Abstract
Membranous nephropathy (MN) is the most common cause of nephrotic syndrome in adults, with an uncertain clinical outcome. The characterization of the phospholipase A2 receptor (PLA2R) as the major target antigen in primary MN and the detection of circulating autoantibodies in these patients is a major advance in understanding this disease. To test whether PLA2R antibody levels reflect disease activity or clinical outcome, we performed a prospective multicenter study of 133 adult patients with primary MN and detectable serum PLA2R antibodies who had not received immunosuppressive therapy. Patients were followed ≤24 months. PLA2R antibody levels associated with clinical disease activity (proteinuria) in patients with immunosuppressive therapy (n=101) or supportive care (n=32). Within 3 months, immunosuppressive therapy led to a sustained 81% reduction in PLA2R antibody levels paralleled by a 39% reduction in proteinuria. Patients who experienced remission of proteinuria after 12 months had significantly lower PLA2R antibody levels at the time of study inclusion compared with patients with no remission. Patients with high PLA2R antibody levels achieved remission of proteinuria significantly later than patients with low PLA2R antibody levels. PLA2R antibody levels fell over time in patients with spontaneous remission but remained elevated in patients who did not show a reduction in proteinuria. Multivariable Cox regression analysis confirmed PLA2R antibody level as an independent risk factor for not achieving remission of proteinuria. We conclude that a decrease in PLA2R antibody level is associated with a decrease of proteinuria in patients with primary MN.
Since the landmark discovery that circulating autoantibodies against the phospholipase A2 receptor (PLA2R) are specific for patients with primary membranous nephropathy (MN) completely new paradigms for the diagnosis and clinical care of these patients are possible.1 These are urgently needed because the clinical outcome of patients with primary MN varies and ranges from spontaneous clinical remissions to end stage renal failure.2,3 Because of the absence of reliable predictors of clinical outcome, the best documented methods to predict outcome and hence make a decision to treat patients with an immunosuppressive agent or maintain them on supportive medications currently require prolonged follow-up measurements of proteinuria.4,5 Furthermore, in patients who receive immunosuppressive therapy, the intensity and duration of the treatment currently depend on changes in proteinuria, which do not necessarily reflect the severity or activity of the immunologic disease. On the other hand, patients who clinically do not respond to immunosuppressive agents may have insufficient therapy and still have active immunologic disease. A marker that reflects immunologic disease activity in real time and indicates clinical outcome could substantially improve the care of these patients. The availability of recently developed and easily applicable assays to measure PLA2R antibody levels in the serum6–8 makes it possible to study patients prospectively and to analyze whether PLA2R antibody levels are related to disease activity. To address this question, we conducted a multicenter open prospective study in patients with biopsy-proven MN.
Results
Clinical Baseline Characteristics
We screened 163 patients with biopsy-proven MN for the presence of PLA2R antibodies. Of these patients, 133 individuals were positive for PLA2R antibodies and were included in this study. Patients were followed for up to 24 months after recruitment. As summarized in Table 1, the majority of patients were men (75.9%). At the time of study inclusion (and first serum measurement), the mean age of the patients was 54.4±15.2 years. The time from renal biopsy until the first measurement of the PLA2R antibody levels was 1.2±1.5 months. Almost all patients (127 of 133) were treated with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. Most patients received diuretics (114 of 133) and lipid-lowering drugs (87 of 133) and 61 of 133 of patients were treated with anticoagulants. A detailed analysis of the baseline characteristics and the analyzed parameters is summarized in Table 1. While in the study, 101 patients received immunosuppressive agents (Figure 1). The initial immunosuppressive agent was cyclosporine A in most patients (n=53) (40 of which received cyclosporine A in combination with steroids). One patient received tacrolimus. Thirty-four patients were treated with cyclophosphamide (32 of which received cyclophosphamide in combination with steroids) and one patient received chlorambucil and steroids. Nine patients were treated with rituximab. Three patients received steroids alone. Thirty-two patients remained on supportive care only.
Table 1.
Clinical baseline characteristics of the patients and PLA2R antibody levels at the time of study inclusion and at the start of immunosuppression
| Characteristics | Whole Patient Cohort | Initial Immunosuppressive Therapy | |||
|---|---|---|---|---|---|
| None (No IS) | CNI | Alk | RTX | ||
| Patients (n) | 133 | 32 | 54 | 35 | 9 |
| Sex, ratio of men to women (% men) | 101/32 (76) | 25/7 (78) | 42/12 (78) | 27/8 (77) | 5/4 (56) |
| Age (yr) | 54.4±15.2 | 57.1±12.1 | 51.4±17.4 | 56.2±13.1 | 55.2±15.5 |
| Time from renal biopsy to first serum measurement (mo) | 1.2±1.5 | 1.0±1.4 | 1.2±1.4 | 1.3±1.6 | 1.4±2.0 |
| Time from first serum measurement to start of IS treatment (mo) | — | — | 2.4±3.2 | 3.0±3.2 | 2.8±3.7 |
| Proteinuria (g/24 h) | |||||
| At the time of first serum measurement | 9.6±5.0 | 7.4±3.2 | 9.9±5.4a | 11.8±5.4a | 8.5±4.2 |
| At 3 mo (no IS) or start of IS | — | 7.3±3.0 | 10.2±5.8a | 10.9±5.1a | 8.2±4.1 |
| Serum albumin (g/L) | |||||
| At the time of first serum measurement | 23.7±4.1 | 25.0±3.5 | 22.8±4.6a | 23.8±3.4 | 24.0±3.3 |
| At 3 mo (no IS) or start of IS | — | 26.9±4.6 | 23.3±4.8a | 23.7±4.2a | 24.8±7.3 |
| Serum creatinine (mg/dl) | |||||
| At the time of first serum measurement | 1.2±0.6 | 1.1±0.6 | 1.2±0.5 | 1.3±0.6 | 1.2±0.4 |
| At 3 mo (no IS) or start of IS | — | 1.3±0.9 | 1.2±0.5 | 1.3±0.6 | 1.2±0.5 |
| PLA2R antibody level (total IgG ELISA) | |||||
| At the time of first serum measurement | 282±355 | 218±276 | 334±383 | 310±411 | 109±92 |
| At 3 mo (no IS) or start of IS | — | 137±165 | 240±250 | 311±439 | 133±130 |
| PLA2R antibody level (IgG4 ELISA) | |||||
| At the time of first serum measurement | 40±53 | 32±45 | 52±63 | 39±49 | 15±11 |
| At 3 mo (no IS) or start of IS | — | 16±23 | 38±47a | 39±52 | 23±30 |
There were no significant differences in any of the clinical characteristics between patients treated with calcineurin inhibitors, alkylating agents, or rituximab at the first serum measurement (study inclusion) or at the start of immunosuppression. There were no significant changes in the PLA2R antibody levels, proteinuria, or any other clinical characteristic from the time of first serum measurement to the start of immunosuppression. There were statistically significant differences in proteinuria, PLA2R antibody levels, and serum albumin between patients on supportive care only and patients who received immunosuppression (P<0.05). —, not applicable; IS, immunosuppression; CNI, calcineurin inhibitor; Alk, alkylating agent; RTX, rituximab.
P<0.05.
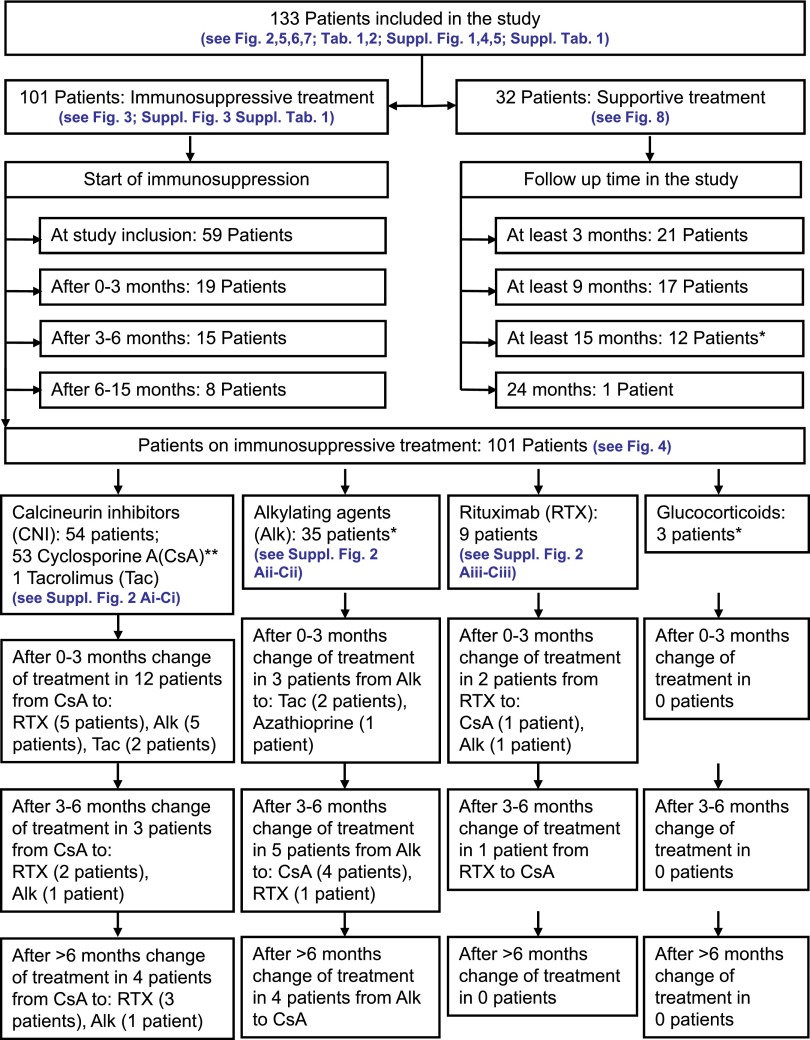
Figure 1.
Flow chart of patients included in the study and their follow-up. Of the 133 patients included in the study, 101 receive immunosuppressive treatment and 32 are treated with supportive care only. Immunosuppressive treatment is started at the time of inclusion in 59 patients and 3–15 months after study inclusion in the remaining 42 patients. After 0–3 months, immunosuppressive treatment is switched from one agent to another in 17 patients. After 3–6 months, immunosuppressive agents are changed in an additional nine patients. After >6 months, eight patients received a different immunosuppressant. *Five patients for whom no ELISA PLA2R antibody levels are available at the time of study inclusion.
Proteinuria, PLA2R Antibody Levels, and Serum Albumin
Findings from All 133 Patients
Considering the goal of our study to correlate clinical changes (proteinuria) with serum PLA2R antibody levels, Figure 2 shows a clear association between both parameters. Over the follow-up period, there was a steady decrease in proteinuria that was statistically significant (P<0.001 for all time points), starting already at 3 months after patient recruitment. This decrease in proteinuria was accompanied by a steady and statistically significant increase of serum albumin (P<0.001 for all time points). The decrease in PLA2R antibody serum levels was associated with a decline in proteinuria and an increase in serum albumin. Within 3 months of observation, proteinuria fell by 25% in the 133 patients. This was paralleled by a significant 45% decrease in PLA2R antibody levels. This was due to the statistically significant decrease of PLA2R antibody levels by 82% and of proteinuria by 43% in the 59 patients in whom immunosuppressive treatment was started at study inclusion. In the 74 patients with no immunosuppressive treatment in the first 3 months of the study, PLA2R antibody levels and proteinuria did not change significantly. There was no difference between the changes of total IgG or IgG4 PLA2R antibody levels. All of these findings were seen with both ELISA and indirect immunofluorescence test techniques (Figure 2, Supplemental Figure 1, respectively). Median values of PLA2R antibody levels measured by ELISA are presented as Supplemental Table 1.
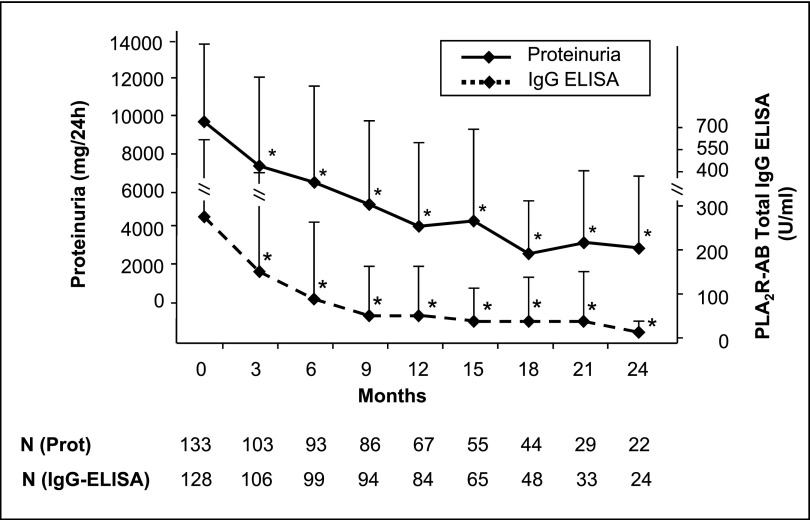
Figure 2.
Proteinuria and PLA2R antibody levels of all patients included in the study. Over the 24-month follow-up period, proteinuria (solid line) and PLA2R antibody levels (dashed line) constantly decrease over time. The decrease is already significant at 3 months, yet the decrease of PLA2R antibody levels is more pronounced than the decrease of proteinuria at 3 months. The bars show the SD values of proteinuria and PLA2R antibody levels. Time 0 shows data at the time of the first PLA2R measurement. N gives the number of patients for whom data were available at the different time points. *P<0.05, statistically significant difference between the single time point and the start of the study.
Patients Receiving Immunosuppressive Treatment
In the 101 patients who were treated with an immunosuppressant, the average time from the first measurement of the PLA2R antibody levels to the start of immunosuppression was 2.6±3.2 months. There were no significant changes in proteinuria or antibody levels between the time of first serum measurement (study inclusion) and the start of therapy (Table 1). There was also no statistical significant difference in antibody levels and proteinuria between the different immunosuppressive treatment groups (Table 1), even though patients who received rituximab had numerically lower levels. Patients who received immunosuppression during the follow-up had higher proteinuria and PLA2R antibody levels at study inclusion compared with patients who received supportive treatment only. Within 3 months after the start of an immunosuppressive therapy, PLA2R antibody levels fell by 69%–81% (depending on the method of PLA2R antibody analysis) and proteinuria fell by 38.8% (Figure 3). During the further follow-up, proteinuria consistently fell by approximately 17%–21% every 3 months for the first 12 months, whereas antibody levels remained low. Thus, there was a remarkable time lag between the rather rapid fall in antibody levels at 3 months and the protracted reduction in proteinuria. Serum albumin steadily increased in those patients over time.
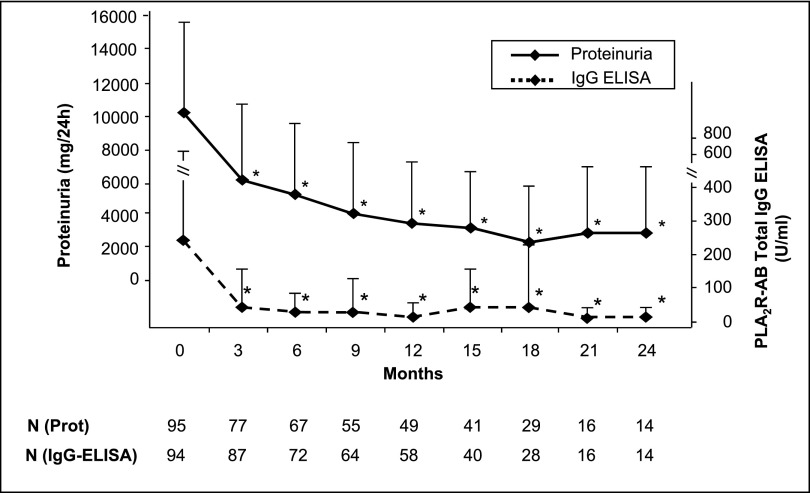
Figure 3.
Proteinuria and PLA2R antibody levels of patients treated with immunosuppressive therapy. Within 3 months after the start of the immunosuppressive therapy (0 months), proteinuria decreases by 39% and PLA2R antibody levels by 81%. Proteinuria continuously declines during the further follow-up, whereas PLA2R antibody levels remained low. The bars show the SD values of proteinuria and PLA2R antibody levels. Time 0 shows data at the time of the start of immunosuppression. *P<0.05, statistically significant difference between the single time point and the start of immunosuppression (0 months).
Effects of Individual Immunosuppressants
We could analyze 101 patients who were treated with an immunosuppressant (Figure 1, Supplemental Figure 2). Clinical characteristics of patients treated with calcineurin inhibitors, alkylating agents, or rituximab were not significantly different at the start of immunosuppression (Table 1). In these patients, PLA2R antibody levels, proteinuria, and serum albumin did not significantly change from the time of study inclusion until the start of immunosuppressive treatment (Table 1, Supplemental Figure 3). In all three treatment groups (calcineurin inhibitors, alkylating agents, and rituximab), PLA2R antibody levels fell significantly (by 83%–96%) within 3 months (Figure 4, Supplemental Figure 4).
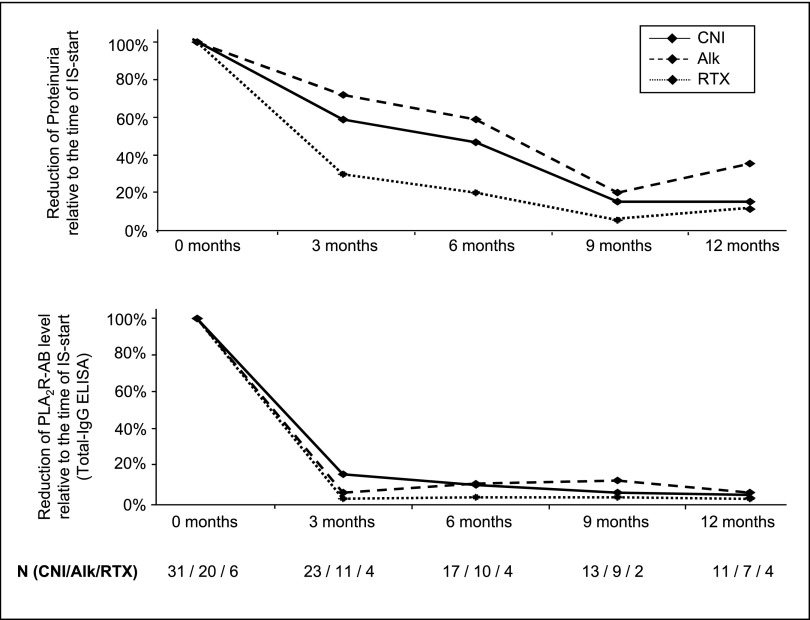
Figure 4.
Changes in PLA2R antibody levels and proteinuria after immunosuppressive therapy with all three treatment protocols (calcineurin inhibitors [CNI], alkylating agents [Alk], rituximab [RTX]) in patients treated with only one treatment regime. Data are presented as the relation of PLA2R antibody levels or proteinuria at the given time point (3, 6, 9, or 12 months) to the time of start of immunosuppression (Time 0). Data are presented for patients treated with calcineurin inhibitors, alkylating agents, or rituximab, in whom immunosuppressive therapy is not changed throughout the study follow-up and for whom both PLA2R antibody levels and proteinuria for the given time points are available. There is no significant difference in the decrease in PLA2R antibody levels and in the decrease in proteinuria between the three treatment groups. The decrease of PLA2R antibody levels is more rapid and is followed by the decrease in proteinuria. The average dosage of cyclosporine A is 187±98 mg/d at 3 months, 203±88 mg/d at 6 months, 197±81 mg/d at 9 months, and 185±67 mg/d at 12 months. The average cumulative dose of cyclophosphamide is 6.6±5.9 g at 3 months, 15.0±12.5 g at 6 months, 17.3±18.8 g at 9 months, and 31.9±33.5 g at 12 months. Patients receive on average 1.6±1.0 g rituximab. Time 0 shows data at the time of the start of immunosuppression. N gives the number of patients for which data are available for the times of follow-up.
PLA2R Antibody Levels, Renal Function, and Response of Proteinuria
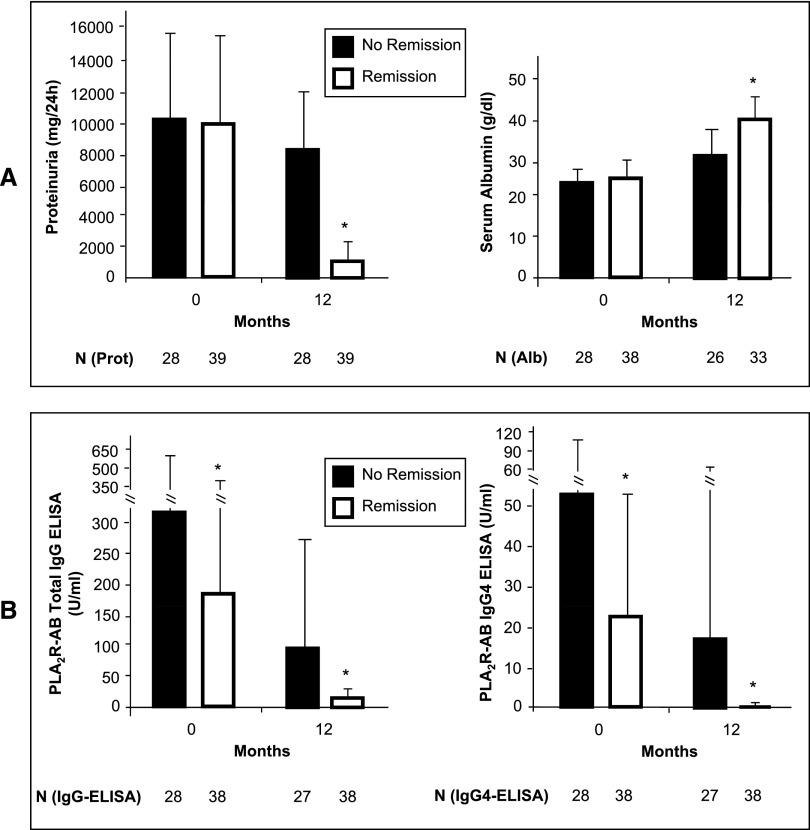
The levels of PLA2R antibodies were correlated with proteinuria and serum creatinine at the time of study inclusion. No correlation was detected between total IgG or IgG4 PLA2R antibody levels measured with either method and renal functional parameters (Supplemental Figure 5). Patients were divided in two groups according to the changes in proteinuria during 12-month follow-up (remission, no remission) to assess the potential role of PLA2R antibody levels on clinical response (proteinuria). As summarized in Table 2, 67 patients could be followed for 12 months. Of these individuals, 39 patients reached remission and 28 patients did not reach remission of proteinuria. There were no significant differences in sex, age, proteinuria, serum albumin, serum creatinine, or the percentage of patients receiving immunosuppressive therapy between the two groups at the time of study inclusion. When the groups were compared according to the PLA2R antibody levels at the time of study inclusion, the antibody levels were significantly higher in the group who did not experience remission compared with the patients who experienced remission of proteinuria. This difference was observed for total IgG and IgG4 subclass PLA2R antibody levels. After 12 months, patients who reached a remission of proteinuria had significantly lower PLA2R antibody levels compared with patients who did not reach remission of proteinuria (Figure 5).
Table 2.
Clinical baseline characteristics and PLA2R antibody levels at the time of study inclusion of patients reaching remission or no remission of proteinuria after 12 months
| Characteristic | Remission | No Remission | P Value |
|---|---|---|---|
| Patients (n) | 39 | 28 | — |
| Sex ratio of men to women (% men) | 29/10 (74.4) | 22/6 (78.6) | — |
| Age (yr) | 55.2±16.3 | 55.8±12.7 | 0.88 |
| Time from renal biopsy to first serum measurement (mo) | 1.0±1.4 | 1.4±1.6 | 0.36 |
| Proteinuria (mg/24 h) | 9732±5429 | 10022±5164 | 0.83 |
| Serum albumin (g/L) | 24.2±4.3 | 24.0±3.3 | 0.78 |
| Serum creatinine (mg/dl) | 1.4±0.6 | 1.1±0.5 | 0.13 |
| Patients on immunosuppressive therapy (%) | 33 (85) | 23 (82) | — |
| PLA2R antibody level (total IgG ELISA) | 179±207 | 311±297 | 0.04 |
| PLA2R antibody level (IgG4 ELISA) | 23±30 | 54±56 | 0.01 |
For 67 patients of the whole study cohort (treated and not-treated with immunosuppression), proteinuria after 12 months was available. After 12 months of follow-up, 39 patients reached remission and 28 patients did not reach remission of proteinuria. Patients with no remission of proteinuria after 12 months had significantly higher PLA2R antibody levels at the time of study inclusion compared with patients who experienced remission of proteinuria. This difference was seen for total IgG and IgG4 subclass antibody levels. Patients with or without remission of proteinuria after 12 months did not show differences in sex, age, proteinuria, serum albumin, or serum creatinine at the time of study inclusion. There was no difference in the percentage of patients who received immunosuppressive therapy between the groups. —, not applicable.
Figure 5.
Proteinuria, serum albumin, and PLA2R antibody levels in patients with remission or no remission of proteinuria after 12 months. Patients who reach a remission of proteinuria after 12 months have statistically significant lower proteinuria than patients who do not have a remission in proteinuria. In the patients with a reduction in proteinuria, serum albumin levels normalize after 12 months, but remain lower in the patients who did not have remission. In patients with remission of proteinuria, antibody levels fall during the follow-up and are significantly lower than in patients who do not have a remission in proteinuria. Time 0 refers to the time of study inclusion and first serum measurement. The bars show the SD values. *P<0.05.
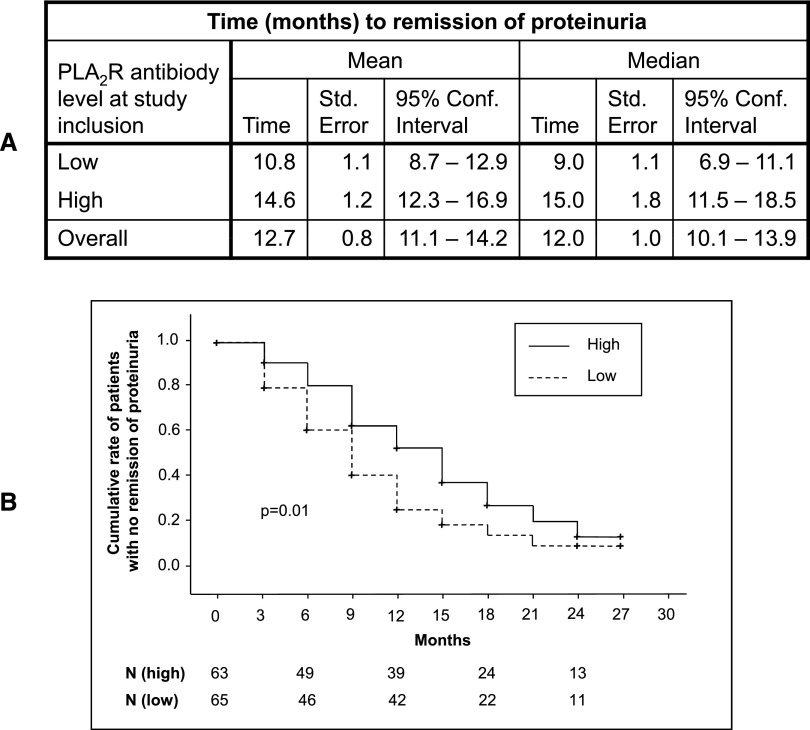
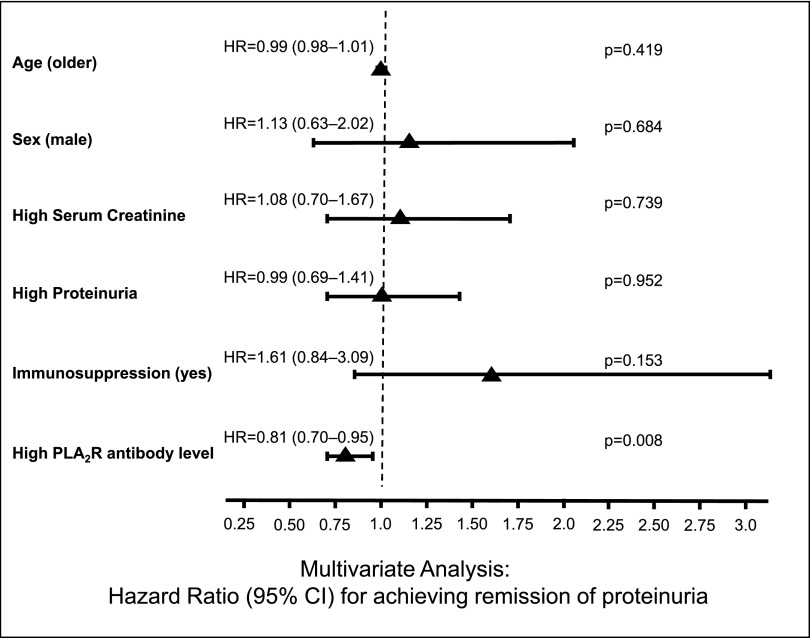
Patients were divided in two groups depending on PLA2R antibody levels at study inclusion (Figure 6). Patients with PLA2R antibody levels lower than the median PLA2R antibody level at study inclusion reached remission of proteinuria significantly faster than patients in whom PLA2R antibody levels at study inclusion were higher than the median PLA2R antibody levels. A multivariable Cox regression analysis identified PLA2R antibody levels as a risk factor for not achieving remission of proteinuria (Figure 7, Supplemental Figure 6).
Figure 6.
Time to achievement of remission of proteinuria in patients with high versus low PLA2R antibody levels at study inclusion. (A) Mean and median time to remission of proteinuria. The 128 patients for whom ELISA PLA2R antibody levels are available are divided in two groups. In patients in the low group, PLA2R antibody levels at study inclusion are lower than the median PLA2R antibody level. In patients in the high group, PLA2R antibody levels at study inclusion are higher than the median PLA2R antibody levels. For five patients, there are no ELISA PLA2R antibody levels available at the time of study inclusion and they are not included in this analysis. (B) Kaplan–Meier analysis. Patients with low PLA2R antibody levels at study inclusion (low group) reach remission of proteinuria significantly faster than patients with high PLA2R antibody levels at study inclusion (high group). N gives the number of patients at the different time points.
Figure 7.
Multivariable Cox regression analysis. Total IgG PLA2R antibody levels measured by ELISA are identified as risk factors for not achieving a remission of proteinuria in the 128 patients for whom ELISA PLA2R antibody levels at are available at the study start. Hazard ratios for achieving a remission of proteinuria are expressed per natural logarithm unit of serum creatinine, proteinuria, and PLA2R antibody levels measured by ELISA per unit of age and dichotomized for sex and treatment.
We analyzed proteinuria and PLA2R antibody levels in patients who reached a complete or partial remission of proteinuria after 18 and 24 months. PLA2R antibody levels in all 11 patients who reached complete remission of proteinuria after 18 months, and in all 6 patients with complete remission after 24 months, were no longer detectable. At both time points, patients with complete remission of proteinuria had lower PLA2R antibody levels compared with patients with partial remission of proteinuria, whereas patients without remission of proteinuria had the highest PLA2R antibody levels (Supplemental Figure 7).
Patients not Receiving Immunosuppressive Treatment
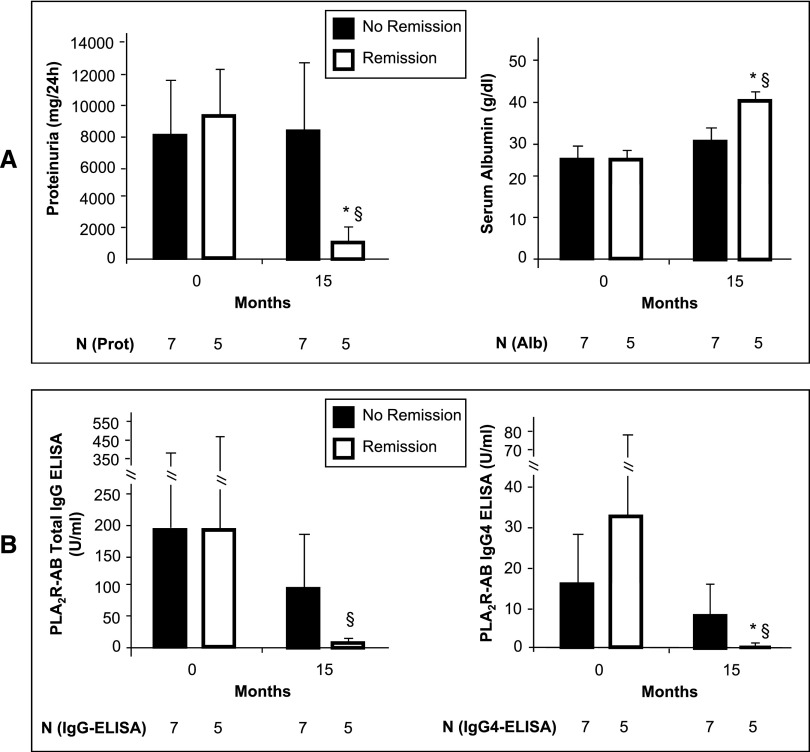
During the follow-up period, 32 patients did not receive immunosuppressive therapy. In the first 3 months after the study start, PLA2R antibody levels fell by 37% in these patients. Twelve of these patients were followed up to 15 months (Figure 8). Five patients reached a remission of proteinuria that averaged 1.2±1.0 g/24 h at 15 months. In these patients with clinical remission of disease, PLA2R antibody levels fell significantly by 15 months compared with the time of study inclusion. In contrast, seven patients did not experience clinical remission. Their PLA2R antibody levels did not fall significantly over time and were significantly higher at 15 months than levels in patients who experienced remission of proteinuria. At the time of inclusion in this study, PLA2R antibody levels and proteinuria of patients who had a remission of the disease were not different from patients with no remission. All changes in PLA2R antibody levels were seen with both methods applied for total IgG as well as for the IgG4 subclass. The remaining 20 patients in this subgroup did not reach the 15-month follow-up (Figure 1).
Figure 8.
Proteinuria and PLA2R antibody levels in 12 patients who do not receive immunosuppressive therapy and complete 15 months of follow-up. (A) Proteinuria and serum albumin in patients with remission or no remission of proteinuria after 15 months. Twelve patients not on immunosuppressive therapy could be analyzed for a 15-month follow-up period. Five of these patients reach a remission of proteinuria over the 15-month follow-up period. In these patients, proteinuria after 15 months is significantly lower than at the time of study inclusion (*P<0.05) and compared with the seven patients who did not have a remission in proteinuria (§P<0.05). In the patients with the reduction in proteinuria serum albumin levels normalized after 15 months, however, remained below normal in the patients who did not have remission. The bars show the SD values of proteinuria and serum albumin. (B) PLA2R antibody levels in patients with remission or no remission of proteinuria after 15 months. At the time of study inclusion, PLA2R antibody levels (both total IgG and IgG4 subclass) are not different between the patients who experience remission and those who do not show significant improvement in proteinuria after 15 months. In those patients with remission of proteinuria, PLA2R antibody levels (both total IgG and IgG 4 subclass) fall during the follow-up and are significantly lower after 15 months compared with patients who did not show remission of proteinuria (§P<0.05). Time 0 shows data at the time of the first PLA2R measurement. The bars show the SD values.
Discussion
The recent availability of sensitive and specific assays for serial measurements of PLA2R antibody levels in the follow-up of patients with primary MN allows the serial monitoring of immunologic and clinical activity in these patients.
We performed a prospective study in a large cohort of patients with MN to test whether PLA2R antibody levels may serve as markers of clinical activity at the time of diagnosis and during follow-up. We assessed total IgG and IgG4 subclass PLA2R antibody levels to determine whether there is a potential difference in the response because it has been originally described that IgG4 antibodies may be the pathogenetic subclass in primary MN.1 There were 133 patients who were included in the study and observed for up to 24 months. When followed over time, these patients showed a steady decrease in proteinuria that was inversely related to an increase in serum albumin. In the total patient cohort, there was a significant decrease in proteinuria and PLA2R antibody levels within the first 3 months of observation due to the decrease in the 59 patients in whom immunosuppressive treatment was started at study inclusion. There were 101 patients who received immunosuppressive therapy, which was started on average 2.6 months after the first serum measurement. In 42 patients who received immunosuppression later in the course of the study, no significant changes in antibody levels and proteinuria were detected between the time of first serum measurement and the start of immunosuppression. PLA2R antibody levels fell by 81% within 3 months after immunosuppressive therapy. This was paralleled by an approximately 39% decrease in proteinuria after 3 months. Whereas antibody levels remained low, proteinuria continuously fell over time, which shows that reduction in proteinuria has a time lag compared with reduction in antibody levels. This pattern of a relatively steeper fall of PLA2R antibody levels confirms data from a retrospective analysis in patients treated with rituximab,9 has been found in individual patients,6,10 and has earlier been shown in an animal model of MN.11 The data also show that proteinuria fell even though some circulating antibody remained detectable in the blood. This finding fits observations made in the passive Heymann nephritis model of MN, in which the onset and the degree of proteinuria require the amount of deposited antipodocyte antibodies to exceed a threshold level, and proteinuria persists for extended periods of time even after antibody deposition has ceased.12 Our findings that there were no differences between the changes in total IgG or IgG4 subclass PLA2R antibody levels does not support the notion that IgG4 antibody levels might be a better parameter for immunologic disease response.7,13 In the group of patients who received immunosuppressive therapy, the relative reduction in the antibody levels after 3 months was greater than in patients with spontaneous remission and in the total cohort of 133 patients. This suggests that the immunosuppressants actively reduced the antibody levels and led to the clinical response. Because this was an open study and the treating physicians decided on the therapy, different immunosuppressive protocols were applied. Most patients received calcineurin inhibitors, followed by alkylating agents and rituximab. The doses of the immunosuppressants that our patients received were very similar to doses reported in earlier protocols.14–16 When the three immunosuppressive regimens were compared, the initial pretreatment antibody levels and proteinuria as well as the fall in antibody levels and in proteinuria after start of immunosuppressive therapy were not different between the treatment groups (Figure 4). The reduction in proteinuria in the calcineurin inhibitor treatment group might be partially due to effects on podocyte biology or renal hemodynamics17; however, we could not detect statistically significant differences between the patients receiving calcineurin inhibitors and other treatment groups. Because our study was not controlled, we cannot draw definitive conclusions regarding the efficacy of the individual protocols or resolve ongoing discussions about the best immunosuppressive approach to treat patients with primary MN.18,19
To eventually define a potential pathogenetic role of PLA2R antibody levels, they were correlated with proteinuria and serum creatinine at the time of study inclusion. We did not find any correlation and cannot confirm earlier observations by others, who showed a positive correlation between proteinuria and PLA2R antibody levels at a defined time point.7 Our findings, however, suggest that levels of PLA2R antibodies may influence clinical response. Patients who experienced remission of proteinuria after 12 months had significantly lower PLA2R antibody levels at study inclusion (Table 2). There were no significant differences between the groups in proteinuria or serum albumin and in the relative number of patients who received immunosuppressive therapy. This excludes an eventual bias of immunosuppressive therapy on proteinuria. Patients who had a remission of proteinuria after 12 months had significantly lower PLA2R antibody levels (Figure 5). Patients with low PLA2R antibody levels at study inclusion reached remission of proteinuria significantly faster than patients with high PLA2R antibody levels (Figure 6). These findings suggest that patients with higher antibody levels may need a longer time to reach spontaneous remission or may need more intensive immunosuppressive therapy to reach complete or partial remission. This is further supported by the fact that patients with no remission of proteinuria after 18 months have higher PLA2R antibody levels compared with patients with partial remission, whereas patients with complete remission have the lowest PLA2R antibody levels.
Approximately one third of patients with MN may experience a spontaneous clinical remission of proteinuria, which is associated with good clinical outcome.2,3 Because these patients would be unnecessarily treated with an immunosuppressive regimen, it would be very helpful to have a marker to identify these patients. Because our study had an open design, we cannot finally predict how many patients would in fact have experienced spontaneous clinical remission, but we did observe patients who went into spontaneous remission within a 15-month follow-up period. In parallel with the decrease in proteinuria in patients with spontaneous remission, PLA2R antibody levels also decreased and were significantly lower compared with the time of study inclusion and compared with patients who remained nephrotic. Thus, spontaneous clinical remission is associated with a decrease in PLA2R antibody levels. In summary, our data show that a decrease in PLA2R antibody levels is associated with a fall in proteinuria. PLA2R antibody levels may serve as useful biomarkers for immunologic and clinical activity of patients with primary MN.
Concise Methods
Patients and Study Design
This prospective multicenter open clinical study included 133 consecutive patients with the histologic diagnosis of MN and a positive PLA2R antibody test result in the serum. The serum test for the presence of PLA2R antibodies had to be performed within 6 months after renal biopsy. Only patients with a proteinuria of ≥3.5 g/24 h and a serum albumin of ≤30 g/L were included. No immunosuppressive therapy was allowed before inclusion in this study. All other medications were allowed. After study inclusion, the treating physicians decided on the therapeutic strategy without any recommendations. In the case of a decision for an immunosuppressive therapy, the dose and the duration of the applied agents had to be documented. PLA2R antibody levels, 24-hour protein excretion, serum creatinine, and serum albumin levels were measured at 3-month intervals. This study was approved by the local ethics committee of the Chamber of Physicians in Hamburg and was conducted in accordance with the ethical principles stated by the Declaration of Helsinki. Informed consent was obtained from all participating patients.
PLA2R Antibody Measurement
Serum levels of total IgG and IgG4-subclass PLA2R antibody were measured by two methods: an indirect immunofluorescence test, which was previously published and validated,6 and an ELISA test, which was recently developed by EUROIMMUN AG (Lübeck, Germany).8 According to the manufacturer, the ELISA results were considered positive at a level >20 U/ml for IgG PLA2R antibodies and >0.259 U/ml for IgG4 PLA2R antibodies.
Proteinuria and Serum Albumin Levels
Proteinuria is given as the total 24-hour excretion and serum albumin is given in grams per liter. Remission of proteinuria was defined as proteinuria of <3.5 g/24 h and at least 50% reduction from the time of inclusion. Complete remission of proteinuria was defined as proteinuria<0.5g/24 h.
Statistical Analyses
Data are given as the mean±SD and as the median for nonparametric data. Statistical significance was defined as P<0.05. Multivariable Cox regression analysis was performed. In the multivariate analysis, we included and adjusted for all clinical parameters that might influence the remission of proteinuria in patients with MN (age, sex, proteinuria, serum creatinine, serum albumin, and immunosuppressive treatment). Hazard ratios are expressed per natural logarithm unit of serum creatinine, proteinuria, and PLA2R antibody levels measured by ELISA or per unit of age, and are dichotomized for sex and treatment. Statistical analyses were performed using SSPS software.
Disclosures
R.S. received educational honoraria from Roche.
Supplementary Material
Acknowledgments
The authors thank Eugen Kinzler, Daniela Bergleiter, and Catharina Fürstenau for their technical assistance; Dr. William G, Couser (University of Washington, Seattle, WA) for the critical reading and comments to the manuscript; and Eik Vettorazzi for his assistance in the statistical analysis of the data.
The following colleagues participated in the recruitment of the patients for this study (in alphabetical order): F. Aedtner (Halberstadt), A. Altrogge (Hamburg), D. Amir-Kabirian (Hamburg), L. Arndt (Buchholz), P. Arnold (Siegen), H. Baberg (Berlin-Buch), P. Beck (Langenfeld), J. Beckermann (Vechta), M.L. Beckmann (Bocholt), S. Beckmann (Herford), T. Benzing (Köln), M. Bieringer (Berlin), T. Bödefeld (Diepholz), J. Böhler (Wiesbaden), K. Böhmer (Nürnberg), J. Bramstedt (Bremerhaven), J. Braun (Dingolfing), R. Brunkhorst (Hannover), M. Bruns (Hanau), E. Büssemaker (Münster), M. Busch (Jena), E.M. Burth (Paderborn), W. Clasen (Münster), E.G. Dannemann (Gelsenkirchen), A. Daul (Essen), T. David-Walek (Kiel), F. Dellanna (Düsseldorf), K. Dieckmann (Flensburg), A. Dillmann (Bremen), J. Donauer (Freiburg), D. Duvigneau (Hamburg), K.U. Eckhardt (Erlangen), M. Falcke (Hamburg), G. Feyerabend (Reinbek), W. Filejski (Hamburg), J. Floege (Aachen), M. Frank (Pforzheim), J. Galle (Lüdenscheid), T. Gerhardt (Bonn), P. Gerke (Lübeck), J. Gerth (Zwickau), M. Gödel (Freiburg), U. Göttmann (Mannheim), S. Grosser (Hamburg), U. Haberstroh (Limburg), F. Hagenah (Offenburg), A. Hamadeh (Höxter), M. Heckel (Kronach), P. Heering (Solingen, Hochtritt Celle), B. Hohenstein (Dresden), M. Hohmann (Vechta), M. Hollenbeck (Bottrop), B. Hörnig (Berlin), T. Huber (Freiburg), W. Jabs (Berlin), J. Jacobsen (Dortmund), J. Jacobsen (Hamburg), H. Kämpf (Sonneberg), F. Keller (Ulm), M. Ketteler (Coburg), F. Kiziler (Bochum), R. Kleinecke (Bamberg), M. Kohnle (Mettmann), A. Korschanowski (Cuxhaven), B. Kortus-Götze (Marburg), I. Krenz (Hamburg), M. Kube (Hamburg), A. Kühns (Hamburg), F. Kunigk (Pinneberg), D. Lange (Heilbad Heiligenstadt), F. Lange-Hüsken (Hamburg), C. Laube (Bremen), O. Laue (Langenhagen), T. Leingärtner (Regensburg), J. Lepenies (Berlin), O. Loke (Lüdenscheid), G. Loley (Osnabrück), G. Lorenz (Melle), C. Marx (Nordhausen), S. Mees (Hamburg), R. Melching (Gütersloh), K. Meßtorff (Stade), T. Mettang (Wiesbaden), T. Meyer (Hamburg), G. Meyer-Sundhaußen (Berlin), G. Oberle (Langenhagen), F. Özcan (Dortmund), M. Oppermann (Perleberg), H.J. Pavenstädt (Münster), B. Pfalzer (Hamburg), J. Potratz (Rotenburg Wümme), F. Reichenberger (Cottbus), E. Rensinghoff (Bochum), M. Riedasch (Coesfeld), P.M. Rob (Lübeck), M. Rudnicki (Innsbruck), D. Schaumann (Hameln), P. Schilken (Paderborn), H. Schlee (Weißenfels), D. Schmiedel (Bad Nenndorf), F.J. Schmitz (Minden), R. Schneidenbacah (Hamburg), O. Schnegelsberg (Buchholz), J. Schnierda (Waldshut-Tiengen), A. Schnitzler (Lüneburg), M. Schüler (Erlangen), M. Schulte-Vorwick (Unna), J. Seyfried (Pforzheim), B. Stiasny (Schwabach), J. Siegmund (Hamburg), E. Siwek-Orman (Arnstadt), A. Stahn (Hamburg), H.B. Steinhauer (Cottbus), E. Sturm (Hamburg), K. Toussaint (Hamburg), W. Treiber (Neuwied), M. Vischedyk (Paderborn), M. Volsek (Essen), K. von Appen (Hamburg), A. Voßkühler (Bottrop), A.K. Wagner (Hamburg), M. Weber (Bovenden), S. Weiner (Trier), M. Weiß (Hamburg), F. Wiedemann (Nahgold), G. Wirtz (Kamen), G. Wolf (Jena), T. Wollweber (Wandersloh), and H.G. Wullstein (Hamburg).
This work is supported by grants from the Else-Kroener-Fresenius Stiftung (to R.S.) and the Deutsche Forschungsgemeinschaft (KFO 228 Z project to E.H. and STA193/9-1 and STA193/9-2).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Phospholipase A2 Receptor Antibodies in Membranous Nephropathy: Unresolved Issues,” on pages 1137–1139.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013040430/-/DCSupplemental.
References
- 1.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schieppati A, Mosconi L, Perna A, Mecca G, Bertani T, Garattini S, Remuzzi G: Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med 329: 85–89, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Polanco N, Gutiérrez E, Covarsí A, Ariza F, Carreño A, Vigil A, Baltar J, Fernández-Fresnedo G, Martín C, Pons S, Lorenzo D, Bernis C, Arrizabalaga P, Fernández-Juárez G, Barrio V, Sierra M, Castellanos I, Espinosa M, Rivera F, Oliet A, Fernández-Vega F, Praga M, Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Española de Nefrología : Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol 21: 697–704, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cattran DC, Pei Y, Greenwood CM, Ponticelli C, Passerini P, Honkanen E: Validation of a predictive model of idiopathic membranous nephropathy: Its clinical and research implications. Kidney Int 51: 901–907, 1997 [DOI] [PubMed] [Google Scholar]
- 5.van den Brand JAJG, Hofstra JM, Wetzels JFM: Prognostic value of risk score and urinary markers in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 7: 1242–1248, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoxha E, Harendza S, Zahner G, Panzer U, Steinmetz O, Fechner K, Helmchen U, Stahl RA: An immunofluorescence test for phospholipase-A₂-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant 26: 2526–2532, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Hofstra JM, Debiec H, Short CD, Pellé T, Kleta R, Mathieson PW, Ronco P, Brenchley PE, Wetzels JF: Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1735–1743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dähnrich C, Komorowski L, Probst C, Seitz-Polski B, Esnault V, Wetzels JF, Hofstra JM, Hoxha E, Stahl RA, Lambeau G, Stöcker W, Schlumberger W: Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin Chim Acta 421: 213–218, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Beck LH, Jr, Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, Cosio FG, Cattran DC, Salant DJ: Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol 22: 1543–1550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stahl R, Hoxha E, Fechner K: PLA2R autoantibodies and recurrent membranous nephropathy after transplantation. N Engl J Med 363: 496–498, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Pruchno CJ, Burns MM, Schulze M, Johnson RJ, Baker PJ, Alpers CE, Couser WG: Urinary excretion of the C5b-9 membrane attack complex of complement is a marker of immune disease activity in autologous immune complex nephritis. Am J Pathol 138: 203–211, 1991 [PMC free article] [PubMed] [Google Scholar]
- 12.Salant DJ, Darby C, Couser WG: Experimental membranous glomerulonephritis in rats. Quantitative studies of glomerular immune deposit formation in isolated glomeruli and whole animals. J Clin Invest 66: 71–81, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanigicherla D, Gummadova J, McKenzie EA, Roberts SA, Harris S, Nikam M, Poulton K, McWilliam L, Short CD, Venning M, Brenchley PE: Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int 83: 940–948, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Ponticelli C, Altieri P, Scolari F, Passerini P, Roccatello D, Cesana B, Melis P, Valzorio B, Sasdelli M, Pasquali S, Pozzi C, Piccoli G, Lupo A, Segagni S, Antonucci F, Dugo M, Minari M, Scalia A, Pedrini L, Pisano G, Grassi C, Farina M, Bellazzi R: A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol 9: 444–450, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Cattran DC, Greenwood C, Ritchie S, Bernstein K, Churchill DN, Clark WF, Morrin PA, Lavoie S, Canadian Glomerulonephritis Study Group : A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Kidney Int 47: 1130–1135, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, Rambaldi A, Marasà M, Remuzzi G: Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1416–1425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appel GB: Rituximab in membranous nephropathy: Is it a first-line treatment? J Am Soc Nephrol 23: 1280–1282, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Waldman M, Austin HA, 3rd: Treatment of idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1617–1630, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.