Abstract
The mechanism of vascular calcification in CKD is not understood fully, but may involve collagen deposition in the arterial wall upon osteo/chondrocytic transformation of vascular smooth muscle cells (VSMCs). Increased levels of circulating angiopoietin-2 correlate with markers of CKD progression and angiopoietin-2 regulate inflammatory responses, including intercellular and vascular adhesion and recruitment of VSMCs. Here, we investigate the potential role of angiopoietin-2 in the pathogenesis of arterial stiffness associated with CKD. In a cohort of 416 patients with CKD, the plasma level of angiopoietin-2 correlated independently with the severity of arterial stiffness assessed by pulse wave velocity. In mice subjected to 5/6 subtotal nephrectomy or unilateral ureteral obstruction, plasma levels of angiopoietin-2 also increased. Angiopoietin-2 expression markedly increased in tubular epithelial cells of fibrotic kidneys but decreased in other tissues, including aorta and lung, after 5/6 subtotal nephrectomy. Expression of collagen and profibrotic genes in aortic VSMCs increased in mice after 5/6 subtotal nephrectomy and in mice producing human angiopoietin-2. Angiopoietin-2 stimulated endothelial expression of chemokines and adhesion molecules for monocytes, increased Ly6Clow macrophages in aorta, and increased the expression of the profibrotic cytokine TGF-β1 in aortic endothelial cells and Ly6Clow macrophages. Angiopoietin-2 blockade attenuated expression of monocyte chemokines, profibrotic cytokines, and collagen in aorta of mice after 5/6 subtotal nephrectomy. This study identifies angiopoietin-2 as a link between kidney fibrosis and arterial stiffness. Targeting angiopoietin-2 to attenuate inflammation and collagen expression may provide a novel therapy for cardiovascular disease in CKD.
Cardiovascular disease (CVD) is a major cause of morbidity and mortality in patients with CKD.1–4 Arteriosclerosis characterized by arterial stiffness is the major vascular complication.5,6 The independent predictive value of arterial stiffness for CVD has been well demonstrated in different populations.7,8 Although arterial stiffness is a hallmark of the aging process, many other factors such as endothelial dysfunction, local or systemic inflammation, and genetic factors are also implicated in the pathogenesis of arterial stiffness.9–11 Arterial stiffness in patients with CKD is characterized by arterial intima-media hypertrophy resulting from alterations of the intrinsic properties of the arterial wall.3,12 Increased arterial stiffness is observed in the early stages of CKD.6,9 Arterial stiffness is accelerated in patients with CKD compared with age-, sex-, and pressure-matched controls, suggesting unique CKD-related factors leading to such a complication.6,9,12
Traditional risk factors in patients with CKD, such as hypertension, diabetes, and hyperlipidemia, account for the increased CVD morbidity and mortality in part; however, actual mortality rates exceed expected rates even though these traditional risk factors are controlled.13 Much attention has been paid to the vascular calcification and functional alteration caused by disturbed mineral metabolism in patients with CKD.14,15 Although the pathogenesis of vascular calcification has not yet been fully elucidated, it likely involves a transformation of vascular smooth muscle cells (VSMCs) into a synthetic phenotype, leading to arterial stiffness by deposits of collagen I– and collagen III–rich extracellular matrix (ECM) in the arterial wall.12,16,17 Treatments correcting the mineral disturbance by phosphate binders, however, have not shown improvement in CVD mortality.18
Cross-talk among endothelial cells, VSMCs, and other cells through humoral and mechanical mechanisms regulates vascular function in health and disease.16,19 In these humoral factors, increased levels of circulating angiopoietin 2 (Angpt2) are correlated with scores of coronary, carotid, and peripheral artery diseases in dialysis patients.20,21 Recent evidence further demonstrates that the levels of circulating Angpt2 have a reverse correlation with GFR, have a positive correlation with albuminuria, and predict long-term mortality in patients with CKD.22–24 Angiopoietin-1 (Angpt1) and Angpt2 are ligands of the Tie-2 receptor, a family of growth factors specific for the vascular endothelium identified after discovery of vascular endothelial growth factor-A (VEGF-A).25–28 In addition to angiogenesis, Angpt2 is an important regulator in numerous pathophysiologic processes, including inflammation.29 Of note, circulating Angpt2 levels are correlated with systemic markers of microinflammation in patients with CKD.23,24 Angpt1-mediated Tie-2 activation is required to maintain quiescent endothelium.30 In contrast, Angpt2 destabilizes quiescent endothelium and primes it to respond to exogenous stimuli, thereby modulating activities of inflammatory (TNF-α) and angiogenic (VEGF-A) cytokines.29,31 In mice undergoing femoral artery ligation, Angpt2 is shown to induce intercellular and vascular adhesion molecules, macrophage infiltration, and VSMC recruitment, thereby promoting arteriogenesis and blood flow recovery.32 Hence, it is reasonable to speculate that dysregulation of the Angpt/Tie-2 system in favor of Angpt2 may affect vascular remodeling through endothelial cells and VSMCs.
Results
Plasma Angpt2 Correlated with Arterial Stiffness Independently in Patients with CKD
A total of 416 patients were stratified into three subgroups (CKD stages 3–5) based on their eGFR (Table 1). No difference was noted in the proportion of patients with diabetes mellitus, hypertension, smoking, dyslipidemia, or statin use. No difference in serum high-sensitivity C-reactive protein was noted in the subgroups (P=0.26). An angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB) was prescribed in more than half of the patients in CKD stages 3 and 4; however, calcium channel blockers and β-blockers were prescribed more often in later CKD stages. Pulse wave velocity (PWV) was measured to assess arterial stiffness and was not different in the CKD subgroups (P=0.81).
Table 1.
Clinical characteristics of patients among CKD stages
| Characteristic | All (N=416) | CKD Stage | P Value | ||
|---|---|---|---|---|---|
| 3 (n=187) | 4 (n=111) | 5 (n=118) | |||
| Age (yr) | 63 (54–71) | 63 (53–70) | 66 (55–73) | 61 (53–71) | 0.37 |
| Men | 62.2 | 76.5 | 56.8 | 45.8 | <0.001 |
| Diabetes | 38.0 | 37.4 | 36.0 | 40.7 | 0.75 |
| Hypertension | 86.5 | 86.1 | 88.3 | 85.6 | 0.81 |
| Smoker | 10.8 | 12.8 | 11.7 | 6.8 | 0.24 |
| Dyslipidemia | 31.3 | 36.9 | 26.1 | 27.1 | 0.08 |
| Body mass index (kg/m2) | 24.2 (22.1–27.0) | 25.0 (22.5–27.2) | 23.9 (21.9–27.3) | 23.6 (21.3–25.8) | 0.03 |
| eGFR (ml/min per 1.73 m2) | 27.3 (13.7–41.2) | 42.4 (36.8–49.3) | 23.6 (19.4–26.3) | 9.4 (7.3–12.0) | 0.001 |
| Creatinine (mg/dl) | 2.3 (1.7–4.3) | 1.6 (1.5–1.9) | 2.6 (2.3–3.0) | 5.5 (4.6–7.3) | 0.001 |
| Albumin (g/dl) | 4.5 (4.2–4.7) | 4.6 (4.3–4.8) | 4.5 (4.2–4.7) | 4.4 (4.1–4.6) | 0.001 |
| Calcium (mmol/L) | 2.32 (2.25–2.42) | 2.37 (2.29–2.45) | 2.32 (2.25–2.40) | 2.27 (2.17–2.36) | 0.001 |
| Phosphate (mg/dl) | 3.8 (3.4–4.5) | 3.4 (3.1–3.8) | 3.8 (3.5–4.3) | 4.9 (4.2–5.5) | 0.001 |
| Calcium phosphate producta | 35.9 (31.4–41.9) | 32.3 (29.4–36.1) | 35.8 (32.1–40.0) | 43.9 (38.8–49.2) | 0.001 |
| Urine albumin/creatinine ratio (mg/g) | 343.5 (66.5–811.0) | 117.5 (25.0–549.0) | 396.5 (110.0–878.0) | 655.0 (297.0–1289.5) | 0.001 |
| Hemoglobin (g/dl) | 11.4 (9.8–13.5) | 13.3 (11.7–14.5) | 11.1 (9.8–12.3) | 9.8 (8.9–11.0) | 0.001 |
| Total cholesterol (mg/dl) | 194 (168–216) | 195 (167–215) | 191 (171–215) | 195 (168–218) | 0.96 |
| HDL cholesterol (mg/dl) | 43 (39–51) | 43 (39–50) | 45 (39–52) | 43 (37–51) | 0.44 |
| Triglyceride (mg/dl) | 138 (99–193) | 140 (96–195) | 143 (104–211) | 127.5 (93–172) | 0.29 |
| hsCRP (mg/dl) | 0.12 (0.07–0.22) | 0.11 (0.07–0.23) | 0.13 (0.07–0.28) | 0.11 (0.06–0.18) | 0.26 |
| Medication [%] | |||||
| ACE inhibitor/ARB | 64.7 | 71.1 | 73.0 | 46.6 | <0.001 |
| Calcium channel blocker | 44.4 | 32.6 | 51.4 | 56.8 | <0.001 |
| β-blocker | 38.9 | 31.2 | 45.9 | 44.1 | 0.02 |
| α-Blocker | 22.8 | 22.5 | 24.3 | 22.0 | 0.91 |
| Diuretic | 32.5 | 30.5 | 36.0 | 32.2 | 0.61 |
| Statin | 24.8 | 27.8 | 21.6 | 22.9 | 0.42 |
| Acetylsalicylic acid | 16.8 | 17.6 | 13.5 | 18.6 | 0.54 |
| PWV (cm/s) | 1086.3 (983.8–1193.5) | 1080.0 (977.0–1212.5) | 1087.5 (987.0–1164.0) | 1105.5 (1006.0–1194.0) | 0.81 |
Continuous and categorical variables are expressed as the median (interquartile range) and percentage, respectively. hsCRP, high-sensitivity C-reactive protein.
Calcium phosphate product is calcium×phosphate×4.
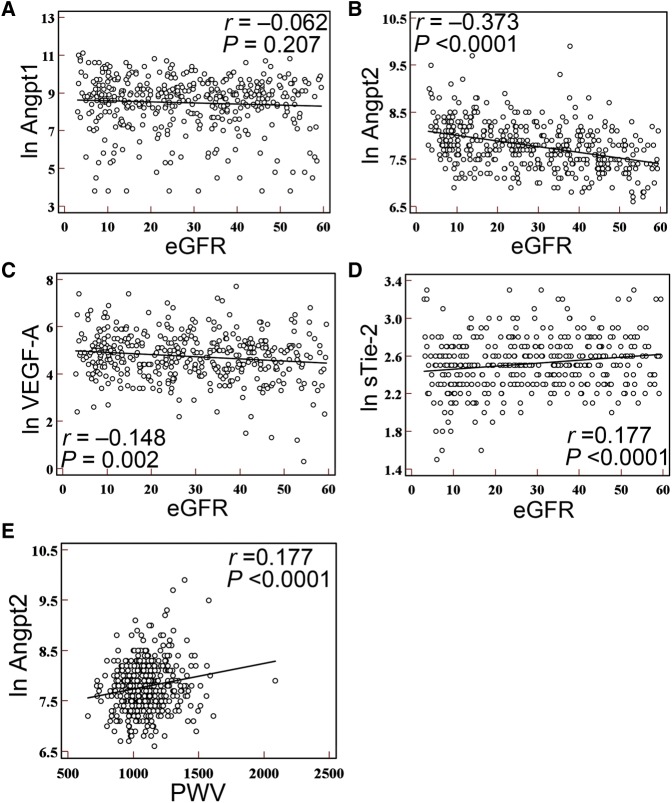
Plasma levels of Angpt1 were not different in CKD subgroups (P=0.45; Table 2), and were not correlated with eGFR (r=–0.06, P=0.21; Figure 1A). Increased plasma levels of Angpt2 and VEGF-A were noted when CKD progressed (P<0.001 for Angpt2 and P=0.01 for VEGF-A; Table 2). A negative correlation with eGFR was noted for Angpt2 (r=–0.37, P<0.001; Figure 1B) and VEGF-A (r=–0.15, P=0.002; Figure 1C). A weak correlation was found between plasma Angpt2 and VEGF-A (r=0.14, P=0.003). Plasma levels of the endogenous Angpt antagonist, soluble Tie-2 receptor (sTie-2), were decreased in CKD (P=0.01; Table 2) and correlated positively with eGFR (r=0.18, P<0.001; Figure 1D). Subgroup analyses did not reveal a stronger correlation between angiotrophic growth factors and eGFR.
Table 2.
Plasma levels of angiotrophic growth factors
| Growth Factor (pg/ml) | All (N=416) | CKD Stage | P Value | ||
|---|---|---|---|---|---|
| 3 (n=187) | 4 (n=111) | 5 (n=118) | |||
| Angpt1 | 6673.5 (2732.5–12,118.0) | 6487.9 (2455.6–11,727.1) | 7081.5 (3505.4–11,507.0) | 6599.2 (2605.8–14,588.4) | 0.45 |
| Angpt2 | 2354.5 (1716.0–3280.9) | 2026.4 (1501.9–2674.6) | 2420.5 (1850.4–3279.2) | 3080.6 (2122.3–4178.3) | <0.001 |
| VEGF-A | 124.5 (66.0–203.9) | 104.3 (60.8–177.7) | 119.1 (71.2–239.9) | 139.0 (82.3–225.8) | 0.01 |
| sTie-2 | 12.4 (10.3–14.7) | 12.6 (10.7–15.0) | 12.4 (10.1–14.7) | 11.9 (9.6–14.0) | 0.01 |
Data are expressed as the median (interquartile range).
Figure 1.
Plasma levels of Angpt2 increase and positively correlate with PWV in patients with CKD. (A–D) Univariate regression analysis shows the linear correlation of eGFR with plasma levels of Angpt1 (A), Angpt2 (B), VEGF-A (C), and sTie-2 (D). (E) Univariate regression analysis shows the linear correlation of PWV with plasma levels of Angpt2 in patients with CKD. The plasma levels of Angpt1, Angpt2, VEGF-A, and sTie-2 are expressed as the natural logarithm (ln).
Univariate regression analysis demonstrated a positive correlation between plasma Angpt2 and PWV (r=0.18, P<0.001; Figure 1E). After adjusting for age and traditional risk factors, a significant association remained (models 1 and 2) (Table 3). Further adjustment for nontraditional risk factors, including calcium phosphate product and medication use, was performed in model 3; the results confirmed that plasma Angpt2 levels were independently associated with PWV (Table 3). Because plasma levels of Angpt2 were significantly higher in CKD stage 5, subgroup analyses revealed a stronger positive correlation between plasma Angpt2 and PWV in CKD stage 5 (r=0.32, P<0.001), but not in CKD stage 3 or 4. Multivariate regression analyses confirmed that plasma Angpt2 levels were more strongly associated with PWV in CKD stage 5 (Supplemental Table 1).
Table 3.
Multivariate-adjusted regression analyses of plasma Angpt2 and PWV
| Analysis | PWV | ||
|---|---|---|---|
| Regression Coefficient β (×10−5) | P Value | 95% Confidence Interval (×10−5) | |
| Univariate | |||
| Angpt2 | 1.65 | <0.001 | 0.87 to 2.4 |
| Multivariate | |||
| Model 1 | 1.25 | 0.001 | 0.54 to 1.96 |
| Model 2 | 0.96 | 0.01 | 0.25 to 1.67 |
| Model 3 | 0.86 | 0.02 | 0.12 to 1.61 |
PWV was log-transformed. Model 1, is adjusted for Angpt2 plus age. Model 2 is adjusted for the variables in model 1 plus traditional risk (e.g., age, sex, hypertension, diabetes, smoking, dyslipidemia); Model 3 is adjusted for the variables in model 2 plus nontraditional risk factors (e.g., calcium phosphate product, medications including ACE inhibitor, ARB, statin, calcium channel blocker, β-blocker).
Angpt2 Increased in Animal Models of CKD
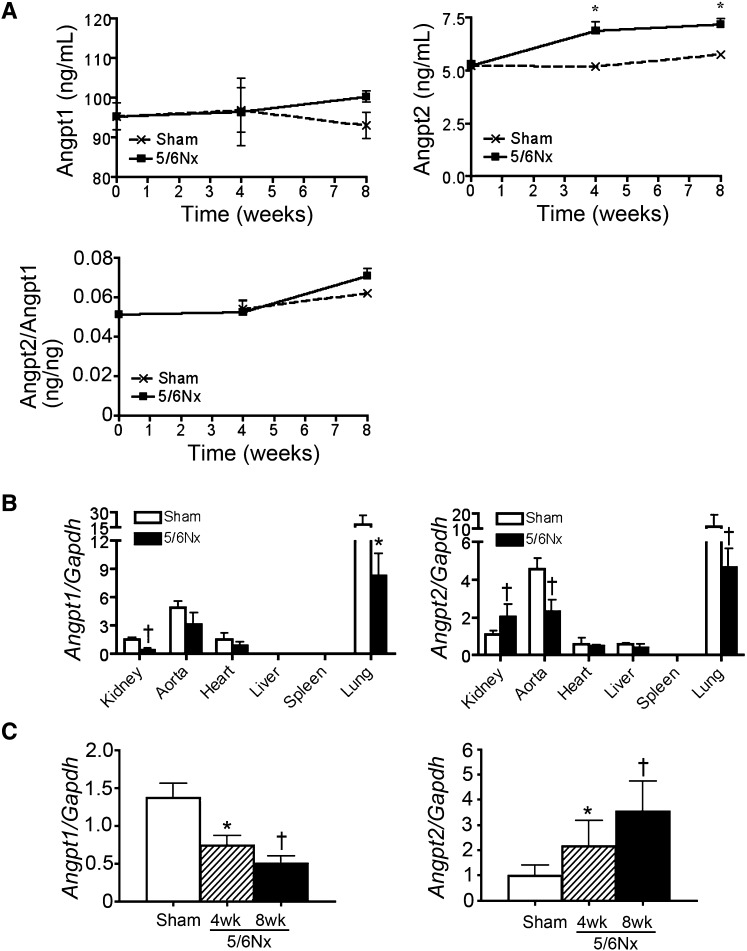
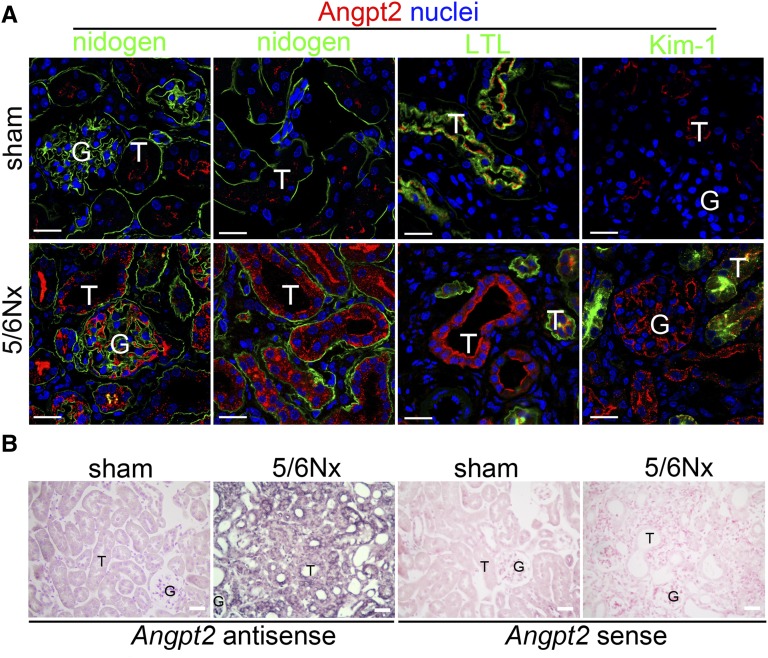
To identify the pathologic consequence of plasma Angpt2 in arterial stiffness of patients with CKD, we first studied CD1 mice after a 5/6 subtotal nephrectomy (5/6Nx). Plasma levels of BUN and creatinine increased after 5/6Nx, but BP did not change (Supplemental Figure 1, A and B). Collagen fibril stained by picrosirius red confirmed renal fibrosis (Supplemental Figure 1C). A significant increase of plasma Angpt2 was noted in mice after 5/6Nx; however, plasma Angpt1, Angpt2/Angpt1 ratio, and VEGF-A were not changed (Figure 2A and Supplemental Figure 2). Quantitative PCR (qPCR) of different tissues, including kidney, aorta, heart, liver, spleen, and lung, from mice 8 weeks after sham operation showed that the lung expressed the highest levels of Angpt1 and Angpt2 transcripts (Figure 2B). In 5/6Nx mice, the Angpt2 transcripts increased in the kidney but decreased in the other tissues, including the aorta and lung (Figure 2B). In contrast, the Angpt1 transcripts were decreased in the kidney as well as in the other tissues of 5/6Nx mice (Figure 2B). The qPCR time course for the kidney confirmed the decreasing Angpt1 and increasing Angpt2 transcripts during disease progression (Figure 2C). Angpt2 protein was not detected by immunofluorescence in renal tubular epithelial cells of kidneys after sham operation despite positive staining observed on the luminal surface of the Lotus tetragonolobus lectin–stained brush border of proximal tubules (Figure 3A). The staining of Angpt2 was markedly increased in renal tubular epithelial cells and glomeruli after 5/6Nx (Figure 3A, Supplemental Figure 3). In situ hybridization reconfirmed the expression of Angpt2 in renal tubular epithelial cells, glomeruli, and interstitial cells in 5/6Nx kidneys (Figure 3B).
Figure 2.
Angpt2 is increased in plasma and kidney after 5/6Nx. (A) Plasma levels of Angpt1 and Angpt2 and the ratio of plasma Angpt2/Angpt1 after 5/6Nx. (B) qPCR for transcripts of Angpt1 and Angpt2 in different organs 8 weeks after 5/6Nx. (C) qPCR time course of kidney for transcripts of Angpt1 and Angpt2. Expression levels are normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH). n=10 per time point. *P<0.05; †P<0.01 versus sham at each time point.
Figure 3.
Angpt2 is increased in injured renal tubular epithelial cells after 5/6Nx. (A) Confocal microscopic images showing Angpt2 expression in injured renal tubular epithelial cells and glomeruli after 5/6Nx. (B) In situ hybridization showing increased transcripts of Angpt2 in injured renal tubular epithelial cells, glomeruli, and interstitial cells after 5/6Nx. The specificity of in situ hybridization is confirmed using a sense RNA probe. T, renal tubular epithelial cell; G, glomeruli; LTL, L. tetragonolobus lectin; Kim-1, kidney injury molecule-1. Scale bar, 20 μm in A; 25 μm in B.
We then studied the second mouse model with unilateral ureteral obstruction (UUO). Plasma creatinine (sham 0.23±0.02 mg/dl and UUO 0.22±0.02 mg/dl) and BP (Supplemental Figure 4A) did not change at day 14 after UUO surgery; however, plasma levels of Angpt2 increased significantly (Supplemental Figure 4B). Both transcripts and proteins of Angpt2 increased in UUO kidneys (Supplemental Figure 4, C and D). In contrast, Angpt1 decreased in UUO kidneys. The transcripts of Angpt2 did not change in tissues other than UUO kidneys and aorta (Supplemental Figure 4E). In situ hybridization demonstrated Angpt2 expression in injured tubular epithelial cells, glomeruli, and interstitial cells of UUO kidneys (Supplemental Figure 4F). Immunofluorescence reconfirmed Angpt2 staining in renal tubular epithelial cells and glomeruli of UUO kidneys (Supplemental Figure 5).
Increased Transcripts of Profibrotic and Proinflammatory Genes in Aorta of Mice after 5/6Nx
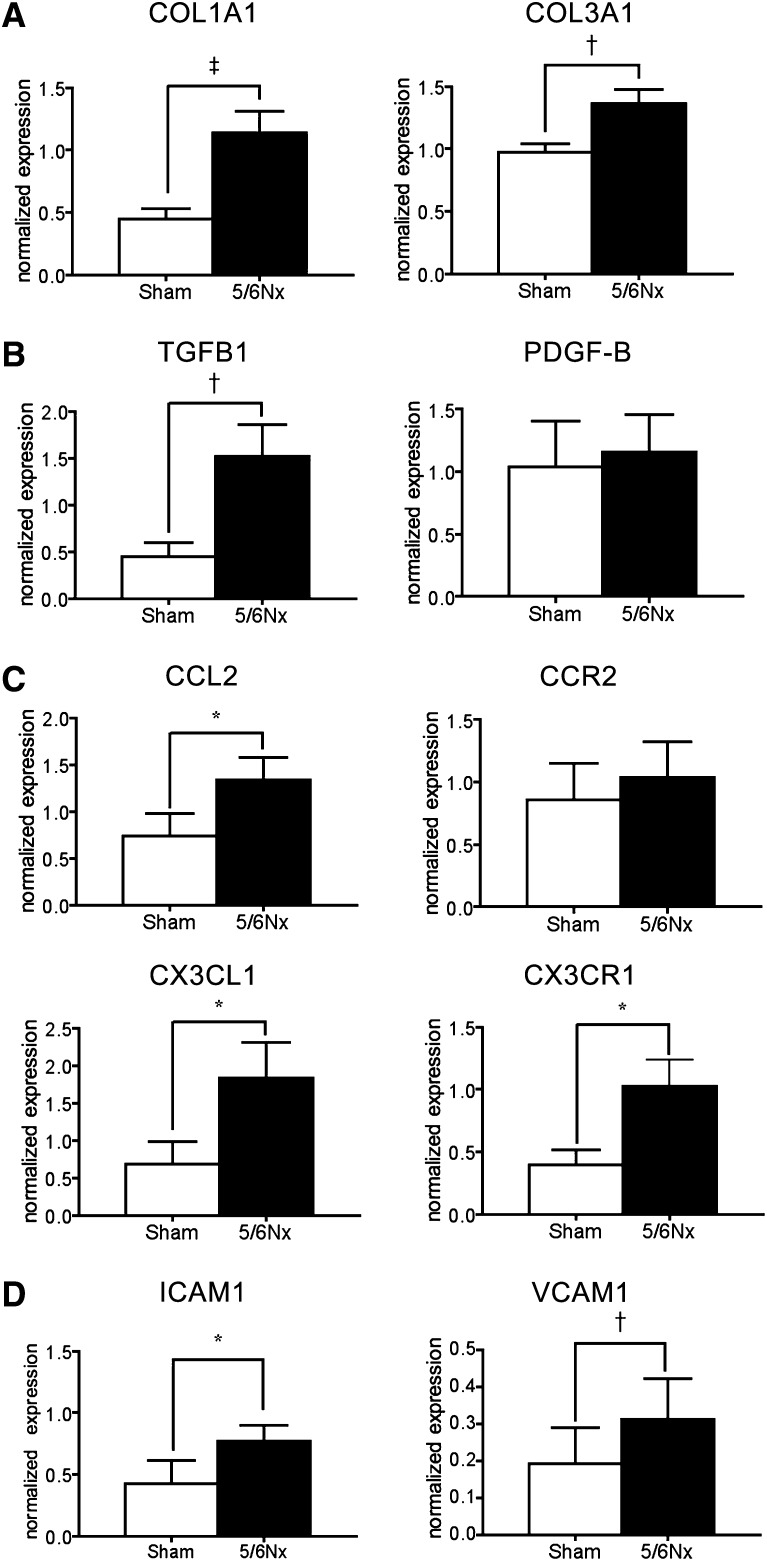
In addition to renal fibrosis and increased plasma Angpt2, qPCR showed an increase of α1 type I collagen (COL1A1) and α1 type III collagen (COL3A1) in thoracic aortas of 5/6Nx mice (Figure 4A). The transcripts of profibrotic growth factor transforming growth factor-β1 (TGFB1), proinflammatory chemokines C-C motif ligand 2 (CCL2), C-X3-C ligand 1 (CX3CL1), chemokine receptor CX3C chemokine receptor 1 (CX3CR1), intercellular adhesion molecule-1 (ICAM1), and vascular cell adhesion molecule-1 (VCAM1) were also increased (Figure 4, B to D). However, the increase of platelet-derived growth factor-B (PDGF-B) and C-C motif receptor 2 (CCR2) was not significant (Figure 4, B and C).
Figure 4.
Increased transcripts of profibrotic and proinflammatory genes in aorta of mice after 5/6Nx. (A–D) qPCR for transcripts of COL1A1 and COL3A1 (A), TGFB1 and PDGF-B (B), CCL2, CCR2, CX3CL1, and CX3CR1 (C), and ICAM1 and VCAMA1 (D) in aortas of mice after 5/6Nx. Expression levels are normalized by glyceraldehyde 3-phosphate dehydrogenase. n=15 per group. *P<0.05; †P<0.01; ‡P<0.001.
Angpt2 Increased Collagen Transcripts in Aortic VSMCs In Vivo
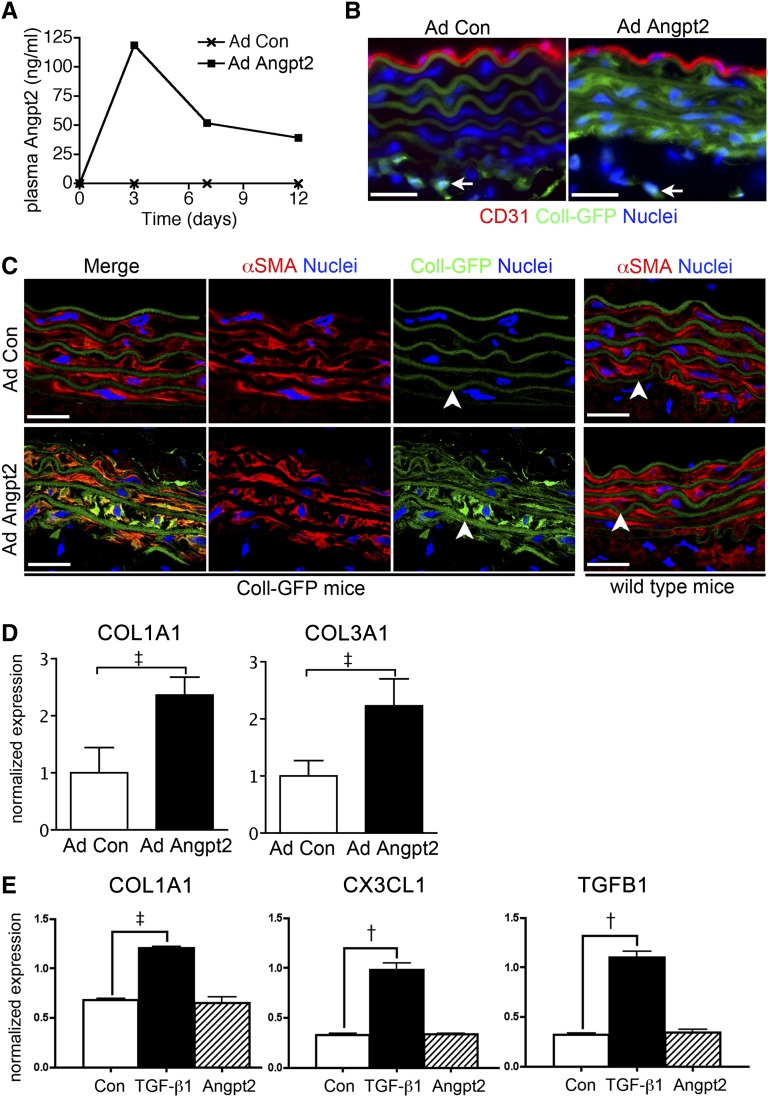
To study the role of increased plasma Angpt2 in arterial stiffness, we overexpressed Angpt2 in reporter mice expressing enhanced green fluorescent protein (GFP) under the regulation of the COL1A1 promoter and enhancer Coll-GFPTg. Coll-GFPTg mice received a single intravenous administration of adenovirus expressing human Angpt2 (AdAngpt2) or control adenovirus (AdCon) and were analyzed 12 days later. Administration of AdAngpt2, but not AdCon, led to robust expression of circulating human Angpt2 (Figure 5A). Plasma levels of Angpt1 and VEGF-A did not change. Marked increase of Coll-GFP expression, suggesting increase of COL1A1 transcript, in α-smooth muscle actin plus VSMCs of the aorta but not in CD31+ endothelial cells, was noted in mice with AdAngpt2 (Figure 5, B and C). By contrast, only perivascular fibroblasts in adventitia were noted to express Coll-GFP in mice with AdCon (Figure 5B). qPCR further confirmed the increase of COL1A1 and COL3A1 transcripts in the aorta of mice with AdAngpt2 (Figure 5D). We have titrated AdAngpt2 to raise the circulating Angpt2 comparable to that found in 5/6Nx mice at day 3 after administration; however, human Angpt2 in plasma and Coll-GFP in aortic VSMCs were not detectable at day 12 or 28. Aortic VSMCs were purified and cultured by sorting PDGF-β (PDGFRβ)+;CD31 cells from single cell preparation of aortas (Supplemental Figure 6). In vitro culture of aortic VSMCs stimulated by recombinant Angpt2 did not show upregulation of COL1A1 (Figure 5E), suggesting an indirect effect of Angpt2 on collagen expression in vivo.
Figure 5.
Aortic VSMCs generate collagen transcripts in mice producing human Angpt2. (A) Plasma levels of human Angpt2 in Coll-GFPTg mice administered with AdAngpt2 and AdCon. (B and C) Immunofluorescence images showing that α-SMA+ aortic VSMCs generate COL1A1 transcripts, as indicated by increased Coll-GFP, in mice with AdAngpt2. Coll-GFP expression in adventitial fibroblasts does not change (arrows in B). C57BL/6 wild-type mice serve as the negative control for GFP. Autofluorescence from elastic fibers is indicated (arrowheads in C). (D) qPCR showing increased transcripts of COL1A1 and COL3A1 in aorta of mice with AdAngpt2. (E) qPCR showing increased transcripts of COL1A1, CX3CL1, and TGFB1 in primary cultured VSMCs stimulated by recombinant TGF-β1 (5 ng/ml), but not by Angpt2 (500 ng/ml), for 24 hours. Angpt2 of different concentrations shows the same results. Expression levels are normalized by glyceraldehyde 3-phosphate dehydrogenase. †P<0.01; ‡P<0.001. αSMA, α-smooth muscle actin. Scale bar, 20 μm in C.
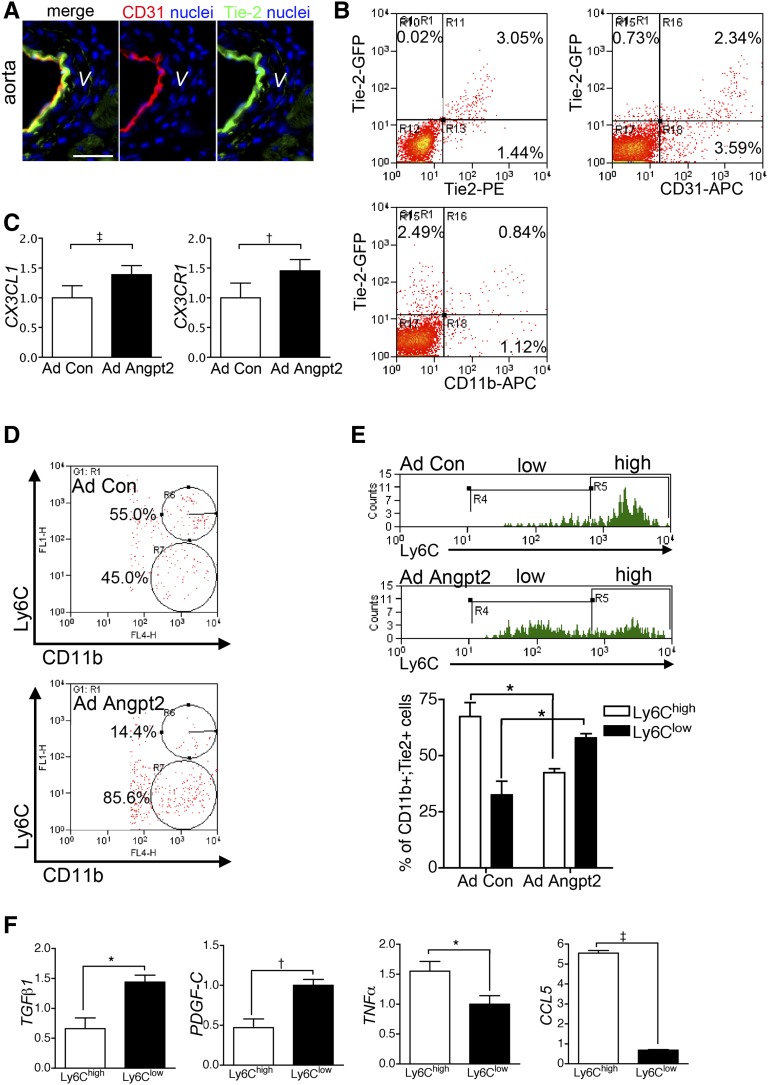
Angpt2 Induced Discrete Subpopulation of Macrophages Defined by Ly6C in Aorta
The Tie-2 receptor was expressed in CD31+ endothelial cells and to a lesser extent in CD11b+ macrophages (Figure 6, A and B). Aortic VSMCs did not express Tie-2 (Figure 6A). We hence hypothesized that endothelial cells and macrophages were a possible direct target of Angpt2. qPCR of thoracic aorta showed that transcripts of CX3CL1 and cognate receptor CX3CR1 were increased in mice with AdAngpt2 (Figure 6C). Flow cytometric analyses of single cell preparation of the aortas revealed that the Ly6Clow subpopulation of CD11b+ macrophages was increased in mice with AdAngpt2 (Figure 6D). Most of the CD11b+;Tie-2+ macrophages in aortas of control mice expressed Ly6Chigh; however, the Ly6Clow;CD11b+;Tie-2+ macrophages increased in mice with AdAngpt2 (Figure 6E). To understand whether there was any functional significance of these two macrophage subpopulations, macrophages of thoracic aortas were labeled and sorted by Ly6C expression and assessed for transcripts of M1 and M2 inflammatory cytokines. Ly6Chigh macrophages exhibited markers of M1-biased activation with high levels of TNFα and CCL5 (Figure 6F). By contrast, Ly6Clow macrophages exhibited markers of M2-biased cytokines TGFB1 and PDGF-C (Figure 6F), suggesting Ly6Clow macrophages as one of the cell sources producing profibrotic cytokines to affect VSMCs.
Figure 6.
Angpt2 induces discrete subpopulation of macrophages defined by Ly6C in aorta. (A) Immunofluorescence images showing that Tie-2 receptor is mainly expressed in CD31+ endothelial cells of the aorta from normal control mice. (B) FACS analysis showing that the Tie-2 receptor is also expressed in CD11b+ macrophages of the aorta from normal control mice. (C) qPCR showing increased transcripts of chemokine and chemokine receptors in aorta of mice with AdAngpt2. (D and E) FACS analysis showing that Ly6Clow subpopulation of CD11b+ macrophages is induced in aorta of mice with AdAngpt2. (F) qPCR of M1- and M2-biased cytokines of sorted CD11b+ macrophages defined by Ly6C expression. Expression levels are normalized by glyceraldehyde 3-phosphate dehydrogenase. *P<0.05; †P<0.01; ‡P<0.001. Scale bar, 20 μm in A.
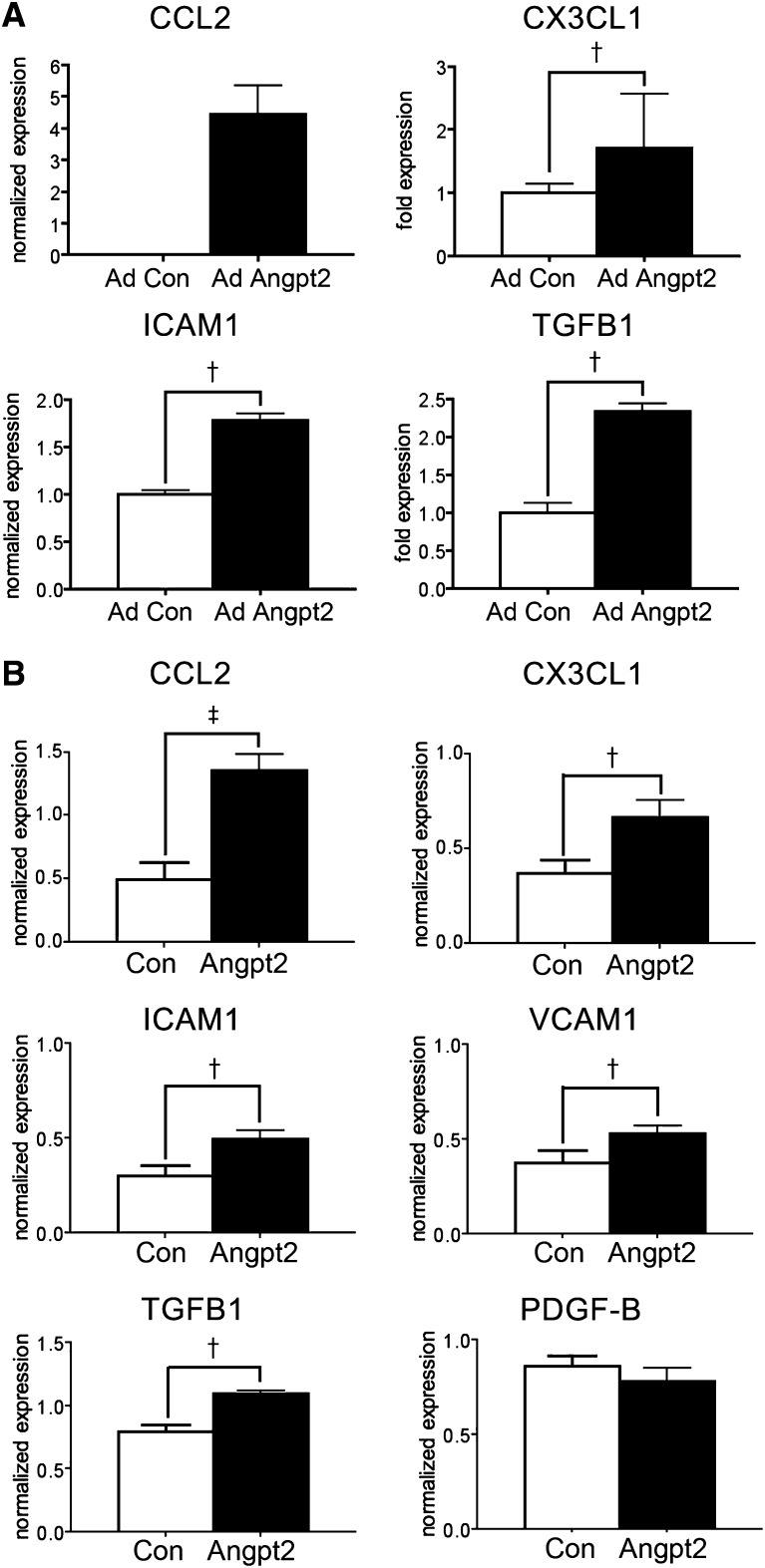
Angpt2 Induced Proinflammatory Chemokines and Adhesion Molecules in Endothelial Cells
To understand the effect of Angpt2 on aortic endothelial cells in mice with AdAngpt2, CD31+;Tie-2+;CD11b–endothelial cells of thoracic aortas were sorted. qPCR showed low or undetectable expression of CCL2, CX3CL1, and ICAM1 in endothelial cells of control mice, nevertheless upregulation was noted in mice with AdAngpt2 (Figure 7A). Furthermore, the expression of TGFB1 was also markedly increased (Figure 7A). Aortic endothelial cells were purified and cultured with purity>90% (Supplemental Figure 7). Recombinant Angpt2 could increase expression of CCL2, CX3CL1, ICAM1, VCAM1, and TGFB1 in cultured endothelial cells (Figure 7B, Supplemental Figure 8).
Figure 7.
Angpt2 induces proinflammatory chemokines and adhesion molecules in endothelial cells. (A) qPCR showing increased transcripts of CCL2, CX3CL1, ICAM1, and TGFB1 in FACS sorted aortic endothelial cells from mice with AdAngpt2. (B) qPCR for the transcripts of chemokines, adhesion molecules, and profibrotic cytokines in primary cultured aortic endothelial cells stimulated with recombinant Angpt2 (500 ng/ml) for 24 hours. Expression levels are normalized by glyceraldehyde 3-phosphate dehydrogenase. †P<0.01; ‡P<0.001.
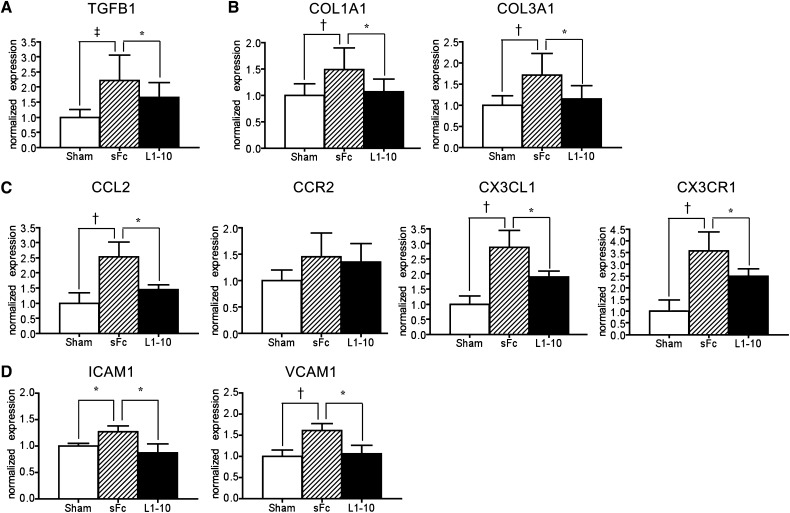
Blocking Angpt2 Decreased Expression of Profibrotic and Proinflammatory Cytokines in Aorta of 5/6Nx Mice
To study the effect of altered Angpt2 signaling within the aorta of mice after 5/6Nx, we initiated injections of L1-10, a specific Angpt2 inhibitor, from days 28 to 56 after surgery, when plasma Angpt2 increased (Figure 2A). The upregulation of TGFB1, COL1A1, COL3A1, CCL2, CX3CL1, CCR2, CX3CR1, ICAM1, and VCAM1 in thoracic aorta of 5/6Nx mice was inhibited by L1-10 treatment (Figure 8). Plasma levels of Angpt1, Angpt2, VEGF-A, and tail cuff BP in 5/6Nx mice were not affected by L1-10 treatment (Supplemental Figures 9 and 10).
Figure 8.
Blocking Angpt2 with recombinant protein L1-10 attenuates the transcripts of profibrotic and proinflammatory genes in aorta of mice after 5/6Nx. (A–D) qPCR for the transcripts of TGFB1 (A), COL1A1 and COL3A1 (B), CCL2, CCR2, CX3CL1, and CX3CR1 (C), and ICAM1 and VCAM1 (D) in aortas of sham, 5/6Nx receiving sFc or L1-10. Expression levels are normalized by glyceraldehyde 3-phosphate dehydrogenase. n=15 per group. *P<0.05; †P<0.01; ‡P<0.001.
Discussion
Angpt2 mainly exists as a multimeric protein that is neither detectable in urine from apparently healthy individuals nor cleared by dialysis.20 Therefore, decreased glomerular filtration cannot account for the increase of circulating Angpt2. Kidney transplantation is noted to attenuate, not to normalize, the increase of Angpt2.20 Correction of uremic toxins by renal transplantation is considered the main reason because systemic induction of endothelial Angpt2 expression by uremic toxins or asymmetric dimethylarginine is possible.22 Excess endothelial Angpt2 secretion has been proposed as a consequence of decreased nitric oxide bioavailability in the presence of high asymmetric dimethylarginine levels in patients with CKD.22 However, it is possible to reduce the Angpt2 through immunosuppressive therapy in patients with kidney transplantation.20 In addition, many triggers, such as angiotensin II, TNF-α, and reactive oxygen species, have been shown to stimulate Angpt2 secretion by endothelial cells.33 Our data that Angpt2 expression increased in fibrotic kidneys but decreased in the other tissues including aorta and lung of 5/6Nx mice argue against the systemic endothelial Angpt2 synthesis induced by uremic toxins or asymmetric dimethylarginine. By contrast, Angpt2 did not change in the tissues other than kidney and aorta in UUO mice, suggesting that the uremic toxin might have an inhibitory effect on Angpt2 expression in tissues other than the kidney in 5/6Nx mice. The fact that Angpt2 increased expression in renal tubular epithelial cells and glomeruli of CKD mice supports the fibrotic kidney as one of the possible sources of circulating Angpt2 in patients with CKD. However, our data could not conclude the increased Angpt2 transcripts in fibrotic kidney enough for the increase of circulating Angpt2. Further studies will be required to understand the underlying mechanisms that induce Angpt2 upregulation in renal tubular epithelial cells and glomeruli of fibrotic kidneys, as well as the significance of increased Angpt2 expression in the fibrotic kidney. Similar to our observation in fibrotic kidneys, airway epithelial cells have been shown to increase Angpt2 in chronic asthmatic mice.34 The increased expression of Angpt2 in the airway epithelium correlates with the severity of airway remodeling.
In our CKD cohort, the plasma levels of Angpt2 were independently correlated with worsening of arterial stiffness measured by PWV. Because the levels of circulating Angpt2 can predict CVD mortality in patients with CKD,20–23 our data may suggest that Angpt2 increases the risk of CVD and mortality in patients with CKD through arterial stiffness. Although a previous study by David et al. did not find an association between circulating Angpt2 and PWV,23 most of their patients were undergoing dialysis therapy; thus, other factors might play a role and reduce the effect of Angpt2 in arterial stiffness. In contrast with the results of David et al., our subgroup analysis demonstrated stronger positive correlation between plasma Angpt2 and PWV in CKD stage 5 without dialysis therapy, suggesting the importance of plasma levels and the duration of exposure to Angpt2 for arterial stiffness in CKD. In addition to our observation, the association of increased circulating Angpt2 with arteriosclerosis has been shown in patients with type 2 diabetes.35 Angpt2 is further suggested to play a role in the development of unstable carotid plaque.36
Although the pathogenesis of arterial stiffness involves many mechanisms, VSMC transformation and local inflammation are the pathologic hallmarks.10,17,37 During the development of arterial stiffness, VSMCs change from the physiologic contractile phenotype to the pathophysiologic synthetic phenotype with production of ECM components including collagen, fibronectin, elastin, and laminin.16,17 Our data demonstrated that the expression of COL1A1 was increased in aortic VSMCs as reported by the GFP in situ in Coll-GFPTg mice with AdAngpt2. We further demonstrated that increased transcripts of COL1A1 and COL3A1 in the aorta of CKD mice were attenuated by Angpt2 blockade. However, increased transcripts of COL1A1 and COL3A1 genes in the aorta are the surrogate markers of arterial stiffness. Further studies are required to elucidate that the inhibition of Angpt2 in CKD mice can be translated to the attenuation of arterial stiffness using applicable measurement in mice or other laboratory animals.
Consistent with previous reports, Tie-2 receptors were expressed in endothelial cells and a population of monocytes/macrophages, but not in VSMCs.38 Our data also showed that recombinant Angpt2 did not induce collagen expression in primary culture of VSMCs. Hence, the in vivo stimulation of collagen expression in aortic VSMCs by Angpt2 was rather a paracrine effect from neighboring cells. This paracrine effect of endothelial cells on VSMCs was supported by that endothelial cells in mice with AdAngpt2 and cultured endothelial cells stimulated by recombinant Angpt2 increased TGF-β1 that has been shown to stimulate collagen production of VSMCs.17 In addition to the cytokines for VSMCs, our data also showed the upregulation of monocyte chemokines and adhesion molecules in endothelial cells by Angpt2. We also observed that increased Angpt2 was associated with an increased subpopulation of Ly6Clow macrophages. Although the biologic significance of our experiments using artificially high levels of Angpt2 produced by adenovirus in vivo or by adding recombinant Angpt2 in cell cultures might be different from that in CKD, the results that surrogate markers of arterial stiffness were comparable in mouse models induced by AdAngpt2 and 5/6Nx and that these surrogate markers in aorta of 5/6Nx mice were attenuated by Angpt2 blockade emphasized the role of elevated Angpt2 in arterial stiffness of patients with CKD. Current evidence suggests that Angpt2 can sensitize endothelial cells to inflammatory cytokines, leading to an increase of local permeability and recruitment of inflammatory monocytes/macrophages into the vascular wall.29 Angpt2 can also stimulate the alternatively activated macrophages that express CCL17 and the mannose receptor.38 CCL17-expressed macrophages can drive atherosclerosis.37 Indeed, we have shown that Ly6Clow macrophages are expressed by CCL17 and CCL22 expressed and are profibrotic in the kidney.39,40 Therefore, longstanding Angpt2 signaling might lead to endothelial cell activation and chronic inflammation, thereby promoting collagen-rich ECM production of VSMCs and arterial stiffness in patients with CKD. Our data were further supported by a study that demonstrates the crucial role of Angpt2 in recruitment and proliferation of VSMCs in arteriogenesis in mice after acute femoral artery ligation.32 The difference between proarteriogenic effects in an acute model of occlusion-revascularization and proarteriosclerotic effects in a chronic model of arterial stiffness needs further study. This difference in their roles in kidney regeneration and fibrosis also exists in macrophages.37,39–41 Finally, we need to emphasize that the exact receptor and mechanism by which Angpt2 induces arterial stiffness in CKD requires further study, although inhibition of Angpt2 by L1-10 can attenuate the surrogate markers.
In conclusion, plasma Angpt2 is associated with arterial stiffness in patients with CKD. Increased Angpt2 may induce inflammatory and fibrotic signaling of endothelial cells and macrophages to stimulate collagen-rich ECM synthesis by VSMCs. This study identifies a link between the fibrotic kidney and arterial stiffness through Angpt2. Angpt2 blockade may represent a novel therapy for cardiovascular protection in CKD.
Concise Methods
Patients
This cross-sectional study enrolled adult patients with CKD stages 3–5 (defined by an eGFR using the six-variable Modification of Diet in Renal Disease study equation) from December 2006 to December 2007.42 CKD stages 3–5 were defined as eGFR measurements of 30–59 ml/min per 1.73 m2, 15–29 ml/min per 1.73 m2, and <15 ml/min per 1.73 m2, respectively. Patients were excluded if they had infection, malignancy, pregnancy, kidney transplantation, or dialysis. Hypertension was classified in patients who had a BP >140/90 mmHg or who were taking antihypertensive medication. Diabetes was defined by history and blood glucose values (using the American Diabetes Association criteria) or by use of hypoglycemic medication. Dyslipidemia was diagnosed in patients who had fasting total cholesterol≥200 mg/dl, LDL cholesterol≥130 mg/dl, or triglyceride≥200 mg/dl or who were using lipid-lowering medication. The study protocol was approved by the National Taiwan University Hospital (201105023RC) and adhered to the Declaration of Helsinki. All patients gave written informed consent.
PWV Measurement
PWV was measured with the patient in a supine position after 15 minutes of rest using an automatic waveform analyzer (Colin VP-1000; Omeron Inc., Kyoto, Japan) as previously described.43 PWV was calculated from the distance between two arterial recording sites divided by transit time. The transit time between the heart and ankle arterial pressure wave was determined by the wave front velocity theory. The time difference was the period between the S2 heart sound and the initial point of tibial arterial pressure wave. The distance between the heart and ankle was automatically calculated based on the patient’s height. Left and right PWVs were averaged in each patient for subsequent analyses.
Animals
Coll-GFPTg mice generated on a C57BL/6 background were as previously described.44 In brief, 3.2 kb of the COL1A1 promoter and enhancer with the open reading frame of enhanced GFP yielded the highest levels of GFP expression when COL1A1 transcripts were generated. Tie-2-GFPTg mice on a FVB/N background were obtained from The Jackson Laboratory (Bar Harbor, ME).
Animal Models of Kidney Fibrosis
UUO was performed in adult male C57BL/6 mice as previously described.44 A 5/6Nx was performed in adult male CD1 mice in two stages under anesthesia as previously described.45 At the first stage, the upper and lower poles of the left kidney were partially resected via a left flank incision. After 1 week (week 0), the entire right kidney was removed via a right flank incision. Sham surgery was performed by manipulation of the renal pedicle only in age-matched, background-matched mice used as controls. L1-10 (4 mg/kg body weight; Amgen, Inc., Thousand Oaks, CA) or soluble Fc fragment (4 mg/kg body weight; Bethyl laboratories Inc., Montgomery, TX) was injected intraperitoneally every other day according to a previously reported method.32 All animal studies were carried out under a protocol approved by the Institutional Animal Care and Use Committee of National Taiwan University.
Tissue Preparation and Histology
Mouse aorta and cultured cells were prepared and stained as previously described.44 Primary antibodies against the following proteins were used for immunolabeling: F4/80, CD31, Kim-1 (eBioscience, San Diego, CA), Nidogen, Tie-2 (Santa Cruz Biotechnology, Santa Cruz, CA), Angpt2 (Calbiochem, Darmstadt, Germany), VEGFR2 (Cell Signaling Technology, Beverly, MA), L. tetragonolobus lectin–fluorescein (Vector Laboratories, Burlingame, CA), and α-smooth muscle actin-Cy3 (Sigma-Aldrich, St. Louis, MO). Fluorescence conjugated affinity-purified secondary antibody labeling (1:400–1:800; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), colabeling with 4′,6-diamidino-2-phenylindole, mounting with Vectashield/4′,6-diamidino-2-phenylindole, and image capture and processing were carried out as previously described.44 The specificity of Angpt2 staining was confirmed by mixing the primary antibody with recombinant mouse Angpt2 (100 ng; R&D Systems, Minneapolis, MN) before applying it to the tissue section.
FACS Analyses
Thoracic aorta was diced and incubated at 37°C for 1 hour with Liberase (0.5 mg/ml; Roche Applied Science, Indianapolis, IN) and DNase (100 U/ml; Roche Applied Science) in HBSS. After centrifugation, cells were resuspended in FACS buffer and incubated with antibodies against Ly6-C-FITC, Ly6G-PE-Cy7 (BD Biosciences, San Jose, CA), Tie-2-PE, CD31-APC, CD11b-APC, and NK1.1-PerCP-Cy5.5, B220-PE-Cy7 (eBioscience). After washing with FACS wash buffer and resuspension in 200 μl FACS buffer, cells were analyzed using a BD FACSCalibur flow cytometer (BD Biosciences) as previously described.39
Analytic Experiments
Details of analytic protocols (ELISA, RT-PCR, Western blot analysis, in situ hybridization) and adenovirus preparation are available in the Supplemental Material.
Statistical Analyses
Because some of the continuous clinical variables and plasma levels of angiotrophic growth factors were not normally distributed, differences in CKD subgroups were analyzed using the Kruskal–Wallis test. Differences in categorical variables were compared using the chi-squared test. Log transformation was performed for variables to assess the correlation by the Pearson test. Univariate regression analysis was performed for the association between Angpt2 and PWV, followed by multivariate regression analyses with regard to potential confounders. In the first model, multivariate regression analyses between Angpt2 level and PWV were adjusted for age. Second, we adjusted for traditional risk factors such as diabetes, hypertension, sex, smoking, hyperlipidemia, and biomarkers that were statistically significantly associated with PWV in univariate analyses. We then used stepwise regression methods to examine independent risk factors. The backward and forward selection methods yielded the same set of independent risk factors. In the full model, we kept all of the traditional risk factors and additionally adjusted with nontraditional risk factors such as calcium and phosphate product and medications with ACE inhibitors/ARBs, statins, calcium channel blockers, and β-blockers. All analyses were conducted using Stata software (version 9; StataCorp, College Station, TX). Statistical significance was evaluated by an unpaired t test or one-way ANOVA in animal and cell experiments. A two-sided P<0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Dr. Christopher D. Kontos (Duke University Medical Center, Durham, NC) for Ad Angpt2, Amgen, Inc., for L1-10, the Department of Medical Research of National Taiwan University Hospital for equipment support, and the Cell Sorting Core Facility of the First Core Laboratory and the Transgenic Mouse Core Facility in the Center for Genomic Medicine, National Taiwan University College of Medicine.
F.-C.C. is supported by grants from the National Science Council (102-2314-B-002-113) and the Mrs. Hsiu-Chin Lee Kidney Research Foundation. S.-L.L. is also supported by grants from the National Science Council (99-2628-B-002-013, 101-2321-B-002-060, 101-2314-B-002-084, 102-2628-B-002-015, and 102-2321-B002-045).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013050542/-/DCSupplemental.
References
- 1.Levin A, Foley RN: Cardiovascular disease in chronic renal insufficiency. Am J Kidney Dis 36[Suppl 3]: S24–S30, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ: Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16: 489–495, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Briet M, Collin C, Karras A, Laurent S, Bozec E, Jacquot C, Stengel B, Houillier P, Froissart M, Boutouyrie P, Nephrotest Study Group : Arterial remodeling associates with CKD progression. J Am Soc Nephrol 22: 967–974, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, Anderson A, Go AS, Shlipak MG, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 23: 1725–1734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 103: 987–992, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Upadhyay A, Hwang S-J, Mitchell GF, Vasan RS, Vita JA, Stantchev PI, Meigs JB, Larson MG, Levy D, Benjamin EJ, Fox CS: Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 20: 2044–2053, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J: Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 113: 664–670, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ: Arterial stiffness and cardiovascular events: The Framingham Heart Study. Circulation 121: 505–511, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townsend RR, Wimmer NJ, Chirinos JA, Parsa A, Weir M, Perumal K, Lash JP, Chen J, Steigerwalt SP, Flack J, Go AS, Rafey M, Rahman M, Sheridan A, Gadegbeku CA, Robinson NA, Joffe M: Aortic PWV in chronic kidney disease: A CRIC ancillary study. Am J Hypertens 23: 282–289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Bussel BC, Henry RM, Schalkwijk CG, Dekker JM, Nijpels G, Stehouwer CD: Low-grade inflammation, but not endothelial dysfunction, is associated with greater carotid stiffness in the elderly: The Hoorn Study. J Hypertens 30: 744–752, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Mäki-Petäjä KM, Elkhawad M, Cheriyan J, Joshi FR, Ostör AJK, Hall FC, Rudd JHF, Wilkinson IB: Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation 126: 2473–2480, 2012 [DOI] [PubMed] [Google Scholar]
- 12.London GM, Guerin AP, Marchais SJ, Pannier B, Safar ME, Day M, Metivier F: Cardiac and arterial interactions in end-stage renal disease. Kidney Int 50: 600–608, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Muntner P, He J, Hamm L, Loria C, Whelton PK: Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 13: 745–753, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK: Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12: 2131–2138, 2001 [DOI] [PubMed] [Google Scholar]
- 15.London GM, Guérin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Mëtivier F: Mineral metabolism and arterial functions in end-stage renal disease: Potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol 18: 613–620, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Tsai MC, Chen L, Zhou J, Tang Z, Hsu TF, Wang Y, Shih YT, Peng HH, Wang N, Guan Y, Chien S, Chiu JJ: Shear stress induces synthetic-to-contractile phenotypic modulation in smooth muscle cells via peroxisome proliferator-activated receptor α/delta activations by prostacyclin released by sheared endothelial cells. Circ Res 105: 471–480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YM, Wu KD, Tsai TJ, Hsieh BS: Pentoxifylline inhibits PDGF-induced proliferation of and TGF-beta-stimulated collagen synthesis by vascular smooth muscle cells. J Mol Cell Cardiol 31: 773–783, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Jamal SA, Fitchett D, Lok CE, Mendelssohn DC, Tsuyuki RT: The effects of calcium-based versus non-calcium-based phosphate binders on mortality among patients with chronic kidney disease: A meta-analysis. Nephrol Dial Transplant 24: 3168–3174, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Lemström KB, Krebs R, Nykänen AI, Tikkanen JM, Sihvola RK, Aaltola EM, Häyry PJ, Wood J, Alitalo K, Ylä-Herttuala S, Koskinen PK: Vascular endothelial growth factor enhances cardiac allograft arteriosclerosis. Circulation 105: 2524–2530, 2002 [DOI] [PubMed] [Google Scholar]
- 20.David S, Kümpers P, Hellpap J, Horn R, Leitolf H, Haller H, Kielstein JT: Angiopoietin 2 and cardiovascular disease in dialysis and kidney transplantation. Am J Kidney Dis 53: 770–778, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Shroff RC, Price KL, Kolatsi-Joannou M, Todd AF, Wells D, Deanfield J, Johnson RJ, Rees L, Woolf AS, Long DA: Circulating angiopoietin-2 is a marker for early cardiovascular disease in children on chronic dialysis. PLoS ONE 8: e56273, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.David S, Kümpers P, Lukasz A, Fliser D, Martens-Lobenhoffer J, Bode-Böger SM, Kliem V, Haller H, Kielstein JT: Circulating angiopoietin-2 levels increase with progress of chronic kidney disease. Nephrol Dial Transplant 25: 2571–2576, 2010 [DOI] [PubMed] [Google Scholar]
- 23.David S, John SG, Jefferies HJ, Sigrist MK, Kümpers P, Kielstein JT, Haller H, McIntyre CW: Angiopoietin-2 levels predict mortality in CKD patients. Nephrol Dial Transplant 27: 1867–1872, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Chang FC, Lai TS, Chiang CK, Chen YM, Wu MS, Chu TS, Wu KD, Lin SL: Angiopoietin-2 is associated with albuminuria and microinflammation in chronic kidney disease. PLoS ONE 8: e54668, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gale NW, Yancopoulos GD: Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev 13: 1055–1066, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, Kutschera S, Bartels S, Appak S, Besemfelder E, Terhardt D, Chavakis E, Wieland T, Klein C, Thomas M, Uemura A, Goerdt S, Augustin HG: Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest 122: 1991–2005, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, Quaggin SE: Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest 121: 2278–2289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woolf AS, Gnudi L, Long DA: Roles of angiopoietins in kidney development and disease. J Am Soc Nephrol 20: 239–244, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG: Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 12: 235–239, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD: Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 6: 460–463, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD: Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277: 55–60, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Tressel SL, Kim H, Ni CW, Chang K, Velasquez-Castano JC, Taylor WR, Yoon YS, Jo H: Angiopoietin-2 stimulates blood flow recovery after femoral artery occlusion by inducing inflammation and arteriogenesis. Arterioscler Thromb Vasc Biol 28: 1989–1995, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinsky DJ, Naka Y, Liao H, Oz MC, Wagner DD, Mayadas TN, Johnson RC, Hynes RO, Heath M, Lawson CA, Stern DM: Hypoxia-induced exocytosis of endothelial cell Weibel-Palade bodies. A mechanism for rapid neutrophil recruitment after cardiac preservation. J Clin Invest 97: 493–500, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makinde TO, Agrawal DK: Increased expression of angiopoietins and Tie2 in the lungs of chronic asthmatic mice. Am J Respir Cell Mol Biol 44: 384–393, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim HS, Lip GY, Blann AD: Angiopoietin-1 and angiopoietin-2 in diabetes mellitus: Relationship to VEGF, glycaemic control, endothelial damage/dysfunction and atherosclerosis. Atherosclerosis 180: 113–118, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Post S, Peeters W, Busser E, Lamers D, Sluijter JP, Goumans MJ, de Weger RA, Moll FL, Doevendans PA, Pasterkamp G, Vink A: Balance between angiopoietin-1 and angiopoietin-2 is in favor of angiopoietin-2 in atherosclerotic plaques with high microvessel density. J Vasc Res 45: 244–250, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Weber C, Meiler S, Döring Y, Koch M, Drechsler M, Megens RTA, Rowinska Z, Bidzhekov K, Fecher C, Ribechini E, van Zandvoort MAMJ, Binder CJ, Jelinek I, Hristov M, Boon L, Jung S, Korn T, Lutz MB, Förster I, Zenke M, Hieronymus T, Junt T, Zernecke A: CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T-cell homeostasis in mice. J Clin Invest 121: 2898–2910, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coffelt SB, Tal AO, Scholz A, De Palma M, Patel S, Urbich C, Biswas SK, Murdoch C, Plate KH, Reiss Y, Lewis CE: Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res 70: 5270–5280, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Lin SL, Castaño AP, Nowlin BT, Lupher ML, Jr, Duffield JS: Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol 183: 6733–6743, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Castaño AP, Lin SL, Surowy T, Nowlin BT, Turlapati SA, Patel T, Singh A, Li S, Lupher ML, Jr, Duffield JS: Serum amyloid P inhibits fibrosis through Fc gamma R-dependent monocyte-macrophage regulation in vivo. Sci Transl Med 1: 5ra13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS: Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 107: 4194–4199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Kuo HK, Chen CY, Liu HM, Yen CJ, Chang KJ, Chang CC, Yu YH, Lin LY, Hwang JJ: Metabolic risks, white matter hyperintensities, and arterial stiffness in high-functioning healthy adults. Int J Cardiol 143: 184–191, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Lin SL, Kisseleva T, Brenner DA, Duffield JS: Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leelahavanichkul A, Yan Q, Hu X, Eisner C, Huang Y, Chen R, Mizel D, Zhou H, Wright EC, Kopp JB, Schnermann J, Yuen PS, Star RA: Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse model. Kidney Int 78: 1136–1153, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.