T1cells and randomized to four treatment groups: untreated control (CON), cyclophosphamide (CYP) treated (100 mg/kg), and CYP + AIT (at 75 × 106 cells/mouse) with B/I-activated DLN cells from 4T1-sensitized BALB/c mice cultured in IL-2 or IL-7/15. CYP was given 3 days after tumor inoculation and cultured lymphocytes were infused i.v. the following day. Mean tumor sizes ± SE are charted over time. Numbers to the right indicate the number of mice with complete tumor regression per total number of mice in each group
With infusion of smaller numbers of cells, however, only cells grown in IL-7/15 induced complete regression of 4T1 tumors in most or all of the tumor-bearing mice (Fig. 8). As shown, treatment with the same numbers of cells grown in IL-2 did not have a significant effect compared with CYP alone. All the IL-7/15 AIT groups were significantly different from CON and from CYP groups. In Fig. 8a, F(1,251) = 81.9 P < 0.001 vs CON; F(1,251) = 19.98 and 46.10 for 20 and 40 million cells vs CYP alone, respectively. In Fig. 8b, for IL-7/15 AIT groups at 25 and 50 million cells versus CON, F (1,167) = 127.24 and 108.58, respectively, P < 0.0001; versus CYP alone F(1,167) = 58.83 and 36.6, respectively, P < 0.0001. Neither of the IL-2 AIT groups were significantly different compared to CON or CYP in either experiment. IL-7/15 at 20 or 40 million cells were significantly different from IL-2 AIT groups at equivalent cell doses in both experiments [In Fig. 8a, F(1,251) = 92.35 for 20 million and 37.95 at 40 millions, both P < 0.0001; in Fig. 8b, F(1,167) = 71.57and 27.88 at 25 and 50 million cells, P < 0.0001 and P = 0.0002, respectively].
Fig. 8.
Adoptive immunotherapy versus 4T1 subcutaneous tumors with graded doses of T cells activated with B/I and cultured in IL-2 or IL-7/15. Recipient BALB/c mice were injected s.c. in the flank with 25,000 4T1 cells and randomized to four treatment groups: untreated control (CON), cyclophosphamide (CYP) treated (100 mg/kg), and CYP + AIT with B/I-activated DLN cells from 4T1-sensitized BALB/c mice cultured in IL-2 or IL-7/15 at varying doses of cells/mouse (20 or 40 × 106 in a and 25 or 50 × 106 in b). CYP was given 3 days after tumor inoculation and cultured lymphocytes were infused i.v. the following day. Mean tumor sizes ± SE are charted over time. Numbers to the right indicate the number of mice with complete tumor regression per total number of mice in each group
Even with doses as low as 9 million IL-7/15-cultured cells per mouse, 2/5 mice were cured, with a tumor growth curve for the overall group that was similar to that obtained with 75 million IL-2-grown cells (data not shown).
Memory function in cured mice and in naïve mice not previously exposed to tumor
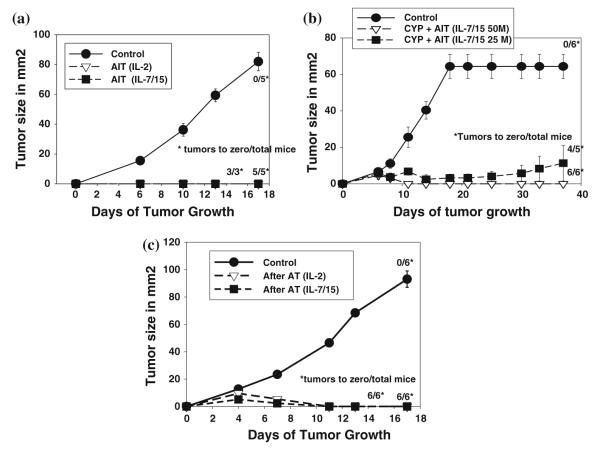
When mice that had been cured and did not develop metastases over a 2-month period after AIT with either IL-2 or IL7/15-cultured T cells were rechallenged with 4T1 tumor cells, most of the cured mice were completely resistant to tumor growth, compared to none of the controls (Fig. 9a, b). Prior to the experiment shown in Fig. 9a, the mice challenged were initially cured by AIT with 75 × 106 cells grown either in IL-2 or IL-7/15. Three out of three is for the group that had received IL-2 cultured cells (only three out of six mice remained tumor-free at 2 months), and 5/5 is for group that had received cells cultured in IL-7/15 (5/7 remained tumor-free at 2 months). Both the groups were significantly different from CON [F(1,33) = 144.7 and 179.0 for IL-2 and IL-7/15 AIT vs CON, respectively, P < 0.0001]. In the experiment depicted in Fig. 9b, mice had been cured with AIT using cells cultured in IL-7/15, at either 25 or 50 × 106 cells per mouse. All but one mouse initially treated with AIT using IL-7/15 cultured cells were tumor-free at 2 months. Both the groups were significantly different from CON [F(1,82) = 37.9 and 158.00 for IL-7/15 AIT at 25 and 50 million cells vs CON, respectively, P < 0.0001]. It is worth noting that AIT, especially with IL-7/15 cultured cells, induced not only regression of the primary tumors but also long-term cures in most of the treated mice used in these challenge experiments. Without immunotherapy, and even with AIT using IL-2 cultured cells, most of these mice died within 1 month from meta-static disease, with or without progression or recurrence of the primary tumor.
Fig. 9.
Adoptive immunotherapy with B/I-activated DLN cells from 4T1-sensitized mice provides long-term resistance to tumor challenge. (a, b) BALB/c mice that had been cured of s.c. 4T1 tumors by AIT and had not developed recurrence or metastases after 2 months were challenged with inoculation of 50,000 4T1 tumor cells again, as were a new set of six control mice. No CYP was given to any mice prior to or after rechallenge. c Naïve mice received either no lymphoid cells or 4T1-sensitized DLN cells activated with B/I and expanded in either IL-2 or IL-7/15. No CYP was given. Three weeks after infusion of lymphocytes, mice were challenged s.c. with 50,000 4T1 tumor cells. Mean tumor sizes ± SE are charted over time. Numbers to the right indicate the number of mice with complete tumor regression per total number of mice in each group
In order to determine whether this immunologic memory response was actually a function of the adoptively transferred cells or reflected sensitization of the host T cells by the inoculated tumor, we adoptively transferred B/I-activated 4T1 DLN cells into naïve mice, and the naïve mice and control mice were inoculated with 4T1 tumor cells 3 weeks later. None of these mice received CYP, cytokines, nor were they ever exposed to tumor antigen prior to the challenge. As shown in Fig. 9c, mice that received T cells grown in either IL-2 or IL-7/15 were completely resistant to tumor growth. Both the groups were significantly different from CON [F(1,65) = 109.83 for both IL-2 and IL-7/15 vs CON, P < 0.001].
Discussion
As we have shown previously in the B16 melanoma system, with either of the wild type or TcR transgenic T cells, we now show in a mammary carcinoma that culturing B/I-activated lymphocytes from tumor-sensitized mice in IL-7/15 results in a much higher yield of viable cells than that of the same cells cultured in IL-2. This results from greater proliferation and higher cell viability. Moreover, cells grown in IL-7/15 continue to expand in numbers during a second week in culture, without restimulation via the antigen receptor pathway, while similar cells grown in IL-2 stop proliferating beyond 1 week in culture. These results are consistent with those of Klebanoff et al., who showed that culturing antigen-sensitized T cells in IL-15 prior to AIT produced many more numbers of cells in vivo than the number of cells cultured in IL-2 [25, 26]. However, they did not comment on the relative growth of cells in vitro in the two different cytokines. The finding that IL-7 combined with IL-15 was markedly more effective than IL-2 is consistent with the suggestion made previously that, even though both IL-7 and IL-15 have roles in the maintenance and self-renewal of memory precursors, effector CD8+ T cells actually need both IL-7 and IL-15 for optimal proliferation and to become long-lived memory cells [32].
The data shown here contrast with those of Carrio et al., who showed that IL-2 induced greater proliferation of CTL than IL-7 or IL-15 alone [33]. The contradictory results may be related to different methods of T cell activation. Carrio et al. activated naïve T cells in vitro with splenocytes pulsed with specific peptide plus IL-2, while our protocol employs pharmacological activation with B/I of T cells previously sensitized by tumor antigen in vivo. It is possible that T cell activation with B/I produces stronger and more prolonged TcR pathway signaling, which would increase the capacity of sensitized T cells to respond to homeostatic cytokines, such as IL-7 and IL-15 [34, 35]. However, in agreement with Carrio et al. and in contrast to what we found in the B16 melanoma model, we did observe a shift away from IFN-γ production for cells grown in IL-7/15 versus those grown in IL-2; this is thought to be characteristic of differentiation toward Tcm cells and away from terminally differentiated effector cells [28, 32]. The greater antitumor activity of the IL-7/15 cells in vivo, despite lower effector function, agrees with findings of other authors who have suggested that T cells with greater effector functions, such as cytotoxicity and IFN-γ release, may actually be less effective at inducing tumor regression than memory T cells with lower effector function activity [25, 26, 28]. The greater antitumor activity for cells grown in IL-7/15 may also be related to the higher proportion of CD8+ cells than what was found for cells grown in IL-2, as we have shown previously that these cells are predominantly responsible for tumor regression in this model [30]. The higher proportion of CD8high cells after growth in IL-7/15 (Fig. 4c) may also help to explain the greater antitumor activity, since CD8high T cells have higher avidity for target cells than CD8low T cells and are favored by exposure to IL-15 [29]. The selective survival and proliferation of T cells with higher avidity TcR in the presence of IL-15 may also contribute to greater antitumor activity in vivo.
Melchionda et al. showed that IL-7 and IL-15 were comparable to or better than IL-2 at expanding antigen-sensitized CD8+ T cells when given as an adjuvant in vivo along with immunization [27]. However, they found no additive or synergistic effect when IL-7 and IL-15 were combined. In our in vitro B/I activation model, we have shown that the combination of IL-7 and IL-15 has an additive effect on expansion, increasing T cell numbers 7–10-fold over IL-2 and leading to growth that was prolonged at least 2-fold longer in culture. We found that this difference in expansion resulted partly from increased cell viability in IL-7/15, which is consistent with the previously reported protective roles of IL-7 and IL-15 against apoptosis versus the propensity for IL-2 to cause activation-induced cell death [17, 23, 36–40].
Although it has been shown that T cells grown in IL-15 persisted longer after adoptive transfer and provided better protection against tumor challenge than cells grown in IL-2, IL-15-expanded T cells were actually less effective at inducing regression of established tumors in a model using OVA-transfected EL-4 [33, 41]. However, Klebanoff et al. found that IL-15-cultured pmel-1 cells were more effective at inducing regression of established s.c. B16 melanomas than T cells cultured in IL-2. This was related to a shift toward a Tcm phenotype, greater proliferation of IL-15 cultured cells in vivo, and increased trafficking to lymphoid tissues [25, 26]. In our previously reported results with a melanoma model, we did observe a moderate shift toward a greater number of CD62L+ cells when B/I-activated T cells were cultured in IL-7/15 versus IL-2, but the IL-7/15 cells secreted higher levels of IFN-γ in response to tumor antigen and were somewhat more effective at inducing tumor regression than IL-2–cultured cells; but this difference was not as dramatic as the earlier results of Klebanoff et al., or those reported here. This could relate to a number of differences, including the use of antigen-sensitized versus naïve pmel-1 T cells, the use of B/I to reactivate the cells in vitro, treating pulmonary metastases rather than s.c. tumors, or the combined use of IL-7 and IL-15 instead of IL-15 alone. It is possible that the ability of T cells to traffic to lymphoid organs, which has been shown to be favored by the use of IL-15 and the Tcm phenotype, may not be as important for treatment of melanoma pulmonary metastases as it would be for s.c. solid tumors, or for response to a vaccine. Trafficking of cells grown in different cytokines will be explored in our future studies. In the results shown here, however, we found that T cells grown in IL-7/15 developed a higher proportion of cells with a memory phenotype and secreted less IFN-γ than cells grown in IL-2 and were dramatically more effective against established s.c. tumors in vivo. This result agrees with the previously reported data suggesting that Tcm with less effector function may be more effective for AIT [25, 26].
Preliminary studies have been reported with human PBL from melanoma patients vaccinated with a peptide tumor antigen to examine the relative merits of using alternate cytokines to expand T cells for AIT [42]. These studies did not show consistently any major differences in the yield or phenotypes of cells grown in IL-7, IL-15, or combinations compared to IL-2. This finding does not necessarily mean that results similar to what we are reporting here would not be achieved with human T cells, since this may depend on the source of cells, the prior sensitization, and the method of in vitro activation. In addition, the concentration of IL-2 used in the reported human studies was 300 IU/ml, versus 40–80 IU/ml (which we have found to be the optimal concentration for expansion of B/I-activated cells) in our experiments. This concentration also maintains antigen specificity more reliably than higher doses. We also used lower concentrations of IL-7 and IL-15 than those that were used in the human studies. In this mammary carcinoma model, using wild-type tumor-sensitized T cells, we found that IL-7/15-cultured cells were markedly more effective in vivo than cells grown in IL-2 on a per cell basis. Even if the IL-7/15-cultured cells did not function better in vivo, the increased yield of viable cells would still offer a major advantage. This may be of critical importance for clinical use, especially when the number of starting antigen-sensitized T cells is limited. This would likely be the case with vaccine-draining lymph nodes, an approach we plan to apply clinically. The comparatively simple regimen described here, with a modest dose of CYP followed by infusion of T cells cultured for short periods of time, may be particularly applicable to clinical use. Future experiments will examine more detailed comparisons of the in vivo functions, phenotypes, and fates after adoptive transfer of cells grown in IL-7/15 versus those grown in IL-2.
Contributor Information
Esther Cha, Department of Physiology and Biophysics, Virginia Commonwealth University’s Medical College of Virginia, Richmond, VA, USA estherhyunwoocha@gmail.com.
Laura Graham, Division of Surgical Oncology, Department of Surgery and the Massey Cancer Center, Virginia Commonwealth University’s Medical College of Virginia, Richmond, VA, USA lgraham2@mcvh-vcu.edu.
Masoud H. Manjili, Department of Microbiology and Immunology and the Massey Cancer Center, Virginia Commonwealth University’s Medical College of Virginia and the Massey Cancer Center, Richmond, VA, USA mmanjili@vcu.edu
Harry D. Bear, Division of Surgical Oncology, Department of Surgery and the Massey Cancer Center, Virginia Commonwealth University’s Medical College of Virginia, Box 980011, Richmond, VA 23298-0011, USA
References
- 1.Chang AE, Li Q, Jiang G, Sayre DM, Braun TM, Redman BG. Phase II trial of autologous tumor vaccination, anti-CD3-activated vaccine-primed lymphocytes, and interleukin-2 in stage IV renal cell cancer. J Clin Oncol. 2003;21:884–890. doi: 10.1200/JCO.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Nijhuis EWP, Wiel-van Kemenade EVD, Figdor CG, Van Lier RAW. Activation and expansion of tumour-infiltrating lymphocytes by anti-CD3 and anti-CD28 monoclonal antibodies. Cancer Immunol Immunother. 1990;32:245–250. doi: 10.1007/BF01741708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander JP, Kudoh S, Melsop KA, Hamilton TA, Edinger MG, Tubbs RR, Sica D, Tuason L, Klein E, Bukowski RM, Finke JH. T-cells infiltrating renal cell carcinoma display a poor proliferative response even though they can produce interleukin 2 and express interleukin 2 receptors. Cancer Res. 1993;53:1380–1387. [PubMed] [Google Scholar]
- 4.Correa MR, Ochoa AC, Ghosh P, Mizoguchi H, Harvey L, Longo DL. Sequential development of structural and functional alterations in T cells from tumor-bearing mice. J Immunol. 1997;158:5292–5296. [PubMed] [Google Scholar]
- 5.Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205–2211. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 6.Morse MA, Clay TM, Lyerly HK. Current status of adoptive immunotherapy of malignancies. Expert Opin Biol Ther. 2002;2:237–247. doi: 10.1517/14712598.2.3.237. [DOI] [PubMed] [Google Scholar]
- 7.Tuttle TM, Inge TH, Bethke KP, McCrady CW, Pettit GR, Bear HD. Activation and growth of murine tumor-specific T-cells which have in vivo activity with bryostatin 1. Cancer Res. 1992;52:548–553. [PubMed] [Google Scholar]
- 8.Tuttle TM, Bethke KP, Inge TH, McCrady CW, Pettit GR, Bear HD. Bryostatin 1-activated T cells can traffic and mediate tumor regression. J Surg Res. 1992;52:543–548. doi: 10.1016/0022-4804(92)90126-k. [DOI] [PubMed] [Google Scholar]
- 9.Tuttle TM, McCrady CW, Inge TH, Salour M, Bear HD. γ-interferon plays a key role in T-cell-induced tumor regression. Cancer Res. 1993;53:833–839. [PubMed] [Google Scholar]
- 10.Tuttle TM, Fleming MF, Hogg PS, Inge TH, Bear HD. Low-dose cyclophosphamide overcomes metastasis-induced immunosuppression. Ann Surg Oncol. 1994;1:53–58. doi: 10.1007/BF02303541. [DOI] [PubMed] [Google Scholar]
- 11.Yee C. Adoptive T cell therapy—immune monitoring and MHC multimers. Clin Immunol. 2003;106:5–9. doi: 10.1016/s1521-6616(02)00015-3. [DOI] [PubMed] [Google Scholar]
- 12.Chin CS, Miller CH, Graham L, Parviz M, Zacur S, Patel B, Duong A, Bear HD. Bryostatin 1/ionomycin (B/I) ex vivo stimulation preferentially activates L-selectinlow tumor-sensitized lymphocytes. Int Immunol. 2004;16:1283–1294. doi: 10.1093/intimm/dxh130. [DOI] [PubMed] [Google Scholar]
- 13.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 14.Kazanietz MG, Lewin NE, Gao F, Pettit GR, Blumberg PM. Binding of [26-3H]bryostatin 1 and analogs to calcium-dependent and calcium-independent protein kinase C isozymes. Mol Pharmacol. 1994;46:374–379. [PubMed] [Google Scholar]
- 15.Pettit GR, Herald SL, Doubek DL, Arnold E, Clardy J. Isolation and structure of bryostatin 1. J Am Chem Soc. 1982;104:6846–6848. [Google Scholar]
- 16.Chatila T, Silverman L, Miller R, Geha R. Mechanisms of T cell activation by the calcium ionophore ionomycin. J Immunol. 1989;143:1283–1289. [PubMed] [Google Scholar]
- 17.Le HK, Graham L, Miller CH, Kmieciak M, Manjili MH, Bear HD. Incubation of antigen-sensitized T lymphocytes activated with bryostatin 1 + ionomycin in IL-7 + IL-15 increases yield of cells capable of inducing regression of melanoma metastases compared to culture in IL-2. Cancer Immunol Immunother. 2009;58:1565–1576. doi: 10.1007/s00262-009-0666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 19.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 20.Oh S, Berzofsky JA, Burke DS, Waldmann TA, Perera LP. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc Natl Acad Sci USA. 2003;100:3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waldmann TA. The biology of interleukin-2 and inter-leukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 22.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 23.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–623. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 24.Haux J, Johnsen AC, Steinkjer B, Egeberg K, Sundan A, Espevik T. The role of interleukin-2 in regulating the sensitivity of natural killer cells for Fas-mediated apoptosis. Cancer Immunol Immunother. 1999;48:139–146. doi: 10.1007/s002620050558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior anti-tumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8(+) T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ra-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chin CS, Graham LJ, Hamad GG, George KR, Bear HD. Bryostatin/ionomycin-activated T cells mediate regression of established tumors. J Surg Res. 2001;98:108–115. doi: 10.1006/jsre.2001.6181. [DOI] [PubMed] [Google Scholar]
- 31.Parviz M, Chin CS, Graham LJ, Miller C, Lee C, George K, Bear HD. Successful adoptive immunotherapy with vaccinesensitized T cells, despite no effect with vaccination alone in a weakly immunogenic tumor model. Cancer Immunol Immun-other. 2003;52:739–750. doi: 10.1007/s00262-003-0405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 33.Carrio R, Bathe OF, Malek TR. Initial antigen encounter programs CD8+ T cells competent to develop into memory cells that are activated in an antigen-free, IL-7- and IL-15-rich environment. J Immunol. 2004;172:7315–7323. doi: 10.4049/jimmunol.172.12.7315. [DOI] [PubMed] [Google Scholar]
- 34.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 35.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Keller AM, Borst J. Control of peripheral T cell survival: a delicate division of labor between cytokines and costimulatory molecules. Hum Immunol. 2006;67:469–477. doi: 10.1016/j.humimm.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Sprent J, Cho JH, Boyman O, Surh CD. T cell homeostasis. Immunol Cell Biol. 2008;86:312–319. doi: 10.1038/icb.2008.12. [DOI] [PubMed] [Google Scholar]
- 38.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Roychowdhury S, May KF, Jr, Tzou KS, Lin T, Bhatt D, Freud AG, Guimond M, Ferketich AK, Liu Y, Caligiuri MA. Failed adoptive immunotherapy with tumor-specific T cells: reversal with low-dose interleukin 15 but not low-dose interleukin 2. Cancer Res. 2004;64:8062–8067. doi: 10.1158/0008-5472.CAN-04-1860. [DOI] [PubMed] [Google Scholar]
- 40.Van PL, Refaeli Y, Lord JD, Nelson BH, Abbas AK, Baltimore D. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–288. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- 41.Rolle CE, Carrio R, Malek TR. Modeling the CD8+ T effector to memory transition in adoptive T-cell antitumor immunotherapy. Cancer Res. 2008;68:2984–2992. doi: 10.1158/0008-5472.CAN-07-3040. [DOI] [PubMed] [Google Scholar]
- 42.Liu S, Riley J, Rosenberg S, Parkhurst M. Comparison of common gamma-chain cytokines, interleukin-2, interleukin-7, and interleukin-15 for the in vitro generation of human tumor-reactive T lymphocytes for adoptive cell transfer therapy. J Immunother. 2006;29:284–293. doi: 10.1097/01.cji.0000190168.53793.6b. [DOI] [PubMed] [Google Scholar]