Abstract
Intralysosomal cystine crystal accumulation, due to mutations in the CTNS gene, is a hallmark of nephropathic cystinosis, but the role of these crystals in disease pathogenesis remains unclear. We hypothesized that, similar to other host-derived crystalline moieties, cystine crystals can induce IL-1β production through inflammasome activation. Thus, we investigated the proinflammatory effects of cystine crystals in primary human PBMCs. LPS-primed PBMCs stimulated with cystine crystals secreted IL-1β in a dose-dependent manner. Similarly to IL-1β secretion induced by other crystalline inflammasome activators, cystine crystal–induced IL-1β secretion required activation of caspase-1. Additionally, exogenous cystine crystals were internalized by monocytes, and inhibition of phagocytosis, cathepsin B leakage, generation of reactive oxygen species, and potassium efflux reduced cystine crystal–induced IL-1β secretion. Patients with cystinosis had higher levels of circulating IL-1β and IL-18 compared with controls. Analysis of inflammasome-related gene expression in PBMCs from patients with cystinosis revealed a significant increase in IL-1β and CASP-1 transcript levels compared with controls. Moreover, knockout of cystinosin in mice led to significant increases in serum IL-18 levels and kidney expression of inflammasome-related genes (Casp-1, Pycard, Il-18, Il18r1, Il1r1, and Il1rl2). Taken together, these data demonstrate that cystine crystals are endogenous inflammasome-activating stimuli, suggesting a novel role for cystine crystals in the pathogenesis of nephropathic cystinosis.
Keywords: chronic kidney disease, genetic renal disease, immunology
Cystinosis is a rare autosomal recessive disorder caused by mutations in CTNS gene, encoding for the lysosomal cystine proton cotransporter cystinosin.1 It is characterized by the lysosomal accumulation of cystine, which leads to the formation of cystine crystals within various organs, including kidneys, brain, cornea, intestine, and bone marrow.2 The exact role of intralysosomal crystal accumulation in the pathogenesis of cystinosis remains unclear. On the basis of the increasing body of evidence demonstrating the role of host-derived crystalline moieties (associated with danger, damage, or cell death) in inflammasome activation,3 we have investigated whether cystine crystals are able to elicit an inflammatory response via inflammasome activation.
Inflammasomes are a group of intracellular protein complexes that recognize a diverse set of inflammation-inducing stimuli (pathogen-associated molecular patterns and damage-associated molecular patterns) and control the caspase-1–dependent proteolytic maturation and release of the proinflammatory cytokines IL-1β and IL-18.4,5 The NLRP3 inflammasome is the best characterized inflammasome, formed by the adaptor protein ASC, pro–caspase-1, and NLRP3.6 NLRP3 is activated by endogenous crystals or particle formats, including monosodium urate, calcium phosphate, hydroxyapatite, silica, asbestos, and cholesterol crystals.7–12 Although the exact mechanism by which NLRP3 is activated is still unknown, NLRP3 activation by crystalline structures has been demonstrated to require phagolysosomal destabilization, generation of reactive oxygen species (ROS), and potassium efflux.13
To understand whether the inflammasome is activated by cystine crystals, we investigated whether cystine crystals induce IL-1β secretion from cultured PBMCs. For this purpose, cystine crystals were added to LPS-primed PBMCs and incubated for 6 hours. As shown in Figure 1A, addition of cystine crystals induced dose-dependent IL-1β secretion, similarly to crystals of monosodium urate (positive control).9 Treatment of PBMCs with cystine crystals alone, in the absence of LPS priming, did not result in detectable IL-1β production (not shown), excluding the possibility of microbial contaminations in these crystal preparations. Western blotting of cell culture supernatants confirmed that cystine crystals induced mature IL-1β secretion and CASP-1 activation, as demonstrated by the presence of the mature cleaved form of IL-1β and activated caspase-1 in the supernatants of cystine crystal–stimulated cells (Figure 1A, bottom).
Figure 1.
Cystine crystals induce IL-1β secretion in LPS-primed human PBMCs and are internalized by monocytes in vitro. (A) Primary human PBMCs were primed with LPS (100 ng/ml) for 2 hours and then incubated with increasing concentration of cystine crystals or monosodium urate crystals (MSU) for 6 hours. Supernatants were collected and IL-1β was measured by ELISA. Data are representative of three independent experiments performed in duplicate and are expressed as means±SD (top). Supernatants (SN) were also analyzed by Western blotting using antibodies detecting the mature form of IL-1β and the activated caspase-1 form (bottom). (B) Monocytes isolated by adherence from freshly isolated PBMCs were prestimulated with LPS (100 ng/ml) for 2 hours and then incubated with cystine crystals for 6 hours. At the end of incubation, cells were stained with anti–wheat germ agglutinin (WGA) lectin (green) to label plasma cell membranes and Hoechst stain (blue) to label nuclei. Cells were imaged by confocal fluorescent microscopy. (b–e) Single section performed on the central focal plane, showing wheat germ agglutinin staining of cell membranes (b), cell phase contrast (c), wheat germ agglutinin and Hoechst staining (d), and merge image (e) visualizing cystine crystals enveloped by cell membranes. Cystine crystals are indicated by arrows. Scale: 50 μm (a) and 10 μm (b–e).
Because crystalline inflammasome activators need to be phagocytized by phagocytes to elicit IL-1β secretion,7,12 we used confocal reflection microscopy to explore whether cystine crystals were internalized by mononuclear adherent cells. Cell membranes were stained with anti–heat germ agglutinin antibodies. Small hexagonal or rectangular cystine crystals were found in LPS-primed cells (Figure 1B) and in unstimulated cells (not shown), enveloped by the plasma membrane.
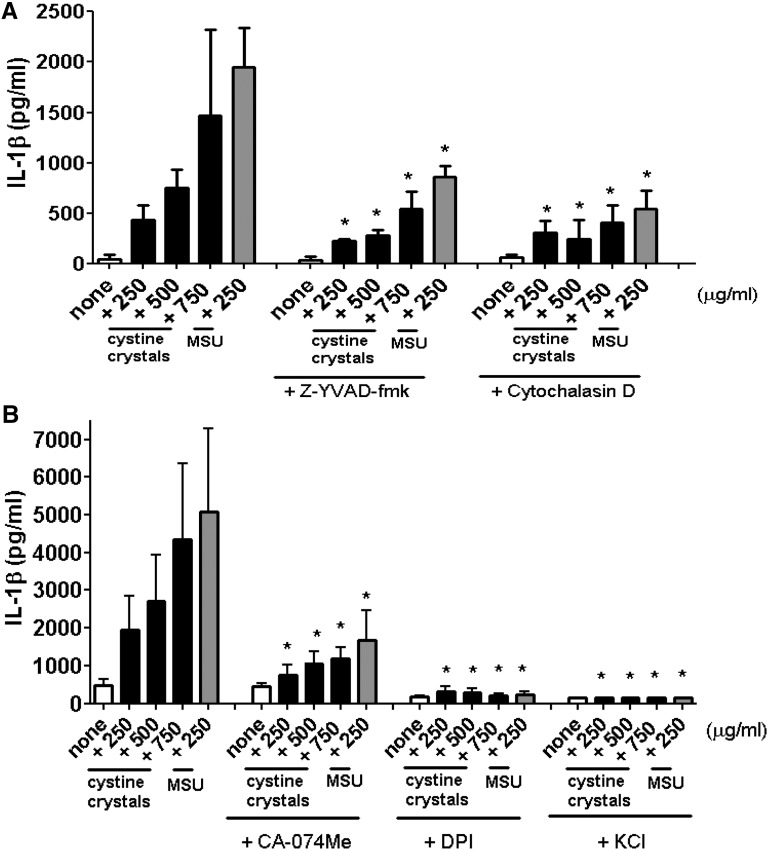
To confirm the role of inflammasome activation in cystine crystal–induced IL-1β secretion, we used z-YVAD-fmk, a specific caspase-1 inhibitor. IL-1β production decreased markedly (Figure 2A), confirming the involvement of an inflammasome-mediated pathway. We then examined whether the phagocytic function of mononuclear cells was required for cystine crystal–induced activation of inflammasome. Inhibition of actin polymerization with cytochalasin D effectively blocked cystine crystal–induced IL-1β secretion (Figure 2A) but did not affect ATP-induced IL-1β production (not shown). On the basis of the evidence that lysosomal destabilization and ROS production are involved in NLRP3 inflammasome activation,14 pretreatment of PBMCs with the cathepsin B inhibitor CA-074Me and diphenyleneiodonium chloride, a chemical inhibitor of ROS production, were performed. As showed in Figure 2B, both inhibitors abrogated the secretion of IL-1β induced by cystine crystals. Furthermore, we assessed the requirement of potassium efflux in cystine crystal–induced inflammasome activation by incubating human PBMCs with high extracellular KCl concentration. IL-1β secretion was completely inhibited when potassium efflux was prevented (Figure 2B). Together these data indicate that the stimulatory effect of cystine crystals on IL-1β production depended on cathepsin B leakage, ROS production, and K+ efflux, similarly to known crystalline inflammasome activators.12
Figure 2.
Cystine crystal–induced IL-1β secretion requires caspase-1 activation, actin polymerization, lysosomal protease activity, ROS generation, and potassium efflux. PBMCs were primed with LPS (100 ng/ml) for 2 hours, incubated for 30 minutes in the presence or absence of (A) the CASP-1 inhibitor z-YVAD-fmk (5 μM) or phagocytosis inhibitor cytochalasin D (5 μM) or (B) cathepsin B inhibitor CA-074Me (10 μM) or diphenyleneiodonium chloride (DPI) (20 μM) or 130mM KCl. Cystine crystals or monosodium urate crystals (MSU) were added for 6 hours. Supernatants were collected and IL-1β was measured by ELISA. Data are representative of three independent experiments performed in duplicate and are expressed as means±SD. *P<0.05 compared with cells stimulated in the absence of inhibitors.
Having demonstrated the effect of cystine crystals on inflammasome activation by in vitro studies, we focused on the physiopathologic relevance of our findings. To this end, we investigated patients with cystinosis and Ctns−/− mice. First, we measured IL-1β and IL-18 levels in plasma collected from patients with cystinosis. Levels of both circulating IL-1β and IL-18 were significantly higher in patients than controls (P=0.05 and P<0.0001, respectively) (Figure 3, A and B). When cystinotic patients were compared with patients with familial Mediterranean fever, a known genetic autoinflammatory disorder characterized by inflammasome deregulation,15 circulating IL-1β levels were similar in the two cohorts of patients, while IL-18 levels were significantly higher in cystinotic patients than in those with FMF (P=0.002). Moreover, IL-1β transcript levels were significantly increased (P=0.0003) in PBMCs isolated from cystinotic patients (Figure 3C) compared with controls. Interestingly, we observed a positive correlation between intracellular cystine levels at the time of blood sampling and IL-1β mRNA expression in freshly isolated PBMCs (Spearman r=0.645; P=0.03) (Figure 3D). In addition, we found a significant increase (P=0.03) in CASP-1 mRNA expression in cystinotic patients compared with controls (Figure 3E) but no differences in NLRP3 or TNF-α (a gene whose expression is unrelated to inflammasome activation) expression (Figure 3, F and G). Circulating IL-1β levels were higher in pretransplant patients than in patients who had undergone transplantation; circulating IL-18 levels were significantly higher both in pretransplant patients and transplant recipients than in controls (Supplemental Figure 1, A and B). Although we did not observe a significant correlation between patients’ GFR and IL-1β levels (r=−0.07; P=0.82), we found a trend for a negative correlation between GFR and IL-18 levels (r=−0.52; P=0.08). Age and IL-1β levels were correlated (r=−0.67; P=0.03); age and IL-18 levels were not (r=−0.18; P=0.57). We have no clear explanation for this observation, which is apparently not related to whether the patient has undergone transplantation or not. Of note, no correlations between age and both IL-1β and IL-18 levels were observed in our cohort of controls. Of notice, all patients were treated with cysteamine, which depletes intracellular cystine content.
Figure 3.
In cystinotic patients, circulating IL-1β and IL-18 levels are increased and inflammasome-related gene expression is upregulated compared with controls. (A and B) IL-1β and IL-18 were measured by ELISA in plasma from cystinotic patients, patients with FMF, and controls. Black horizontal lines represent the median values. (C) Quantitative real-time PCR analysis of IL-1β in freshly isolated PBMCs obtained from cystinotic patients, patients with FMF, and controls. (D) Positive correlation of IL-1β mRNA levels in PBMCs freshly isolated from cystinotic patients with intracellular cystine levels at time of blood sampling. (E–G) Real-time PCR analysis of gene products involved in IL-1β regulation, including CASP-1 and NLRP3, and of the nonrelated cytokine TNF-α in freshly isolated PBMCs obtained from cystinotic patients and controls. The results are expressed as arbitrary units (AU) obtained after normalization with the housekeeping gene GAPDH. Black horizontal lines represent the median values. *P<0.05; **P<0.01.
Finally, to support our results obtained in humans, we investigated the activation of the inflammasome in the mouse model of nephrophatic cystinosis,16 the Ctns−/− mouse, by performing a gene expression profiling on kidney, spleen, liver, and muscle tissues from 16-month-old mice. In kidneys, but not in the other tissues analyzed, we observed a significant upregulation of some of the genes involved in the inflammasome activation pathway, including Casp1, Pycard (Asc), Il18, Il18r1, Il1r1, and Il1rl2 in Ctns−/− mice compared with wild-type (WT) mice (Figure 4A). We confirmed these data by real time PCR on another group of 15-/17-month-old mice (Figure 4, B–D). To verify whether the inflammasome pathway was also upregulated in an early phase of the disease, we analyzed Il18, Casp1, and Pycard gene kidney expression in 5-/7-month-old mice. The expression of these genes was significantly upregulated even in the younger Ctns−/− mice compared with WT mice (Figure 4, B–D). We measured IL-1β and Il-18 levels in sera collected from 5-/7- or 15-/17-month-old Ctns−/− mice and compared them with those of age-matched WT mice. Serum IL-18 levels were significantly higher in both groups of Ctns−/− mice compared with WT mice (Figure 4E), while IL-1β was not detectable in the mice tested.
Figure 4.
Ctns −/− mice have increased inflammasome-related gene expression in kidneys and higher circulating IL-18 levels compared with WT mice. (A) Total kidney RNA extracts were analyzed using microarray analysis. Gene expression changes between Ctns−/− and WT kidneys are expressed as fold change. The genes listed are significant at the nominal 0.001 level of the univariate test. (B–D) Real-time PCR analyses of gene products involved in inflammasome pathway in kidneys from 5-/7-month-old and 15-/17-month-old WT or Ctns−/− mice. The results are expressed as arbitrary units (AU) and obtained after normalization with the housekeeping gene Gapdh. Black horizontal lines represent the median values. (E) IL-18 was measured by ELISA in sera from 5-/7-month-old and 15-/17-month-old WT or Ctns−/− mice. Black horizontal lines represent the median values. *P<0.05; **P<0.01.
In this study, we have shown that phagocytized cystine crystals can elicit an inflammatory reaction by triggering IL-1β secretion by inflammatory cells, demonstrating that they represent an endogenous danger signal sensed by inflammasomes. Intralysosomal accumulation of cystine in tissues is the hallmark of cystinosis, but the exact role played by these crystals in the pathogenesis of the disease is still a matter of debate. Our data suggest that cystine crystals may not be inert but rather a trigger of inflammation, as recently demonstrated for calcium oxalate crystals in nephrocalcinosis.17
Elevated circulating levels of IL-1β and IL-18 in cystinotic patients and elevated inflammasome-related gene expression both in PBMCs from patients and in kidney tissue from Ctns−/− mice support the hypothesis that inflammasome activation may contribute to the development of the interstitial inflammation and fibrosis leading to ESRD observed in cystinotic patients and mice. Interestingly, the inflammasome-regulated cytokines IL-1β and IL-18 are implicated in several animal models or human forms of CKD.18–20
In this study, we used LPS to prime the inflammasome because the nature of the immunostimulatory priming signals in cystinosis are unknown. Indeed, on the basis of the evidence that pattern recognition receptors can also be activated by host-derived nonmicrobial stimuli (i.e., sterile inflammation),21 we hypothesize that, in vivo, the priming of the inflammasome can be provided by endogenous molecules that are released during cellular injury (especially in kidneys) and inflammasome activation is triggered by cystine crystals.
Which cells express functional inflammasomes in the kidney is largely unknown. Several studies demonstrated that proximal tubular epithelial cells (PTECs) and mesangial cells are involved in the release of chemokines, such as monocyte chemotactic protein-1, and cytokines, such as IL-6, TNF, and IL-1β22; hence, they should express all necessary components of the inflammasome caspase-1/IL-1β/IL-18 axis.
On the basis of this evidence and on our results, we propose a speculative model in which lysosomal cystine crystal accumulation, acting as a “stress signal,” induces lysosomal leakage, mitochondrial oxidation stress, and possibly inflammasome activation in PTECs. In turn, PTECs produce higher amounts of proinflammatory cytokines and chemokines, which subsequently promote infiltration of inflammatory cells. Eventually, extracellular minute cystine crystals and intracellular cystine crystals trigger activation of inflammasome also in infiltrating inflammatory cells (macrophages and dendritic cells). Notably, cystine crystals were observed in interstitial cells and macrophages of most organs, particularly in kidneys, of Ctns−/− mice and of cystinotic individuals.16,23 Consistent with this observation, we found significant upregulation of inflammasome-related genes in kidneys of Ctns−/− mice.
Compared with other forms of Fanconi syndrome associated with low-molecular-weight protein urinary excretion, such as Lowe syndrome and Dent disease, cystinosis is characterized by a more rapid progression to ESRD. Biopsies show progressive interstitial fibrosis and development of swan-neck tubular strictures, which are believed to be due to early apoptotic events of tubular cells in the S1 segment. It may well be that inflammation caused by cystine crystals is responsible for these early changes; positive effects on renal function of cysteamine,24 which depletes intralysosomal cystine content, would support this hypothesis.
According to our findings, therapeutic strategies that dampen cystine crystal–triggered inflammasome activation may complement conventional therapies used in cystinosis treatment.
Concise Methods
Preparation of l-Cystine Crystals
The hexagonal form was crystallized from a supersaturated solution prepared by adding 70 mg of l-cystine to 100 ml of deionized water and heating under reflux at 100°C for 20 minutes with stirring, as described by Rimmer et al.25 The crystals were collected and evaporated to dryness using a vacuum concentrator, autoclaved, and stored at −20°C until use.
Human Cell Culture
PBMCs were obtained from buffy coats of healthy donors after centrifugation over Ficoll-Hystopaque (Pharmacia, Uppsala, Sweden) gradients as described. Freshly isolated PBMCs were suspended in RPMI 1640 (Gibco) supplemented with 10% human serum, seeded in 96-well plates at a density of 5.0×105 cells/well and incubated overnight at 37°C. The next day, PBMCs were stimulated with 100 ng/ml LPS (Escherichia coli serotype 055:B5; Sigma-Aldrich) for 2 hours, followed by incubation with cystine crystals or MSU (Alexis Biochemicals, San Diego, CA) for 6 hours. In some experiments, cells were preincubated 30 minutes before crystal addition with the caspase-1 inhibitor z-YVAD-fmk (Alexis Biochemicals), cytochalasin D (Sigma-Aldrich), cathepsin B inhibitor CA-074Me (Calbiochem), diphenyleneiodonium chloride (ENZO Life Sciences), or 130 mM KCl.
Confocal Reflection Microscopy
PBMCs were incubated overnight at 37°C. The next day, nonadherent cells were removed and mononuclear adherent cells were incubated with 100 ng/ml LPS for 2 hours, followed by incubation with cystine crystals for 6 hours. At the end of incubation, cells were fixed for 20 minutes in 4% paraformaldehyde in PBS and immunostained for wheat germ agglutinin. The nuclei were stained with Hoechst stain. Confocal images were acquired using the Olympus Fluoview FV1000 confocal microscope equipped with FV10-ASW version 2.0 software.
ELISA and Western Blotting
After stimulation, cell culture media were collected and human IL-1β was measured using commercial ELISA kits (R&D Systems, Minneapolis, MN). The assays were performed according to the manufacturer’s instructions. The detection limit of the assay was 7.8 pg/ml.
Human and mouse circulating IL-1β and IL-18 levels were measured using human IL-1β/IL-1F2 Quantikine HS ELISA Kit (R&D Systems) and human or mouse IL-18 ELISA kit (Medical and Biologic Laboratories, Nagoya, Japan), according to the manufacturer’s instructions. The detection limits for these assays were 0.023 pg/ml, 25 pg/ml, and 25.6 pg/ml, respectively.
For Western blotting, cell culture supernatant proteins were resolved by 15% SDS-PAGE. Proteins were then transferred to nitrocellulose membranes (Amersham Life Sciences, Little Chalfont, UK), and probed with polyclonal rabbit antibody to human IL-1β and caspase-1 (Cell Signaling Technology, Beverly, MA) using standard procedures.
RNA Isolation and Quantitative Real-Time PCR
Total RNA was extracted from 1×106 PBMCs or mouse kidney tissues using Qiagen-RNeasy Mini kit (Qiagen, Valencia, CA), and cDNA was obtained using the Superscript Vilo kit (Invitrogen). Real-time PCR assays were performed using TaqMan Universal PCR Master mix (Applied Biosystems) and the following gene expression assays: human IL-1β, CASP-1, NLRP3, PYCARD (ASC), TNF-α (Applied Biosystem) and mouse Casp1, Pycard (Asc) and Il18. Gene expression data were normalized using human or mouse GAPDH (Applied Biosystems) as endogenous controls. Data are expressed as arbitrary units (AU), determined using the 2−Δct method.
Intracellular Cystine Measurement
HPLC with fluorescence detection assay on polymorphonuclear leukocytes isolated from cystinotic patients has been performed.26
Gene Expression Microarray Analysis
Animals were housed and studied according to the National Institutes of Health’s Guidelines for the Care and Use of Laboratory Animals. Sixteen-month-old C57BL/6 WT (n=6) and Ctns−/− (n=6) mice16 were euthanized, and their liver, muscle, spleen, and kidney immediately removed and stored at −80°C in RNA Later (Life Technologies, Carlsbad, CA). Tissues were subsequently ground using Precellys 24 (Bertin Technologies, Rockville, MD), and RNA was isolated using the Qiagen AllPrep DNA/RNA Mini kit (Qiagen). The RNA was run on a Bioanalyzer (Agilent Technologies, Santa Clara, CA) for quantification and quality assessment. The Ambion WT Expression Kit was used to generate double-stranded biotinylated cDNA, and the Affymetrix HT WT Terminal Labeling Kit was used to prepare the cDNA for hybridization to Affymetrix GeneChip Mouse Gene 1.1 ST arrays (Affymetrix, Santa Clara, CA). The double-stranded biotinylated cDNA from each tissue for each mouse was run on individual Affymetrix GeneChip. The data were collected as CEL files, and quality control analysis was performed with Affymetrix Expression Console. Normalized signal intensities were generated with Robust Multichip Average, which uses a background adjustment and quantile normalization strategy.27 Genes without an average log2-transformed signal above 6 in the WT or Ctns−/−group were removed from further analysis. Class comparisons of variance by two-way t tests for two-sample comparisons (P<0.001) were performed using BRB-ArrayTools (http://linus.nci.nih.gov/BRB-ArrayTools.html) to identify the set of significantly differentially expressed genes between WT and Ctns−/− mice in each tissue.
Cystinotic Patients
After signing an informed consent form, 14 patients were included in this study. Patients were aged 5–28 years. Eight patients were pretransplant (median age, 7.5 years [IQR, 6–11 years]) and 6 had undergone transplantation (median age, 28.0 years [IQR, 20.5–28 years]). Cystinosis had been diagnosed between ages 9 months and 4 years; in all patients the diagnosis was based on the demonstration of elevated cystine levels in leukocytes and on the demonstration of homozygous or compound heterozygous mutations of the CTNS gene. All patients were treated with cysteamine bitartrate. At the time of blood sample collection, no patient had evidence of infection or inflammation, and none had increased C-reactive protein. Similarly, all the patients with FMF (n=23; median age, 10.0 years [IQR, 9–13 years]) were in a symptom-free period and were receiving colchicine treatment at the time of blood collection. Patients hospitalized for planned minor surgical procedures with an age similar to that of pretransplant cystinotic patients (n=27; median age, 9.0 years [IQR, 6–13 years]; P>0.5) were used as controls. Blood samples were collected before surgery. The patients did not have evidence of infection or inflammation or increased C-reactive protein at the time of sample collection. All blood samples were taken after patients provided informed consent. Institutional Ethical Committee approved the study.
Statistical Analyses
Data are presented as means±SDs or as median and IQRs. We performed group comparisons using the nonparametric Mann-Whitney U test. To assess the correlations between variables, we calculated the Spearman rank correlation coefficient (r). All statistical analyses were performed using GraphPad Prism IV software. P<0.05 was considered to represent statistically significant differences.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Corinne Antignac for kindly providing the Ctns−/− mouse model. We thank Elena Levtchenko for critical reading of the manuscript.
S.C. and F.E. were supported by the Cystinosis Research Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Proximal Tubule in Cystinosis: Fight or Flight?,” on pages 1131–1132.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013060653/-/DCSupplemental.
References
- 1.Gahl WA, Thoene JG, Schneider JA: Cystinosis. N Engl J Med 347: 111–121, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Wilmer MJ, Emma F, Levtchenko EN: The pathogenesis of cystinosis: Mechanisms beyond cystine accumulation. Am J Physiol Renal Physiol 299: F905–F916, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Cassel SL, Joly S, Sutterwala FS: The NLRP3 inflammasome: A sensor of immune danger signals. Semin Immunol 21: 194–198, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strowig T, Henao-Mejia J, Elinav E, Flavell R: Inflammasomes in health and disease. Nature 481: 278–286, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Schroder K, Tschopp J: The inflammasomes. Cell 140: 821–832, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Leemans JC, Cassel SL, Sutterwala FS: Sensing damage by the NLRP3 inflammasome. Immunol Rev 243: 152–162, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E: Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9: 847–856, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin C, Frayssinet P, Pelker R, Cwirka D, Hu B, Vignery A, Eisenbarth SC, Flavell RA: NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc Natl Acad Sci U S A 108: 14867–14872, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J: Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Pazár B, Ea HK, Narayan S, Kolly L, Bagnoud N, Chobaz V, Roger T, Lioté F, So A, Busso N: Basic calcium phosphate crystals induce monocyte/macrophage IL-1β secretion through the NLRP3 inflammasome in vitro. J Immunol 186: 2495–2502, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E: NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464: 1357–1361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J: Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320: 674–677, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathinam VA, Vanaja SK, Fitzgerald KA: Regulation of inflammasome signaling. Nat Immunol 13: 333–332, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tschopp J, Schroder K: NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 10: 210–215, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Savic S, Dickie LJ, Battellino M, McDermott MF: Familial Mediterranean fever and related periodic fever syndromes/autoinflammatory diseases. Curr Opin Rheumatol 24: 103–112, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Cherqui S, Sevin C, Hamard G, Kalatzis V, Sich M, Pequignot MO, Gogat K, Abitbol M, Broyer M, Gubler MC, Antignac C: Intralysosomal cystine accumulation in mice lacking cystinosin, the protein defective in cystinosis. Mol Cell Biol 22: 7622–7632, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulay SR, Kulkarni OP, Rupanagudi KV, Migliorini A, Darisipudi MN, Vilaysane A, Muruve D, Shi Y, Munro F, Liapis H, Anders HJ: Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J Clin Invest 123: 236–246, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitching AR, Turner AL, Wilson GR, Semple T, Odobasic D, Timoshanko JR, O’Sullivan KM, Tipping PG, Takeda K, Akira S, Holdsworth SR: IL-12p40 and IL-18 in crescentic glomerulonephritis: IL-12p40 is the key Th1-defining cytokine chain, whereas IL-18 promotes local inflammation and leukocyte recruitment. J Am Soc Nephrol 16: 2023–2033, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Sugiyama M, Kinoshita K, Kishimoto K, Shimazu H, Nozaki Y, Ikoma S, Funauchi M: Deletion of IL-18 receptor ameliorates renal injury in bovine serum albumin-induced glomerulonephritis. Clin Immunol 128: 103–108, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Wu H, Craft ML, Wang P, Wyburn KR, Chen G, Ma J, Hambly B, Chadban SJ: IL-18 contributes to renal damage after ischemia-reperfusion. J Am Soc Nephrol 19: 2331–2341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen GY, Nuñez G: Sterile inflammation: Sensing and reacting to damage. Nat Rev Immunol 10: 826–837, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gould SE, Day M, Jones SS, Dorai H: BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int 61: 51–60, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Broyer M, Guillot M, Gubler MC, Habib R: Infantile cystinosis: A reappraisal of early and late symptoms. Adv Nephrol Necker Hosp 10: 137–166, 1981 [PubMed] [Google Scholar]
- 24.Markello TC, Bernardini IM, Gahl WA: Improved renal function in children with cystinosis treated with cysteamine. N Engl J Med 328: 1157–1162, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Rimer JD, An Z, Zhu Z, Lee MH, Goldfarb DS, Wesson JA, Ward MD: Crystal growth inhibitors for the prevention of L-cystine kidney stones through molecular design. Science 330: 337–341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilfix BM, Blank DW, Rosenblatt DS: Novel reductant for determination of total plasma homocysteine. Clin Chem 43: 687–688, 1997 [PubMed] [Google Scholar]
- 27.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP: Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.