Abstract
♦ Background: One of the most common and severe complications affecting peritoneal dialysis (PD) patients is exit-site infection of the peritoneal catheter; it is therefore of vital importance to prevent it. This complication has a negative impact on the success of the technique. In spite of this, there are no clear guidelines concerning how to take care of the exit site. The objective of this study was to assess the efficacy of polyhexanide in preventing exit-site infection over a 12-month period.
♦ Methods: We designed a single-center, prospective, open-labeled, randomized controlled clinical trial with parallel groups. Requirements for participation in the study included implantation of the peritoneal catheter at least six weeks before entering the study and no infectious complications requiring either hospital admission or antibiotic treatment for at least three months before entering into the study. Patients were randomized to be daily cured as follows: Group A: traditional care with saline serum and povidone-iodine; and Group B: polyhexanide solution. Exit sites were evaluated at baseline and every four to six weeks or if any event occurred, according to the Twardowski criteria.
♦ Results: Of the 60 included patients, 46 completed the 12-month follow-up period. Six underwent transplantation, five died and three were transferred to hemodialysis (HD). The treatment was well tolerated, with no side effects nor abandonments due to such effects. Throughout the study period, six patients (20%) undergoing traditional care and only two (6,7%) receiving polyhexanide developed an exit-site infection (p = 0.032). There were a total number of 12 infections; nine occurred in patients following the traditional approach and only three in patients treated with polyhexanide (p = 0.037). The germs responsible for the infections were: S. aureus (six cases), Corynebacterium jeikeium (two cases) and P. aeruginosa (one case) in the saline serum and povidone-iodine group and P. aeruginosa (three cases) in the polyhexanide group. The mean rate of exit-site infection was 1 episode/36.6 patient-months for the traditional care group and 1 episode/102.7 patient-months for the polyhexanide group (p = 0.017). Patients following the traditional treatment required fewer days to get infected than those using polyhexanide (p = 0.033; log rank: 4.2).
♦ Conclusions: These results show that using polyhexanide is efficient for the prevention of exit-site infections. Patients treated with this product suffer from fewer infections and need more time to become infected. Polyhexanide application is painless, no allergies have been described and it is well tolerated by patients. We therefore propose that it may be used routinely from now on for the care of healthy exit site.
Key words: Polyhexanide, exit-site infection, peritoneal dialysis
One of the most common and severe complications derived from the peritoneal dialysis (PD) technique is the infection of the exit site of the peritoneal catheter. Exit-site infections are responsible for 20% of peritoneal infections, provoke 20% of catheter removals (1) and are implicated in the transfer to hemodialysis (HD) in 15 - 20% of patients (2). These data show that it is of vital importance to prevent exit-site infections.
The approach to exit-site care must be holistic and it should encompass: care before catheter insertion; immediate care (intraoperatory and post-implantation), and, finally, aftercare, i.e., performed on healthy, healed, chronic (more than 6 weeks) exit sites, which is where more dissimilarities exist (3). In these cases, care should include daily cleaning with soap, until healed, with different antiseptic solutions and topical antibiotics application.
The use of povidone-iodine effectively reduces exit-site infection incidence for up to 140 days after catheter insertion (4) (evidence level II); however, it causes the degradation of polyurethane catheters and skin alterations due to its cytotoxic effect (5), but no evidence of damage from these exit-site preparations has been shown for the commonly used silicone-based catheters.
Mupirocin reduces Staphylococcus aureus (S. aureus) infection, as well as peritoneal infections caused by this organism (6,7), although it is not useful against gram-negative bacteria such as Pseudomonas aeruginosa (P. aeruginosa), it degrades polyurethane catheters (8), and may cause resistances (9).
The common use of other antibiotics such as topical ciprofloxacin, which reduces exit-site infections caused by S. aureus and P. aeruginosa, as well as the topical use of gentamicin, seem to lead to good results, although some authors refer possible manifestations of resistances and infections caused by uncommon organisms, such as Corynebacterium spp (10), Mycobacteria, or fungus (11).
Currently, polyhexanide is considered the treatment of choice for wound cleansing because it achieves microorganism removal thanks to a physical-chemical selective effect which does not affect proper cells (12,13). Its efficacy has been proven against S. aureus, responsible for 60 - 70% of exit-site infections (1), P. aeruginosa, coagulase-negative staphylococci, and fungus, and it features a highly decontaminant effect against bacterial biofilms (14-16). Polyhexanide application is painless and is well accepted by patients, since they don’t have to modify the healing procedures learnt during training. It features a good cutaneous tolerance (no allergies described) and there is neither presence of absorption nor cytotoxicity. Polyhexanide has proven it favors cicatrization when healing wounds (immediate care) and it is also an excellent product against pathogens (exit-site infection care) (15). It has been proven that there is still room for improvement in the care of healthy, chronic exit sites and that the success of PD depends in no small measure on them. For that reason we have decided to use Polyhexanide, which has a successful history in the treatment of chronic wounds.
The aim of the present study is to assess the efficacy of polyhexanide compared to the standard cure used (0.9% saline serum and povidone-iodine (2,11,17)) in preventing exit-site infections.
Patients, Materials and Methods
The primary study outcome was the analysis of the exit-site infection rates in the groups, with the hypothesis that polyhexanide would be more effective than a traditional treatment in preventing exit-site infections. Other outcomes analyzed included overall catheter infection and peritonitis rates, causative organisms, catheter removals as a result of infection, and time to first catheter infection, but the study was not designed to detect a difference in these outcomes. Patients were followed from the first day of study until censored at renal transplant, transfer off PD or to another program, death on PD, patient request for withdrawal from study, or the end of the study. All patients on the PD program from Hospital Universitario Central de Asturias (Oviedo) were assessed for the present study.
Inclusion criteria were: 1) Patients older than 18 years of both genders in PD program in which a peritoneal catheter had been implanted at least six weeks before; 2) Absence of infectious complications which had required either hospital admission or antibiotic treatment at least three months before entering the study; 3) Absence of known reaction or contingent polyhexanide intolerance; 4) The patient or representatives had signed the informed consent of the study.
Exclusion criteria were: 1) Presence of exit-site infection at randomization time; 2) History of bad adherence to treatment and/or medical advice; 3) Withdrawal of the informed consent.
Study Design
A single-center, prospective, open-labeled, randomized controlled clinical trial with parallel groups was designed to study the efficacy and safety of polyhexanide in preventing exit-site infections. Patient enrollment was from March 2009 to June 2009 and they were followed until June 2010. Sixty-five patients were analyzed and 60 met the inclusion and exclusion criteria and were included in the study.
Patients were randomized to be daily cured as follows: Group A: traditional care with 0.9% saline serum and povidone-iodine [SEDEN (Spanish Society of nephrological nursery) guidelines] (18); Group B: polyhex anide solution.
In the traditional care group, routine exit-site care by the patient consisted of daily washing with antibacterial soap, thorough drying, and application of 0.9% saline serum and povidone-iodine around the catheter exit site using a cotton swab. In group B, patients replaced povidone-iodine with polyhexanide solution. Catheters were anchored with tape and a small gauze dressing to prevent exit-site trauma.
Patients were considered as incident when they entered the study within three months of initiating PD. All others were considered prevalent. All patients used the “traditional care” protocol before the study.
This study was performed according to Good Clinical Practices Guidelines and was approved by the Ethical Board of the Institution. All the patients included signed their informed consent. Randomization was performed by means of a randomization code via random number table, StatXact5 (Cytel Inc, Cambridge, MA, USA).
Exit-site infection was defined as the presence of purulent drainage, with or without erythema of the skin at the catheter-epidermal interface plus a positive culture (19). Those patients with positive culture at initial evaluation were excluded even if the exit-site appearance was normal, in order to avoid any confounding data. Samples for cultures were obtained by introducing a dry cotton swab into the catheter hub and then rotating the swab across a 1 cm area around the exit site.
The PD catheter used in all cases was a surgically implanted Swan-neck (double Dracon, multi-perforated silicone catheter; Fresenius Medical Care, Bad Homburg, Germany). As per protocol, we use cephazoline, 2 grams, prior to the insertion of the peritoneal catheter.
Stages of Development (Chronogram)
As a previous step to the study, a nasal culture and an exit-site culture were obtained from each patient with a swab as transport medium, following the hospital’s guidelines. Nasal carriers of S. aureus were treated according to routine clinical guides (intranasal mupirocin application twice a day for seven days). Patients who met the inclusion criteria were assigned to a random cure. At the start of the trial, exit sites were assessed according to the Twardowski criteria (modified) (20) by an observer who supervised all the assessments with the aid of photographic support. Afterwards, evaluations were performed every four to six weeks or if any event occurred. Culture sampling was carried as per protocol or whenever the appearance of the exit site required it.
Sample Size Estimation
The sample size was estimated at 56 participants in each arm, on the basis of an accrual period of 6 months and a follow-up period of 12 months, and an instantaneous hazard ratio of 0.041 for the “traditional care” group versus 0.020 for the polyhexanide group to achieve a power of 81%, with an α of 0.05 (21).
Statistical Analysis
Continuous variables were expressed as averages and standard deviation, and categorical values, as percentages. The basal values from both groups were compared by Student t-test and chi-square as required. Previously, the Kolmogorov-Smirnov test had been performed to check for normal distribution. Those patients who did not finish the study due to renal transplantation, transfer to HD, or death were censored at this time from the longitudinal study, although their basal results were analyzed by intention to treat. Kaplan-Meier and Cox proportional hazard models for survival free of infection analysis were used.
Results
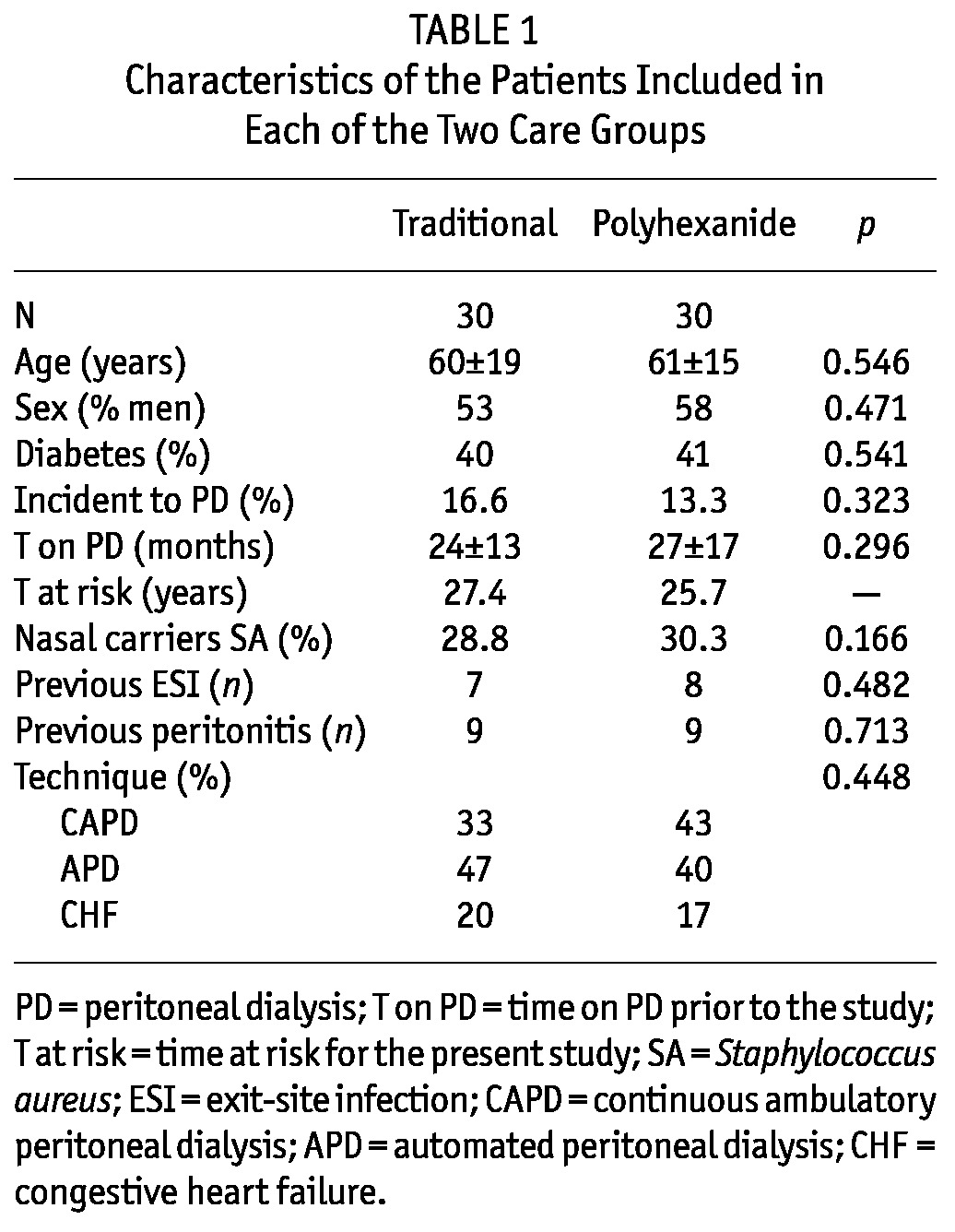
Finally, 60 patients met the inclusion and exclusion criteria and were included in the study. Four patients were excluded because of exit-site infection at randomization time and others did not sign the informed consent. Table 1 shows the baseline characteristics of the patients according to the treatment group. Both groups were homogeneous, with no significant differences between them. Patients in the study used continuous ambulatory PD (CAPD), automated PD (APD) and finally, there were patients with refractory congestive heart failure treated with a single nocturnal two-liter exchange of icodextrin.
TABLE 1.
Characteristics of the Patients Included in Each of the Two Care Groups
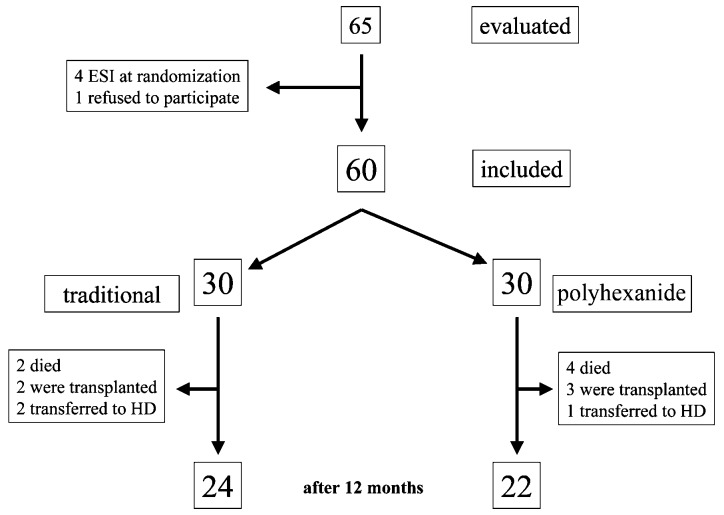
Of the 60 included patients, 46 completed the 12-month follow-up period. Six underwent transplantation, five died and three were transferred to HD. The treatment was well tolerated, with neither side effects nor abandonments due to such effects.
Exit-site care with polyhexanide was associated with a lower risk of exit-site infection. Throughout the study period, six patients (20%) undergoing traditional care and only two (6.7%) receiving polyhexanide developed an exit-site infection (p = 0.032). There were a total number of 12 infections; nine occurred in patients following the traditional approach and only three in patients treated with polyhexanide (p = 0.037) (Figure 1). The mean rate of exit-site infection was 0.328 per year (1 episode/36.6 patient-months) for the traditional care group and 0.117 per year (1 episode/102.7 patient-months) for the polyhexanide group (p = 0.017) (Table 2).
Figure 1 —
Flow of the patients during follow-up. ESI = exit-site infection; HD = hemodialysis.
TABLE 2.
Exit-Site Infections During the Follow-Up Period.
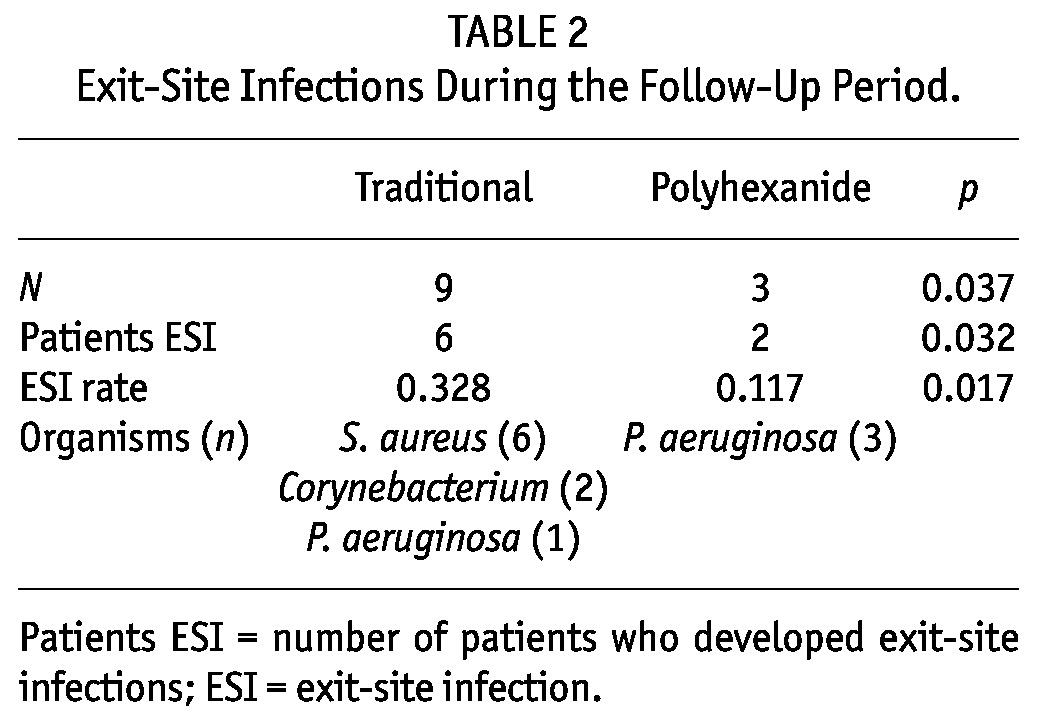
The agents responsible for the infections were:
0.9% saline serum and povidone-iodine group: S. aureus (6 cases), Corynebacterium jeikeium (2 cases) and P. aeruginosa (1 case).
Polyhexanide group: P. aeruginosa (3 cases).
No significant differences regarding the risk of exit-site infection were found associated with the age of the patient, sex, presence of diabetes, or time on PD. Infected patients were similarly distributed between those using CAPD and those using APD (4 patients in each group). Those patients on the PD program due to heart failure refractory to diuretics did not develop any exit-site infection, whereas CAPD and APD patients showed about the same infection incidence rates. No differences were found in exit-site infection when incident and prevalent PD patients were analyzed separately (0.323 vs 0.331 episodes per year in the traditional care group and 0.114 vs 0.020 episodes per year in the polyhexanide group).
Peritonitis rates were lower using polyhexanide compared with traditional care, (0.37 vs 0.51 per year; p = 0.036). Both gram-negative and gram-positive rates were lower in the polyhexanide group. There were no episodes of P. aeruginosa peritonitis in either group. There were no catheter removals as a result of infection in either treatment group.
Finally, we analyzed the time to first exit-site infection. We confirmed via a Kaplan-Meyer test that those patients following the traditional treatment required less days to develop an infection than those using polyhexanide (p = 0.033; log rank: 4.2) (Figure 2); and Cox proportional hazards model revealed that the risk of exit-site infection was four times greater in the “traditional” cure [HR (95%CI): 3.942 (1.098 - 14.149)].
Figure 2 —
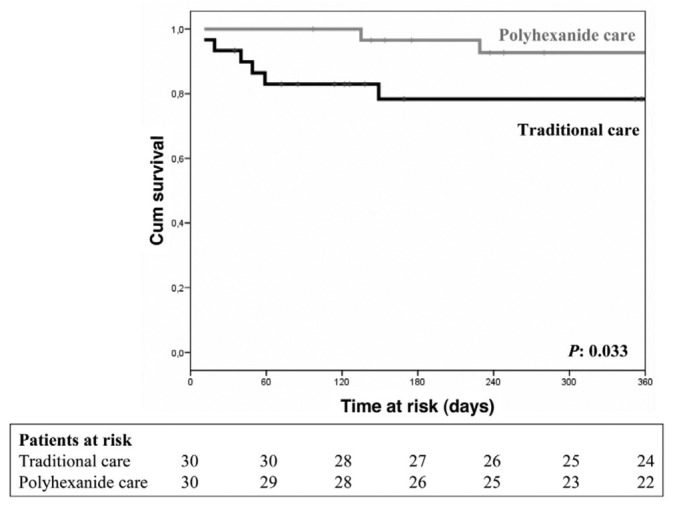
Period of time free of infection of the exit site. The black line represents patients who received the traditional treatment; the grey line represents patients who received the polyhexanide treatment (p=0.033; log rank: 4.2).
Discussion
In this study we present the results of a prospective, open-label, randomized clinical trial which compares the efficacy of two types of treatments to ensure healthy exit sites of PD catheters. These results show that the use of polyhexanide reduces the risk of infection.
Some of the most common complications caused by exit site have to do with infectious processes. Advances in PD techniques, especially those related to connectivity, have led to an important reduction of peritonitis incidence. While antibiotic cream/ointment at the exit site lowers infectious risk, the use raises the specter of resistance and alternative approaches would be preferable.
Patients with exit-site/tunnel infections also present a higher incidence of peritonitis (22), and its diagnosis is not as clear as other cases of peritonitis not related to the exit-site pathology (23). Thus, the scientific community should welcome every activity which may lead to the reduction of exit-site infection rates.
Polyhexanide is becoming more and more used in the prevention and care of chronic wounds (12), with a proven efficacy against the pathogens most commonly responsible for exit-site infections, such as S. aureus and P. aeruginosa (16). We were able to verify that the use of polyhexanide in patients with peritoneal catheter led to a reduction in the number of infections and, if an infection did appear, it would do so at later stages.
Infected patients were similarly distributed between those using CAPD and those using APD. Patients with heart failure did not suffer from any infection. This could be explained by the fact that these patients only underwent a single exchange per day. However, it did not seem that exit-site infection risk had to do with the number of exchanges, so maybe the risk depended on patient-specific factors. It is known that uremia produces an immunosuppression state which may account for these differences.
On the other hand, it should be noted that in patients who used polyhexanide and got infected, the agent was P. aeruginosa in the three cases (2 patients). It has been published that polyhexanide is effective against P. aeruginosa (16,24) and it has been used for pre-surgical antisepsis and antiseptic treatment of skin, wounds, and mucous membranes based on internationally accepted standards. Although it might be a question of hazard, we would like to explain the clinical characteristics of these patients. Case 1: Female, 58 years old; she had already experienced previous infections caused by this agent, which had responded quickly and satisfactorily to the specific antibiotic treatment; for that reason, the removal of her PD catheter had been postponed; she suffered from two exit-site infections. Case 2: Male, 70 years old; he was infected during a long hospital admission due to a polytraumatism; it should be noted that this situation favors Pseudomonas infections (25).
An aspect that must be taken into account when dealing with a product which looks for new applications is the cost its use would entail. Although a cost-utility analysis was not done, we took into account the cost of the two treatments. Surprisingly enough, using polyhexanide is less expensive than performing the traditional approach with povidone-iodine. Usage-related costs should be added to those associated with the antibiotic treatments required by the greater number of exit-site infections present in patients receiving traditional care. It should be mentioned that povidone-iodine cannot be used in patients allergic to iodine and that monitoring of the thyroid function of patients using this product for a long time is recommended. Consequently, it is interesting to have the possibility of using a product able to prevent exit-site infections, such as polyhexanide, which does not impose such limitations. For all of the above reasons, it seems the use of polyhexanide in exit-site infection prevention should be an option to be taken into account in all PD units.
The historical concern of all PD teams worldwide has been to reduce the number of peritoneal infections, and this objective has to do undoubtedly with a reduction of exit-site infections. The year before performing this protocol, the peritoneal infection rate in our unit was slightly less than one each 24 months (within the average value described in the literature). We hope this study may help us take a step towards reducing that rate. It would also be interesting to study the efficacy of polyhexanide in the prevention of exit-site infection of HD catheters.
This study is limited by sample size, although statistical power was achieved. PD is not the “dominant” modality of dialysis in Europe. In Spain, only around 5% of patients under renal replacement therapies are in PD programs (26). Peritoneal dialysis units therefore include a small number of patients. This is a preliminary study and longer and larger studies are needed to confirm these data and to generalize the use of polyhexanide. It would be very interesting to promote a multicenter study in order to confirm our data, although the possibility of inter-observer variations in the aspect of the exit site could bias the results.
To sum up, we conclude that polyhexanide is efficient for the prevention of exit-site infections and that it may be used routinely from now on for the care of healthy exit sites. We should further study the efficacy of polyhexanide in the prevention and treatment of exit-site infections caused by Pseudomonas. If a reduction of infections related to this agent could be confirmed, it would present a major advantage, since it would imply a reduction of the high morbidity associated with such infections and of the frequent need of catheter removals. Together with this point, additional studies are warranted to investigate the effectiveness of polyhexanide in HD catheter care.
Disclosures
The authors declare no potential conflict of interest relevant to this article. Part of these data belong to Baxter S.L. funds as we received the Nephrological Nursing Investigation Baxter award 2010.
Acknowledgments
Dra. María Varela (Oficina de Investigación Biomédica del Principado de Asturias/Ficyt) for preliminary review of the manuscript. Consorcio de Apoyo a la Investigación Biomédica en Red (CAIBER) for its continuous support. The Plastic and Reconstructive Surgery Nursing staff of our Hospital for their knowledge.
References
- 1. Montenegro J. Prevention and treatment of peritoneal catheter exit-site infection. Nefrología 1999; 19:502–7 [Google Scholar]
- 2. Castro Notario MJ. Chapter 34. In: Manual práctico de diálisis peritoneal. Badalona, Spain: SEN-SEDEN; January 2005: 293–7 [Google Scholar]
- 3. Vijt D, Castro MJ, Endall G, Lindley E, Elseviers M, EDTNA/ERCA Research Board. Post insertion catheter care in peritoneal dialysis (PD) centres across Europe: results of the Post Insertion Project of the Research Board. EDTNA ERCA J 2004; 30:42–7 [DOI] [PubMed] [Google Scholar]
- 4. Oltra-Rodríguez E. Efectividad clínica de los distintos abordajes del cuidado de salida del catéter de diálisis peritoneal. Instituto Joanna Briggs, Instituto de salud Carlos III, Consejería de salud y servicios sanitarios del Principado de Asturias. Enferm Clin 2006; 16:53–4 [Google Scholar]
- 5. Rao SP, Oreopoulos DG. Unusual complication of a polyurethane PD catheter. Perit Dial Int 1997; 17:440–1 [PubMed] [Google Scholar]
- 6. Piraino B, Bernasrdini J, Florio T, Fried L. Staphylococcus aureus prophylaxis and trends in gram-negative infection in peritoneal dialysis patients. Perit Dial Int 2003; 23:456–9 [PubMed] [Google Scholar]
- 7. Tacconelli E, Carmeli Y, Aizer A, Ferreira G, Foreman MG, D’Agata EM. Mupirocin prophylaxis to prevent staphylococcus aureus infection in patients undergoing dialysis: a meta-analysis. Clin Infect Dis 2003; 37:1629–38 [DOI] [PubMed] [Google Scholar]
- 8. Riu S, Ruiz C, Martinez-Vea A, Peralta C, Oliver J. Spontaneous rupture of polyurethane peritoneal catheter. A possible deleterious effect of mupirocin ointment. Nephrol Dial Transplant 1998; 13:1870–1 [DOI] [PubMed] [Google Scholar]
- 9. Annigeri R, Conly J, Vas S, Dedier H, Prakashan KP, Bargman JM, et al. Emergence of mupirocin-resistant Staphylococcus aureus in chronic peritoneal dialysis patients using mupirocin prophylaxis to prevent exit-site infection. Perit Dial Int 2001; 21: 554–9 [PubMed] [Google Scholar]
- 10. Teixidó J, Arias N, Tarrats L, Romero R. The microbial pattern of the catheter exit-site infection in peritoneal dialysis: A non-diphtheria Corynebacteria emergence? Nefrología 2007; 27:350–8 [PubMed] [Google Scholar]
- 11. Minguela Pesquera JI, Ruiz de Gauna R, Muñoz González RI. Complicaciones del túnel y orificio de salida del catéter peritoneal. In: Montenegro J, Correa-Rotter R, Riella MC. Tratado de Diálisis Peritoneal. Barcelona, Spain: Elsevier; 2009: 259–72 [Google Scholar]
- 12. Carville K, Cuddigan J, Fletcher J, Fuchs J. Principios de las mejores prácticas: La infección de las heridas en la práctica clínica. Consenso internacional. London: MEP Ltd, 2008. www.mepltd.co.uk [Google Scholar]
- 13. Kramer A, Daeschlein G, Kammerlander G, Andriessen A, Aspöck C, Bergemann R, et al. Consensus recommendation on wound antisepsis. Zeitschrift Wundheilung 2004; 3:110–20 [Google Scholar]
- 14. Martínez Cuervo F, López Moreno JS. Uso del Prontosan en el cuidado UPP. Ulcus clínica 2006; 4:6–9 [Google Scholar]
- 15. Martínez Cuervo F. La limpieza de la herida: paso imprescindible en el cuidado UPP. Ulcus clínica 2006; 3:6–11 [Google Scholar]
- 16. Grupo Nacional para el estudio y asesoramiento en úlceras por presión y heridas crónicas. Recomendaciones sobre la utilización de antisépticos en el cuidado de heridas crónicas. Documento VIII. December 2002. www.gneaupp.org
- 17. Sansone G, Cirugeda A, Bajo MA, Del Peso G, Sánchez Tomero JA, Alegre L, et al. Clinical practice protocol update in peritoneal dialysis—2004. Nefrología 2004; 24:410–44 [PubMed] [Google Scholar]
- 18. Castro Notario MJ. Cuidados del orificio de salida del catéter peritoneal. In: Coronel F, Montenegro J, Selgas R, Celadilla O, Tejuca M. Manual práctico de Diálisis Peritoneal. Badalona, Spain: Ed Atrium Comunicación Estratégica; 2005: 293–7 [Google Scholar]
- 19. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. International Society for Peritoneal Dialysis. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423 [DOI] [PubMed] [Google Scholar]
- 20. De Miguel B, Arias Suárez N, Gascó Senet A, López Miravalles A, Ruiz Carbonell M, Teixidó J. Catéter peritoneal: valoración del orificio según criterios de Twardowski, modificados. Rev Soc Esp Enferm Nefrol 1997; 1er trimestre:12–4 [Google Scholar]
- 21. Montenegro J, Saracho R, Aguirre R, Martínez I, Iribar I, Ocharán J. Exit-site care with ciprofloxacin otologic solution prevents polyurethane catheter infection in peritoneal dialysis patients. Perit Dial Int 2000; 20:209–14 [PubMed] [Google Scholar]
- 22. Abraham G, Savin E, Blake P, Dombros N, Símbolos K. Natural history of exit-site infection in patients on CAPD. Perit Dial Bull 1988; 3:211–6 [Google Scholar]
- 23. Martin CM, Brier ME, Golper TA. Outcome of single organism peritonitis in peritoneal dialysis: gram negatives versus gram positives in the Network 9 Peritonitis Study. Kidney Int 1997; 52:524–9 [DOI] [PubMed] [Google Scholar]
- 24. Koburger T, Hübner NO, Braun M, Siebert J, Kramer A. Standardized comparison of antiseptic efficacy of triclosan, PVP-iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J Antimicrob Chemother 2010; 65:1712–9 [DOI] [PubMed] [Google Scholar]
- 25. Sekiguchi J, Asagi T, Miyoshi-Akiyama T, Kasai A, Mizuguchi Y, Araake M, et al. Outbreak of multidrug-resistant Pseudomonas aeruginosa in community hospitals in Japan. J Clin Microbiol 2007; 45:979–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stel VS, van de Luijtgaarden MW, Wanner C, Jager KJ, on behalf of the European Renal Registry Investigators. The 2008 ERA-EDTA registry annual report—a précis. NDT plus 2011; 4:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]