Abstract
Ischemia reperfusion injury (IRI) causes tissue and organ injury, in part, through alterations in tissue blood flow and the production of reactive oxygen species. The cell surface receptor signal-regulatory protein-α (SIRP-α) is expressed on inflammatory cells and suppresses phagocytosis, but the function of SIRP-α in IRI has not been determined. We reported previously that the matricellular protein thrombospondin-1 is upregulated in IRI. Here, we report a novel interaction between thrombospondin-1 and SIRP-α on nonphagocytic cells. In cell-free experiments, thrombospondin-1 bound SIRP-α. In vascular smooth muscle cells and renal tubular epithelial cells, treatment with thrombospondin-1 led to phosphorylation of SIRP-α and downstream activation of Src homology domain 2–containing phosphatase-1. Thrombospondin-1 also stimulated phosphorylation of p47phox (an organizer subunit for nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 1/2) and increased production of superoxide, both of which were abrogated by knockdown or antibody blockade of SIRP-α. In rodent aortic rings, treatment with thrombospondin-1 increased the production of superoxide and inhibited nitric oxide–mediated vasodilation in a SIRP-α–dependent manner. Renal IRI upregulated the thrombospondin-1–SIRP-α signaling axis and was associated with increased superoxide production and cell death. A SIRP-α antibody that blocks thrombospondin-1 activation of SIRP-α mitigated the effects of renal IRI, increasing blood flow, suppressing production of reactive oxygen species, and preserving cellular architecture. A role for CD47 in SIRP-α activation in these pathways is also described. Overall, these results suggest that thrombospondin-1 binding to SIRP-α on nonphagocytic cells activates NADPH oxidase, limits vasodilation, and promotes renal IRI.
Thrombospondin-1 (TSP1) is a secreted matricellular protein produced by platelets, endothelial and vascular smooth muscle cells (VSMCs), and nonvascular cells.1 TSP1 transduces signals from the extracellular to cellular components of tissues through binding to cell surface receptors, including the integrins, CD36 and CD47.2 We and others have shown that TSP1 levels are increased in plasma and in conditions associated with decreased blood flow, such as ischemia reperfusion injury (IRI),3 atherosclerosis,4 pulmonary hypertension,5,6 and sickle cell anemia.7
Signal regulatory protein-α (SIRP-α) is a cell surface receptor expressed on phagocytic and neuronal cells and activated through interactions with the cell surface protein CD47, by growth factors or integrin signaling.8,9 SIRP-α controls cell responses through the recruitment and phosphorylation of Src homology domain 2–containing phosphatase-1 (SHP1) and -2 (SHP2).10 SIRP-α is classified as an inhibitory cell receptor, and SIRP-α–mediated signaling suppresses macrophage phagocytosis.11 However, little is known about the role of SIRP-α in vascular cells and IRI.
Loss of nitric oxide (NO) signaling, including decreased NO bioavailability, is a major contributor to cardiovascular disease.12 NO reacts rapidly with the reactive oxygen species (ROS) superoxide anion (O2·−) which dramatically limits its biologic effect.13 This interaction becomes important after ischemia reperfusion, where pathologic ROS production, including O2·−, is increased. We have shown that TSP1 inhibits NO signaling5 and limits blood flow,14–16 but the exact mechanisms are still unclear.
Our data demonstrate that TSP1 stimulates phosphorylation of nonphagocytic SIRP-α and stimulates NADPH oxidase (Nox)–mediated O2·− production and that SIRP-α phosphorylation is absent upon CD47 deletion. In arteries, TSP1 inhibits NO-mediated vasodilation through SIRP-α–dependent stimulation of ROS. IRI upregulates renal TSP1–SIRP-α signaling, increases pathologic ROS production, and promotes cell death. Disruption of TSP1–SIRP-α signaling inhibits O2·− production, promotes vasodilation, improves blood flow, and limits IRI.
Results
TSP1 Engages and Phosphorylates Nonphagocytic SIRP-α
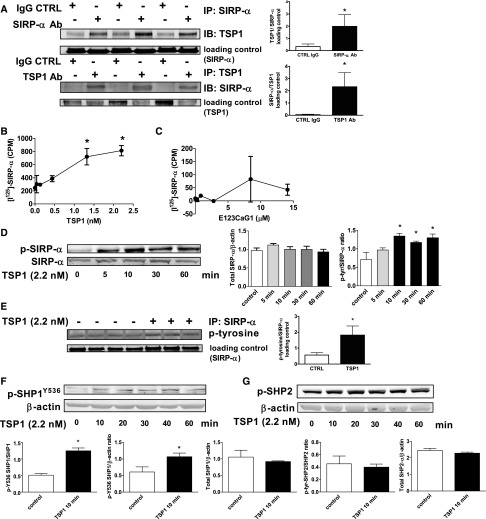
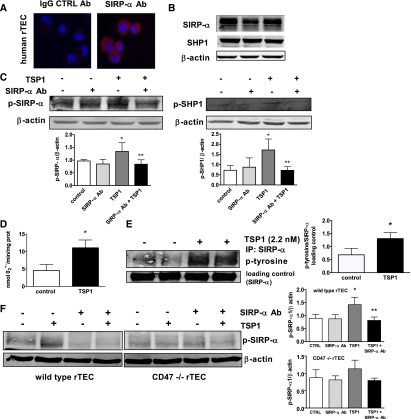
TSP1 can interact with several cell surface receptors,1 including CD47, as we have reported.17 However, it is not known whether TSP1 can interact with or signal through SIRP-α. In arterial VSMC lysates, SIRP-α was coprecipitated by a TSP1 monoclonal antibody and, conversely, TSP1 was coprecipitated by a SIRP-α monoclonal antibody (Figure 1A). An isotype-matched control IgG antibody did not coprecipitate SIRP-α (Figure 1A) or TSP1. In cell-free preparations, low concentrations of immobilized TSP1 bound soluble human SIRP-α (Figure 1B). In contrast, the signature domain of TSP1 (E123CaG1), which contains the C-terminal of TSP1 and binds CD47,17 did not bind to SIRP-α (Figure 1C). Extending these observations to cell culture systems, where endogenous TSP1 production was minimized by restricting serum and growth factors, we treated arterial VSMCs with exogenous TSP1 (2.2 nmol/L) and assessed SIRP-α phosphorylation. TSP1 phosphorylated SIRP-α within 10 minutes, and this persisted for at least 60 minutes (Figure 1D). Because these experiments used a general phospho-tyrosine antibody, we confirmed our results by immunoprecipitating for SIRP-α and then probing for changes in tyrosine phosphorylation (Figure 1E). Finally, TSP1 treatment under these conditions did not alter total SIRP-α protein levels (Figure 1D, densitometry presented).
Figure 1.
TSP1 binds to and activates nonphagocytic SIRP-α and its downstream signal transducer SHP1. (A) Coimmunoprecipitation in arterial VSMCs of TSP1 and SIRP-α. Immunoprecipitation was with monoclonal Ab to TSP1, SIRP-α, or an isotype-matched control IgG antibody. Results presented are representative of six separate experiments. Plastic wells coated with TSP1 (B) or the recombinant domain of TSP1 containing the C-terminal (E123CaG1, C) at the indicated concentrations were incubated with 125I-SIRP-α at room temperature. Bound radioactivity was quantified and data are presented as the mean±SEM. of three separate experiments. VSMCs were incubated in basal medium with TSP1 (2.2 nmol/L) for the indicated time points, cell lysate prepared, protein separated by SDS-PAGE electrophoresis, membranes probed with a phospho-tyrosine antibody (D) or lysates immunoprecipitated with a SIRP-α Ab, protein separated via SDS-PAGE electrophoresis, and nitrocellulose membranes probed with Ab to total SIRP-α and to phosphorylated tyrosine residues (E), as well as phosphorylated SHP1 (Y536) (F) and p-SHP2 (G). Densitometry is presented as mean ratios of p-tyr-SIRP-α to total SIRP-α and total SIRP-α to β actin (±SEM) (D), p-SHP1 to total SHP1, p-SHP1 to β actin, and total SHP1 to β actin (±SEM) (F), and p-SHP2 to total SHP2 and total SHP2 to β actin (±SEM) (G). Representative data from four independent experiments are presented. *Statistically significant difference (P<0.05 compared with untreated).
TSP1 Activates a Downstream Target of SIRP-α
Upon phosphorylation, SIRP-α activates the Src homology-2 (SH2) domain containing protein phosphatases SHP1 and/or SHP2.18 We tested whether TSP1 activates these downstream signal transducers in smooth muscle cells. Arterial VSMCs preincubated under growth factor-free and serum-free conditions (for 24 hours) and treated with TSP1 (2.2 nmol/L) displayed phosphorylation of SHP1 in a temporal fashion similar to that of SIRP-α (Figure 1F). Treatment of VSMCs with TSP1 did not result in SHP2 phosphorylation (Figure 1G) and did not alter total SHP1 or SHP2 protein levels within the time course of the experiment (Figure 1, F and G, densitometry presented).
Treatment of VSMCs with the NO Donor Sodium Nitroprusside Does Not Stimulate Phosphorylation of SIRP-α or SHP1
The NO donor S-nitroso-N-acetylpenicillamine was reported to activate SHP1,19 and our laboratory reported that TSP1 regulates NO signaling in vascular cells.20–22 We were interested in exploring what role exogenous NO could play in our studies. We tested this in VSMCs by determining SIRP-α phosphorylation in the presence of sodium nitroprusside (SNP), a prodrug that is metabolized by VSMCs to NO.23,24 Once again, TSP1 phosphorylated SIRP-α and SHP1, whereas treatment with SNP had no effect on SIRP-α or SHP1 phosphorylation (Supplemental Figure 1, A and B).
TSP1-Stimulated Phosphorylation of VSMC SIRP-α May Not Completely Depend on CD47 Activation
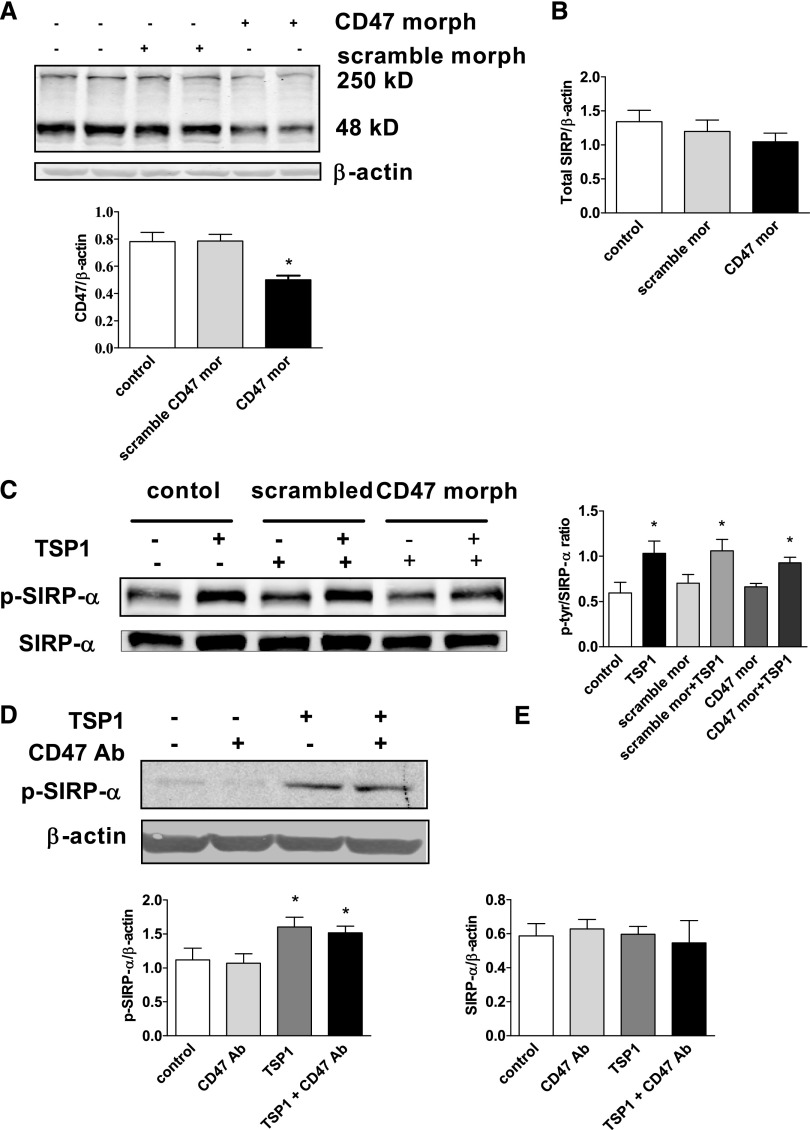
CD47 and SIRP-α are expressed on vascular cells,25 and SIRP-α activation requires CD47 in phagocytic cells.25,26 This conclusion is based, in part, on studies using a TSP1-derived peptide (peptide 4N1K, KRFYVVMWKK) that was reported to interact specifically with CD47.27 However, the validity of this contention is not clear because peptide 4N1K has effects on CD47 null cells.28 Nevertheless, to test whether CD47 is required for TSP1 activation of SIRP-α, we first treated cells with a CD47-targeting morpholino oligonucleotide to block mRNA translation.29 Western blot confirmed suppression of CD47 protein (by 37%) (Figure 2A). A control nonspecific morpholino (scrambled) did not alter total CD47 expression in treated cells (Figure 2A), and neither the CD47 targeting nor the control morpholinos significantly changed total SIRP-α expression (Figure 2B, densitometry presented). TSP1 in CD47 gene-suppressed cells still phosphorylated SIRP-α (Figure 2C). In further experiments, we used a CD47 monoclonal antibody (Ab) that blocks TSP1 binding to CD47,15–17, 30 as we reported, and also blocks CD47 interaction with SIRP-α.31 Treating VSMCs with CD47-blocking Ab had no effect on basal or TSP1-mediated SIRP-α phosphorylation (Figure 2D) and did not alter total SIRP-α protein levels (Figure 2E, densitometry presented). These data, although interesting, cannot exclude a possible role for CD47 in TSP1-mediated phosphorylation of SIRP-α in VSMCs. That is, there is the possibility of cross-talk, especially in light of CD47 null data in rTEC (vide infra).
Figure 2.
TSP1-stimulated phosphorylation of VSMC SIRP-α may not depend completely on CD47 activation. (A) VSMCs were treated with a CD47-specific (10 μM) or scrambled oligonucleotide morpholino as per the manufacturer instructions. Western blot analysis of CD47 was performed. Densitometry is presented as the mean ratio of CD47 to β actin (±SEM). (B) Densitometry of Western blots from untreated (control), scrambled, and CD47 morpholino treated VSMC is presented as the mean ratio of total SIRP-α to β actin (±SEM). (C) VSMCs were pretreated with a CD47-specific or scrambled morpholino, then incubated in basal medium with TSP1 (2.2 nmol/L) for 10 minutes, lysate prepared and Western blots performed. Densitometry is presented as the mean ratio of p-SIRP-α to SIRP-α (±SEM). (D) VSMCs were incubated in basal medium, treated with a CD47-blocking Ab (1 μg/ml) for 20 minutes followed by TSP1 (2.2 nmol/L) for 10 minutes and cell lysate prepared. Western blot analysis of p-SIRP-α was performed. Densitometry is presented as the mean ratio of p-SIRP-α to β actin (±SEM). (E) Densitometry of Western blots from CD47 Ab and TSP1-treated VSMCs is presented as the mean ratio of total SIRP-α to β actin (±SEM). For all measurements: *statistically significant difference (P<0.05) compared with untreated and control morpholino-treated, and representative examples of four independent experiments are presented.
TSP1 Activation of SIRP-α Does Not Require β Integrins
TSP1 has several functional domains, and we reported that TSP1, via its C-terminal domain, binds CD47.17 To test whether β integrin could be mediating SIRP-α phosphorylation, we used the TSP1-based peptide 753 (M.W. 685, sequence-acAELDVP) that is derived from the N-terminal domain of the protein and is known to activate integrins.32 Treating VSMCs with peptide 753 (10 µmol/L, 10 minutes) phosphorylated SIRP-α (Supplemental Figure 2A) without changing total SIRP-α protein levels (Supplemental Figure 2C, densitometry presented). The N-terminal domain of TSP1 is known to engage β integrins.32 To explore the role of β integrins in this process, we treated VSMCs with a β integrin–blocking Ab (clone Ha2/5, BD Biosciences) followed by TSP1 (2.2 nmol/L), and determined SIRP-α phosphorylation. Interestingly, TSP1-stimulated phosphorylation of SIRP-α was not altered by β integrin Ab blockade (Supplemental Figure 2B). Under basal conditions, treatment of VSMCs with the β integrin Ab alone did not increase SIRP-α phosphorylation and had no effect on total SIRP-α protein levels (Supplemental Figure 2B, densitometry presented). Peptide 753–mediated activation of SIRP-α was also not inhibited with the β integrin–blocking Ab (Supplemental Figure 2C). In contrast, a monoclonal SIRP-α Ab blocked TSP1-mediated phosphorylation of both SIRP-α (data not shown) and the downstream signal transducer SHP-1 (Supplemental Figure 2D).
TSP1-Stimulated O2·− Production in VSMCs Requires SIRP-α
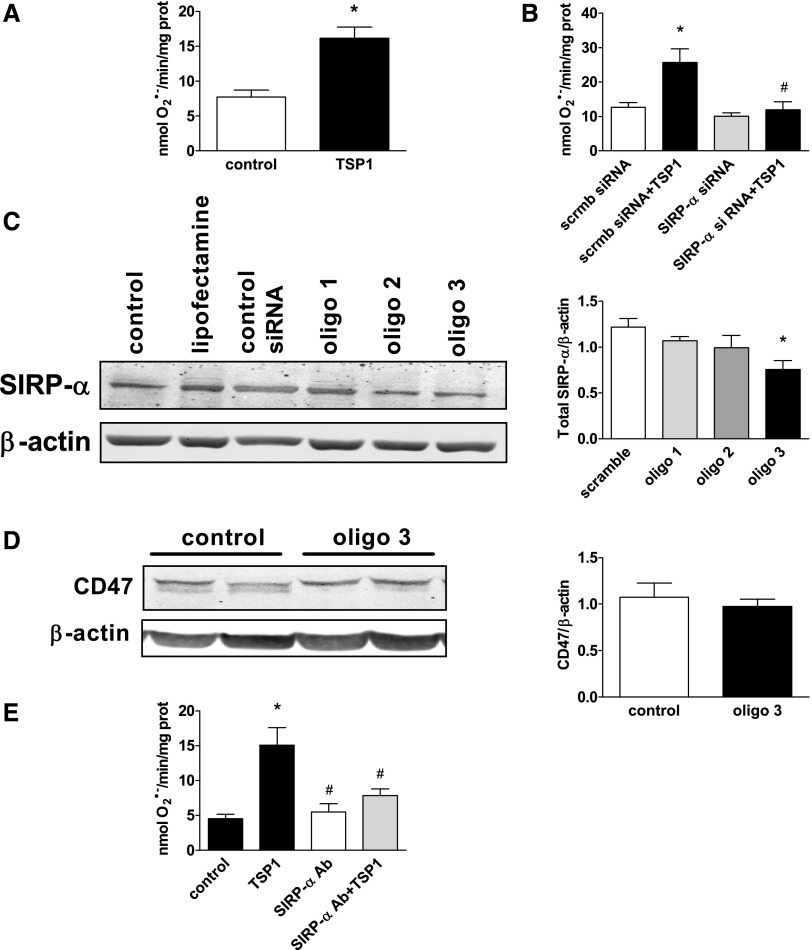
We have reported concurrent upregulation of TSP1 and ROS in ischemia33 and IRI.3,15 To determine whether TSP1 is capable of stimulating O2·− production in VSMC via activation of SIRP-α, we used a specific small interfering RNA (siRNA) (oligo 3) to suppress SIRP-α, then treated cells with TSP1 (2.2 nmol/L for 60 minutes) and determined O2·− levels via a cytochrome c reduction assay. TSP1 significantly stimulated VSMC O2·− production (Figure 3A). Gene suppression of SIRP-α using siRNA abrogated TSP1-stimulated O2·− generation (Figure 3B). Conversely, a control siRNA (scrmb siRNA) had no effect on TSP1-stimulated O2·− production (Figure 3B). Western blot analysis confirmed a reduction in SIRP-α expression by the targeting siRNA (40%) (Figure 3C). Treatment of VSMCs with the SIRP-α siRNA (oligo 3) did not change CD47 protein expression (Figure 3D). Corroborating these data, TSP1-stimulated O2·− production was inhibited by more than half in cells treated with a SIRP-α Ab (Figure 3E).
Figure 3.
TSP1-stimulated O2·− production in VSMCs requires SIRP-α. (A) VSMCs were incubated with vehicle or TSP1 (2.2 nmol/L) for 60 minutes. Superoxide production was measured in the 28,000g membrane fraction and calculated from the initial linear rate of superoxide dismutase–inhibitable cytochrome c reduction. Data represent the rate of superoxide production (±SEM). *Statistically significant difference (P<0.05) compared with untreated. (B) VSMCs were transfected with SIRP-α siRNA (oligo 3) or a control scrambled (scrmb) siRNA and incubated with TSP1 (2.2 nmol/L) for 60 minutes. Superoxide production was measured as described above. Data represent the rate of superoxide production (±SEM). *Statistically significant difference (P<0.05) compared with scrmb; #statistically significant difference (P<0.05) compared with scrmb siRNA+TSP1. (C) VSMCs were transfected with several SIRP-α or scrambled (scrmb) siRNA oligos. Western blot analysis was performed for SIRP-α. A representative blot from three separate experiments is presented. Densitometry is presented as the mean ratio of total SIRP-α to β actin (±SEM). *Statistically significant difference (P<0.05) compared with scrambled control. (D) VSMCs were transfected with SIRP-α (oligo 3) or control scrambled siRNA. Western blot analysis was performed for CD47. Densitometry is presented as the mean ratio of total CD47 to β actin (±SEM) from three experiments. (E) VSMCs were treated with a SIRP-α–blocking Ab (1 μg/ml) for 10 minutes, followed by TSP1 (2.2 nmol/L) for 60 minutes. Superoxide production was measured. Data represent the rate of superoxide production (±SEM). *Statistically significant difference (P<0.05) compared with untreated control; #statistically significant difference (P<0.05) compared with TSP1 treated.
TSP1, via SIRP-α, Phosphorylates the Nox1/2 Organizer Subunit p47phox
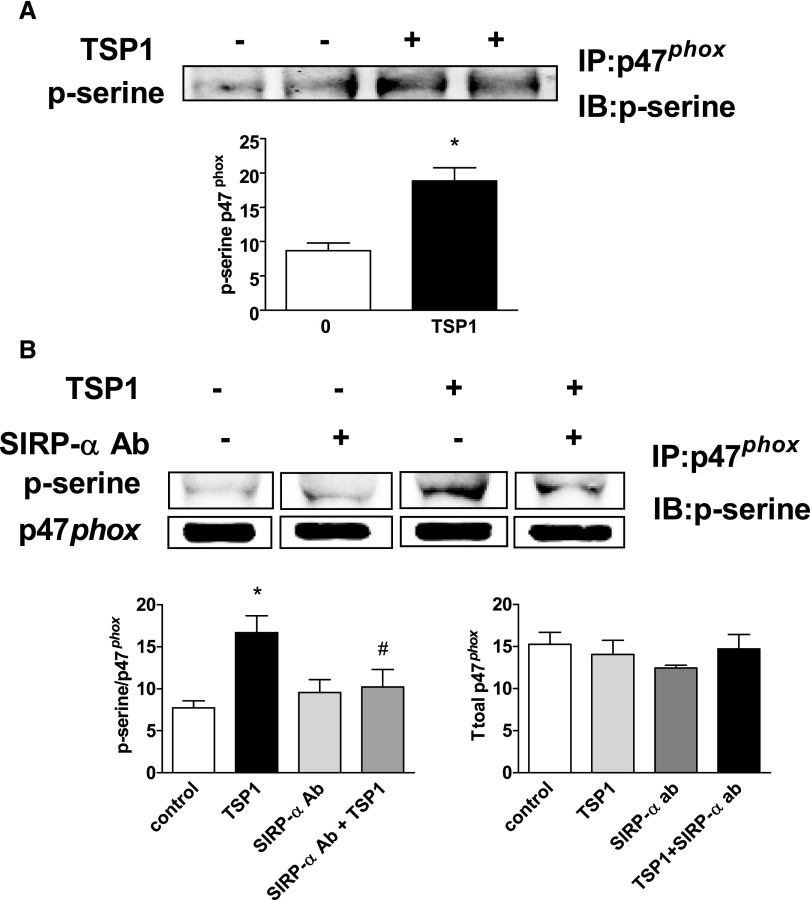
A major NADPH oxidase family member found in rodent large vessel arterial VSMCs is Nox1.34 Activation of Nox1 (and Nox2) and subsequent enzymatic O2·− formation occur on assembly of several subunit proteins, including p47phox (reviewed by Lassègue and Griendling35). This latter process requires serine phosphorylation of p47phox.36 TSP1 treatment (2.2 nmol/L) increased p47phox phosphorylation in VSMCs (Figure 4A). Treatment with the SIRP-α–blocking Ab reduced TSP1-mediated phosphorylation of p47phox (Figure 4B), suggesting that SIRP-α is upstream of TSP1-mediated phosphorylation of the Nox subunit.
Figure 4.
TSP1, via SIRP-α, phosphorylates the Nox1/2 organizer subunit p47phox. (A) VSMCs were treated in minimal medium with TSP1 (2.2 nmol/L) for 60 minutes or (B) a SIRP-α–blocking Ab (1 μg/ml) for 10 minutes with or without TSP1 (2.2 nmol/L) for 60 minutes, cell lysates prepared, immunoprecipitated with a p47phox Ab, resolved via gel electrophoresis, and blots probed with a phospho-serine Ab. A representative blot from four separate experiments is presented. Densitometry is presented as the mean ratio of total p-serine to p47phox (±SEM). *Statistically significant difference (P<0.05) compared with untreated control; #statistically significant difference (P<0.05) compared with TSP1 treated. Densitometry of Western blot of total p47phox expression from indicated treated cell groups not significantly different from control.
TSP1–SIRP-α Signaling Stimulates VSMC O2·− Production and Inhibits Vasodilation
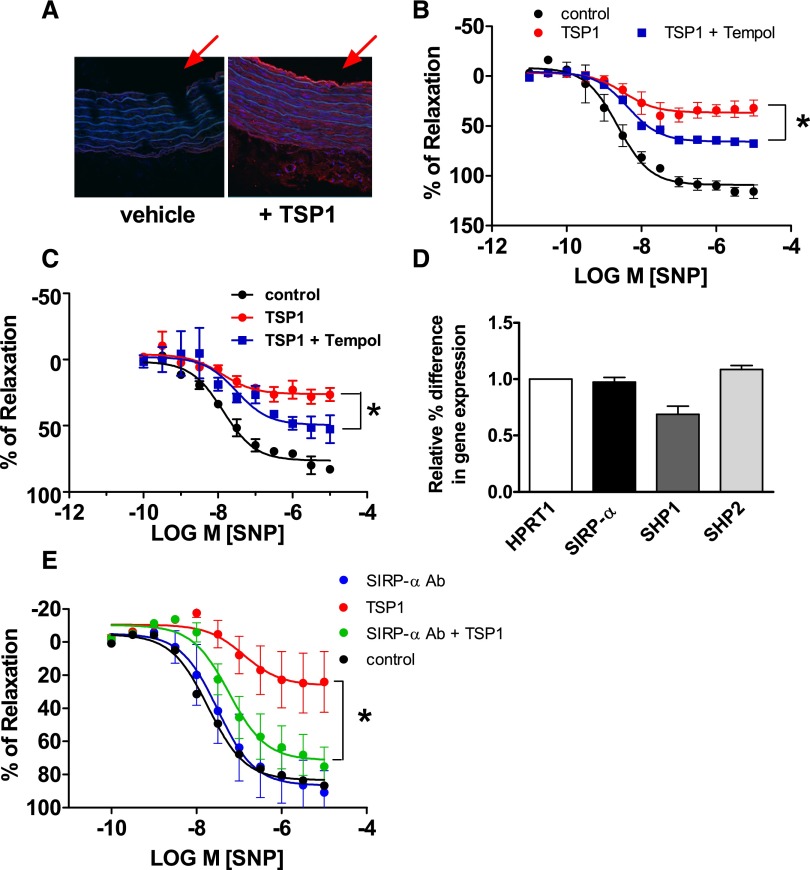
TSP1 is a large, 480-kDa protein found in the blood of healthy persons at 100-picomolar concentrations21 and at low nanomolar concentrations in disease.7 It is unknown whether TSP1 can cross the subendothelial barrier to gain access to the arterial VSMC compartment. To test this, aortic endothelium-intact arterial rings were incubated with TSP1 (2.2 nmol/L) for 60 minutes and tissue sections cut from the middle of the vessels prepared. TSP1-treated arterial rings showed increased TSP1 on both luminal endothelial cells and throughout the subendothelial VSMC compartment (Figure 5A; arrows indicate vessel lumen).
Figure 5.
TSP1, via SIRP-α, stimulates VSMC O2·− production and inhibits vasodilation. (A) Murine aortic segments were freshly harvested from wild-type male C57BL/6 mice, incubated in standard myograph buffer with TSP1 (2.2 nmol/L, 60 minutes), and tissue sections prepared for immunofluorescence. Representative images of three independent experiments are presented. TSP1 appears red. The red arrows highlight the vessel lumen. Murine (B) and rat (C) aortic rings were prepared from freshly harvested thoracic aortas. The endothelium was removed and the resulting arterial rings were incubated with vehicle, TSP1 (2.2 nmol/L, 60 minutes), or TSP1+Tempol (30 μM) and constricted with phenylephrine (3×10−7 M). Endothelium-independent vasodilation was stimulated by SNP (10−9 to 10−5 M). Data represent the means±SEM of four experiments. *Statistically significant difference (P<0.05) in relaxation between TSP1 versus TSP1 + Tempol. (D) RT-PCR assessment of SIRP-α, SHP1, and SHP2 mRNA of murine thoracic aorta. Data were normalized to the housekeeping gene HPRT1 and represent the means±SEM of five separate samples. (E) Murine thoracic aortas were harvested and endothelial-free arterial rings were incubated with vehicle, TSP1 (2.2 nmol/L, 60 minutes), or TSP1+a SIRP-α–blocking Ab (1 μg/ml) and constricted with phenylephrine (3×10−7 M). Endothelium-independent vasodilation was stimulated by SNP (10−9 to 10−5 M). Data represent the means±SEM of four experiments. *Statistically significant difference (P<0.05) in relaxation between TSP1 versus TSP1+SIRP-α Ab.
NO stimulates VSMC relaxation and arterial vasodilation.20 NO also rapidly interacts with O2·− to generate peroxynitrite,37 limiting the bioavailability of NO. On the basis of our finding that TSP1 can activate SIRP-α to increase O2·− production in arterial VSMCs, we hypothesized that this might, in part, account for our previous observation that TSP1 inhibits NO-mediated arterial vasodilation.5 We tested whether TSP1 inhibited arterial vasodilation via stimulation of VSMC O2·− production in endothelial-free arterial rings, using SNP, which, on conversion to NO, stimulates vasodilation.38 In both murine (Figure 5B) and rat (Figure 5C) endothelial-free arterial rings, TSP1 (2.2 nmol/L) inhibited SNP-mediated vasodilation whereas the O2·− scavenger Tempol39 significantly attenuated this effect. Interestingly, Tempol alone also decreased SNP-mediated vasodilation (data not shown). It is unknown whether the SIRP-α–SHP1 signaling cascade is present in rodent arteries. Real-time PCR confirmed the presence of SIRP-α, SHP1, and SHP2 in arterial rings (Figure 5D). To investigate the role SIRP-α plays in controlling vascular tone, we treated endothelial-free arteries with the SIRP-α–blocking Ab. Importantly, TSP1-mediated inhibition of NO-stimulated vasodilation was attenuated in vessels treated with the SIRP-α–blocking Ab (Figure 5E).
TSP1 Stimulates Phosphorylation of SIRP-α and O2·− Production in Human Renal Tubular Endothelial Cells
Renal tubular epithelial cells (rTECs) express Noxs,40,41 including Nox1 and 2,42,43 and are a target of cell injury driven by O2·−.3 To determine whether TSP1–SIRP-α–mediated stimulation of O2·− is a general finding and extends to other parenchymal cells, we treated human rTECs with TSP1. Human rTECs expressed SIRP-α and SHP1 (Figure 6, A and B), and TSP1 (2.2 nmol/L) treatment promoted rapid phosphorylation of SIRP-α and SHP1 (Figure 6C) concurrent with stimulating increased O2·− production (Figure 6D). On the other hand, TSP1-stimulated phosphorylation of SIRP-α and SHP1 was blocked in rTECs treated with a SIRP-α Ab (Figure 6C). In these experiments a general phospho-tyrosine antibody was again employed. Therefore we confirmed these results by immunoprecipitating for SIRP-α and probing for changes in tyrosine phosphorylation. Here, too, TSP1 treatment increased p-SIRP-α expression in human rTECs (Figure 6E). Having previously reported rTEC express CD47,3 we tested whether CD47 is required for TSP1-mediated SIRP-α phosphorylation in rTECs. Wild-type (CD47+/+) and null (CD47−/−) rTECs obtained from kidneys of the respective strains of mice were pre-treated with a SIRP-α antagonist Ab (1 μg/ml) for 15 minutes followed by exogenous TSP1 (2.2 nmol/L) for 60 minutes. TSP1 treatment stimulated SIRP-α phosphorylation in wild-type rTECs (Figure 6F), which was inhibited by SIRP-α Ab treatment. However, 2.2 nmol/L TSP1 treatment did not increase p-SIRP-α levels in CD47 null rTEC (Figure 6F). Total SIRP-α and SHP1 protein levels did not vary between wild-type and CD47 null rTEC or treatment groups (data not shown). rTEC undergo apoptotic cell death post IRI44 and a TSP1-derived peptide is known to promote endothelial cell death.45 We tested whether TSP1, via SIRP-α, promoted rTEC apoptosis. Cells were treated with TSP1 (2.2 nmol/L) for 24 hours, and apoptosis and cell viability were assessed. Under these conditions TSP1 did not stimulate apoptosis or decrease cell viability (Supplemental Figure 3, A and B).
Figure 6.
TSP1 stimulates SIRP-α phosphorylation and O2·− production in human rTECs. (A) Human rTECs were stained for SIRP-α (red) and nuclear protein (blue). Representative images are presented at a magnification of ×20. (B) Human rTECs were cultured in basal medium, lysate prepared and analyzed for total SIRP-α and SHP1 protein expression. A representative blot from three separate experiments is presented. (C) Human rTECs were incubated with vehicle or TSP1 (2.2 nmol/L) with or without a SIRP-α Ab (1 μg/ml) for 60 minutes, and protein expression of p-SIRP-α and p-SHP1 was determined. Data represent the mean ratios of target protein to β actin (±SEM). *Statistically significant difference (P<0.05) compared with untreated. **Statistically significant difference (P<0.05) compared with TSP1 treated. (D) Human rTECs in minimal medium were treated with TSP1 (2.2 nmol/L) for 45 minutes and O2·− production measured as described previously. Data represent the rate of superoxide production (±SEM). *Statistically significant difference (P<0.05) compared with untreated. (E) Human rTECs were incubated in basal medium with TSP1 (2.2 nmol/L), cell lysate prepared and immunoprecipitated with a SIRP-α Ab, protein separated via SDS-PAGE electrophoresis, and nitrocellulose membranes probed with Ab to total SIRP-α and to phosphorylated tyrosine residues. A representative blot from three separate experiments is presented. Data represent the mean ratios of target protein to SIRP-α (± SEM). *Statistically significant difference (P<0.05) compared with untreated (control). (F) Wild-type and CD47 null murine rTECs were treated with TSP1 (2.2 nmol/L) with or without a SIRP-α Ab (1 μg/ml) for 60 minutes and protein expression of p-SIRP-α determined. A representative blot from three separate experiments is shown. Data are presented as the mean ratio of target protein to β actin (±SEM). *Statistically significant difference (P<0.05) compared with untreated (control). **Statistically significant difference (P<0.05) compared with TSP1 treated.
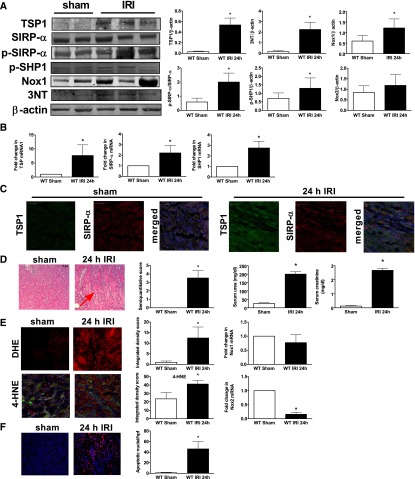
TSP1–SIRP-α Signaling Is Upregulated in Renal IRI Concurrent with Increased ROS Production, Decreased Blood Flow, and Increased Tissue Injury
IRI is a major cause of cellular and organ dysfunction and, in transplantation, of graft failure.46,47 Pathologic ROS contribute significantly to this process,48,49 and ROS mitigation is a therapeutic goal for limiting tissue injury in several conditions, including myocardial infarction, stroke, and organ transplantation.50 To test whether TSP1–SIRP-α signaling is upregulated in IRI, we challenged mice with 20 minutes of renal ischemia followed by 24 hours of reperfusion. Our data demonstrate concordant upregulation of renal TSP1, p-SIRP-α, and p-SHP1 protein (Figure 7A) and SIRP-α and SHP1 mRNA (Figure 7B) after IRI. Immunofluorescent staining of renal corticomedullary sections after IRI demonstrated increased TSP1 expression in the renal tubules compared with sham control (Figure 7C). SIRP-α was expressed in both proximal and distal tubules regardless of injury state. Analysis of merged images suggests colocalization of TSP1 and SIRP-α in renal tubules after IRI. Histology confirmed significant renal tubular injury and decreased renal function (as assessed by elevated serum urea and creatinine) in wild-type kidneys and mice after IRI (Figure 7D). As we reported previously,3 real-time laser Doppler analysis demonstrated marked deterioration of renal blood flow in post-IRI animals compared with sham-operated mice (data not shown) and greater cell death on TUNEL staining (Figure 7F). Assessment of ROS using the cell-permeable agent dihydroethidium (DHE) demonstrated increased fluorescence in renal tissue after IRI (Figure 7E). Because DHE can be oxidized by cells to several fluorescent products, only one of which is specific for ethidium-O2·− interaction,51 we also evaluated tissue oxidative stress using 4-hydroxynonenal (4-HNE), a stable aldehyde formed by the degradation of polyunsaturated fatty acids during lipid peroxidation.52 4-HNE immunostaining was greater after IRI and was minimally detected in renal tissue sections from sham-operated mice (Figure 7E). A byproduct of O2·− reacting with NO is the reactive nitrogen species peroxynitrite (ONOO−), which can adversely alter protein function through nitration.53 Consistent with increased in vivo O2·− and reactive nitrogen species, renal tissue levels of 3-nitrotyrosine were increased after IRI (Figure 7A). Interestingly, Nox1 protein expression was increased (Figure 7A, blot and densitometry) and Nox2 remained unchanged (Figure 7A, densitometry alone), while Nox1 mRNA levels were unchanged whereas Nox2 mRNA levels were decreased post IRI in wild-type kidneys compared with sham controls (Figure 7E).
Figure 7.
TSP1-SIRP-α signaling is upregulated in renal IRI concurrent with increased ROS production, decreased blood flow, and increased tissue injury. Male C57BL/6 mice were challenged with 20 minutes of unilateral renal ischemia and 24 hours of reperfusion (n=8) or sham surgery (n=6) and Western blot for (A) TSP1, SIRP-α, p-SIRP-α, p-SHP1, Nox1, Nox2, 3-nitrotyrosine, and β actin was performed. Data shown are mean±SEM. *Statistically significant (P<0.001) IRI compared with sham-operated kidneys. (B) RT-PCR analysis of renal mRNA expression of the indicated genes in IRI and sham-operated kidneys. Data shown are mean±SEM. *Statistically significant (P<0.05) IRI compared with sham. (C) Representative kidney tissue sections from IRI and sham-operated mice stained for TSP1 and SIRP-α. TSP1 colored green; SIRP-α colored red; nuclei colored blue. Scale bar, 50 μm (magnification ×20). (D) Quantitative analysis and photomicrographs of tubular damage and neutrophil invasion in juxtamedullalary sections from IRI and sham-operated mice are shown (magnification, ×200; arrow highlights tubular injury); serum urea and creatinine from the same. Data shown are means ± SEM. *Statistically significant (P<0.05) IRI compared with sham. (E) Representative tissue sections and quantitative analysis from IRI and sham-operated mice stained with DHE or 4-HNE and visualized by microscopy (magnification, ×200). RT-PCR analysis of Nox1 and Nox2 mRNA from sham and post-IRI kidneys. Data shown are means±SEM. *Statistically significant (P<0.05) IRI compared with sham. (F) Representative tissue sections and quantitative analysis from IRI and sham-operated mice stained by TUNEL assay and visualized by microscopy (magnification, ×200). *Statistically significant (P<0.05) IRI compared with sham.
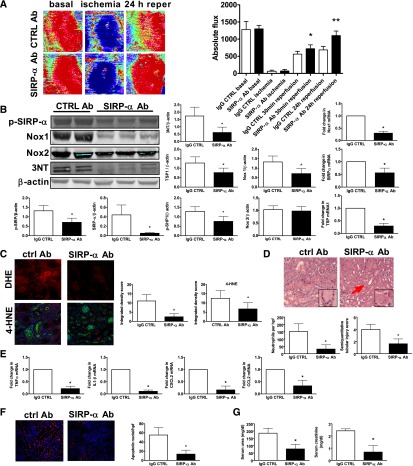
Disrupting TSP1–SIRP-α Signaling Suppresses Nox1 Transcription and Oxidative Stress, Increases Reperfusion Blood Flow, and Limits Renal IRI Injury
To further assess the role of the TSP1–SIRP-α signaling cascade in promoting renal IRI, we treated mice with the SIRP-α–blocking Ab that we have shown herein prevents TSP1-stimulated phosphorylation of SIRP-α and inhibits subsequent O2·− production. Following IRI, SIRP-α Ab treated mice demonstrated restoration of kidney blood flow to near preischemic levels after 24 hours (Figure 8A), concurrent with decreased p-SIRP-α, p-SHP1, total SIRP-α, Nox1 and 3-nitrotyrosine protein expression (protein and/or densitometry shown) and decreased TSP1, SIRP-α and Nox1 mRNA (Figure 8B). Nox2 protein levels were unchanged in both treatment groups. In IRI-challenged animals, treatment with a SIRP-α Ab decreased oxidative stress as quantified by DHE and 4-HNE immunofluorescence (Figure 8C), concordant with less renal tubular injury and neutrophil infiltration (Figure 8D), decreased proinflammatory cytokine and chemokine transcript expression (CCL2, CXCL2, IL1-β, TNF-α) (Figure 8E), and less cell death (as detected by TUNEL staining with results in kidneys from SIRP-α Ab–treated animals [Figure 8F] approaching levels found in sham-operated kidneys [Figure 7E]). From a functional perspective, serum urea and creatinine levels were lower after IRI in animals treated with the SIRP-α Ab compared with control IgG Ab–treated animals (Figure 8G). Previously we reported that CD47 null mice were protected from renal IRI.3 Therefore, we treated CD47 null mice with the SIRP-α antagonist Ab and challenged them with renal IRI. Interestingly, we found no further improvement in serum urea and creatinine levels in SIRP-α Ab–treated CD47 null mice after IRI compared with untreated CD47 null animals (Supplemental Figure 4).
Figure 8.
Disrupting TSP1–SIRP-α signaling suppresses Nox1 transcription and oxidative stress, increases reperfusion blood flow, and limits renal IRI injury. Wild-type mice were treated with a SIRP-α–blocking Ab or isotype control (CTRL) Ab and challenged with renal ischemia and 24-hour reperfusion (n=8 per group). (A) Laser Doppler images of renal blood flow. Red indicates blood flow. Results represent the means±SEM of four measurements from five mice in each group. *Statistically significant difference (P<0.001) for SIRP-α compared with isotype control at 30 minutes; **statistically significant difference (P<0.001) for SIRP-α compared with isotype control at 24 hours of reperfusion. (B) Western and RT-PCR analysis was performed for the indicated proteins and genes. Data shown are means±SEM, with representative Western blots.*Statistically significant difference (P<0.001) for SIRP-α compared with isotype control Ab. (C) Quantification and representative tissue sections from SIRP-α or control Ab treated mice after renal IRI stained with DHE or 4-HNE and visualized by microscopy (magnification, ×200) (n=5 per group). (D) Representative tissue sections and analysis of renal tubular damage and neutrophilic invasion from SIRP-α and isotype control Ab–treated mice (magnification, ×200; inset ×400). Data shown are means±SEM, *statistically significant difference (P<0.001) for SIRP-α compared with control Ab. Arrows highlight rTEC death and cast formation. (E) RT-PCR analysis of inflammatory cytokines and chemokines. Data shown are means±SEM. *Statistically significant difference (P<0.001) for SIRP-α compared with control Ab. (F) Quantification and tissue sections from control or SIRP-α Ab–treated mice after renal IRI mice stained by TUNEL assay (magnification, ×200). Data shown are means±SEM (n=6 per group). *Statistically significant difference (P<0.001) for SIRP-α compared with control Ab. (G) Serum urea and creatinine from mice. Data shown are means±SEM (n=6 per group). *Statistically significant difference (P<0.05) for SIRP-α compared with control Ab.
Discussion
In a myeloid leukemia cell line, SIRP-α inhibits expression of the Nox2 subunit and is associated with decreased hydrogen peroxide production.54 However, nonphagocytic SIRP-α has not previously been linked directly to pathologic ROS production. Our present results implicate a role for SIRP-α in the stimulation of Nox-mediated O2·− production in multiple nonphagocytic cell types. The data indicate that cells treated with a physiologically relevant concentration of TSP1 demonstrate a SIRP-α–dependent increase in O2·− production. Either siRNA knockdown or antibody blockade of SIRP-α inhibited both TSP1-mediated phosphorylation of the Nox organizer subunit p47phox and O2·− production without changing basal ROS production, consistent with SIRP-α playing an active role in stimulating O2·− production.
Signaling through SIRP-α is reported to occur via interactions with integrins,55 growth factors and CD47.56,57 In myeloid cells, SIRP-α phosphorylation has been demonstrated in the absence of CD47.55 Our data indicate that in nonmyeloid cells, TSP1 stimulates the phosphorylation of SIRP-α and its downstream effector SHP1 through a process that may depend on interaction with CD47. Gene suppression of CD47 by 37% and CD47 monoclonal Ab blockade did not prevent TSP1-mediated activation of SIRP-α in VSMCs. It is still possible that a residual amount of CD47 in gene-silenced cells is still sufficient to mediate TSP1-induced SIRP-α phosphorylation. Moreover, the pharmacologic properties of the employed CD47 Ab has to be more fully characterized using protein binding experiments to determine whether the Ab inhibits TSP1–SIRP-α binding or the Ab modifies CD47–SIRP-α interaction. Interestingly, treatment of murine CD47 null rTEC with 2.2 nmol/L TSP1 for 60 minutes did not stimulate SIRP-α phosphorylation. Also, CD47 null mice treated with a SIRP-α antibody did not exhibit further improvement in serum creatinine after renal IRI compared with isotype control-treated animals. Together, these data suggest an interaction between TSP1 and SIRP-α, and they also suggest an important role of CD47. Indeed, our previous studies showed that TSP1 activates Nox1-dependent ROS production in a CD47-dependent manner.58 In conjunction with the current data, it would appear that TSP1 could mediate its ROS-inducing effect via several mechanisms, including (1) parallel activation of CD47 and SIRP-α with these two pathways exhibiting cross-talk or (2) activation of CD47 followed by downstream activation of SIRP-α. Future studies are required to further delineate the precise molecular mechanisms by which TSP1 stimulates ROS generation.
In human umbilical vein endothelial cells, angiotensin II–stimulated ROS production was associated with increased SHP1 activity.59 We now find in two distinct nonphagocytic cell types, VSMCs and rTECs, that TSP1 treatment stimulates SIRP-α and downstream SHP1 and increased O2·− production. VSMCs and rTECs, depending on the vascular bed, express both Nox1 and Nox2, and the assembly of both of these enzyme complexes is promoted by the serine phosphorylation of the p47phox subunit.60,61 TSP1 phosphorylated p47phox, whereas a SIRP-α Ab blocked TSP1-mediated phosphorylation of this essential Nox organizing subunit, and both the SIRP-α–blocking Ab and SIRP-α siRNA abrogated TSP1-stimulated O2·− production. These data identify SIRP-α as a new, physiologically important activator of Nox-derived O2·− production in vascular and epithelial cells. Further work will define the dominant Nox isoform responsible for TSP1–SIRP-α–induced ROS following renal IRI.
We have reported that TSP1 limits arterial vasodilation via inhibition of NO signaling.14,62 Results from our present study suggest that TSP1 inhibits arterial vasodilation, in part by stimulating pathologic O2·− production. The O2·− scavenger Tempol decreased TSP1-mediated inhibition of NO-driven vasodilation in murine and rat arteries, supporting a role for TSP1 in promoting arterial O2·− production. A SIRP-α Ab reversed TSP1-mediated inhibition of vasodilation, placing SIRP-α upstream in this process. These data obtained using whole arteries are unlikely to be merely the result of TSP1-mediated inhibition of NO bioavailability because before analysis, vessels were rendered endothelial free and thus lacked an endogenous source of NO. Importantly, these data are, to our knowledge, the first to characterize SIRP-α as an acute regulator of arterial vasodilation.
IRI is characterized by defects in tissue blood flow, increased thrombosis, pathologic ROS production,63 and cell death.64 We herein identify SIRP-α as a new receptor for the matricellular protein TSP1. These results are also the first to identify a soluble ligand of SIRP-α. TSP1, on engaging SIRP-α in nonphagocytic cells, stimulates NADPH oxidase–derived O2·− production. Contrary to previous work (reviewed by Barclay et al.2 and Matozaki et al.65), we identify SIRP-α as an activating, rather than inhibitory, receptor in two nonphagocytic cell types, specifically VSMCs and rTECs. In vivo, TSP1, SIRP-α, and SHP1 were induced by and promoted IRI. Consistent with this notion, blocking TSP1–SIRP-α signaling with a SIRP-α monoclonal Ab inhibited IRI-mediated expression of these proteins, lowered O2·− production in cells and tissues, restored NO-mediated vasodilation (in ex vivo vessel preparations), augmented reperfusion blood flow, preserved end-organ integrity, and improved renal function after IRI. Consistent with our previous findings, CD47 is likely to play a parallel and supportive role in this process. Which of the two pathways predominates is still open to question.
Concise Methods
Detailed methods are available are available in the Supplemental Material.
Reagents and Cells
Human rTECs and rat aortic VSMCs were purchased from Lonza (Switzerland) and maintained in recommended medium. Cells were used between passages 3 and 7. TSP1 was purchased from Athens Research & Technology (Athens, GA). CD47 morpholino oligonucleotides and corresponding mismatched control morpholino were purchased from GeneTools, Inc. (Philmonth, Oregon). The TSP1-derived peptide 753 was synthesized by Dr. Henry C. Krutzsch and kindly provided by Dr. David D. Roberts (National Cancer Institute, National Institutes of Health, Bethesda, MD). The SIRP-α monoclonal Ab (clones C20 for treatment applications and A1 for Western blot) and monoclonal CD47 Ab (clone OX101) were purchased from Santa Cruz Biotechnology, and the β integrin–blocking Ab (clone Ha2/5) was purchased from BD Biosciences.
Animals
Male C57BL/6 wild-type mice and Sprague–Dawley rats were obtained from The Jackson Laboratory (Bar Harbor, ME) and Taconic (Hudson, NY), respectively. All studies were performed using protocols approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and in accordance with National Institutes of Health guidelines.
Murine rTEC Cultures
Kidneys from age-matched male wild-type and CD47 null mice were excised and digested, and primary rTEC were harvested and cultured as previously described66 with several minor modifications.
Protein-Binding Assays
Recombinant SIRP-α was labeled using NaI125 by the iodogen method as we previously published.17 Immulon 2HB Removawells (Thermo, Franklin, MA) were coated with 50 μl of TSP1 at several concentrations for 16–20 hours at 4°C. Nonspecific binding was blocked by incubating the wells with DPBS with Ca2+ and Mg2+ and 1% BSA. Radiolabeled SIRP-α was then added and incubation conducted for 2 hours at room temperature. Following extensive washing, bound radioactivity was quantified.
Coimmunoprecipitation
Immunoprecipitation was performed as we have described, with minor modification.67
Western protein analysis
Tissue or cells were homogenized in ice-cold lysis buffer. Supernatants were collected and lysates quantified using a Bradford assay (Bio-Rad, Hercules, CA). Thirty micrograms of total protein were resolved by SDS-PAGE and transferred onto nitrocellulose membrane (Bio-Rad). In blots for CD47, nonreducing Laemmli buffer was used with 8% SDS-PAGE. Blots were probed with primary antibody to the respective proteins and visualized on an Odyssey Imaging System (Licor, Lincoln, NE). The intensity of the bands was quantified using ImageJ.
RNA Extraction and Quantification by Real-Time PCR
Total RNA was extracted using Qiagen RNeasy Mini Kits (Qiagen, Hilden, Germany) as per the manufacturer’s instructions. RNA was quantified using the Take3 Gen5 spectrophotometer (BioTek, Winooski, VT). One microgram of RNA was treated with DNase I (amplification grade; Invitrogen, Carlsbad, CA) and then reverse-transcribed using the Superscript III First Strand Synthesis Supermix (Invitrogen). cDNA was amplified using Platinum Quantitative PCR SuperMix-UDG (Invitrogen) in 10-µl volumes in triplicate with gene-specific primers and probed on the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA), according to the manufacturer’s instructions.
Assessment of p47phox Phosphorylation
We determined the phosphorylation of p47phox (critical cytosolic subunit of Nox) in p47phox immunoprecipitates using an antiphosphoserine Ab as described.68 Briefly, cells were lysed and protein from the cytosolic fraction incubated with anti-p47phox Ab. Immune complexes were recovered with Protein G Plus-Agarose. Immunoprecipitates were then subjected to Western blotting with a phospho-serine Ab.
Measurement of O2·− via Cytochrome c Reduction
Cells were suspended in 200 µl of ice-cold disruption buffer. The suspension was lysed by five freeze/thaw cycles and passed through a 30-gauge needle five times.58 The cell lysate was centrifuged at 1000g for 10 minutes at 4°C to remove intact cells, nuclei, and debris. The supernatant was transferred to another Eppendorf tube and was centrifuged at 28,000g for 15 minutes at 4°C to collect the membrane fraction pellet, which was resuspended in 50 µl disruption buffer. Membrane fractions (2 µg/well) were added to cytochrome c–containing oxidase assay buffer. After a 5-minute baseline measurement, NADPH (180 µM) was added to initiate the reaction. O2·– was measured as the initial linear rate of superoxide dismutase–inhibitable cytochrome c reduction.69
siRNA Transfection
Cells were plated the day before transfection to achieve 30%–50% confluence in DMEM medium containing 10% fetal calf serum without antibiotics. The siRNA transfections were performed by using Lipofectamine 2000 (Invitrogen) in Opti-MEM according to the manufacturer’s instructions.
Morpholino Oligonucleotide Protein Suppression of CD47
Cells were seeded onto 60-mm2 plates in DMEM medium containing 10% fetal calf serum. CD47 morpholino was transfected with a final concentration of 10 µM using the delivery agent Endoporter (6 µl/ml; Genetools, Philomath, OR) according to protocol instructions.
Tissue Histology and Microscopy
Kidneys embedded in paraffin were sectioned at 3 µm and stained with hematoxylin and eosin by standard methods. Markers of tubular damage (tubular dilatation, cell necrosis, infarction, and cast formation) were scored in “blinded” fashion on randomly selected corticomedullary fields (magnification, ×200). Light microscopy images were acquired under identical settings using a Zeiss Axiovert 40CFL microscope and Axiovision software, version 4.8 (Carl Zeiss, Oberkochen, Germany).
Immunofluorescence Staining of Arterial Rings, Human rTECs, and Murine Kidneys
Cryostat sections of vessels were washed with PBS, followed by 0.5% BSA in PBS. Sections were blocked with 2% BSA solution. The slides were incubated at room temperature with primary anti-TSP1 Ab. Slides were washed with BSA solution and incubated with a CY3 goat anti-rabbit secondary Ab in combination with the F-actin dye rhodamine phalloidin. For rTEC, cytospins were generated and fixed with ethanol. Slides were blocked with 5% goat serum and then incubated overnight at 4°C with SIRP-α Ab. Nuclei were stained with Hoechst dye. Fluorescent images were captured with an Olympus Fluoview 1000 confocal microscope (software version 1.7a). Cryostat sections (5 µm) of mouse kidneys were fixed for 20 minutes with 2% paraformaldehyde. They were then washed, blocked with 2% donkey serum in BSA, incubated with combined primary antibodies for TSP1 and SIRP-α, washed and incubated with secondary antibody. Nuclei were stained with Hoescht dye. After rinses with PBS, sections were coverslipped with Gelvatol mounting media. Fluorescent images were captured with an Olympus Fluoview 1000 confocal microscope (software version 2.01).
Cell Apoptosis and Viability Assays
Human rTECs were grown to 80% confluence, treated with TSP1 (2.2 nmol/L) with or without SIRP-α antibody (1 μg/ml) for 24 hours, lysates prepared, proteins then separated by SDS-PAGE, transferred to nitrocellulose membrane and probed for caspase-3. Cell viability was assessed in a 96-well plate using the LIVE/DEAD Viability/Cytotoxicity kit (Molecular Probes). Calcein (0.5 µM) and ethidium homodimer-1 (2 µM) were added to the cells for 30 minutes and fluorescence read using a microplate reader as per manufacturer instructions.
Arterial Myography
Myography of arterial segments was performed as previously published5,70 with minor modifications. Animals were anesthetized with pentobarbital (50 mg/kg intraperitoneally). Thoracic aortas of mice and rats were cleared of adherent adipose tissue and excised. The endothelial layer was removed by gently rubbing the luminal side of the vessel along the rough surface of a blunt needle. Arterial segments were mounted on myograph pins (Danish Myo Technology, Atlanta, GA) in 5-ml incubation buffer maintained at 37°C, pH 7.4, gassed with 95% O2 and 5% CO2, and brought to an optimal resting tension. Viability of the vessels was ascertained by a contractile response to potassium chloride. Phenylephrine (Sigma-Aldrich) concentration-response curves (10−9 to 10−5 M) were generated by measuring contraction plateaus at each concentration. In precontracted vessels, endothelium-independent vasodilation to SNP (Sigma-Aldrich; 10−10 to 10−5 M) with or without the indicated treatments was then tested.
Detection of ROS in Renal Tissue Sections
ROS in renal tissue sections was assessed using several techniques. The cell-permeable agent dihydroethidium was applied to unfixed frozen sections that were incubated in a light-protected humidified chamber at 37°C for 30 minutes, washed with PBS, and mounted with fluorescent mounting medium. Cryosections of kidneys were washed with PBS, followed by 0.5% BSA in PBS. Sections were blocked with 2% BSA solution. They were incubated at room temperature with primary antibody for 4-HNE. Slides were incubated with CY3 goat anti-rabbit secondary antibody (Jackson ImmunoResearch) in combination with F-actin dye rhodamine phalloidin. Nuclei were stained with Hoescht dye. Fluorescent images were captured with an Olympus Fluoview 1000 confocal microscope (software version 1.7a).
IRI
Mice were anesthetized using isoflurane and oxygen titrated to effect, and body temperature was maintained at 36°C with the aid of a rectal temperature probe, warming pad, and warming lamp. A microaneurysm clip was placed to occlude the left, or both, renal pedicles for 20 minutes, after which the clip was removed. The abdomen was closed with 5/0 monofilament suture. Mice were used for laser Doppler experiments 30 minutes or 24 hours after reperfusion, and then euthanized. Blood was collected and kidney tissue snap frozen, placed in RNAlater, embedded in optimal cutting temperature compound, or fixed in 10% neutral buffered formalin.
Laser Doppler Blood Flow Analysis
Real-time kidney perfusion and blood flow was measured using laser Doppler imaging (MoorLDI-2λ; Moor Instruments, Devon, UK). Briefly, animals were anesthetized and core temperature maintained at 36°C. Organ blood flow was assessed at baseline, in response to ischemia and reperfusion at 30 minutes and 24 hours. Results are expressed as the percentage change from baseline control of the region of interest.
In Vivo SIRP-α Ab Treatment
Age-matched male wild-type C57BL/6 or CD47 null mice were randomly assigned to receive a SIRP-α monoclonal Ab (clone C20; Santa Cruz Biotechnology; 0.4 µg/g body weight intraperitoneally in 100 µl sterile PBS) or an IgG isotype-matched control Ab (Santa Cruz Biotechnology) 90 minutes before surgery.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism software. Data were analyzed by one-way ANOVA followed by the Tukey test for multiple comparisons. For grouped analysis, data were analyzed by two-way ANOVA followed by Bonferroni post hoc test. A P value of <0.05 was assumed to indicate a statistically significant difference.
Disclosures
J.S.I. is chair of the Scientific Advisory Boards of Vasculox, Inc. (St. Louis, MO), and Radiation Control Technologies, Inc. (Rockville, MD), and holds equity interests in the same.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant R01-HL108954, American Heart Association (AHA) grant 11BGIA7210001 (J.S.I.), and NIH grant R01-HL079207 (P.J.P.); AHA award 10POST3030009 and K99-HL114648 (G.C.S.); and a C.J. Martin Award and AHA award 13POST14520003 (N.M.R.). This work was also supported by the Institute for Transfusion Medicine, the Hemophilia Center of Western Pennsylvania, and the Vascular Medicine Institute (J.S.I., P.J.P.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013040433/-/DCSupplemental.
References
- 1.Lawler J: Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med 6: 1–12, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H, Herndon ME, Lawler J: The cell biology of thrombospondin-1. Matrix Biol 19: 597–614, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Rogers NM, Thomson AW, Isenberg JS: Activation of parenchymal CD47 promotes renal ischemia-reperfusion injury. J Am Soc Nephrol 23: 1538–1550, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riessen R, Kearney M, Lawler J, Isner JM: Immunolocalization of thrombospondin-1 in human atherosclerotic and restenotic arteries. Am Heart J 135: 357–364, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD, Isenberg JS: Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res 88: 471–481, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moura R, Tjwa M, Vandervoort P, Cludts K, Hoylaerts MF: Thrombospondin-1 activates medial smooth muscle cells and triggers neointima formation upon mouse carotid artery ligation. Arterioscler Thromb Vasc Biol 27: 2163–2169, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Novelli EM, Kato GJ, Ragni MV, Zhang Y, Hildesheim ME, Nouraie M, Barge S, Meyer MP, Hassett AC, Gordeuk VR, Gladwin MT, Isenberg JS: Plasma thrombospondin-1 is increased during acute sickle cell vaso-occlusive events and associated with acute chest syndrome, hydroxyurea therapy, and lower hemolytic rates. Am J Hematol 87: 326–330, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A: A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 386: 181–186, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Stofega MR, Argetsinger LS, Wang H, Ullrich A, Carter-Su C: Negative regulation of growth hormone receptor/JAK2 signaling by signal regulatory protein alpha. J Biol Chem 275: 28222–28229, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Gresham HD, Dale BM, Potter JW, Chang PW, Vines CM, Lowell CA, Lagenaur CF, Willman CL: Negative regulation of phagocytosis in murine macrophages by the Src kinase family member, Fgr. J Exp Med 191: 515–528, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamao T, Noguchi T, Takeuchi O, Nishiyama U, Morita H, Hagiwara T, Akahori H, Kato T, Inagaki K, Okazawa H, Hayashi Y, Matozaki T, Takeda K, Akira S, Kasuga M: Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS-1. J Biol Chem 277: 39833–39839, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Chirkov YY, Horowitz JD: Impaired tissue responsiveness to organic nitrates and nitric oxide: A new therapeutic frontier? Pharmacol Ther 116: 287–305, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Wink DA, Cook JA, Pacelli R, Liebmann J, Krishna MC, Mitchell JB: Nitric oxide (NO) protects against cellular damage by reactive oxygen species. Toxicol Lett 82-83: 221–226, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD: Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood 109: 1945–1952, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isenberg JS, Maxhimer JB, Powers P, Tsokos M, Frazier WA, Roberts DD: Treatment of liver ischemia-reperfusion injury by limiting thrombospondin-1/CD47 signaling. Surgery 144: 752–761, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maxhimer JB, Shih HB, Isenberg JS, Miller TW, Roberts DD: Thrombospondin-1/CD47 blockade following ischemia-reperfusion injury is tissue protective. Plast Reconstr Surg 124: 1880–1889, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, Roberts DD: Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem 284: 1116–1125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barclay AN: Signal regulatory protein alpha (SIRPalpha)/CD47 interaction and function. Curr Opin Immunol 21: 47–52, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palen DI, Belmadani S, Lucchesi PA, Matrougui K: Role of SHP-1, Kv.1.2, and cGMP in nitric oxide-induced ERK1/2 MAP kinase dephosphorylation in rat vascular smooth muscle cells. Cardiovasc Res 68: 268–277, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Isenberg JS, Frazier WA, Roberts DD: Thrombospondin-1: A physiological regulator of nitric oxide signaling. Cell Mol Life Sci 65: 728–742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD: Regulation of nitric oxide signalling by thrombospondin 1: Implications for anti-angiogenic therapies. Nat Rev Cancer 9: 182–194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD: Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci U S A 102: 13141–13146, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer RF, Lasseter KC: Drug therapy. Sodium nitroprusside. N Engl J Med 292: 294–297, 1975 [DOI] [PubMed] [Google Scholar]
- 24.Reves JG, Sheppard LC, Wallach R, Lell WA: Therapeutic uses of sodium nitroprusside and an automated method of administration. Int Anesthesiol Clin 16: 51–88, 1978 [DOI] [PubMed] [Google Scholar]
- 25.Maile LA, Badley-Clarke J, Clemmons DR: The association between integrin-associated protein and SHPS-1 regulates insulin-like growth factor-I receptor signaling in vascular smooth muscle cells. Mol Biol Cell 14: 3519–3528, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP: Role of CD47 as a marker of self on red blood cells. Science 288: 2051–2054, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Maile LA, Clemmons DR: Integrin-associated protein binding domain of thrombospondin-1 enhances insulin-like growth factor-I receptor signaling in vascular smooth muscle cells. Circ Res 93: 925–931, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Tulasne D, Judd BA, Johansen M, Asazuma N, Best D, Brown EJ, Kahn M, Koretzky GA, Watson SP: C-terminal peptide of thrombospondin-1 induces platelet aggregation through the Fc receptor gamma-chain-associated signaling pathway and by agglutination. Blood 98: 3346–3352, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Heasman J: Morpholino oligos: Making sense of antisense? Dev Biol 243: 209–214, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD: Increasing survival of ischemic tissue by targeting CD47. Circ Res 100: 712–720, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Vernon-Wilson EF, Kee WJ, Willis AC, Barclay AN, Simmons DL, Brown MH: CD47 is a ligand for rat macrophage membrane signal regulatory protein SIRP (OX41) and human SIRPalpha 1. Eur J Immunol 30: 2130–2137, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Calzada MJ, Zhou L, Sipes JM, Zhang J, Krutzsch HC, Iruela-Arispe ML, Annis DS, Mosher DF, Roberts DD: Alpha4beta1 integrin mediates selective endothelial cell responses to thrombospondins 1 and 2 in vitro and modulates angiogenesis in vivo. Circ Res 94: 462–470, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Isenberg JS, Romeo MJ, Maxhimer JB, Smedley J, Frazier WA, Roberts DD: Gene silencing of CD47 and antibody ligation of thrombospondin-1 enhance ischemic tissue survival in a porcine model: Implications for human disease. Ann Surg 247: 860–868, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton CA, Brosnan MJ, Al-Benna S, Berg G, Dominiczak AF: NAD(P)H oxidase inhibition improves endothelial function in rat and human blood vessels. Hypertension 40: 755–762, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Lassègue B, Griendling KK: NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 30: 653–661, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J, Kleinberg ME: Activation of the phagocyte NADPH oxidase protein p47(phox). Phosphorylation controls SH3 domain-dependent binding to p22(phox). J Biol Chem 274: 19731–19737, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Squadrito GL, Pryor WA: The formation of peroxynitrite in vivo from nitric oxide and superoxide. Chem Biol Interact 96: 203–206, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Medina P, Segarra G, Martínez-León JB, Vila JM, Otero E, Lluch S: Relaxation and cGMP formation in response to sildenafil and sodium nitroprusside in saphenous veins from normotensive and hypertensive patients. Am J Hypertens 15: 798–802, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Simonsen U, Christensen FH, Buus NH: The effect of tempol on endothelium-dependent vasodilatation and blood pressure. Pharmacol Ther 122: 109–124, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Djamali A, Vidyasagar A, Yagci G, Huang LJ, Reese S: Mycophenolic acid may delay allograft fibrosis by inhibiting transforming growth factor-beta1-induced activation of Nox-2 through the nuclear factor-kappaB pathway. Transplantation 90: 387–393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Y, Yang S, Huang J, Ruan S, Zheng Z, Lin J: Tgf-β1 induces autophagy and promotes apoptosis in renal tubular epithelial cells. Int J Mol Med 29: 781–790, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Khanna AK, Xu J, Baquet C, Mehra MR: Adverse effects of nicotine and immunosuppression on proximal tubular epithelial cell viability, tissue repair and oxidative stress gene expression. J Heart Lung Transplant 28: 612–620, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Simão S, Fraga S, Jose PA, Soares-da-Silva P: Oxidative stress plays a permissive role in alpha2-adrenoceptor-mediated events in immortalized SHR proximal tubular epithelial cells. Mol Cell Biochem 315: 31–39, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Du C, Wang S, Diao H, Guan Q, Zhong R, Jevnikar AM: Increasing resistance of tubular epithelial cells to apoptosis by shRNA therapy ameliorates renal ischemia-reperfusion injury. Am J Transplant 6: 2256–2267, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Guo N, Krutzsch HC, Inman JK, Roberts DD: Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res 57: 1735–1742, 1997 [PubMed] [Google Scholar]
- 46.Halloran PF, Homik J, Goes N, Lui SL, Urmson J, Ramassar V, Cockfield SM: The “injury response”: a concept linking nonspecific injury, acute rejection, and long-term transplant outcomes. Transplant Proc 29: 79–81, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Gobé G, Willgoss D, Hogg N, Schoch E, Endre Z: Cell survival or death in renal tubular epithelium after ischemia-reperfusion injury. Kidney Int 56: 1299–1304, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M, et al. : Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem 268: 18532–18541, 1993 [PubMed] [Google Scholar]
- 49.Li C, Jackson RM: Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol 282: C227–C241, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Chatterjee PK: Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: A comprehensive review. Naunyn Schmiedebergs Arch Pharmacol 376: 1–43, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Laurindo FR, Fernandes DC, Santos CX: Assessment of superoxide production and NADPH oxidase activity by HPLC analysis of dihydroethidium oxidation products. Methods Enzymol 441: 237–260, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Ormsby A, Oja-Tebbe N, Pagano PJ: Gene transfer of NAD(P)H oxidase inhibitor to the vascular adventitia attenuates medial smooth muscle hypertrophy. Circ Res 95: 587–594, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Nediani C, Raimondi L, Borchi E, Cerbai E: Nitric oxide/reactive oxygen species generation and nitroso/redox imbalance in heart failure: From molecular mechanisms to therapeutic implications. Antioxid Redox Signal 14: 289–331, 2011 [DOI] [PubMed] [Google Scholar]
- 54.van Beek EM, Zarate JA, van Bruggen R, Schornagel K, Tool AT, Matozaki T, Kraal G, Roos D, van den Berg TK: SIRPα controls the activity of the phagocyte NADPH oxidase by restricting the expression of gp91(phox). Cell Rep 2: 748–755, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Johansen ML, Brown EJ: Dual regulation of SIRPalpha phosphorylation by integrins and CD47. J Biol Chem 282: 24219–24230, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Brown EJ, Frazier WA: Integrin-associated protein (CD47) and its ligands. Trends Cell Biol 11: 130–135, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Maile LA, Allen LB, Hanzaker CF, Gollahon KA, Dunbar P, Clemmons DR: Glucose regulation of thrombospondin and its role in the modulation of smooth muscle cell proliferation. Exp Diabetes Res 2010: 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Csányi G, Yao M, Rodríguez AI, Al Ghouleh I, Sharifi-Sanjani M, Frazziano G, Huang X, Kelley EE, Isenberg JS, Pagano PJ: Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler Thromb Vasc Biol 32: 2966–2973, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sohn HY, Raff U, Hoffmann A, Gloe T, Heermeier K, Galle J, Pohl U: Differential role of angiotensin II receptor subtypes on endothelial superoxide formation. Br J Pharmacol 131: 667–672, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Briones AM, Tabet F, Callera GE, Montezano AC, Yogi A, He Y, Quinn MT, Salaices M, Touyz RM: Differential regulation of Nox1, Nox2 and Nox4 in vascular smooth muscle cells from WKY and SHR. J Am Soc Hypertens 5: 137–153, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Kawahara T, Ritsick D, Cheng G, Lambeth JD: Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J Biol Chem 280: 31859–31869, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Isenberg JS, Wink DA, Roberts DD: Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res 71: 785–793, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Baud L, Ardaillou R: Involvement of reactive oxygen species in kidney damage. Br Med Bull 49: 621–629, 1993 [DOI] [PubMed] [Google Scholar]
- 64.Padanilam BJ: Cell death induced by acute renal injury: A perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol 284: F608–F627, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Matozaki T, Murata Y, Okazawa H, Ohnishi H: Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol 19: 72–80, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ: TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 117: 2847–2859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bauer PM, Bauer EM, Rogers NM, Yao M, Feijoo-Cuaresma M, Pilewski JM, Champion HC, Zuckerbraun BS, Calzada MJ, Isenberg JS: Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc Res 93: 682–693, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Touyz RM, Yao G, Schiffrin EL: c-Src induces phosphorylation and translocation of p47phox: Role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 23: 981–987, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Csányi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egaña L, Lopes LR, Jackson HM, Kelley EE, Pagano PJ: Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med 51: 1116–1125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cascino T, Csanyi G, Al Ghouleh I, Montezano AC, Touyz RM, Haurani MJ, Pagano PJ: Adventitia-derived hydrogen peroxide impairs relaxation of the rat carotid artery via smooth muscle cell p38 mitogen-activated protein kinase. Antioxid Redox Signal 15: 1507–1515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.