Abstract
♦ Introduction: Escherichia coli (E. coli) peritonitis is a frequent, serious complication of peritoneal dialysis (PD). The extended-spectrum β-lactamase (ESBL)-producing E. coli peritonitis is associated with poorer prognosis and its incidence has been on continuous increase during the last decades. However, the clinical course and outcomes of E. coli peritonitis remain largely unclear.
♦ Methods: All of the E. coli peritonitis episodes that occurred in our dialysis unit from 2006 to 2011 were reviewed. The polymicrobial episodes were excluded.
♦ Results: In total, ninety episodes of monomicrobial E. coli peritonitis occurred in 68 individuals, corresponding to a rate of 0.027 episodes per patient-year. E. coli was the leading cause (59.2%) of monomicrobial gram-negative peritonitis. ESBL-producing strains accounted for 35.5% of E. coli peritonitis. The complete cure rate and treatment failure rate of E. coli peritonitis were 77.8% and 10.0% respectively. Patients with preceding peritonitis had a higher risk of ESBL production as compared to those without peritonitis history [odds ratio (OR): 5.286; 95% confidence interval (CI): 2.018 - 13.843; p = 0.001]. The risk of treatment failure was significantly increased when the patient had a baseline score of Charlson Comorbidity Index (CCI) above 3 (OR: 6.155; 95% CI: 1.198 - 31.612; p = 0.03), or had diabetes mellitus (OR: 8.457; 95% CI: 1.838 - 38.91; p = 0.006), or hypoalbuminemia (≤ 30g/l) on admission (OR: 13.714; 95% CI: 1.602 - 117.428; p = 0.01). Prolonging the treatment course from 2 to 3 weeks or more reduced the risk of relapse and repeat significantly (p < 0.05).
♦ Conclusions: E. coli peritonitis remains a common complication of PD. The clinical outcomes of E. coli peritonitis are relatively favorable despite the high ESBL rate. A history of peritonitis is associated with increased risk for ESBL development. The severity of baseline comorbidities, the presence of diabetes mellitus and hypoalbuminemia at admission are associated with poor outcomes.
Key words: Escherichia coli, extended-spectrum β-lactamase, outcomes, peritonitis, peritoneal dialysis
Peritoneal dialysis (PD) possesses advantages, such as being more hemodynamically stable and having fewer life style limitations, as compared to hemodialysis in treating end-stage renal disease (ESRD) patients, without compromise in the long-term survival. However, peritonitis remains a major threat to a successful and sustainable PD program (1,2). It results in about 18% of infection-related mortality and is probably the most common cause of technique failure in PD (3). Mainly due to the improvements in connectology of PD made during the last decades and the application of topical antibiotics as prophylaxis against exit-site infection, peritonitis caused by gram-positive pathogens in PD has decreased markedly (4). However, the incidence of gram-negative peritonitis has not decreased to the same extent, and its proportion increased consequently (5-7).
Escherichia coli (E. coli) is one of the most common organisms that cause gram-negative peritonitis in PD patients (5,8,9), and is associated with a high probability of mortality and technique failure (10). Moreover, the virulence of E. coli was reported to get more severe than previously found, which had led to even worse outcomes in PD patients with E. coli peritonitis (11). In addition, the extended-spectrum β-lactamase (ESBL)-producing strain has made the prognosis of E. coli peritonitis poorer since its emergence in the 1980s and the incidence of ESBL-producing E. coli peritonitis has been on continuous increase (12). However, studies focused on E. coli peritonitis in PD were limited. In the present study, we retrospectively investigated the prevalence, patients’ demography, antibiotic sensitivity pattern and clinical features of E. coli peritonitis in our PD patients over a 6-year period. In particular, factors associated with the ESBL’s development and clinical outcomes were analyzed.
Methods
Study Population
This study was conducted at the First Affiliated Hospital of Sun Yat-sen University, performed in compliance with the ethical principles of the Helsinki Declaration and approved by the Institutional Review Board for human research at Sun Yat-sen University (Guangzhou, China). Written informed consent was obtained from all participating patients. Patients on stable continuous ambulatory PD (CAPD) between 1 January 2006 and 31 December 2011 were enrolled and followed up until the cessation of PD (transfer to hemodialysis, transplantation or death) or the end of the study. Polymicrobial and relapsing episodes of peritonitis were excluded from this study. Data analyzed included demographic information such as age, gender; baseline features such as the severity of comorbidities (indicated by the Charlson Comorbidity Index, CCI), body mass index (BMI) and estimated glomerular filtration rate (eGFR); clinical data for each peritonitis episode, including the PD vintage, serum albumin concentration, white blood cell (WBC) count in peripheral blood and PD effluent (PDE) on admission, and antibiotic sensitivity pattern of isolates and antibiotic regimens used. The history of using antibiotics within three months before the episode of E. coli peritonitis was also investigated.
Definitions
Diagnosis of PD-associated peritonitis was made based on at least two of the following criteria: (1) abdominal pain or cloudiness of PDE; (2) WBC count in PDE > 100/μL with > 50% polymorphonuclear leukocytes; and (3) a positive culture from PDE (13,14). Exit-site infection was defined as the presence of purulent drainage, with or without erythema of the skin at the catheter-epidermal interface. Relapse referred to an episode that occurs within 4 weeks of completion of therapy of a prior episode with the same organism or 1 sterile episode. Repeat was defined as an episode that occurs more than 4 weeks after completion of therapy of a prior episode with the same organism. Recurrence referred to an episode that occurs within 4 weeks of completion of therapy of a prior episode but with a different organism (3). Complete cure was defined as the resolution of peritonitis without relapse or recurrence by antibiotics alone (15). A primary response was termed as resolution of abdominal pain, clarification of the dialysate and WBC count of PDE < 100/μL within 5 days of antibiotic treatment. Failure of the effluent to clear after 5 days of appropriate antibiotic treatment was termed as refractory peritonitis. Peritonitis-related death referred to death of a patient with active peritonitis, or admitted with peritonitis, or within 2 weeks of a peritonitis episode (3). Treatment failure included discontinuation of PD, whether temporary or permanent, and death during peritonitis (12).
Clinical Management
Patients were treated with CAPD with standard solutions (Dianeal (Baxter Healthcare Corporation, Guangzhou, China)) using a twin-bag system during the study period. After a sample of PDE was collected, patients suspected of peritonitis were empirically treated with first-generation cephalosporin (cefazolin or cefradine) and third-generation cephalosporin (ceftazidime) as soon as possible. This first-line regimen, which was recommended by ISPD guidelines (3,16,17), has been continuously practised in our center from 2000 up to the present. The initial antibiotic regimen was evaluated and modified when antibiotic sensitivity results were available. In general, patients were given antibiotic treatment for at least 2 weeks. In severe cases, antibiotic treatment was prolonged appropriately. If antibiotics alone failed to resolve the peritonitis or any fungus was isolated, the catheter would be removed and the patient transferred to hemodialysis temporarily (if reinsertion succeeded) or permanently (if reinsertion failed).
Microbiology Testing
For microbiology tests, 50 mL PDE was centrifuged at 3000 g for 15 min, then the pellet was inoculated in the BacT/Alert anaerobic and aerobic bottles (bioMérieux, Durham, NC, USA). The identities of all isolates and their susceptibilities to certain antibiotics were determined using the Vitek-2 AutoMicrobic system (bioMérieux, St Louis, MO, USA). ESBL production was confirmed by the double disk synergy test before 2007, and by a method using AST-GN13 cards (bioMérieux, St Louis, MO, USA) after 2007. Results were interpreted according to the criteria of the Clinical and Laboratory Standards Institute (18).
Statistical Analysis
Data are expressed as mean ± standard deviation for continuous variables, median and interquartile range for non-parametric data, and frequencies and percentages for categorical variables. Comparison of data between groups was performed using the χ2 test for categorical data, the Student t-test for continuous parametric data and the rank-sum test for continuous non-parametric data. The incidence of peritonitis was analyzed longitudinally using a Poisson regression model. Logistic regression was used to analyze the risk factors for the treatment outcomes. All probabilities were two-tailed. Statistical analysis was performed by SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA). P values < 0.05 were considered statistically significant.
Results
Between January 2006 and December 2011, 1,575 patients received CAPD in our center. They were followed up for 3,289 patient-years. Among them, 498 episodes of peritonitis with results of microbiological culturing were recorded.
E. coli was involved in 95 episodes of peritonitis during this period. After the 5 episodes of polymicrobial peritonitis were excluded, a total of 90 episodes of monomicrobial E. coli peritonitis were identified in 68 individuals. E. coli was the most common cause of the monomicrobial gram-negative peritonitis (90/152, 59.2%). The rate of monomicrobial E. coli peritonitis was 0.027 episodes per patient-year. Between 2006 and 2011, the incidence of overall peritonitis, monomicrobial gram-positive peritonitis and gram-negative peritonitis all decreased, while the monomicrobial E. coli peritonitis rate increased from 0.020 to 0.026 episodes per patient-year (p < 0.05) (Figure 1). As a result, both the proportion of E. coli peritonitis among the total peritonitis and E. coli peritonitis among gram-negative peritonitis increased (details not shown).
Figure 1 —
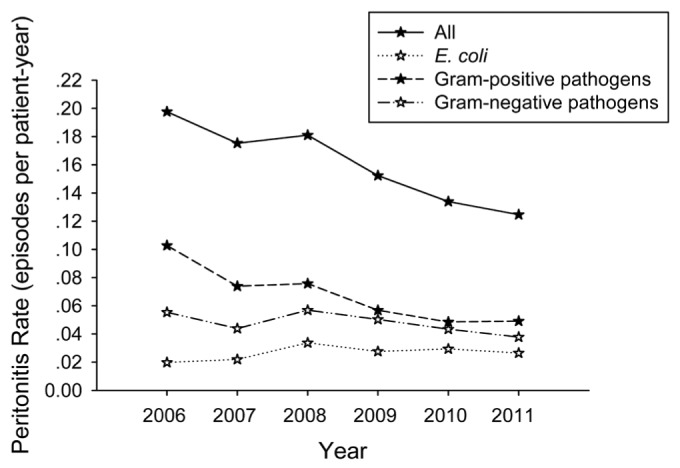
Evolution of PD-related peritonitis incidence over the 6-year study period. A trend of decrease was demonstrated in overall peritonitis (p<0.001), monomicrobial gram-positive peritonitis (p<0.001) and monomicrobial gram-negative peritonitis (p<0.05). By contrast, monomicrobial E. coli peritonitis incidence showed an increasing trend (p<0.05) (analyzed by Poisson regression).
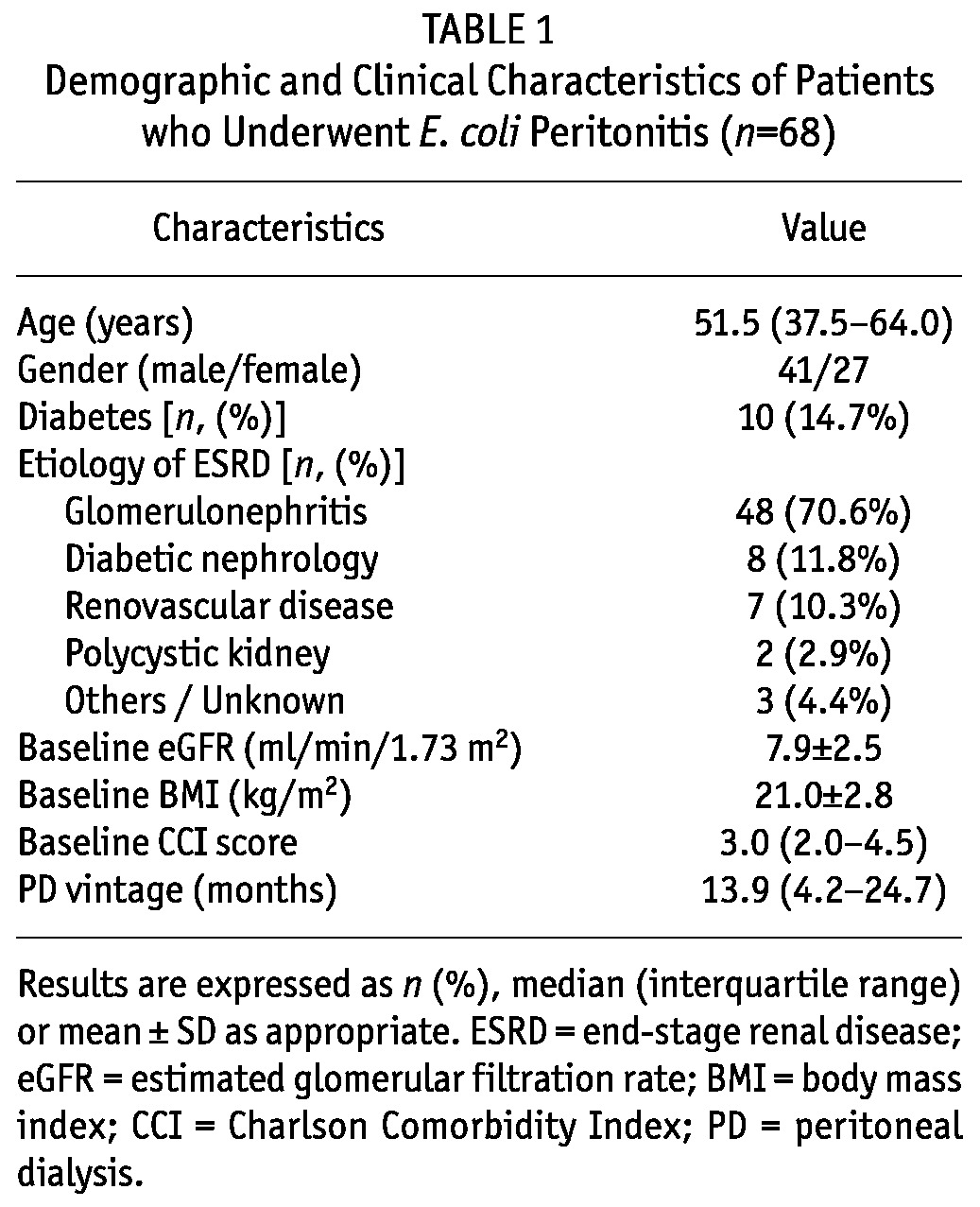
The demographic and clinical characteristics of patients who underwent E. coli peritonitis are shown in Table 1. The median age was 51.5 years [inter-quartile range (IQR): 37.5 - 64.0 years], 14.7% of patients had diabetes and 60.3% were male. The presenting symptoms included fever (64.8%), abdominal pain (94.9%), diarrhea (56.6%), vomiting (36.7%), cloudiness of PDE (100%) and abdominal tenderness (56.3%), which were similar to those caused by non-E. coli peritonitis (details not shown).
TABLE 1.
Demographic and Clinical Characteristics of Patients who Underwent E. coli Peritonitis (n=68)
Of these 68 patients who experienced E .coli peritonitis, 53 experienced 1 episode, 10 experienced 2 episodes, 3 experienced 3 episodes and 2 experienced 4 episodes. There was only one case with concomitant exit-site infection (1.1%) and none had tunnel infection. In 52 (57.8%) episodes, the patients had received antibiotic treatment during a 3-month period preceding the onset of E. coli peritonitis.
Microbiological Investigation
The overall antibiotic resistance in E. coli isolates between 2006 and 2011 is summarized in Table 2. Over this period, E. coli strains remained highly sensitive to carbapenems (including imipenem, meropenem and ertapenem), furadantin, amikacin, cefmetazole and cefoperazone/sulbactam. By contrast, a high proportion of these isolates were resistant to ampicillin, cefazolin and ceftazidime. Moreover, the prevalence of resistance to ampicillin, cefazolin, ceftazidime, as well as cefepime, increased markedly between 2006 and 2011 (p < 0.05) (Figure 2).
TABLE 2.
Prevalence of Antibiotic Resistance in Isolated E. coli Strains (n=90)
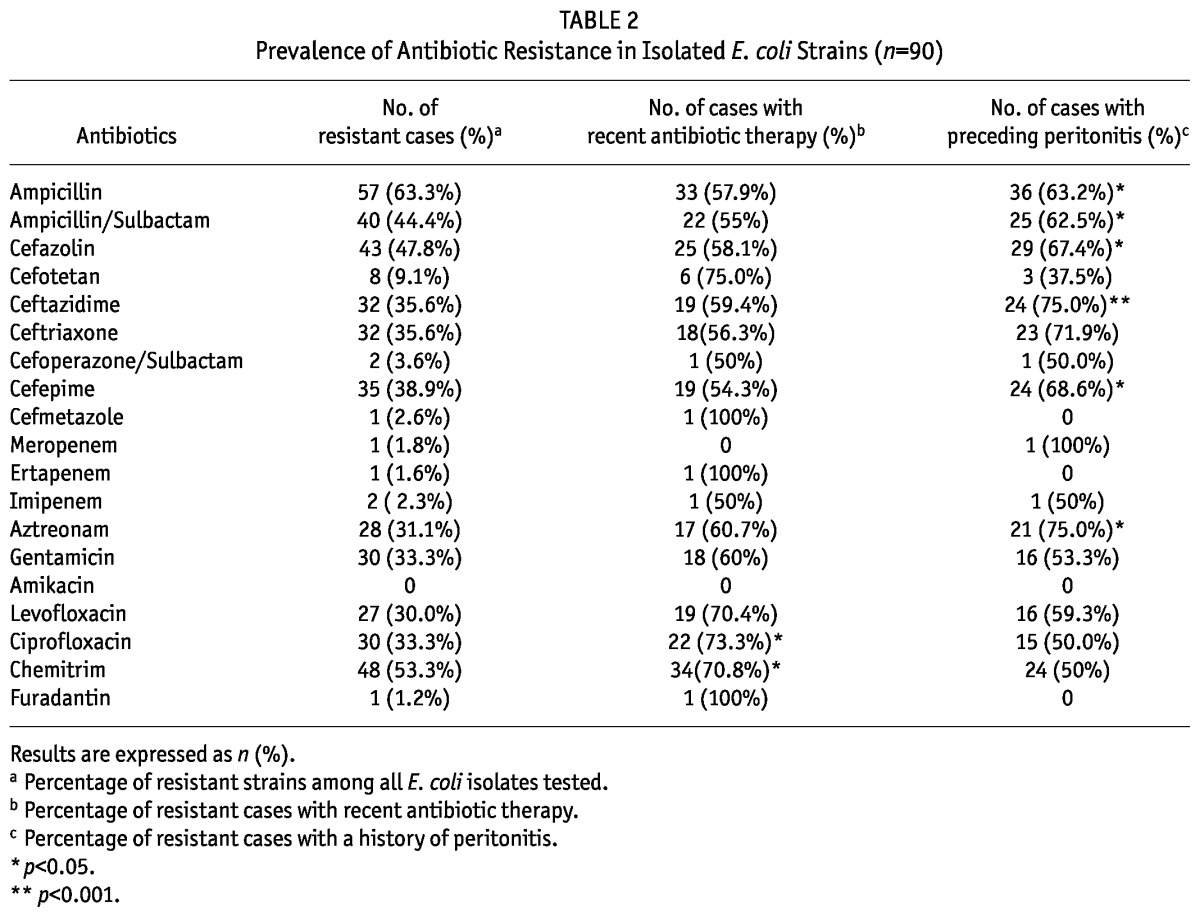
Figure 2 —
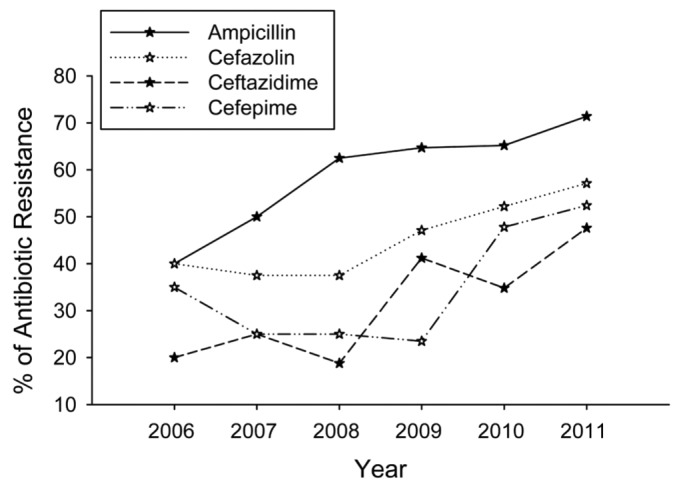
Prevalence of antibiotic resistance among E. coli isolates between 2006 and 2011. An ascending trend was clear in the prevalence of resistance to ampicillin, cefazolin, ceftazidime as well as cefepime (p<0.05) (analyzed by Poisson regression).
Recent antibiotic use was associated with higher prevalence of resistance to ciprofloxacin and chemitrim but not other antibiotics (Table 2). However, a history of peritonitis was associated with significantly higher prevalence of resistance to ampicillin, ampicillin/sulbactam, cefazolin, ceftazidime, cefepime and aztreonam (Table 2).
ESBL-producing strains accounted for 32 (35.6%) of all E. coli peritonitis episodes. The ESBL rate fluctuated from 20% in 2006 to 37.5%, 37.5%, 23.5%, 47.8% and 30%, respectively, in the years 2007 to 2011 (p < 0.05). The frequency of ESBL-producing cases in E. coli peritonitis episodes with or without a history of peritonitis is shown in Figure 3. Twenty-four (53.3%) of 45 episodes with a history of peritonitis developed ESBL as compared to only 8 (17.8%) episodes of those without previous peritonitis (OR: 5.286; 95% CI: 2.018 - 13.843; p = 0.001). Recent antibiotic use had no influence on the ESBL incidence (p = 0.39).
Figure 3 —
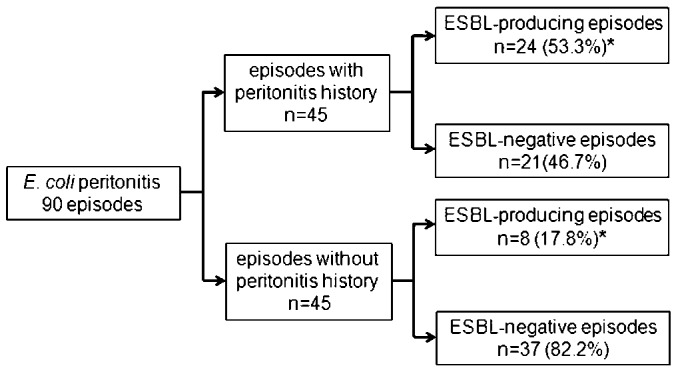
Frequency of ESBL-producing cases in E. coli peritonitis episodes with or without peritonitis history. *p=0.001; odds ratio: 5.286; 95% confidence interval: 2.018-13.843.
Clinical Outcomes
The clinical outcomes of all E. coli episodes are summarized in Figure 4. The subsequent antibiotic regimens and their relation to the clinical outcomes are summarized in Table 3. The primary response, complete cure and treatment failure rate of E. coli peritonitis was 76.7%, 77.8% and 10.0%, respectively. We observed a significantly higher risk of treatment failure when patients had a baseline CCI score above 3.0 (OR: 6.155; 95% CI: 1.198 - 31.612; p = 0.03), or had hypoalbuminemia (≤ 30g/L) on admission (OR: 13.714; 95% CI: 1.602 - 117.428; p = 0.01), or diabetes mellitus (OR: 8.457; 95% CI: 1.838 - 38.91; p = 0.006). The hypoalbuminemia on admission was also associated with significantly lower probability of complete cure (OR: 0.109; 95% CI: 0.027 - 0.436; p = 0.002). The treatment failure rate and complete cure rate were not associated with age, gender, the cause of ESRD, baseline eGFR and BMI, vintage of PD, the presence of ESBL, WBC count in peripheral blood or PDE on admission, recent antibiotic use and the history of peritonitis (p > 0.05). The relation between exit-site infection and clinical outcomes was not analyzed due to the small sample size (one case).
Figure 4 —
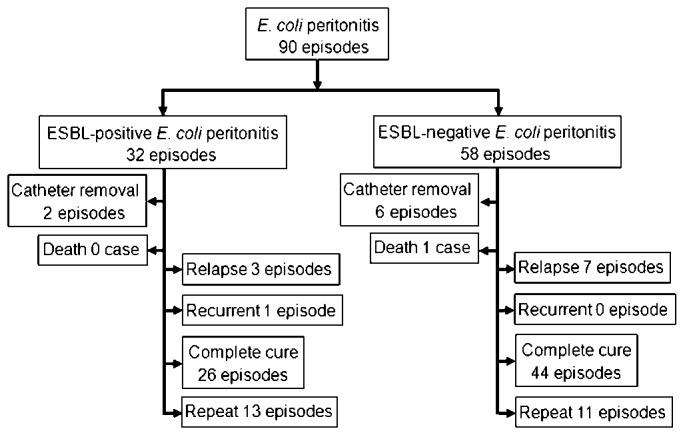
Summary of clinical outcomes of E. coli peritonitis.
TABLE 3.
Subsequent Antibiotic Regimen and its Relation to the Clinical Outcomes
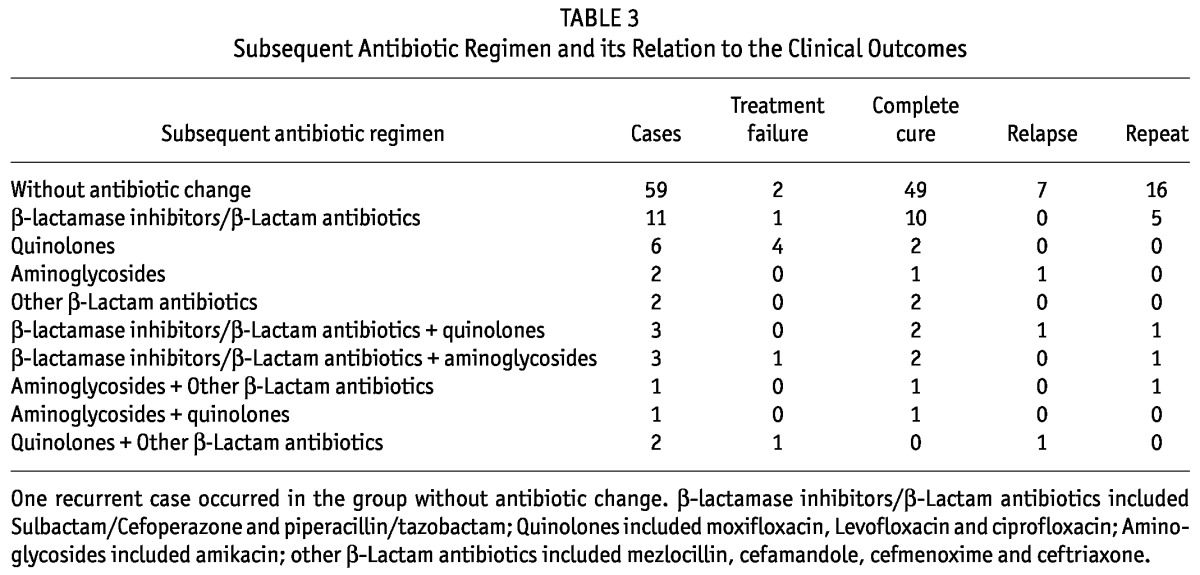
A total of 24 (26.7%) episodes developed repeat and 10 (11.1%) episodes developed relapse. The risk of repeat increased markedly in cases due to ESBL-producing E. coli (OR: 2.923; 95% CI: 1.115 - 7.663; p = 0.029) or in cases with a history of peritonitis (OR: 3.077; 95% CI: 1.153 - 8.214; p = 0.02), but neither had influence on the relapse rate (p > 0.05). Of these 90 episodes, thirty-five episodes received antibiotic treatment for 3 weeks or more (median 25 days, IQR 21 - 36 days) due to a serious presentation of inflammation. For the 55 episodes that received treatment for 2 weeks, nineteen (34.5%) developed repeat and 9 (16.4%) developed relapse. By contrast, only 5 (14.3%) episodes developed repeat and 1 (2.9%) developed relapse in cases that received treatment for 3 weeks or more (p = 0.034 and p = 0.047, respectively).
Discussion
Despite improvements in the connectology and prophylaxis against exit-site infection during the past decades, peritonitis remains a common and serious complication that threatens ESRD patients on PD. E. coli was reported to account for 21% to 43% of all gram-negative peritonitis in PD (7,8). In accordance with, or even surpassing findings in other centers, E. coli was the leading cause (59.2%) of gram-negative peritonitis in our study.
While it has been widely recognized that gram-negative peritonitis, as a whole, is associated with poor outcomes (3), E. coli peritonitis carries an interesting variability in its severity, from relatively favorable outcomes (19,20) to a high incidence of treatment failure despite apparently appropriate antibiotic treatment (11). This notion is further supported by the present study, since the clinical outcomes of E. coli peritonitis in our study were generally better than those reported by Yip et al. (21), who examined 153 episodes of monomicrobial E. coli peritonitis that occurred between 1995 and 2009 in a single center in Hong Kong. Compared with their findings, the primary response rate in our patients was higher (76.7% vs 69.9%) while both the catheter removal rate and peritonitis-related mortality rate in our study were much lower (8.9% vs 19.6% and 1.1% vs 10.5%, respectively).
Generally, the clinical course of infection is determined by the interaction between the host’s defense, the virulence of pathogens and the therapeutic intervention. A variety of host characteristics of affected patients such as age, gender, PD duration, nutritional status and comorbidities were reported to influence the outcomes of peritonitis in PD (22-25), maybe by their effects on the immune system. The presence of exit-site infection was also found to increase the risk of catheter loss and mortality of peritonitis (20,26). In our study, the incidence of exit-site infection was remarkably lower (1.1%) than that reported by other centers (2,15,24), which may be a contributing factor to the relatively better outcomes in our cohort.
Another important factor that may influence outcomes of E. coli peritonitis concerns an intriguing variability in the pathogenicity of this organism (10). As one of the most versatile microorganisms, E. coli can frequently create novel pathotypes or serotypes with different combinations of virulence factors through mobilization of genetic elements between different strains (27). As a result, it comprises a broad population of bacteria that exhibit an extremely high degree of genetic and phenotypic diversity, with only 20% of the genome common to all strains (28). However, to what extent this highly diverse pathotype contributes to the different outcomes of E. coli peritonitis in PD remains mainly unexplored in published literatures (11,12,21,24).
A continuously rising trend in the prevalence of ESBL has been a serious clinical problem worldwide since its emergence in the mid-1980s (12,29,30). ESBLs belong to β-lactamase enzymes, with the ability to hydrolyze β-lactam rings, and are thus capable of resisting β-lactam antibiotics like penicillins, cephalosporins, and monobactams (29). Plasmids encoding ESBLs frequently carry genes responsible for resistance to other antibiotic classes such as aminoglycosides. So, antibiotic options in treating ESBL-producing organisms are extremely limited. E. coli is one of the main ESBL-producing organisms isolated, with the incidence of ESBL-producing E. coli reported varying worldwide from 0 - 1% in Australia (31) to 63.9% in Germany (32). There was a high ESBL prevalence background, from 23.8% to 61.5%, in E. coli strains within our locality according to the reports in Chinese Journals (33-35), which is much higher than those reported from Hong Kong (12.5%) (12) and Thailand (17%) (36).
Previous studies have found an association between recent antibiotic use and the emergence of ESBL (37,38). Furthermore, it was reported that recent use of first- and second-generation cephalosporins was associated with the development of ESBL-producing strains in E. coli peritonitis among CAPD patients (12). Inconsistent with previous reports, the present study did not find an influence of recent antibiotic use on the ESBL development in E. coli peritonitis, even though recent antibiotic use was associated with higher resistance to ciprofloxacin and chemitrim. On the other hand, the history of peritonitis was found to be associated with a significantly higher ESBL proportion.
Generally speaking, the outcomes of infections by ESBL-producing pathogens tend to be severe. Yip et al. found that peritonitis caused by ESBL-producing E. coli resulted in significantly higher treatment failure rates and mortality rates as compared with ESBL-negative E. coli peritonitis (12). Inconsistent with previous findings, there was no significant difference between ESBL-producing cases and ESBL-negative cases in our study with regard to the complete cure (81.3% vs 75.9%, p = 0.56) and treatment failure rates (6.3% vs 12.1%, p = 0.31). This result is not surprising because the antibiotic-resistant capability of pathogens is not always consistent with their virulence (39,40). On the other hand, the cases with ESBL-producing peritonitis in the present study tended to be younger (42.0 vs 54.0, p = 0.15), with a shorter PD duration (7.9 vs 18.0, p = 0.01), less serious comorbidities at baseline (CCI score: 2.0 vs 3.0, p = 0.19) and a smaller proportion of diabetes (6.3% vs 15.5%, p = 0.19) as compared to patients with ESBL-negative peritonitis, which indicates a superiority of immune function in the ESBL-producing cases over ESBL-negative cases in our study.
To our limited knowledge, studies identifying risk factors associated with the clinical outcomes of E. coli peritonitis in PD patients are scarce. Serum albumin is a marker of nutritional status which is tightly associated with the immune function of the patients. It is known from previous studies that a low serum albumin level was significantly associated with catheter removal after peritonitis episodes (41). Our study confirmed that hypoalbuminemia on admission could increase the risk of treatment failure in E. coli peritonitis. Comorbidities are common in the PD population and have been shown to adversely influence the survival of PD patients (42). Moreover, patients with diabetes mellitus are at high risk for non-resolution of peritonitis episodes (4). The present study strongly supports these observations, since the baseline CCI >3.0 and the presence of diabetes mellitus both were significantly associated with treatment failure. These findings highlight the importance of improving comorbidities and nutritional status of PD patients to ameliorate the outcomes of a PD program.
This study has some limitations. As a retrospective study, correct evaluation of the effects of different antibiotic regimens on clinical outcomes was difficult. However, we believe it is a meaningful observation that patients who received antibiotic treatment for 3 weeks or more had a lower risk of relapse and repeat than those treated for 2 weeks, considering the fact that patients treated with the longer course were those who should have been more severe and had worse outcomes. Another limitation is the small sample of treatment failure, which made the multivariate analysis of its predicting factors inappropriate. Finally, the culture-negative peritonitis accounted for 21% of all episodes, which marginally exceeded the upper limit of 20% recommended by ISPD (3,16). The large percentage of culture-negative cases may reflect common antibiotic use prior to microorganism culture. Therefore timely specimen collection should be strengthened in the future to lower the percentage of negative culture.
In conclusion, E. coli accounted for the leading proportion of gram-negative peritonitis in our center. Both its absolute incidence and its percentage among overall peritonitis increased while the incidence of overall peritonitis decreased. Although the ESBL rate was high, clinical outcomes of E. coli peritonitis were relatively favorable when a combination of first- and third-generation cephalosporins was empirically used as standard first-line therapy for PD-related peritonitis. Prolonging the treatment course from 2 weeks to 3 weeks or more can decrease the risk of relapse and repeat of E. coli peritonitis significantly. The severity of comorbidities at baseline, the presence of diabetes mellitus, as well as hypoalbuminemia at admission was significantly associated with treatment failure. These findings suggest that nephrologists should pay more attention to ameliorate the patients’ comorbidities and nutritional status to improve the outcome of CAPD.
Disclosures
The authors have no financial conflicts of interest to declare.
Acknowledgments
Thanks to Ms. Yafang Li, Ms. Juan Wang and Ms. Juan Wu for their assistance in data collection and statistical analysis. This work was supported by grants from U.S. Baxter’s Renal Discoveries Extramural Grant Program, the National Basic Research Program of China (Grant No. 2011CB504000) and Guangdong Natural Science Foundation of China (Grant No. 9151008901000051).
References
- 1. Brown MC, Simpson K, Kerssens JJ, Mactier RA. Peritoneal dialysis-associated peritonitis rates and outcomes in a national cohort are not improving in the post-millennium (2000-2007). Perit Dial Int 2011; 31:639–50 [DOI] [PubMed] [Google Scholar]
- 2. Davenport A. Peritonitis remains the major clinical complication of peritoneal dialysis: the London, UK, peritonitis audit 2002-2003. Perit Dial Int 2009; 29:297–302 [PubMed] [Google Scholar]
- 3. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423 [DOI] [PubMed] [Google Scholar]
- 4. Barretti P, Moraes TM, Camargo CH, Caramori JC, Mondelli AL, Montelli AC, et al. Peritoneal dialysis-related peritonitis due to Staphylococcus aureus: a single-center experience over 15 years. PLoS One 2012; 7:e31780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zelenitsky S, Barns L, Findlay I, Alfa M, Ariano R, Fine A, et al. Analysis of microbiological trends in peritoneal dialysis-related peritonitis from 1991 to 1998. Am J Kidney Dis 2000; 36:1009–13 [DOI] [PubMed] [Google Scholar]
- 6. Piraino B, Bernardini J, Florio T, Fried L. Staphylococcus aureus prophylaxis and trends in gram-negative infections in peritoneal dialysis patients. Perit Dial Int 2003; 23:456–9 [PubMed] [Google Scholar]
- 7. Huang ST, Chuang YW, Cheng CH, Wu MJ, Chen CH, Yu TM, et al. Evolution of microbiological trends and treatment outcomes in peritoneal dialysis-related peritonitis. Clin Nephrol 2011; 75:416–25 [DOI] [PubMed] [Google Scholar]
- 8. Szeto CC, Leung CB, Chow KM, Kwan BC, Law MC, Wang AY, et al. Change in bacterial aetiology of peritoneal dialysis-related peritonitis over 10 years: experience from a centre in South-East Asia. Clin Microbiol Infect 2005; 11:837–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zurowska A, Feneberg R, Warady BA, Zimmering M, Monteverde M, Testa S, et al. Gram-negative peritonitis in children undergoing long-term peritoneal dialysis. Am J Kidney Dis 2008; 51:455–62 [DOI] [PubMed] [Google Scholar]
- 10. Perez-Fontan M, Lueiro F. Escherichia coli peritonitis in patients undergoing peritoneal dialysis: a serious problem that may get worse. Perit Dial Int 2006; 26:174–77 [PubMed] [Google Scholar]
- 11. Valdes-Sotomayor J, Cirugeda A, Bajo MA, Del PG, Escudero E, Sanchez-Tomero JA, et al. Increased severity of Escherichia coli peritonitis in peritoneal dialysis patients independent of changes in in vitro antimicrobial susceptibility testing. Perit Dial Int 2003; 23:450–5 [PubMed] [Google Scholar]
- 12. Yip T, Tse KC, Lam MF, Tang S, Li FK, Choy BY, et al. Risk factors and outcomes of extended-spectrum beta-lactamase-producing E. coli peritonitis in CAPD patients. Perit Dial Int 2006; 26:191–7 [PubMed] [Google Scholar]
- 13. Shukla A, Abreu Z, Bargman JM. Streptococcal PD peritonitis-a 10-year review of one centre’s experience. Nephrol Dial Transplant 2006; 21:3545–9 [DOI] [PubMed] [Google Scholar]
- 14. Keane WF, Alexander SR, Bailie GR, Boeschoten E, Gokal R, Golper TA, et al. Peritoneal dialysis-related peritonitis treatment recommendations: 1996 update. Perit Dial Int 1996; 16:557–3 [PubMed] [Google Scholar]
- 15. Szeto CC, Kwan BC, Chow KM, Lau MF, Law MC, Chung KY, et al. Coagulase negative staphylococcal peritonitis in peritoneal dialysis patients: review of 232 consecutive cases. Clin J Am Soc Nephrol 2008; 3:91–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 2005; 25:107–31 [PubMed] [Google Scholar]
- 17. Keane WF, Bailie GR, Boeschoten E, Gokal R, Golper TA, Holmes CJ, et al. Adult peritoneal dialysis-related peritonitis treatment recommendations: 2000 update. Perit Dial Int 2000; 20:396–411 [PubMed] [Google Scholar]
- 18. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 16th informational supplement. Wayne, PA: CLSI; 2006: M100–S16 [Google Scholar]
- 19. Lye WC, van der Straaten JC, Leong SO, Sivaraman P, Tan SH, Tan CC, et al. Once-daily intraperitoneal gentamicin is effective therapy for gram-negative CAPD peritonitis. Perit Dial Int 1999; 19:357–60 [PubMed] [Google Scholar]
- 20. Bunke CM, Brier ME, Golper TA. Outcomes of single organism peritonitis in peritoneal dialysis: gram negatives versus gram positives in the Network 9 Peritonitis Study. Kidney Int 1997; 52:524–9 [DOI] [PubMed] [Google Scholar]
- 21. Yip T, Tse KC, Ng F, Hung I, Lam MF, Tang SC, et al. Clinical course and outcomes of single-organism Enterococcus peritonitis in peritoneal dialysis patients. Perit Dial Int 2011; 31:522–8 [DOI] [PubMed] [Google Scholar]
- 22. Jarvis EM, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Predictors, treatment, and outcomes of non-Pseudomonas Gram-negative peritonitis. Kidney Int 2010; 78:408–14 [DOI] [PubMed] [Google Scholar]
- 23. Choi P, Nemati E, Banerjee A, Preston E, Levy J, Brown E. Peritoneal dialysis catheter removal for acute peritonitis: a retrospective analysis of factors associated with catheter removal and prolonged postoperative hospitalization. Am J Kidney Dis 2004; 43:103–11 [DOI] [PubMed] [Google Scholar]
- 24. Szeto CC, Chow VC, Chow KM, Lai RW, Chung KY, Leung CB, et al. Enterobacteriaceae peritonitis complicating peritoneal dialysis: a review of 210 consecutive cases. Kidney Int 2006; 69:1245–52 [DOI] [PubMed] [Google Scholar]
- 25. Perez FM, Rodriguez-Carmona A, Garcia-Naveiro R, Rosales M, Villaverde P, Valdes F. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int 2005; 25:274–84 [PubMed] [Google Scholar]
- 26. Gupta B, Bernardini J, Piraino B. Peritonitis associated with exit site and tunnel infections. Am J Kidney Dis 1996; 28:415–9 [DOI] [PubMed] [Google Scholar]
- 27. Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol 2004; 2:123–40 [DOI] [PubMed] [Google Scholar]
- 28. Lukjancenko O, Wassenaar TM, Ussery DW. Comparison of 61 sequenced Escherichia coli genomes. Microb Ecol 2010; 60:708–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malloy AM, Campos JM. Extended-spectrum beta-lactamases: a brief clinical update. Pediatr Infect Dis J 2011; 30:1092–3 [DOI] [PubMed] [Google Scholar]
- 30. Shu JC, Chia JH, Kuo AJ, Su LH, Wu TL. A 7-year surveillance for ESBL-producing Escherichia coli and Klebsiella pneumoniae at a university hospital in Taiwan: the increase of CTX-M-15 in the ICU. Epidemiol Infect 2010; 138:253–63 [DOI] [PubMed] [Google Scholar]
- 31. Bell JM, Turnidge JD, Gales AC, Pfaller MA, Jones RN. Prevalence of extended spectrum beta-lactamase (ESBL)-producing clinical isolates in the Asia-Pacific region and South Africa: regional results from SENTRY Antimicrobial Surveillance Program (1998-99). Diagn Microbiol Infect Dis 2002; 42:193–8 [DOI] [PubMed] [Google Scholar]
- 32. Kohlenberg A, Schwab F, Ruden H. Wide dissemination of extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella spp. in acute care and rehabilitation hospitals. Epidemiol Infect 2012; 140:528–34 [DOI] [PubMed] [Google Scholar]
- 33. Jin G, Zhuo C, Su D, Yuan J, Yang L, Qiu G, et al. Surveillance of antibiotic resistance in clinical isolates from First Affiliated Hospital of Guangzhou Medical College during 2005-2008. Chin J Antibiot J 2009; 34:753–8 [Google Scholar]
- 34. Zhuo C, Su D, Zhong N. Surveillance of antimicrobial resistance in Guangzhou hospitals in 2007. Chin J Lab Med 2009; 32:397–402 [Google Scholar]
- 35. Wang T, Chen Y, Song Z. The trend in the prevalence of hospital-infected Gram-negative bacteria and their antibiotic sensitivity pattern. Guide Chin Med 2008; 6:75–6 [Google Scholar]
- 36. Udomsantisuk N, Nunthapisud P, Tirawatanapong T, Dansuputra M. Molecular characterization of extended spectrum beta-lactamase among clinical isolates Escherichia coli and Klebsiella pneumoniae. J Med Assoc Thai 2011; 94:1504–12 [PubMed] [Google Scholar]
- 37. Cheong HS, Ko KS, Kang CI, Chung DR, Peck KR, Song JH. Clinical significance of infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae blood isolates with inducible AmpC beta-lactamase. Microb Drug Resist 2012; 18:446–52 [DOI] [PubMed] [Google Scholar]
- 38. Nasa P, Juneja D, Singh O, Dang R, Singh A. An observational study on bloodstream extended-spectrum beta-lactamase infection in critical care unit: incidence, risk factors and its impact on outcome. Eur J Intern Med 2012; 23:192–5 [DOI] [PubMed] [Google Scholar]
- 39. Soto SM. Relationship between virulence and antimicrobial resistance in bacteria. Rev Med Microbiol 2009; 20:84–90 [Google Scholar]
- 40. Da SG, Mendonca N. Association between antimicrobial resistance and virulence in Escherichia coli. Virulence 2012; 3:18–28 [DOI] [PubMed] [Google Scholar]
- 41. Yang CY, Chen TW, Lin YP, Lin CC, Ng YY, Yang WC, et al. Determinants of catheter loss following continuous ambulatory peritoneal dialysis peritonitis. Perit Dial Int 2008; 28:361–70 [PubMed] [Google Scholar]
- 42. Davies SJ, Phillips L, Naish PF, Russell GI. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant 2002; 17:1085–92 [DOI] [PubMed] [Google Scholar]


